Abstract
The hepatitis C virus (HCV) virus epidemic is ongoing in the United States and globally. Incidence rates remain high, especially in young adult injection drug users. New outbreaks of HCV in the United States among young adults, in predominantly suburban and rural areas, have emerged and may be fueling an increase in HCV. This paper discusses some key HCV prevention strategies that to date have not been widely researched or implemented, and wherein future HCV prevention efforts may be focused: (1) reducing sharing of drug preparation equipment; (2) HCV screening, and testing and counseling; (3) risk reduction within injecting relationships; (4) injection cessation and “breaks”; (5) scaled-up needle/syringe distribution, HCV treatment, and vaccines, according to suggestions from mathematical models; and (6) “combination prevention.” With ongoing and expanding transmission of HCV, there is little doubt that there is a need for implementing what is in the prevention “toolbox” as well as adding to it. Strong advocacy and resources are needed to overcome challenges to providing the multiple and comprehensive programs that could reduce HCV transmission and associated burden of disease worldwide in people who inject drugs.
Keywords: hepatitis C virus, prevention, injection drug users, syringe access, counseling and testing, harm reduction, HCV treatment, HCV vaccine, combination prevention
In the United States, as in other countries, there is an ongoing epidemic of hepatitis C virus (HCV) infection among young adult injection drug users [1–6]. Outbreaks of HCV in young injectors have recently been reported by the US Centers for Disease Control and Prevention (CDC) [4–7], and new investigations in Wisconsin, Indiana, Virginia, Pennsylvania, Florida, and the Northern Plains (Native American community) (S. Holmberg, personal oral communication, February 2013) have raised serious concerns. These outbreaks are notable for the locations (predominantly suburban and rural areas) and populations (predominantly young white adults aged <30 with a history of previous or concurrent prescription opioid use) [7]. Extraordinary increases in prescription opiate use in the United States and, in particular, among young people, who also have the highest rates of heroin use, are believed to be fueling this emergent HCV epidemic in the United States and elsewhere, including Canada [8, 9]. Effective HCV prevention remains a huge challenge globally, especially young adults. This paper discusses how prevention strategies might be scaled up to prevent HCV infection based primarily on evidence from the UFO Study, an ongoing prospective study in San Francisco, California. Because of its longevity, large sample size, and focus on HCV, this study provides an important platform from which to explore adoption and expansion of HCV prevention approaches in people who inject drugs (PWID) globally.
In 2007, Armstrong et al [10] estimated the number of ever and current noninstitutionalized PWID in the United States at 2.3 million; more than half a million (590 000) were <30 years of age. Because this survey excluded people who were homeless, incarcerated, or hospitalized and those in the military, these numbers represent a lower bound at best. Based on recent research in active young injectors in 20 US cities [11], we estimate that 3%, or just under 20 000 of 590 000, are infected with human immunodeficiency virus (HIV). HCV may infect 10–15 times as many PWID, or upward of 200 000. With annualized HCV incidence estimates in young adult injectors between 8% and 25% [1, 3], >31 000 new HCV infections might occur every year (among the 390 000 susceptible). HCV infection is rapidly acquired after initiation of injecting, and incidence rates are highest among newer injectors, a quarter of whom are infected within 2 years of initiating [12]. Although there is evidence that HCV incidence has declined in recent decades [12], if the number of young injectors increases, as the CDC suggests is occurring in the United States, these gains could be lost. Ongoing and targeted surveillance efforts to enumerate the population at risk and assess the burden of infection are essential steps in targeting and implementing effective HCV prevention in this group.
Globally, 10 million PWID are estimated to be infected with HCV, corresponding with a midpoint prevalence of 67% [13]. This high prevalence, combined with the high infectivity of HCV, presents significant challenges to HCV prevention. HCV is at least 10 times more infectious than HIV: 3%–10% per injection compared to 0.3% for HIV [14]. Moreover, contaminated needles/syringes are not the only vehicle for blood-borne HCV transmission: the virus remains infective in liquid, syringes, and on inanimate surfaces for weeks [15, 16].
