Abstract
Self-control plays an important role in healthy development and has been shown to be amenable to intervention. This article presents a theoretical framework for the emerging area of “brain-training” interventions that includes both laboratory-based direct training methods and ecologically valid school-, family-, and community-based interventions. Although these approaches have proliferated in recent years, evidence supporting them is just beginning to emerge, and conceptual models underlying many of the techniques they employ tend to be underspecified and imprecise. Identifying the neural systems responsible for improvements in self-control may be of tremendous benefit not only for overall intervention efficacy but also for basic science issues related to underlying shared biological mechanisms of psychopathology. This article reviews the neurodevelopment of self-control and explores its implications for theory, intervention, and prevention. It then presents a neurally informed framework for understanding self-control development and change and discusses how this framework may inform future intervention strategies for individuals suffering with psychopathology or drug abuse/dependence, or for young children with delays in cognitive or emotional functioning.
Keywords: self-control, executive function, training, transfer, inferior frontal gyrus
Self-control involves the ability to prevent or override unwanted thoughts, behaviors, and emotions (Muraven, Baumeister & Tice, 1999) and is integral to successful navigation of daily life. Here, we describe a theoretical framework for understanding how self-control develops over time and may be altered with training and discuss the implications of this framework for interventions with children and adolescents. We begin by briefly outlining the protracted developmental course of self-control and its associated vulnerability to environmental influences, as well as its potential for improvement through targeted interventions. We then discuss a model of domain-general self-control, which incorporates a neurobiological perspective and contributes to understanding the potential for training-induced improvements in self-control during development. For the purpose of the present review, we focus on inhibitory control—defined as the ability to override a dominant response in order to enact a subdominant response (Kochanska, Murray, & Harlan, 2000; Rothbart & Posner, 1985)—as an important subcategory of self-control. We also reference executive functioning (EF) as a broader domain encompassing working memory, attention, and self-control (Liew, in press; Miller & Cohen, 2001).1
A key reason to consider inhibitory control as a target of childhood interventions is that it develops gradually over time, with behavioral studies documenting improvements in inhibitory control beginning in early childhood (Dennis, Brotman, Huang, & Gouley, 2007; McClelland et al., 2007; Moilanen, Shaw, Dishion, Gardner, & Wilson, 2010) and continuing through late adolescence (Leon-Carrion, García-Orza, & Pérez-Santamaría, 2004) and even into early adulthood (Bedard et al., 2002; Carver, Livesey, & Charles, 2001; Williams, Ponesse, Schachar, Logan, & Tannock, 1999). Rudimentary forms of inhibitory control are observed as early as the second half of the 1st year of life, when the capacity to inhibit a rewarding action in response to a caregiver's command begins to emerge (Kochanska, Coy, & Murray, 2001; Kochanska, Coy, Tjebkes, & Husarek, 1998). Qualitative shifts and quantitative gains in performance on tasks involving inhibitory control have been observed over the preschool years (from 3 to 4 years of age; Jones, Rothbart, & Posner, 2003; Zelazo, Reznick, & Piñon, 1995) and around the time of transition to kindergarten (from 4 to 5 years of age; Bell & Livesey, 1985; Livesey & Morgan, 1991). Beyond these early developmental shifts, inhibitory control continues to develop between the ages of 6 and approximately 12 years of age (Carver et al., 2001; Williams et al., 1999) and throughout adolescence (Leon-Carrion et al., 2004; Levin et al., 1991) and into early adulthood (Carver et al., 2001; Williams et al., 1999). Improvements over this protracted time period include increases in speed of reaction when inhibiting a response (Bedard et al., 2002; Williams et al., 1999) and decreases in errors when cued to inhibit a prepotent response (Bedard et al., 2002; Carver et al., 2001).
This slow developmental time course for inhibitory control, and for self-control more generally, is attributed to the protracted course of development of underlying neural regions. Although multiple neural regions are involved in self-control, including subcortical regions such as the subthalamic nucleus (STN; Munakata et al., 2011) and regions of the prefrontal cortex (PFC) appears to play a critical role (Godefroy, Lhullier, & Rousseaux, 1996; Robinson, Heaton, Lehman, & Stilson, 1980), particularly the ventrolateral prefrontal cortex (VLPFC; Bunge & Zelazo, 2006) and the presupplementary motor area (preSMA; Munakata et al., 2011). The involvement of these regions in self-control is consistent with the behavioral findings of a protracted developmental course because the PFC is one of the last brain regions to reach maturity: Key developmental processes within it, such as pruning of synapses and myelination, begin prenatally and continue into early adulthood (Bourgeois, Goldman-Rakic, & Rakic, 1994; Chugani, Phelps, & Mazziota, 1987; Diamond, 2002; Gogtay et al., 2004; Huttenlocher & Dabholkar, 1997; Sowell et al., 2004).
In line with this extended period of structural development, research also indicates that functional patterns of brain activity associated with inhibitory control change over the course of development. This literature shows that maturation involves both increasing recruitment of certain prefrontal regions (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Luna et al., 2001; Rubia et al., 2000; Tamm, Menon, & Reiss, 2002) and decreasing prefrontal activation, presumably reflecting increasing specialization in the functioning of prefrontal regions with age (Booth et al., 2003; Casey et al., 1997; Durston et al., 2002). In line with the idea of increasing specialization, developmental increases or decreases in activation during inhibitory-control tasks depend on the specific region of interest and the nature of the task (Luna et al., 2001; Rubia et al., 2000; Tamm et al., 2002; Velanova, Wheeler, & Luna, 2008). For example, in one key region within the VLPFC—the right inferior frontal cortex—adults, compared to children, demonstrate greater levels of activation during successful response inhibition when the overall performance is equated between the two groups (Rubia, Smith, Taylor, & Brammer, 2007). Taken together, the structural and functional MRI literature supports a long developmental time course for inhibitory control, with potential age-related increases in the activation of specific prefrontal regions associated with inhibitory control when the performance is equated.
