Abstract
Most people who inject drugs (PWID) are infected with hepatitis C virus (HCV), and PWID have the highest risk of HCV infection of any risk group. The incidence of HCV infection is 5%–25% per year, demonstrating continued need for HCV infection prevention in PWID. Existing data in chimpanzees and PWID suggest that protective immunity against persistent HCV infection is achievable. Due to the high incidence of infection, PWID are both the most likely to benefit from a vaccine and a population in which vaccine efficacy could be tested. Challenges to testing a vaccine in PWID are significant. However, the first HCV vaccine trial in at-risk HCV-uninfected PWID was initiated in 2012. The results will likely guide future vaccine development and strategies for vaccination of this and other high-risk populations.
Keywords: hepatitis C virus, viral hepatitis, injection drug users, vaccine, hepatitis
The highest prevalences of hepatitis C virus (HCV) infection is reported among people who inject drugs (PWID). In 1991, antibodies to HCV (anti-HCV) were detected in 1203 of 1356 (89%) PWID in Baltimore [1]. In that study and subsequent studies in the same cohort, HCV infection was linked to high-risk drug use practices, but not other exposures, and was highly prevalent even in those who acknowledged only a few years of injection drug use. In a systematic review of the global prevalence of HCV infection among PWID, >80% were infected in 12 countries and the mid-point prevalence was 60–80% for 25 countries (Supplementary Figure 1) [1–5]. They estimated that 10 million PWID had HCV antibodies worldwide (Supplementary Figure 1) [1–5].
ONGOING HCV RISK IN PWID
Hagan et al evaluated new HCV infections in 5 US cities from 2002 to 2004 among 483 PWID aged 15–30 years [6]. HCV incidence was 17.2 cases per 100 person-years and was strongly linked to shared use of drug preparation equipment (but not syringe sharing). An estimated 37% of HCV seroconversions in PWID were due to the sharing of drug preparation equipment, demonstrating that needle sharing is not the only source of risk. In Australia, an HCV incidence of 45.8 per 100 person-years was reported. Infection risk was highest in new injectors (injecting duration <1 year), having a culturally and linguistically diverse background, and injecting cocaine use [7]. In most settings, incidence is especially high when injection drug use begins, perhaps related to PWID being taught to inject by more experienced, HCV-infected PWID sharing their needles or equipment [8].
HCV RISK CAN BE REDUCED, BUT NOT ENOUGH
HCV infections can be prevented among PWID. Hagan et al reviewed 26 studies of various methods to prevent HCV transmission [9]. Interventions using multiple combined strategies reduced risk of seroconversion by 75%. Effective strategies coupled substance abuse treatment and methods to improve the safety of injections. Accordingly, HCV incidence in some settings has declined. In the Baltimore PWID cohort mentioned above, Mehta and coworkers showed that the HCV incidence declined during 4 periods (1988–1989, 1994–1995, 1998, and 2005–2008) [10]. However, the incidence remained robust with 22.0 cases per 100 person-years in the 1988–1989 cohort declining to 7.8 cases per 100 person-years in the 2005–2008 cohort. In Amsterdam, more dramatic reductions in HCV incidence have been reported [11]. Nonetheless, in most settings, HCV incidence remains high among PWID. Emerging data from in vitro studies demonstrated that the virus is more stable in the environment than previously believed [12–14]. In one study, a high HCV inoculum remained infectious in cell culture after storage in bottled water for 3 weeks [14]. In another, high HCV titers were recovered from swabs used during drug use [13]. Collectively, these data indicate the need for measures such as HCV vaccination to help reduce HCV transmission among PWID.
TREATMENT IS A FORM OF PREVENTION
HCV is not transmissible from persons without HCV RNA; thus, is it possible that treatment will reduce the HCV reservoir sufficiently to prevent HCV transmission [15]. However, despite the numerous potentially effective agents for treating HCV in the drug development pipeline, PWID and other marginalized populations at risk for HCV infection are unlikely to be treated in high enough numbers to control this international epidemic. The cost of treatment is substantial, and inadequate access to medical care will limit uptake. None of the agents in testing have been shown to prevent reinfection, further decreasing effectiveness in a population at ongoing high risk of infection. An alternative and potentially effective way to prevent disease from HCV infection is infection prevention through vaccination.