The UFO Study is a large community-based epidemiologic study of HCV infection in young adult injectors in San Francisco, California, ongoing since January 2000 [1, 17]. Young (<30 years), active (report injecting drugs in the prior month), HCV-negative (either antibody to HCV [anti-HCV] or HCV RNA) adults are enrolled in prospective quarterly follow-up to assess incident infection. Details of the study methods have been published previously [1, 17]. As of 1 February 2013, the median age of 1494 participants screened at baseline was 22 years (interquartile range [IQR], 20–25 years); less than half (45.9%) had completed high school; and most (65.2%) were male, were white (76.8%), had been injecting a median of 3.7 years (IQR, 1.3–6.0 years), and reported a median of 20 days injecting per month (IQR, 7–30 days). Of 1494 adults screened, 33% were HCV positive, and among 552 HCV-negative subjects followed prospectively, HCV incidence was 23 per 100 person-years (95% confidence interval [CI], 19.6–26.7). Table 1 shows characteristics, exposures, and HCV incidence in the UFO Study. Incidence of HCV was significantly higher among those who shared injecting equipment, including needles and syringes (Figure 1). Sharing ancillary equipment carried the same excess risk as sharing needles/syringes. Those who shared with more people, and inject more frequently, had a higher risk of infection.
Table 1.
Characteristics, Exposures, and Risk Behaviors and Association With Incident Hepatitis C Virus Infection in Young Injectors in the UFO Cohort Study, San Francisco, California, 2000–2013
| Baseline Characteristic | Total No. | Incident HCV, No. (%) | Incidence per Person-year(95% CI) | Rate Ratio (95% CI) | P Value |
|---|---|---|---|---|---|
| Overall | 554 | 169 (30.5) | 23.1 (19.9–26.8) | ||
| Age, y | |||||
| ≤22 | 236 | 84 (35.6) | 25.8 (20.8–32.0) | 1 | |
| >22 | 318 | 85 (26.7) | 20.9 (16.9–25.9) | .81 (.60–1.10) | .17 |
| Sex | |||||
| Male | 370 | 107 (28.9) | 21.2 (17.5–25.6) | 1 | |
| Female | 180 | 62 (34.4) | 27.8 (21.6–35.5) | 1.31 (.96–1.79) | .09 |
| Years injecting | |||||
| ≤3 | 288 | 88 (30.6) | 22.8 (18.5–28.1) | 1 | |
| >3 | 264 | 81 (30.7) | 23.4 (18.8–29.1) | 1.03 (.76–1.39) | .86 |
| History of drug treatment | |||||
| No | 196 | 54 (27.6) | 19.4 (14.9–25.3) | 1 | |
| Yes | 353 | 114 (32.3) | 25.5 (21.2–30.6) | 1.31 (.95–1.82) | .1 |
| Ceased injecting for ≥3 mo (any during follow-up) | |||||
| No | 411 | 135 (32.9) | 33.3 (28.1–39.4) | 1 | |
| Yes | 143 | 34 (23.8) | 10.4 (7.4–14.6) | .31 (.22–.46) | <.01 |
| Drug injected most days, last mo | |||||
| Speed/cocaine/crack | 188 | 49 (26.1) | 17.5 (13.2–23.1) | 1 | |
| Heroin/heroin mixed | 337 | 119 (35.3) | 29.6 (24.7–35.4) | 1.69 (1.21–2.36) | <.01 |
| Injection frequency, last mo | |||||
| Less than daily | 367 | 92 (25.1) | 17.5 (14.3–21.5) | 1 | |
| Daily | 187 | 77 (41.2) | 37.1 (30.0–46.3) | 2.11 (1.56–2.86) | <.01 |
| No. of injection events, last mo | |||||
| >100 | 129 | 51 (39.5) | 37.3 (28.4–49.1) | 1 | |
| 30–100 | 198 | 72 (36.7) | 30.6 (24.3–38.5) | .82 (.57–1.18) | .27 |
| <30 | 226 | 46 (20.4) | 12.9 (9.7–17.2) | .35 (.23–.52) | <.01 |
| Borrowed used needle, last 3 mo | |||||
| No | 342 | 90 (26.3) | 18.0 (14.6–22.1) | 1 | |
| Yes | 209 | 79 (37.8) | 34.7 (27.8–43.3) | 1.93 (1.43–2.62) | <.01 |
| No. of people borrowed used needle from, last 3 mo | |||||
| 0 | 341 | 90 (26.4) | 18.0 (14.6–22.1) | 1 | <.01 |