The protracted course of development for inhibitory control, along with other forms of self-control, indicates the potential for high levels of plasticity and substantial influence on these systems by the early environment. This susceptibility to environmental impact has both positive and negative implications. For example, early-life adversity has repeatedly been shown to predict poor inhibitory control in children (Beers & De Bellis, 2002; Lengua, Honorado, & Bush, 2007; Lewis, Dozier, Ackerman, & Sepulveda-Kozakowski, 2007; Valiente, Lemery-Chalfant, & Reiser, 2007). Poor inhibitory control is, in turn, associated with downstream maladaptive outcomes. For example, a recent study demonstrated that the association between the history of maltreatment and academic functioning for children in the foster care system was fully mediated by inhibitory control (Pears, Fisher, Bruce, Kim, & Yoerger, 2010). Problems with inhibitory control also appear to be central to a range of childhood mental health disorders, leading researchers to hypothesize that disinhibition is an underlying component of many forms of psychopathology (Nigg, 2000; Schachar & Logan, 1990; Young et al., 2009).
In addition to imparting vulnerability, the protracted development of inhibitory control suggests the positive potential for intervention during periods of plasticity associated with ongoing development. A recent review of “brain training” interventions for children focused on the broader domain of EF, including several studies on inhibitory control (Bryck & Fisher, 2012). This review categorized EF interventions into two primary categories: (a) laboratory-based training focused on specific cognitive processes involved in EF and (b) ecologically valid, contextually based training informed by neurobiological models of EF. Laboratory-based strategies included interventions targeting attention, working memory, or fluid reasoning in controlled settings (e.g., Karbach & Kray, 2009; Mackey, Hill, Stone, & Bunge, 2011; Rueda, Rothbart, McCandliss, Saccomanno, & Posner, 2005; Stevens, Fanning, Coch, Sanders, & Neville, 2008; Thorell, Lindqvist, Nutley, Bohlin, & Klingberg, 2009).
The ecological approach to brain training described by Bryck and Fisher (2012) targeted aspects of EF, such as working memory and effortful control, within school, family, or community settings. Effective programs have targeted EF in school settings and at home by teaching techniques for regulating emotions and behaviors (Diamond, Barnett, Thomas, & Munro, 2007; Raver et al., 2009) and using activities that promote attention and memory (Diamond et al., 2007). Program results include gains in EF domains, such as inhibitory control, and in downstream outcomes, such as academic performance (Diamond et al., 2007; Tominey & McClelland, 2011) and socioemotional functioning (Raver et al., 2009). Another recent review found that the largest effect sizes occurred in EF interventions that featured challenging, prolonged, and increasingly demanding training tasks (Diamond & Lee, 2011). The fact that only one ecologically based intervention (Bruce, McDermott, Fisher, & Fox, 2009) has been shown to have an effect at a neural level underscores the need for specific models explicating the positive impacts of these interventions on EF, and particularly on its underlying neural systems.
Although interventions produce significant gains in the targeted domains, evidence for transfer or generalization of gains across domains and settings at present is limited (Diamond & Lee, 2011). Some exceptions include training programs for working memory that demonstrate associated reduction in ADHD symptomatology (Beck, Hanson, Puffenberger, Benninger, & Benninger, 2010; Klingberg et al., 2005) or improved mathematical ability (Holmes, Gathercole, & Dunning, 2009). One theoretical model predicts a bidirectional relation between EF and emotion regulation but is not yet supported by direct evidence (Ursache et al., in press). A key problem is the lack of precise conceptual models (rooted in neuroscience) for transfer of gains across domains and beyond the laboratory setting (Bryck & Fisher, 2012). This is problematic because difficult-to-treat clinical populations, such as multiproblem youths, are rarely characterized by isolated deficits in a single domain of neuropsychological functioning (Iacono, Malone, & McGue, 2008). This fact suggests that there may be an underlying pathway that links symptoms across domains and that this pathway may be amenable to domain-general improvement through an intervention that specifically targets it.
Future work in this area will benefit from conceptual models that specify how interventions on inhibitory control, self-control, or EF more broadly may generalize to functional improvements across settings. Here, we argue that the strength model (Baumeister & Heatherton, 1996) represents one component of such a theoretical framework (reviewed below), with the other component being recent advances in cognitive and affective neuroscience that have begun to identify a final common pathway for inhibitory-control “strength” at a neural level. Testing this combined social-neurocognitive model of inhibitory control has the potential to advance intervention science in several ways. First, because effective ecological interventions typically involve multiple components (e.g., cognitive, emotional, and behavioral tasks), understanding the mechanisms of change at the neural level may allow for efficient intervention refinement. Having a specific neural model for the effects of an intervention would provide an unambiguous way to identify which components of the intervention do or do not affect the underlying pathway. Second, increasingly limited financial and personnel resources in many contexts necessitate effective, efficient interventions with high likelihood of improving functioning beyond a laboratory setting. In order to test interventions in a manner likely to replicate, intervention design and expected outcomes should be driven by a theoretical model that specifies which behaviors will be affected and the neural mechanisms underlying the behavior change. Understanding these mechanisms has implications both for evaluation of outcomes and for identification of which individuals may benefit from an intervention.
THE STRENGTH MODEL: SELF-CONTROL AS A LIMITED RESOURCE THAT IS SHARED ACROSS BEHAVIORAL, EMOTIONAL, AND COGNITIVE DOMAINS
One source of support for the proposed conceptual framework on the domain generality of self-control (see Figure 1) is derived from theory and research in the social psychology literature on the strength model. In this model, self-control is considered a unitary resource that can be exerted in one of several response domains, including cognitive, affective, and behavioral domains. For example, the ability to focus one's thoughts on the task at hand instead of daydreaming (cognitive), the ability to control one's anger at a demeaning superior at work (affective), and the ability to override a prepotent motor response such as stopping at a green light for a jaywalking pedestrian (behavioral) are conceptualized as being drawn from a shared “domain general” self-control resource.
