Abstract
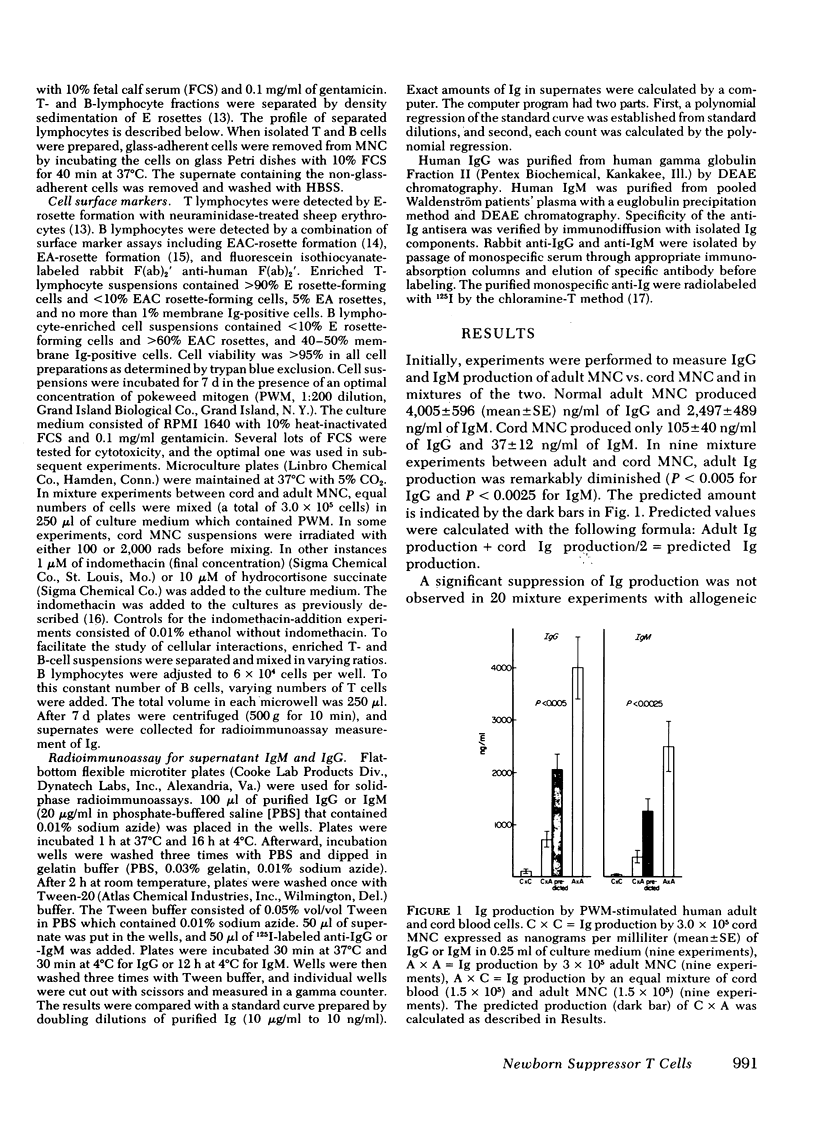
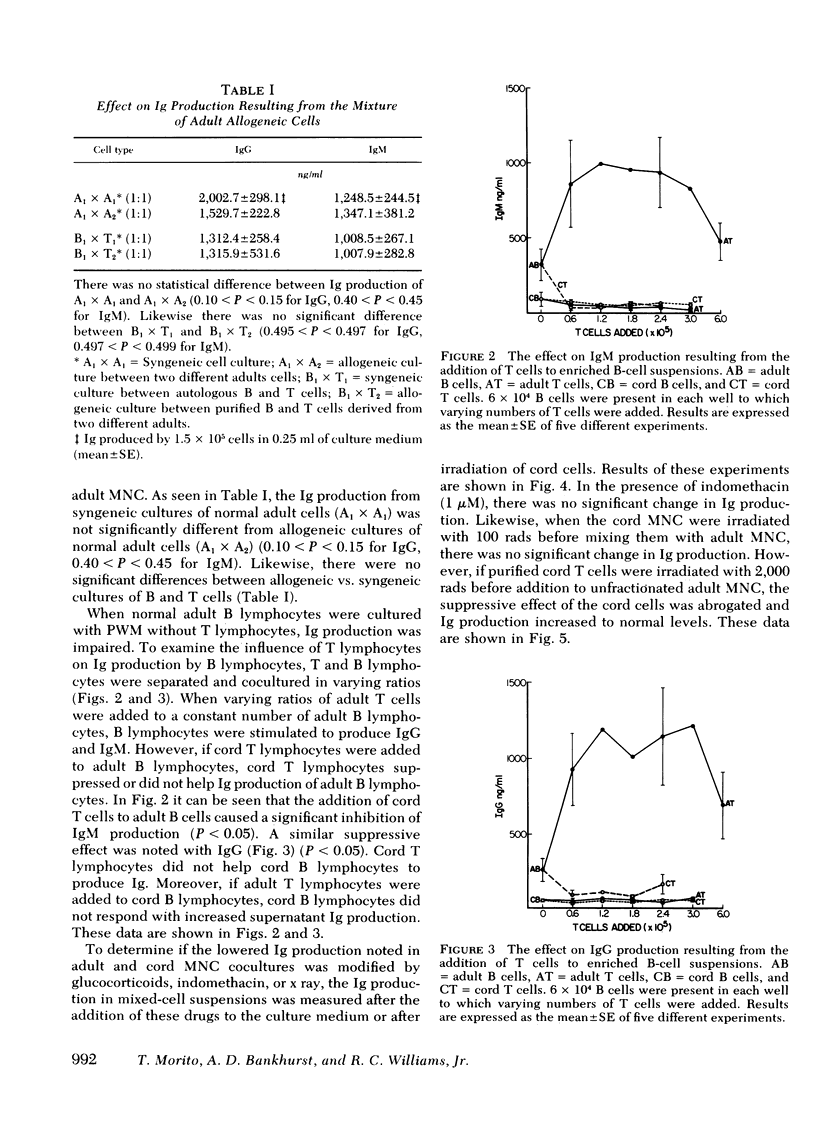
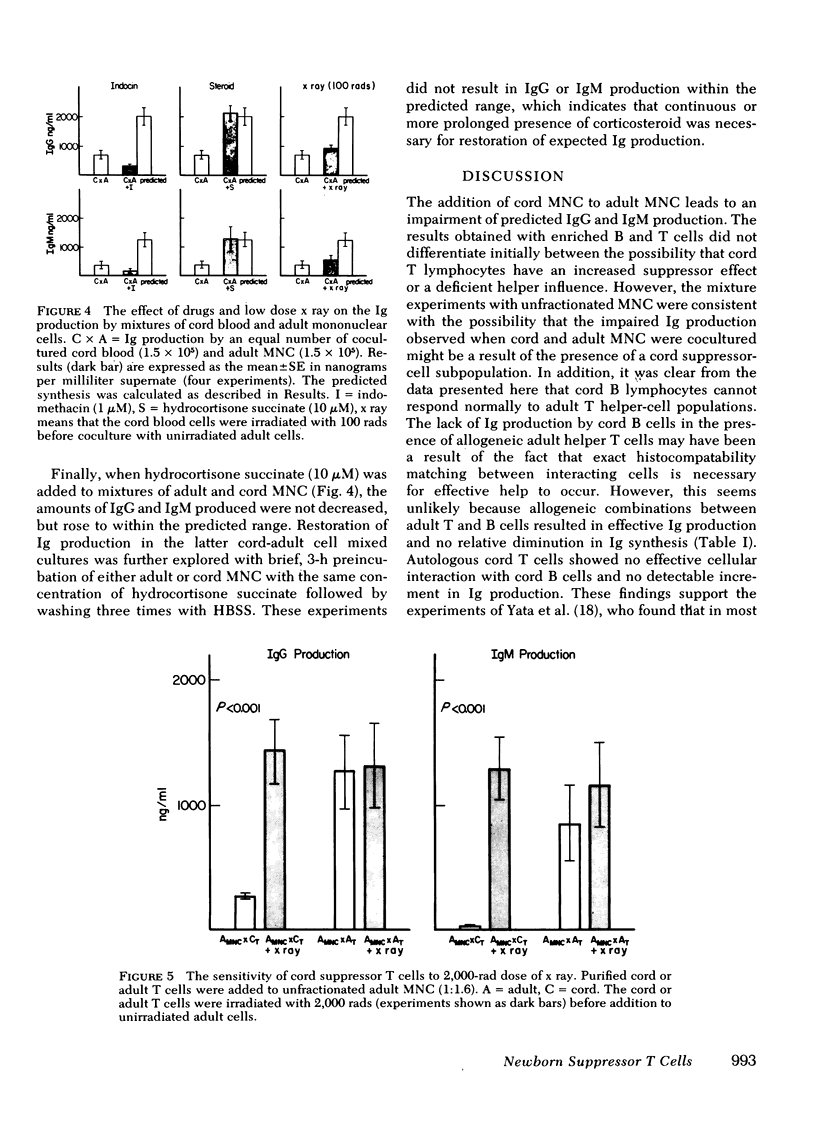
Newborns are unable to produce normal amounts of immunoglobulin despite the presence of circulating lymphocytes with surface immunoglobin (Ig). This study was designed to examine the cellular basis of such impaired Ig synthesis in the newborn infant. An in vitro assay for IgG and IgM synthesis was employed which measured the Ig present in the supernates of pokeweed mitogen-stimulated cord blood and/or adult peripheral mononuclear cells (MNC). Results were as follows: (a) the addition of cord blood MNC to adult MNC suppressed both normal IgG and IgM production; (b) addition of a suspension of adult thymus-derived (T) cells to cord bone marrow-derived (B) cells did not enhance the production of Ig; (c) the addition of cord T cells to adult B cells did not enhance normal Ig production but did significantly depress IgM and IgG synthesis; and (d) irradiation of cord T cells with 2,000 rads removed the suppressive effect of cord T cells on adult MNC. A similar reversal of the suppressive effect exerted by cord MNC was also seen in the presence of 10 microM of hydrocortisone. It appears that the inability of newborn infants to make normal amounts of Ig is a result of a combined B-cell defect and the presence of a steroid-sensitive and radiosensitive suppressor cord T cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankhurst A. D., Hastain E., Husby G., Diaz-Jouanen E., Williams R. C., Jr Human lymphocyte subpopulations defined by double surface markers. J Lab Clin Med. 1978 Jan;91(1):15–23. [PubMed] [Google Scholar]