| 1 | 113 | 39 (34.5) | 31.4 (22.9–42.9) | 1.75 (1.20–2.55) | <.01 |
| ≥2 | 91 | 37 (40.7) | 38.2 (27.7–52.7) | 2.13 (1.45–3.13) | |
| Used a contaminated cooker, last 3 mo | |||||
| No | 364 | 96 (26.4) | 18.4 (15.1–22.5) | 1 | |
| Yes | 188 | 73 (38.8) | 35.6 (28.3–44.8) | 1.94 (1.43–2.63) | <.01 |
| Injected someone's rinse, last 3 mo | |||||
| No | 350 | 90 (25.7) | 18.6 (15.1–22.8) | 1 | |
| Yes | 202 | 79 (39.1) | 32.2 (25.8–40.1) | 1.74 (1.28–2.35) | <.01 |
| Days drank alcohol, last mo | |||||
| 0 | 117 | 36 (30.8) | 23.5 (17.0–32.6) | 1 | |
| 1–14 | 230 | 70 (30.4) | 26.1 (20.7–33.0) | 1.11 (.74–1.66) | .61 |
| ≥15 | 207 | 63 (30.4) | 20.3 (15.8–25.9) | .86 (.57–1.30) | .47 |
| Any sex partner, last 3 mo | |||||
| No | 92 | 27 (29.4) | 19.7 (13.5–28.8) | 1 | |
| Yes | 462 | 142 (30.7) | 23.9 (20.2–28.1) | 1.21 (.80–1.82) | .37 |
| IDU sex partner, last 3 mo | |||||
| No | 257 | 71 (27.6) | 20.7 (16.4–26.2) | 1 | |
| Yes | 297 | 98 (33.0) | 25.2 (20.6–30.7) | 1.21 (.89–1.65) | .21 |
Abbreviations: CI, confidence interval; HCV, hepatitis C virus; IDU, injection drug user.
Figure 1.
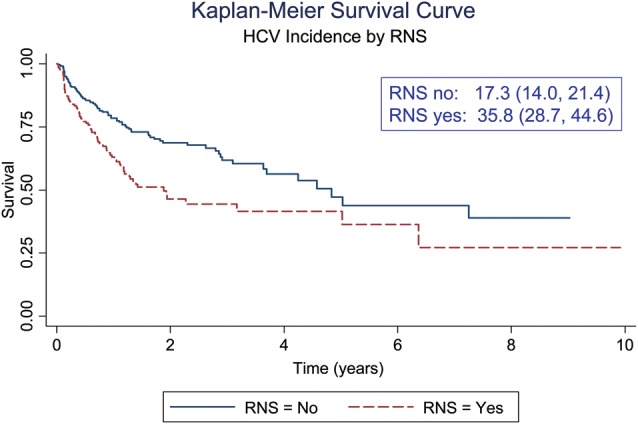
Cumulative incidence of hepatitis C virus infection by receptive needle sharing among young injection drug users in the UFO Study in San Francisco, California, 2000–2013. Abbreviations: CI, confidence interval; HCV, hepatitis C virus; py, person-years; RNS, receptive needle/syringe sharing.
In this review, we identify and discuss 6 key areas of evidence, which to date have not been widely researched or implemented, and wherein future HCV prevention efforts may be focused: (1) sharing of drug preparation equipment; (2) HCV screening, and testing and counseling; (3) injecting relationships; (4) injection cessation and “breaks”; (5) scaled-up needle/syringe distribution, HCV treatment, and vaccines according to suggestions from mathematical models; and (6) “combination prevention.” With ongoing and expanding transmission of HCV, there is little doubt that there is a need to implement what is in the prevention “toolbox,” as well as add to it.
Contribution of Shared Drug Preparation Equipment to HCV Risk
In the past decade, numerous studies have shown elevated risk of HCV in association with shared ancillary drug preparation equipment, and a recent review and meta-analysis of 21 studies quantified risk estimates across several drug equipment sharing behaviors [18]. Consistently, pooled risk ratios for shared use of drug preparation containers, filters, rinse water, and backloading were comparable to those for syringe sharing (1.97; 95% CI, 1.57–2.49). Those authors further estimated the population attributable risk (PAR) percentage in relation to the proportion exposed by equipment category: where the prevalence of syringe sharing is 36%, 25% of HCV seroconversions could be prevented with the elimination of that risk exposure. Because exposure rates for sharing drug preparation equipment are generally higher, the PAR percentage is even higher—up to 43%. These consistent and overwhelming results demonstrate that efforts to reduce exposure to HCV in PWID must incorporate reduction of sharing of drug preparation equipment through messaging, training, and availability of appropriate single-use supplies. Research to implement and scale up this messaging should be prioritized, including in areas with nascent outbreaks and burgeoning epidemics [19].