Figure 1.
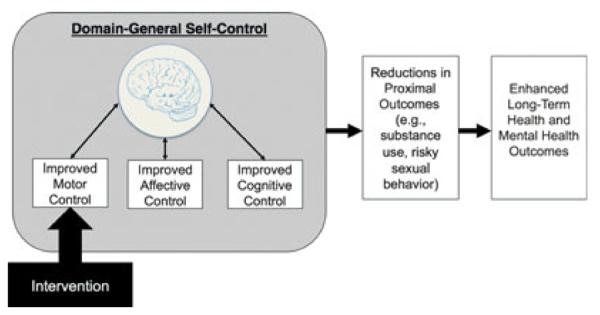
A neurally informed model of domain-general inhibitory control and how it can be applied to intervention.
Note. A set of brain regions (including inferior frontal gyrus, presupplementary motor area, subthalamic nucleus, and basal ganglia) are involved in inhibitory control in the behavioral, affective, and cognitive domains. Successful intervention to modulate one domain will transfer to the others, influencing proximal outcomes related to inhibitory control and, eventually, long-term physical and mental health outcomes.
Baumeister and Muraven proposed that self-control is a domain-general resource that, like a muscle, becomes depleted with exertion (Muraven & Baumeister, 2000). Research supporting the strength model shows that a self-control attempt is more likely to fail when it is preceded by another task that requires exertion of self-control (Muraven et al., 1999). The reduction in subsequent task performance as a result of prior self-control exertion (which is typically interpreted as reflecting a reduction in self-control capacity) was found in a recent meta-analysis of 198 independent studies to have a medium to large effect size of approximately .6 (Hagger, Wood, Stiff, & Chatzisarantis, 2010).
This meta-analysis also found evidence that self-control is domain general. Many studies are explicitly designed to test cross-domain transfer (of depletion) by measuring whether exertions of self-control in one domain result in poorer subsequent performance in other, seemingly unrelated, domains. For example, subjects assigned to regulate their emotions during a distressing video clip (affective self-control) performed more poorly on a subsequent task of physical stamina (behavioral self-control) than did subjects who watched the same video clip but were not instructed to regulate their emotions (Muraven, Tice, & Baumeister, 1998). This type of finding has been replicated at least 130 times (Hagger et al., 2010). In addition to this experimental evidence, correlational data have also supported the notion that self-control is shared across domains in children as young as 3 years old (Wiebe et al., 2011) and throughout early childhood (Willoughby, Wirth, & Blair, in press), and that trait self-control in adults is associated with enhanced functioning in academic, interpersonal, socioemotional, and health domains (de Ridder, Lensvelt-Mulders, Finkenauer, Stok, & Baumeister, 2012).
In addition to positing that self-control is domain general and becomes depleted following exertion, the strength model suggests that self-control may improve with repeated practice over time (i.e., training). Several studies have found evidence that training improves self-control in novel tasks within the same training domain. For example, longitudinal studies show that subjects who practice behavioral self-control for 2–3 weeks (e.g., by monitoring and improving their posture or using their nondominant hand for daily activities) exhibit improvement in other tasks requiring behavioral self-control (e.g., increasing endurance time on squeezing a handgrip; Gailliot, Plant, Butz, & Baumeister, 2007; Muraven, 2010; Muraven et al., 1999).
Most relevant to the present argument, emerging evidence indicates that self-control improvements gained within one domain may transfer to another. For example, one recent study found that subjects who trained for 2 weeks on either behavioral self-control (e.g., using their nondominant hand for everyday tasks such as brushing their teeth, opening doors, and using scissors) or verbal self-control (e.g., not using linguistic colloquialisms, speaking only in complete sentences, and avoiding slang) demonstrated improvement in the affective domain of impulse control relative to participants who received a control training (Finkel, DeWall, Slotter, Oaten, & Foshee, 2009). A second study found that subjects who practiced both cognitive and behavioral self-regulation tasks (a 5-min Stroop task and an antiseptic mouthwash rinse) twice per day for 2 weeks showed improvement in their tolerance for pain on a cold pressor task (Hui et al., 2009).
These studies demonstrate that self-control training can generalize beyond the limited scope of the target task to other response domains. However, they are limited in two key ways. First, and perhaps most importantly, they lack a mechanistic account, grounded in neurophysiology, of how training in one domain might generalize to others. Although there is some evidence that glucose is the biological energy source that becomes depleted following self-control exertion (Gailliot & Baumeister, 2007; Gailliot, Baumeister, et al., 2007), it is unclear whether ingested glucose enters the brain in the time frame suggested by these studies or whether a single act of self-control actually depletes blood glucose in any meaningful way (Beedie & Lane, 2012; Kurzban, 2010). A deeper problem with the glucose hypothesis, even if it is correct, is that it does not provide specificity regarding which brain structures actually consume the glucose (if any), whether those structures are amenable to intervention, and if so, whether intervention gains would transfer across domains. Second, the ability of the studies reviewed above to identify a mechanism for cross-domain transfer is limited because they rely on a multipronged training approach (e.g., both cognitive and behavioral tasks each day during the training phase) instead of a single-domain training and other-domain testing.