- Buckley R. H., Schiff R. I., Amos D. B. Blocking of autologous and homologous leukocyte responses by human alloimmune plasmas: a possible in vitro correlate of enhancement. J Immunol. 1972 Jan;108(1):34–44. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Galili U., Schlesinger M. The formation of stable E rosettes after neuraminidase treatment of either human peripheral blood lymphocytes or of sheep red blood cells. J Immunol. 1974 May;112(5):1628–1634. [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison M. R. Maternal immunocompetence. II. Proliferative responses of maternal lymphocytes in vitro and inhibition by serum from pregnant rats. Scand J Immunol. 1976;5(8):881–889. doi: 10.1111/j.1365-3083.1976.tb03038.x. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lydyard P. M. Suppression of B lymphocyte differentiation by newborn T lymphocytes with an Fc receptor for IgM. Clin Exp Immunol. 1978 Dec;34(3):374–378. [PMC free article] [PubMed] [Google Scholar]
- Kasakura S. A factor in maternal plasma during pregnancy that suppresses the reactivity of mixed leukocyte cultures. J Immunol. 1971 Nov;107(5):1296–1301. [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Lawler S. D., Ukaejiofo E. O., Reeves B. R. Interaction of maternal and neonatal cells in mixed-lymphocyte cultures. Lancet. 1975 Dec 13;2(7946):1185–1187. doi: 10.1016/s0140-6736(75)92663-x. [DOI] [PubMed] [Google Scholar]