Potential Preventive Impact of HCV Testing and Counseling
The rationale for testing and counseling (T&C) populations at risk for HCV includes the potential to alter risk behaviors that impact disease acquisition and transmission. However, limited research exists exploring this hypothesis [20]. In the UFO Study, we investigated the effects of disclosure of new HCV-positive results on risk behavior, including injection and noninjection drug use, lending and sharing injecting equipment, alcohol use, unprotected sex, and symptoms of depression [21]. Outcomes were assessed from data collected 3, 6, and 12 months following T&C and disclosure. Declines in alcohol and noninjection drug use were observed immediately after disclosure, but not sustained beyond 6 months. Injection drug use and depression symptoms did not change, corroborating others’ results [22]. Although there has been substantial research on risk reduction in association with HIV T&C [23], differing disease course, including an extended seronegative viremic window period and spontaneous clearance of HCV, pose important challenges for HCV prevention, and no studies to date have assessed the effects of HCV T&C on risk behavior in HCV-negative susceptible injectors.
Because detectable anti-HCV can take up to 2 months to develop following HCV inoculation [24], the viremic preseroconversion phase, or “window period,” represents a period of high viremia, during which individuals may not know they are positive and may have a high risk of HCV transmission. This window period may present an important prevention opportunity. Despite the lack of direct empirical evidence for higher risk of transmission among PWID during acute HCV infection, testing for and identifying acute HCV in the preseroconversion phase may yield prevention benefits.
Newly Food and Drug Administration–approved rapid HCV point-of-care tests are highly accurate for detection of anti-HCV, are acceptable to PWID [25], and offer a significant means to remove barriers to HCV testing, increasing uptake, opportunities to evaluate HCV T&C in relation to subsequent risk reduction, and access to further diagnosis, care, and treatment.
Injecting Relationships: Potential Targets for Prevention
In addition to “individual” risk, HCV risk is linked to the “social” or relational contexts of injecting. Female sex has been independently associated with an increased risk of HCV infection in some studies [26], but not others [1, 27]. Female injectors, in particular younger ones, demonstrate higher-risk injection behaviors than their male counterparts, including being more likely to engage in needle/syringe borrowing and ancillary equipment sharing, to be injected by others [28], and to be initiated into injecting by male sex partners [29]. Receptive needle/syringe sharing (RNS) is most likely to occur within injecting partnerships where injecting partners are also sex partners, inject together frequently, and pool money to buy drugs [30]. In the UFO Study, RNS with an HCV-infected sex partner was independently associated with a higher risk of HCV infection [17]. Individuals who perceived that their injecting partners were HCV positive had significantly lower odds of RNS than those who thought that their partner was uninfected (0.49; 95% CI, .25–.95) [31]. In analyses of dyadic-level data of HCV-serodiscordant injecting-partner couples, those who lived with and had sex with their injecting partners were at significantly higher risk for both recent RNS and shared cooker use compared to those in partnerships not living together or having sex [32]. These data highlight how HCV risk may be amplified beyond “individual” risk. In the UFO study, both women and men who report injecting with sex partners have the highest risk for incident HCV (adjusted hazard ratio, 2.23; 95% CI, 1.9–2.6).
These data have 2 important implications for potential HCV interventions. First, knowledge of an injecting partner's HCV status can potentially impact risk behavior. Increased HCV testing combined with partner HCV disclosure may reduce equipment sharing and, potentially, HCV transmission. Second, relationship-level intervention strategies should be considered, within both injecting and injecting-sexual partnerships. While a recent study showed couples-based T&C to be effective at reducing behavioral risk (injection risk with primary partners) [33], there is a need to assess the potential impact on HCV infection outcomes.