MECHANISMS FOR IMPROVING SELF-CONTROL ACROSS DOMAINS: INSIGHTS FROM NEUROSCIENCE AND SUPPORTING EVIDENCE
The source of support for the proposed domain-general self-control framework comes from cognitive and affective neuroscience (Figure 1). Neuroscientists have identified several brain regions critical to self-control. A number of functional neuroimaging studies (Aron, Robbins, & Poldrack, 2004; Leung & Cai, 2007) and lesion studies (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Chambers et al., 2006) implicate the right inferior frontal gyrus (rIFG) as one of the primary brain regions for behavioral response inhibition. Many of these studies also find that the dorsal anterior cingulate cortex (dACC), the anterior insula, the preSMA, and subcortical regions such as the STN and the basal ganglia are co-active with rIFG during response inhibition (Aron, Behrens, Smith, Frank, & Poldrack, 2007; Munakata et al., 2011; Wager et al., 2005). Recent studies have suggested that the preSMA and dACC are involved in the detection of potential conflict between the prepotent and desired response (Botvinick, Cohen, & Carter, 2004; Mostofsky & Simmonds, 2008; Nachev, Wydell, ONeill, Husain, & Kennard, 2007), that the rIFG plays a role in representing the mapping between the inhibition cue and stopping (Van Gaal, Ridderinkhof, Scholte, & Lamme, 2010), and that the subcortical structures are important for directly inhibiting the behavioral response (Aron, Durston, et al., 2007; Sharp et al., 2010). Thus, inhibitory control recruits an interacting network of regions, including the rIFG, the preSMA, and others (Aron, Durston, et al., 2007).
The precise roles of each of the regions involved in self-control are still under investigation. One view is that the rIFG is the final common pathway that is shared by all forms of self-control (e.g., Aron et al., 2003; Tabibnia et al., 2011). Another view is that the rIFG and the larger functional subdivision to which it belongs, the ventrolateral PFC, are broadly involved in maintaining and acting upon sets of conditional rules, and that there is a posterior–anterior gradient of increasing rule abstraction with the lateral PFC (Bunge & Zelazo, 2006). Still another is that the rIFG is not necessarily involved in direct inhibition of other regions via inhibitory neurons but instead generates de facto inhibition of undesired responses through increased excitation of desired responses (Miller & Cohen, 2001; Munakata et al., 2011). Thus, for our present purposes, we will focus on rIFG as a representative member of the self-control network while acknowledging that it is only one part of a broader inhibitory control network and that its specific role is yet to be determined. There is consensus that the rIFG is a key component in the network that ultimately inhibits behavior in the service of top-down goals, making this region an excellent candidate target for self-control training interventions.
In addition to the behavioral domain, the rIFG has been implicated in self-control in the cognitive and affective domains. Supporting its involvement in cognitive self-control, the rIFG is recruited when participants report successfully inhibiting thoughts of white bears (Mitchell et al., 2007) and when participants overcome a “belief bias” in which syllogisms that are not true because one of their premises is false must still be judged as logically valid (Goel & Dolan, 2003). Extensive studies have also found rIFG activation during affective self-control (i.e., emotion regulation). The rIFG is recruited when participants downregulate negative emotions (Kim & Hamann, 2007; Ochsner et al., 2004) or overcome emotional distractions (Dolcos & McCarthy, 2006). During displays of negative affective images, activity in rIFG has been shown to be correlated with reduced amygdala activity and diminished self-reported distress (Ochsner et al., 2004; Phan et al., 2005).
The involvement of rIFG in self-control across the affective, behavioral, and cognitive domains makes it a promising candidate to serve as a final common pathway for distinct forms of self-control (Cohen, Berkman, & Lieberman, in press). Indirect evidence that rIFG is a shared locus of self-control—and may be an appropriate target for intervention work—comes from methamphetamine abusers, who demonstrate specific deficits in self-control across motor, cognitive, and affective domains (Monterosso, Aron, Cordova, Xu, & London, 2005; Payer et al., 2008; Salo et al., 2002) and show structural differences in the rIFG in comparison with control subjects (Thompson et al., 2004).
Recent studies have begun to garner direct evidence for the view that rIFG is a shared locus of self-control across domains (Berkman, Burklund, & Lieberman, 2009; Hare, Tottenham, Davidson, Glover, & Casey, 2005; Shafritz, Collins, & Blumberg, 2006). In one such study, Tabibnia et al. (2011) found that performance levels on an affective and a nonaffective inhibitory-control task were correlated with one another and with gray matter intensity in rIFG (specifically, in pars opercularis within the rIFG). In another study, experimenters induced prepotent (i.e., habitual) responses in both the behavioral and affective domains, but only instructed participants to intentionally inhibit behavioral (i.e., motor) responses (Berkman et al., 2009). Replicating previous findings (Aron et al., 2004; Chambers et al., 2006; Leung & Cai, 2007; Wager et al., 2005), this behavioral inhibition increased activity in rIFG. When negative emotional stimuli (unrelated to the task) were displayed during motor inhibition, there was a decrease in amygdala activity relative to trials in which negative stimuli were shown without behavioral inhibition (and even relative to baseline, suggesting that the effect was not merely driven by distraction). Critically, the extent of the amygdala decrease was correlated with the behavioral inhibition-related rIFG increase. This suggests that inhibiting a behavioral response in the presence of a negative emotional stimulus can lead to unintentional “spillover” of response inhibition from the behavioral domain to the affective domain because the rIFG is a global self-control pathway, and thus establishes a potential neural mechanism for the transfer of training-related improvements from one domain of self-control to another.
NEUROLOGICALLY INFORMED INTERVENTIONS FOR CROSS-DOMAIN IMPROVEMENT IN SELF-CONTROL
The work reviewed above provides a foundation for the hypothesis that interventions that generated improvements in a single target domain of inhibitory control might alter responses in the rIFG and, via those functional and/or structural changes, also improve inhibitory control in other domains (see Figure 1). Neuroscience studies have identified several tasks that reliably elicit activation in the rIFG that might be used as part of an intervention aimed at improving global inhibitory-control resources. For example, the stop-signal task (SST) involves (a) developing a prepotent “go” response to rapidly press a button and (b) occasionally (on about 25% of trials) inhibiting the behavioral “go” response when cued by an auditory “stop” signal (Verbruggen & Logan, 2008). The stop-signal response time (SSRT) derived during this task is an estimate of the amount of time needed to produce successful stopping on 50% of the trials, with smaller SSRTs indicating more efficient behavioral response inhibition. Performance on this task has been shown to improve following practice on self-control tasks in other domains (Muraven, 2010) and to recruit activation in the rIFG and other regions involved in inhibitory control (Swick, Ashley, & Turken, 2011), and the task has been adapted for use with children as young as 4 years old (Thorell et al., 2009). Work is currently underway in our laboratory to directly test whether training on a modified version of the SST improves inhibitory control in other domains via changes in rIFG (Morales et al., 2012).