- Nakai H., Morito T., Tanimoto K., Horiuchi Y. Reduced Fc-receptor bearing cells in peripheral bloods of patients with systemic lupus erythematosus and in rheumatoid synovial fluids. J Rheumatol. 1977 Winter;4(4):405–413. [PubMed] [Google Scholar]
- Olding L. B., Benirschke K., Oldstone M. B. Inhibition of mitosis of lymphocytes from human adults by lymphocytes from human newborns. Clin Immunol Immunopathol. 1974 Sep;3(1):79–89. doi: 10.1016/0090-1229(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Olding L. B., Oldstone B. A. Thymus-derived peripheral lymphocytes from human newborns inhibit division of their mothers' lymphocytes. J Immunol. 1976 Mar;116(3):682–686. [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Moretta L. Active thymus derived suppressor lymphocytes in human cord blood. Nature. 1977 Sep 22;269(5626):333–335. doi: 10.1038/269333a0. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Zuckerman J. E., Alpert E., David J. R. Effect of multiparity on human maternal hypersensitivity to foetal antigen. Nature. 1973 Jan 12;241(5385):130–131. doi: 10.1038/241130a0. [DOI] [PubMed] [Google Scholar]
- Siegal F. P., Siegal M., Good R. A. Role of helper, suppressor and B-cell defects in the pathogenesis of the hypogammaglobulinemias. N Engl J Med. 1978 Jul 27;299(4):172–178. doi: 10.1056/NEJM197807272990404. [DOI] [PubMed] [Google Scholar]