Taking a Break: Injection Cessation as a Way of Reducing Exposure to HCV
PWID who stop injecting effectively terminate their risk for HCV [17]. Prospectively, many factors, including younger age, stable housing, lower injection frequency, HIV infection, methadone maintenance treatment, employment, injecting fewer drug types, not having an injecting sex partner, and neighborhood environment have been shown to be associated with shorter time to injection cessation [34, 35]. Understanding drug use trajectories and factors associated with injection cessation are important for HCV prevention; even temporary “breaks” from injecting may also have preventive benefits. In the UFO Study, more than one-quarter (29%) of participants had at least one 3-month injection break. Those who reported recent participation in any drug treatment program, including detoxification, 12-step, or residential, were independently and significantly more likely to report at least 1 of these injection cessation breaks [36]. Although relapse was also common, these short breaks were protective for HCV incidence (rate ratio, 0.31; 95% CI, .22–.46; Table 1). Drug treatment was significantly associated with cessation, consistent with evidence which indicates that a key benefit of opioid substitution treatment (OST) is reduced frequency of injecting [37]. In addition to other benefits [38], increasing access and uptake of OST by young injectors has the potential to reduce HCV infection in this group.
The What-ifs: Mathematical Models That Inform New HCV Prevention Approaches
Kwon et al [39] modeled the effects of sterile syringe availability on the HIV and HCV epidemics among PWID in Australia, including the epidemic stability by number of years of injecting postinfection and the impact of increasing syringe availability. Although they found that HIV was effectively controlled by current syringe distribution, HCV incidence was not, and was likely to remain high. However, the model suggested that doubling the number of syringes distributed, from 30 million to 60 million per year, could potentially halve the number of new HCV infections by reducing the number of times each syringe is used and the frequency of syringe sharing. These findings are significant because Australia has one of the most comprehensive programs for syringe access globally. Currently, legal syringe access programs are operated in only 34 of the 50 US states, Puerto Rico, and the District of Columbia, resulting in large numbers of PWID still unable to access this essential prevention tool.
Within several years, oral interferon-free, once-daily HCV treatment regimens using new direct-acting antivirals will become available [40], raising the prospect of “treatment as prevention” in relation to HCV in PWID. Higher efficacy, low toxicity, and shorter regimens could result in higher uptake, better adherence, and completion of treatment, in contrast to interferon-based regimens. Mathematical models that have explored the potential impact of HCV therapy on HCV infection in injecting populations collectively show that treatment could have positive impacts on HCV prevalence and transmission [41, 42]. Despite their limitations, such as not addressing age-specific effects on transmission, and assumptions regarding injection cessation and treatment, these models are fueling significant optimism.
A prophylactic vaccine for HCV could also significantly impact HCV prevalence and incidence. Our group has modeled several scenarios for impact of a prophylactic HCV vaccine, including by varying levels of vaccine efficacy and delivery strategies [43]: a vaccine with 50%–80% efficacy targeted to high-risk or seronegative PWID at a high vaccination rate had the highest impact. A best-case scenario with 80% efficacy and 1% of high-risk PWID vaccinated per month would reduce HCV incidence from 13.5% to 3.2% at 10 years. HCV vaccine research has made significant progress in recent years and several vaccine candidates have been tested in phase 1 trials [44]. Testing HCV vaccines in PWID is essential as this is the population most affected by HCV globally [45]; the first-ever phase 1/2 trial assessing the immunogenicity and efficacy of a preventive vaccine candidate in active PWID is under way in 2 sites in the United States.
The Evidence for “Comprehensive” or “Combination” Prevention to Reduce and Prevent HCV
There is mounting evidence that comprehensive prevention incorporating high-coverage needle and syringe access programs with OST can impact HCV transmission [19, 46, 47]. In Amsterdam, full participation in harm reduction, including both methadone maintenance and needle exchange programs, was significantly associated with reduced HCV and HIV incidence in a prospective study of PWID [46]. Importantly, this study also showed that each of these modalities alone was not associated with reduced infection rates. In the United Kingdom, Turner et al [47] showed that high coverage of OST combined with syringe distribution program could potentially reduce the odds of a new HCV infection by up to 80%. This study was supported by another model demonstrating that scaled-up OST in combination with increased syringe access could reduce HCV prevalence to <30% over a decade [48]. Finally, in a systematic review and meta-analysis of interventions to reduce HCV, including behavioral interventions, substance use treatment, syringe access, syringe disinfection, and multicomponent interventions, Hagan et al [19] showed that interventions using multiple combined strategies reduced risk of HCV seroconversion by 75% (pooled relative risk, 0.25; 95% CI, .07–.83), as compared to single-method interventions where pooled effects ranged from 0.6 to 1.6.