This neurobiologically informed model of inhibitory control for cross-domain training can be leveraged to inform intervention with young children. As noted previously, inhibitory control represents one core, underlying feature of many mental health disorders in childhood (Nigg, 2000; Schachar & Logan, 1990) and appears to mediate between early adversity and maladjustment (Lewis et al., 2007; Pears et al., 2010). In addition, poor self-control during childhood is associated with negative long-term developmental outcomes, such as increased risk of drug initiation during adolescence (Wills & Stoolmiller, 2002). Although early intervention strategies often aim to address emotional and cognitive self-control and related downstream outcomes during periods of development characterized by high levels of neural plasticity, the level of functioning within these domains during early childhood may make this difficult. Identifying methods for promoting self-control and specific domains of self-control that may be more amendable to intervention in children remains an important area of investigation.
Existing interventions for children provide indirect support for the neurally informed domain-general framework of inhibitory control and illustrate how it could be used to inform further refinements of those interventions. Several relevant interventions are described for the purposes of illustrating this point, but a thorough review of the literature is not provided here. The Promoting Alternative Thinking Strategies (PATHS) curriculum is a school-based intervention for improving social and emotional functioning through a focus on inhibitory control of emotions in social contexts and general awareness of emotions (Kusché & Greenberg, 1994; Riggs, Greenberg, Kusché, & Pentz, 2006). In a sample of second and third graders, the positive transfer effects of the intervention from basic tasks to decreases in internalizing and externalizing at 1-year follow-up appear to be mediated by gains in inhibitory control as assessed through the Stroop test (Riggs et al., 2006). Taking another approach to enhancing emotional and cognitive self-control, Mendelson and colleagues recently reported on a trial of a mindfulness-based intervention for fourth and fifth graders involving yoga, didactics in stress reduction, and guided meditation (Mendelson et al., 2010). Intervention effects highlighting the transfer of skills to new domains included decreases in involuntary engagement with negative emotions and thoughts. However, there were no intervention effects on depression symptoms or social functioning. The authors suggest that if changes in inhibitory control mediate gains in these other domains, such gains may emerge only over time as opposed to immediately following the intervention (Mendelson et al., 2010). Taken together, these results indicate that various intervention strategies for school-aged children, with emphasis on cognitive, emotional, and behavioral self-control, are effective for enhancing self-control and associated outcomes. However, it is difficult to determine which aspects of these interventions most effectively promote gains in self-control or whether they work synergistically to cause improvements.
The framework proposed here might also help explain null effects of similar interventions with younger children on self-control. For example, although an adaptation of the PATHS curriculum for preschoolers in Head Start classrooms was associated with gains in teacher-rated social competence, gains in inhibitory control and other domains of EF were not found (Domitrovich, Cortes, & Greenberg, 2007). Another recent intervention for preschoolers in Head Start classrooms made use of the preschool PATHS curriculum to target social and emotional development while adding a literacy component (Bierman, Nix, Greenberg, Blair, & Domitrovich, 2008). Among several EF measures, only one significant intervention effect was observed. However, initial levels of EF moderated intervention effects such that children with low initial EF demonstrated greater gains in social competence and cognitive skills (Bierman et al., 2008). It may be the case that this intervention bolstered EF only indirectly, benefiting those at the lowest levels, and that more explicit training in EF domains is necessary for younger children to make gains in EF and associated downstream outcomes such as improved academic functioning (Diamond et al., 2007). It may also be the case that inhibitory control in the behavioral domain, as opposed to the cognitive or emotional domain, represents a more accessible intervention target for younger children.
In contrast, evidence suggests that adolescents with lower levels of EF may benefit less from interventions for substance misuse (Buckman, Bates, & Morgenstern, 2008; Fishbein et al., 2006; Riggs & Greenberg, 2009). This contrasting developmental pattern indicates that young children with low EF may be more amenable to intervention compared to their older counterparts, potentially due to higher levels of neural plasticity in the early years or because interventions designed for younger children usually involve more external sources of structure and support. The findings with adolescents suggest the potential for more direct training of EF to allow maximal benefit from additional services. Taken together, these findings clearly underscore the need for tailored treatment of youths with low levels of cognitive self-control (Diamond & Lee, 2011; Riggs & Greenberg, 2009).
Our neurally informed, domain-general model of self-control provides a theoretical and neural basis for examining potential cross-domain effects of individual intervention components focused on particular aspects of self-control. A specific focus on intervention components targeting behavioral self-control will be important inasmuch as this domain may represent a more feasible intervention target for young children with limited cognitive and emotional awareness. In addition to potentially increasing efficiency of interventions by limiting training to fewer self-control domains, this model provides a framework for assessing which individuals may need preliminary training in self-control to benefit from additional services. For children and families requiring a high level of services, initial intervention to improve self-control may simply represent a starting point to allow maximum efficacy of additional intervention strategies. Advances in neuroscience methodologies, including techniques for safely conducting neuroimaging with infants and young children (Pierce, 2011), will allow for testing the involvement of the rIFG and related neural networks in intervention readiness or gains related to self-control. Functional and structural changes in known self-control brain regions would be expected to mediate the transfer of gains from the behavioral to the affective and cognitive domains.