In Conclusion: Then and Now
Research over the past 2 decades has resulted in a substantial evidence base regarding the essential elements of HCV prevention in PWID including access to sterile injection equipment in combination with OST; interventions to reduce risk behavior among individuals and in injecting partnerships; rapid and accurate HCV testing and diagnosis; strategies to encourage injection cessation, including effective drug treatment; and increased access and uptake of HCV treatment. The evidence suggests that all of these elements are needed to impact HCV. Young and new initiates to injecting are critical candidates for interventions to delay or prevent HCV infection. In the United States, for >20 years, public health and political efforts to increase clean syringe/needle availability have been met with ideological, social, and political barriers, effectively thwarting the delivery of one of the most efficacious biomedical technologies for preventing injection-related infections. Expanding HCV prevention and reaching the new generation of young injectors will require dedicated advocacy, pragmatism, and persistence to enable access to all of these technologies. Thirteen years ago, Prof. Dr. Roel Coutinho, from the Amsterdam Public Health Service in the Netherlands, wrote:
“On the basis of the available data, it appears that needle and syringe exchange alone is not sufficient to decrease injecting risk behavior and to lower the incidence of blood borne infections. Drug users can change their risk behavior, but they do so only on an individual basis, if they are ready for it. Access to comprehensive intervention programs that include good medical care, social support, methadone maintenance, and needle exchange, will help them make the decision to use drugs safely and, for some, to stop using drugs altogether.” [49]
Despite an increasing array of evidence-based interventions at our disposal, access and uptake of HCV prevention interventions by PWID remain suboptimal and indeed, dismal, in many settings, including the United States. As Strathdee et al [50] have recently argued, addictophobia or fear, aversion and/or discrimination, apathy or indifference to the suffering of drug users and their right to access prevention, and inattention to key subgroups such as young people and female injectors contribute to the inequities in access to and lack of provision of prevention services for PWID. Injecting drug use is expanding, not only in the United States, but also Eastern Europe, Central Asia, and parts of Africa. The huge populations of PWID infected with HCV in China and Russia [13] reflect the potential for ongoing global expansion of HCV if prevention is not prioritized. Although we know what works, and have evidence for what is needed to expand, both political will and additional resources are needed to overcome challenges and maximize the potential impact of multiple and comprehensive programs to reduce HCV transmission and associated burden of disease worldwide.
Notes
Acknowledgments. The authors acknowledge the helpful contributions from Dr Julie Bruneau (University of Montreal), Dr Holly Hagan (New York University), and Drs John Ward and Scott Holmberg (CDC).
Financial support. The work was supported by the National Institute on Drug Abuse (award numbers R01DA031056 and R01DA016017 to K. P. and J. A. H.). The Kirby Institute is funded by the Australian Government Department of Health and Ageing. L. M. is also supported by a National Health and Medical Research Council Senior Research Fellowship.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institutes of Health, or the Australian government.