CONCLUSION
The neurobiologically informed strength model of self-control presented in this article addresses a core feature of maladaptive and adaptive functioning across the behavioral, emotional, and cognitive domains. The model includes a conceptual and neurological basis for cross-domain effects of brief, targeted interventions. In addition, it provides a basis for continued evaluation of existing interventions with the aim of more thoroughly understanding readiness for, and mechanisms of, change. Such work has the potential to lead to tailoring of interventions for individuals and refinement of interventions to make maximum use of educational and mental health resources. In the current political and social climate of scarce resources combined with increasing awareness of the need for mental health treatment, efficient interventions rooted in solid conceptual and neural frameworks have great potential to augment existing strategies by targeting key neural systems that influence regulatory functioning across domains and settings.
Footnotes
Although self-control has been referred to as a broader category encompassing EF in the developmental literature (e.g., Fox & Calkins, 2003), we use the term self-control and the above definition for consistency with adult research literature that we propose is highly relevant for understanding adaptive development of regulatory functioning. This usage is consistent with that of Liew (in press) and Ursache, Blair, and Raver (in press), who also view self-control as part of EF.
REFERENCES
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. Journal of Neuroscience. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. doi:10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Durston S, Eagle DM, Logan GD, Stinear CM, Stuphorn V. Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. Journal of Neuroscience. 2007;27:11860–11864. doi: 10.1523/JNEUROSCI.3644-07.2007. doi:10.1523/JNEUROSCI.3644-07.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. doi:10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Science. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. doi:10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Heatherton T. Self-regulation failure: An overview. Psychological Inquiry. 1996;7:1–15. [Google Scholar]
- Beck SJ, Hanson CA, Puffenberger SS, Benninger KL, Benninger WB. A controlled trial of working memory training for children and adolescents with ADHD. Journal of Clinical Child & Adolescent Psychology. 2010;39:825–836. doi: 10.1080/15374416.2010.517162. doi:10.1080/15374416.2010.517162. [DOI] [PubMed] [Google Scholar]
- Bedard A-C, Nichols S, Barbosa JA, Schachar R, Logan GD, Tannock R. The development of selective inhibitory control across the life span. Developmental Neuropsychology. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. doi:10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Beedie CJ, Lane AM. The role of glucose in self-control: Another look at the evidence and an alternative conceptualization. Personality and Social Psychology Review. 2012;16:143–153. doi: 10.1177/1088868311419817. [DOI] [PubMed] [Google Scholar]
- Beers SR, De Bellis MD. Neuropsychological function in children with maltreatment-related posttraumatic stress disorder. American Journal of Psychiatry. 2002;159:483–486. doi: 10.1176/appi.ajp.159.3.483. [DOI] [PubMed] [Google Scholar]
- Bell JA, Livesey PJ. Cue significance and response regulation in 3- to 6-year-old children's learning of multiple choice discrimination tasks. Developmental Psychobiology. 1985;18:229–245. doi: 10.1002/dev.420180304. doi:10.1002/dev.420180304. [DOI] [PubMed] [Google Scholar]
- Berkman ET, Burklund L, Lieberman MD. Inhibitory spillover: Intentional motor inhibition produces incidental limbic inhibition via right inferior frontal cortex. NeuroImage. 2009;47:705–712. doi: 10.1016/j.neuroimage.2009.04.084. doi:10.1016/j.neuroimage.2009.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman KL, Nix RL, Greenberg MT, Blair C, Domitrovich CE. Executive functions and school readiness intervention: Impact, moderation, and mediation in the Head Start REDI program. Development and Psychopathology. 2008;20:821–843. doi: 10.1017/S0954579408000394. doi:10.1017/S0954579408000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, et al. Neural development of selective attention and response inhibition. NeuroImage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. doi:10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cerebral Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- Bruce J, McDermott JM, Fisher PA, Fox NA. Using behavioral and electrophysiological measures to assess the effects of a preventive intervention: A preliminary study with preschool-aged foster children. Prevention Science. 2009;10:129–140. doi: 10.1007/s11121-008-0115-8. doi:10.1007/s11121-008-0115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryck RL, Fisher PA. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuro-science, developmental psychology, and prevention science. American Psychologist. 2012;67:87–100. doi: 10.1037/a0024657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, Bates ME, Morgenstern J. Social support and cognitive impairment in clients receiving treatment for alcohol-and drug-use disorders: A replication study. Journal of Studies on Alcohol and Drugs. 2008;69:738–746. doi: 10.15288/jsad.2008.69.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Zelazo PD. A brain-based account of the development of rule use in childhood. Current Directions in Psychological Science. 2006;15:118–121. [Google Scholar]
- Carver AC, Livesey DJ, Charles M. Age related changes in inhibitory control as measured by stop signal task performance. International Journal of Neuroscience. 2001;107:43–61. doi: 10.3109/00207450109149756. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. Journal of Cognitive Neuroscience. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB. Executive “brake failure” following deactivation of human frontal lobe. Journal of Cognitive Neuroscience. 2006;18:444–455. doi: 10.1162/089892906775990606. doi:10.1162/jocn.2006.18.3.444. [DOI] [PubMed] [Google Scholar]
- Chugani H, Phelps M, Mazziota J. Position emission tomography study of human brain functional development. Annals of Neurology. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Intentional and Incidental Self-Control in Ventrolateral PFC. In: Stuss DT, Knight RT, editors. Principles of frontal lobe functions. 2nd ed. Oxford University Press; Oxford, England: in press. [Google Scholar]
- Dennis TA, Brotman LM, Huang K-Y, Gouley KK. Effortful control, social competence, and adjustment problems in children at risk for psychopathology. Journal of Clinical Child and Adolescent Psychology. 2007;36:442–454. doi: 10.1080/15374410701448513. doi:10.1080/15374410701448513. [DOI] [PubMed] [Google Scholar]
- de Ridder DTD, Lensvelt-Mulders G, Finkenauer C, Stok FM, Baumeister RF. Taking stock of self-control: A meta-analysis of how trait self-control relates to a wide range of behaviors. Personality and Social Psychology Review. 2012;16:76–99. doi: 10.1177/1088868311418749. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy, and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. pp. 466–503. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. doi:10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. doi: 10.1126/science.1204529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. Journal of Neuroscience. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. doi:10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domitrovich CE, Cortes RC, Greenberg MT. Improving young children's social and emotional competence: A randomized trial of the preschool “PATHS” curriculum. The Journal of Primary Prevention. 2007;28:67–91. doi: 10.1007/s10935-007-0081-0. doi:10.1007/s10935-007-0081-0. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:F9–F16. doi:10.1111/1467-7687.00235. [Google Scholar]
- Finkel E, DeWall C, Slotter E, Oaten M, Foshee V. Self-regulatory failure and intimate partner violence perpetration. Journal of Personality and Social Psychology. 2009;97:483–499. doi: 10.1037/a0015433. doi:10.1037/a0015433. [DOI] [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27:7–26. [Google Scholar]
- Fishbein DH, Hyde C, Eldreth D, Paschall MJ, Hubal R, Das A, Yung B. Neurocognitive skills moderate urban male adolescents' responses to preventive intervention materials. Drug and Alcohol Dependence. 2006;82:47–60. doi: 10.1016/j.drugalcdep.2005.08.008. doi:10.1016/j.drugalcdep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF. The physiology of willpower: Linking blood glucose to self-control. Personality and Social Psychology Review. 2007;11:303–327. doi: 10.1177/1088868307303030. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF, DeWall CN, Maner JK, Plant EA, Tice DM, Brewer LE, Schmeichel BJ. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. Journal of Personality and Social Psychology. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Plant EA, Butz DA, Baumeister RF. Increasing self-regulatory strength can reduce the depleting effect of suppressing stereotypes. Personality and Social Psychology Bulletin. 2007;33:281–294. doi: 10.1177/0146167206296101. doi:10.1177/0146167206296101. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier C, Rousseaux M. Non-spatial attention disorders in patients with frontal or posterior brain damage. Brain. 1996;119:191–202. doi: 10.1093/brain/119.1.191. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8624681. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan R. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. doi:10.1016/S0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2010;136:495–525. doi: 10.1037/a0019486. doi:10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hare T, Tottenham N, Davidson M, Glover G, Casey B. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. doi:10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12:F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. doi:10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Hui S.-k. A., Wright RA, Stewart CC, Simmons A, Eaton B, Nolte RN. Performance, cardiovascular, and health behavior effects of an inhibitory strength training intervention. Motivation & Emotion. 2009;33:419–434. doi:10.1007/s11031-009-9146-0. [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: Common and specific influences. Annual Review of Clinical Psychology. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- Jones LB, Rothbart MK, Posner MI. Development of executive attention in preschool children. Developmental Science. 2003;6:498–504. doi:10.1111/1467-7687.00307. [Google Scholar]
- Karbach J, Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental Science. 2009;12:978–990. doi: 10.1111/j.1467-7687.2009.00846.x. doi:10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. doi:10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlström K, Westerberg H. Computerized training of working memory in children with ADHD—A randomized, controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. doi:10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Development. 2001;72:1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Tjebkes TL, Husarek SJ. Individual differences in emotionality in infancy. Child Development. 1998;64:375–390. [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kurzban R. Does the brain consume additional glucose during self-control tasks? Evolutionary Psychology. 2010;8:244–259. [PubMed] [Google Scholar]
- Kusché CA, Greenberg MT. The PATHS (promoting alternative thinking strategies) curriculum. Channing-Bete; South Deerfield, MA: 1994. [Google Scholar]
- Lengua LJ, Honorado E, Bush NR. Contextual risk and parenting as predictors of effortful control and social competence in preschool children. Journal of Applied Developmental Psychology. 2007;28:40–55. doi: 10.1016/j.appdev.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Carrion J, García-Orza J, Pérez-Santamaría FJ. Development of the inhibitory component of the executive functions in children and adolescents. International Journal of Neuroscience. 2004;114:1291–1311. doi: 10.1080/00207450490476066. doi:10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Leung H-C, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. Journal of Neuroscience. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. doi:10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Culhane KA, Hartmann J, Evankovich K, et al. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. doi:10.1080/87565649109540499. [Google Scholar]
- Lewis EE, Dozier M, Ackerman J, Sepulveda-Kozakowski S. The effect of placement instability on adopted children's inhibitory control abilities and oppositional behavior. Developmental Psychology. 2007;43:1415–1427. doi: 10.1037/0012-1649.43.6.1415. doi:10.1037/0012-1649.43.6.1415. [DOI] [PubMed] [Google Scholar]
- Liew J. Effortful control, executive functions, and education: Bringing self-regulatory and social-emotional competencies to the table. Child Development Perspectives. in press. [Google Scholar]
- Livesey DJ, Morgan GA. The development of response inhibition in 4- and 5-year-old children. Australian Journal of Psychology. 1991;43:133–137. [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Hill SS, Stone SI, Bunge SA. Differential effects of reasoning and speed training in children. Developmental Science. 2011;14:582–590. doi: 10.1111/j.1467-7687.2010.01005.x. doi:10.1111/j.1467-7687.2010.01005.x. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers' literacy, vocabulary, and math skills. Developmental Psychology. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. doi:10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- Mendelson T, Greenberg MT, Dariotis JK, Gould LF, Rhoades BL, Leaf PJ. Feasibility and preliminary outcomes of a school-based mindfulness intervention for urban youth. Journal of Abnormal Child Psychology. 2010;38:985–994. doi: 10.1007/s10802-010-9418-x. doi:10.1007/s10802-010-9418-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. doi:10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Macrae CN. Separating sustained from transient aspects of cognitive control during thought suppression. Psycholog ical Science. 2007;18:292–297. doi: 10.1111/j.1467-9280.2007.01891.x. doi:10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Moilanen KL, Shaw DS, Dishion TJ, Gardner F, Wilson M. Longitudinal growth and predictors of inhibitory control in early childhood. Social Development. 2010;19:326–347. doi: 10.1111/j.1467-9507.2009.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79:273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morales JI, Berkman ET, Lieberman MD. Improving self-control across domains: Increase emotion regulation ability through motor inhibition training. 2012. Manuscript submitted for publication. [Google Scholar]
- Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. Journal of Cognitive Neuroscience. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. doi:10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O'Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15:453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M. Building self-control strength: Practicing self-control leads to improved self-control performance. Journal of Experimental Social Psychology. 2010;46:465–468. doi: 10.1016/j.jesp.2009.12.011. doi:10.1016/j.jesp.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does selfcontrol resemble a muscle? Psychological Bulletin. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. doi:10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF, Tice DM. Longitudinal improvement of self-regulation through practice: Building self-control strength through repeated exercise. The Journal of Social Psychology. 1999;139:446–457. doi: 10.1080/00224549909598404. doi:10.1080/00224549909598404. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as limited resource: Regulatory depletion patterns. Journal of Personality and Social Psychology. 1998;74:774–789. doi: 10.1037//0022-3514.74.3.774. doi:10.1037/0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Nachev P, Wydell H, ONeill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. doi:10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. doi:10.1037//0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. doi:10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Payer DE, Lieberman M, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug and Alcohol Dependence. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears KC, Fisher PA, Bruce J, Kim HK, Yoerger K. Early elementary school adjustment of maltreated children in foster care: The roles of inhibitory control and caregiver involvement. Child Development. 2010;81:1550–1564. doi: 10.1111/j.1467-8624.2010.01491.x. doi:10.1111/j.1467-8624.2010.01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: A functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. doi:10.1016/j.bio-psych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Pierce K. Early functional brain development in autism and the promise of sleep fMRI. Brain Research. 2011;1380:162–174. doi: 10.1016/j.brainres.2010.09.028. doi:10.1016/j.brainres.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raver CC, Jones SM, Li-Grining C, Zhai F, Metzger MW, Solomon B. Targeting children's behavior problems in preschool classrooms: A cluster-randomized controlled trial. Journal of Consulting and Clinical Psychology. 2009;77:302–316. doi: 10.1037/a0015302. doi:10.1037/a0015302. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Greenberg MT. Neurocognition as a moderator and mediator in adolescent substance misuse prevention. The American Journal of Drug and Alcohol Abuse. 2009;35:209–213. doi: 10.1080/00952990903005940. doi:10.1080/00952990903005940. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Greenberg MT, Kusché CA, Pentz MA. The mediational role of neurocognition in the behavioral outcomes of a social-emotional prevention program in elementary school students: Effects of the PATHS Curriculum. Prevention Science. 2006;7:91–102. doi: 10.1007/s11121-005-0022-1. doi:10.1007/s11121-005-0022-1. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Heaton RK, Lehman RA, Stilson D. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. Journal of Consulting and Clinical Psychology. 1980;48:605–614. doi: 10.1037//0022-006x.48.5.605. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI. Temperament and the development of self-regulation. In: Hartlage L, Telzrow CF, editors. The neuropsychology of individual differences: A developmental perspective. Plenum; New York: 1985. pp. 92–123. [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E. Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. doi:10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. doi:10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD. Impulsivity and inhibitory control in normal development and childhood psychopathology. Developmental Psychology. 1990;26:710–720. doi:10.1037//0012-1649.26.5.710. [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. NeuroImage. 2006;31:468–475. doi: 10.1016/j.neuroimage.2005.11.053. doi:10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Fanning J, Coch D, Sanders L, Neville H. Neural mechanisms of selective auditory attention are enhanced by computerized training: Electrophysiological evidence from language-impaired and typically developing children. Brain Research. 2008;1205:55–69. doi: 10.1016/j.brainres.2007.10.108. doi:10.1016/j.brainres.2007.10.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks NeuroImage. 2011;56:1655–1565. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AA, Poldrack RA, Chakrapani S, Lee B, London ED. Different forms of self-control share a neurocognitive substrate. Journal of Neuroscience. 2011;31:4805–4810. doi: 10.1523/JNEUROSCI.2859-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. Journal of Neuroscience. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorell LB, Lindqvist S, Nutley SB, Bohlin G, Klingberg T. Training and transfer effects of executive functions in preschool children. Developmental Science. 2009;12:106–113. doi: 10.1111/j.1467-7687.2008.00745.x. doi:10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Tominey SLM, McClelland MM. Red light, purple light: Findings from a randomized trial using circle time games to improve behavioral self-regulation in preschool. Early Education & Development. 2011;22:489–519. doi:10.1080/10409289.2011.574258. [Google Scholar]
- Ursache A, Blair C, Raver CC. The promotion of self-regulation as a means of enhancing school readiness and early achievement in children at risk for school failure. Child Development Perspectives. doi: 10.1111/j.1750-8606.2011.00209.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente C, Lemery-Chalfant K, Reiser M. Pathways to problem behaviors: Chaotic homes, parent and child effortful control, and parenting. Social Development. 2007;16:249–267. [Google Scholar]
- Van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VAF. Unconscious activation of the prefrontal no-go network. Journal of Neuroscience. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. doi:10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K, Wheeler ME, Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. doi:10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends in Cognitive Sciences. 2008;12:418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. doi:10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CAC, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. Journal of Experimental Child Psychology. 2011;108:436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, Blair CB. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment. doi: 10.1037/a0025779. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Stoolmiller M. The role of self-control in early escalation of substance use: A time-varying analysis. Journal of Consulting and Clinical Psychology. 2002;70:986–997. doi: 10.1037//0022-006x.70.4.986. doi:10.1037/0022-006X.70.4.986. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, et al. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. doi:10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD, Reznick JS, Piñon DE. Response control and the execution of verbal rules. Developmental Psychology. 1995;31:508–517. doi:10.1037/0012-1649.31.3.508. [Google Scholar]