Supplement sponsorship. This article was published as part of a supplement entitled “Prevention and Management of Hepatitis C Virus Among People Who Inject Drugs: Moving the Agenda Forward,” sponsored by an unrestricted grant from the International Network on Hepatitis in Substance Users (INHSU), The Kirby Institute (University of New South Wales), Abbvie, Gilead Sciences, Janssen-Cilag, and Merck.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis. 2009;200:1216–26. doi: 10.1086/605947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hagan H, Pouget ER, Williams IT, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–85. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 3.Mehta SH, Astemborski J, Kirk GD, et al. Changes in blood-borne infection risk among injection drug users. J Infect Dis. 2011;203:587–94. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Notes from the field: hepatitis C virus infections among young adults—rural Wisconsin, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:358. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002–2009. MMWR Morb Mortal Wkly Rep. 2011;60:537–41. [PubMed] [Google Scholar]
- 6.Christian WJ, Hopenhayn C, Christian A, McIntosh D, Koch A. Viral hepatitis and injection drug use in Appalachian Kentucky: a survey of rural health department clients. Public Health Rep. 2010;125:121–8. doi: 10.1177/003335491012500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Use of enhanced surveillance for hepatitis C virus infection to detect a cluster among young injection-drug users—New York, November 2004–April 2007. MMWR Morb Mortal Wkly Rep. 2008;57:517–21. [PubMed] [Google Scholar]
- 8.Substance Abuse and Mental Health Services Administration. Rockville, MD: SAMHSA: 2012. Results from the 2011 National Survey on Drug Use and Health: summary of national findings. NSDUH Series H-44, HHS Publication No. (SMA) 12-4713 Available at http://www.samhsa.gov/data/NSDUH/2k11Results/NSDUHresults2011.htm#5.5 . Accessed 11 March 2013. [Google Scholar]
- 9.Bruneau J, Roy E, Arruda N, Zang G, Jutras-Aswad D. The rising prevalence of prescription opioid injection and its association with hepatitis C incidence among street-drug users. Addiction. 2012;107:1318–27. doi: 10.1111/j.1360-0443.2012.03803.x. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong GL. Injection drug users in the United States, 1979–2002: an aging population. Arch Intern Med. 2007;167:166–73. doi: 10.1001/archinte.167.2.166. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. HIV infection and HIV-associated behaviors among injecting drug users—20 cities, United States, 2009. MMWR Morb Mortal Wkly Rep. 2012;61:133–8. [PubMed] [Google Scholar]
- 12.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168:1099–109. doi: 10.1093/aje/kwn237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wicker S, Cinatl J, Berger A, Doerr HW, Gottschalk R, Rabenau HF. Determination of risk of infection with blood-borne pathogens following a needlestick injury in hospital workers. Ann Occup Hyg. 2008;52:615–22. doi: 10.1093/annhyg/men044. [DOI] [PubMed] [Google Scholar]
- 15.Paintsil E, He H, Peters C, Lindenbach BD, Heimer R. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202:984–90. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doerrbecker J, Friesland M, Ciesek S, et al. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis. 2011;204:1830–8. doi: 10.1093/infdis/jir535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186:1558–64. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 18.Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction. 2011;107:1057–65. doi: 10.1111/j.1360-0443.2011.03765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140:465–79. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 21.Tsui JI, Vittinghoff E, Hahn JA, Evans JL, Davidson PJ, Page K. Risk behaviors after hepatitis C virus seroconversion in young injection drug users in San Francisco. Drug Alcohol Depend. 2009;105:160–3. doi: 10.1016/j.drugalcdep.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clin Infect Dis. 2002;35:783–8. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- 23.Wolitski RJ, MacGowan RJ, Higgins DL, Jorgensen CM. The effects of HIV counseling and testing on risk-related practices and help-seeking behavior. AIDS Educ Prev. 1997;9:52–67. [PubMed] [Google Scholar]
- 24.Page-Shafer K, Pappalardo BL, Tobler LH, et al. Testing strategy to identify cases of acute hepatitis C virus (HCV) infection and to project HCV incidence rates. J Clin Microbiol. 2008;46:499–506. doi: 10.1128/JCM.01229-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briceno A, Evans J, Hayes B, Hahn J, Page K. Acceptability and implications of rapid HCV test among high risk young injection drug users. Oral presentation at the 2012 National Summit on HIV and Viral Hepatitis Diagnosis, Prevention and Access to Care,; November; Washington, DC. 2013. pp. 26–27. [Google Scholar]
- 26.Maher L, Jalaludin B, Chant KG, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101:1499–508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg CH, Smit C, Bakker M, et al. Major decline of hepatitis C virus incidence rate over two decades in a cohort of drug users. Eur J Epidemiol. 2007;22:183–93. doi: 10.1007/s10654-006-9089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003;80:137–46. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz T, Vlahov D, Edwards V, Conover S, Monterroso ER. Sex-specific differences in circumstances of initiation into injecting-drug use among young adult Latinos in Harlem, New York. City. AIDS Behav. 2002;6:117–22. [Google Scholar]
- 30.Sherman SG, Latkin CA, Gielen AC. Social factors related to syringe sharing among injecting partners: a focus on gender. Subst Use Misuse. 2001;36:2113–36. doi: 10.1081/ja-100108439. [DOI] [PubMed] [Google Scholar]
- 31.Hahn JA, Evans JL, Davidson PJ, Lum PJ, Page K. Hepatitis C virus risk behaviors within the partnerships of young injecting drug users. Addiction. 2010;105:1254–64. doi: 10.1111/j.1360-0443.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris M, Evans J, Yu M, Briceno A, Page K, Hahn J. Risk profiles of injecting partnerships: correlates of receptive syringe and cooker sharing among a cohort of young injection drug users in San Francisco, CA. Oral presentation at the 2012 International AIDS Conference,; July 2012; Washington, DC,. [Google Scholar]
- 33.McMahon JM, Tortu S, Pouget ER, Torres L, Rodriguez W, Hamid R. Effectiveness of couple-based HIV counseling and testing for women substance users and their primary male partners: a randomized trial. Adv Prev Med. 2013:286207. doi: 10.1155/2013/286207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug Alcohol Depend. 2006;83:147–56. doi: 10.1016/j.drugalcdep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Nandi A, Glass TA, Cole SR, et al. Neighborhood poverty and injection cessation in a sample of injection drug users. Am J Epidemiol. 2010;171:391–8. doi: 10.1093/aje/kwp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans JL, Hahn JA, Lum PJ, Stein ES, Page K. Predictors of injection drug use cessation and relapse in a prospective cohort of young injection drug users in San Francisco, CA (UFO Study) Drug Alcohol Depend. 2009;101:152–7. doi: 10.1016/j.drugalcdep.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes T, Hedrich D. Harm reduction among injection drug users—evidence of effectiveness. In: Rhodes T, Hedrich D, editors. Harm reduction—evidence, impacts and challenges. European Monitoring Centre for Drugs and Drug Addiction; 2010. pp. 115–164. [Google Scholar]
- 38.Subramaniam GA, Fishman MJ, Woody G. Treatment of opioid-dependent adolescents and young adults with buprenorphine. Curr Psychiatry Rep. 2009;11:360–3. doi: 10.1007/s11920-009-0054-5. [DOI] [PubMed] [Google Scholar]
- 39.Kwon JA, Iversen J, Maher L, Law MG, Wilson DP. The impact of needle and syringe programs on HIV and HCV transmissions in injecting drug users in Australia: a model-based analysis. J Acquir Immune Defic Syndr. 2009;51:462–9. doi: 10.1097/QAI.0b013e3181a2539a. [DOI] [PubMed] [Google Scholar]
- 40.Asselah T, Marcellin P. Direct acting antivirals for the treatment of chronic hepatitis C: one pill a day for tomorrow. Liver Int. 2012;32(suppl 1):88–102. doi: 10.1111/j.1478-3231.2011.02699.x. [DOI] [PubMed] [Google Scholar]
- 41.Martin NK, Pitcher AB, Vickerman P, Vassall A, Hickman M. Optimal control of hepatitis C antiviral treatment programme delivery for prevention amongst a population of injecting drug users. PLoS One. 2011;6:e22309. doi: 10.1371/journal.pone.0022309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeiler I, Langlands T, Murray JM, Ritter A. Optimal targeting of hepatitis C virus treatment among injecting drug users to those not enrolled in methadone maintenance programs. Drug Alcohol Depend. 2010;110:228–33. doi: 10.1016/j.drugalcdep.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Hahn J, Wylie D, Dill J, et al. Potential impact of vaccination on the hepatitis C virus epidemic in injection drug users. Epidemics. 2009;1:47–57. doi: 10.1016/j.epidem.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maher L, White B, Hellard M, et al. Candidate hepatitis C vaccine trials and people who inject drugs: challenges and opportunities. Vaccine. 2010;28:7273–8. doi: 10.1016/j.vaccine.2010.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–62. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–88. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 48.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107:1984–95. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho RA. Needle exchange, pragmatism, and moralism. Am J Public Health. 2000;90:1387–8. doi: 10.2105/ajph.90.9.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strathdee SA, Shoptaw S, Dyer TP, Quan VM, Aramrattana A. Towards combination HIV prevention for injection drug users: addressing addictophobia, apathy and inattention. Curr Opin HIV AIDS. 2012;7:320–5. doi: 10.1097/COH.0b013e32835369ad. [DOI] [PMC free article] [PubMed] [Google Scholar]