HCV PREVENTION BY VACCINATION
Spontaneous recovery from HCV infection occurs in 25%–30% of primary HCV infections in humans. Given the high incidence of HCV infection among PWID and frequent persistence of drug use, one would expect that nearly all anti-HCV–positive PWID would also be HCV RNA positive. For example, if 20% of infections were cleared and a person were reexposed only 2 additional times during a lifetime of injection drug use, then the prevalence of anti-HCV–positive, RNA-negative PWID should be 0.23, or 0.8%. However, even in settings with high rates of other factors that increase HCV persistence such as human immunodeficiency virus infection and African ancestry, at least 15% of anti-HCV–positive PWID are HCV RNA negative at any time [16, 17]. That finding suggests that those who clear infection for the first time are less likely to have viral persistence the next time they are infected than are persons who have never been infected. Whether that finding indicates the induction of protective immunity or simply that the subset of patients who cleared once are more likely to clear again for fixed genetic reasons required additional studies of chimpanzees and prospective studies of PWID.
Studies in the chimpanzee model of HCV infection suggest that immunity against HCV can be generated both by infection and by vaccination [18–22]. That immunity can be protective against persistent HCV infection even if not sterilizing, as demonstrated by clearance of multiple infections with both homologous and heterologous virus [19–22]. Some chimpanzees were infected with challenges at high infectious doses, suggesting incomplete protection against reinfection. However, where assessed, the kinetics of viral infection were altered in reinfected vs naive animals [21]. Clearance of both homologous and heterologous viral rechallenges was associated with decreased duration and magnitude of viremia, suggesting enhanced efficacy in responding to HCV infection in subsequent exposures [19, 21].
Studies in PWID also demonstrate protective immunity following spontaneous clearance. Two studies have demonstrated a decreased risk of becoming viremic in previously infected PWID compared to naive PWID, even after accounting for risk behavior [17, 23]. Brief viremia could have been missed in these studies with less frequent sampling because detection of virus present very transiently is known to be limited by sampling at longer intervals [24, 25]. However, continuous viremia is easily detected and in both studies, the rates of persistent infection in subsequent HCV exposures were lower than in people infected with HCV for the first time. To reduce the risk of missing brief viremia, another study examined PWID at more frequent intervals. This study found that PWID who cleared an HCV infection with subsequent reinfection with a heterologous hepatitis C virus controlled those new infections 82% of the time [26]. This is a much higher clearance rate than that seen in subjects infected for the first time (27% in the same cohort).
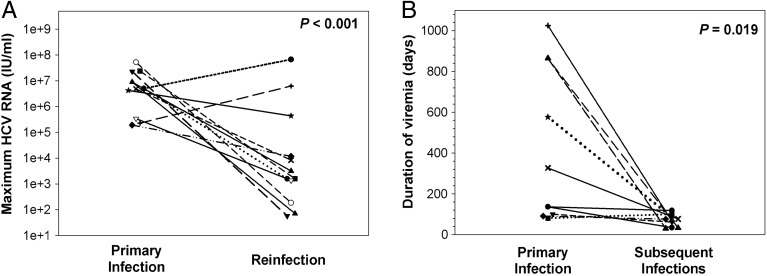
As seen in chimpanzees, reinfected PWID also exhibited decreased duration and magnitude of viremia in subsequent infections compared with the initial infection (Figure 1) [26]. This finding in both chimpanzees and PWID was a very important step toward demonstrating that protective immunity is generated. More rapid and effective control of subsequent than initial infections suggests a role for immunologic memory. The reduction in the magnitude and duration of viremia in subsequent infections in PWID was associated with broadening of cellular immune responses and the generation of cross-reactive humoral responses [26]. The findings of these studies are consistent with development of adaptive immunity that is not sterilizing, but which protects against chronic disease. Because cirrhosis, hepatocellular carcinoma, and the other severe consequences of HCV infection occur exclusively with chronic infection, prevention of chronic infection may be sufficient to prevent disease. Although a precise understanding of aspects of an immune response to HCV necessary to prevent chronic infection is lacking, some key components have been identified.
Figure 1.
Clearance of a primary infection attenuates the infection kinetics of subsequent hepatitis C virus (HCV) infections in injection drug users. A, Maximum HCV RNA concentrations (IU/mL) detected in serum samples obtained during primary and subsequent infections in subjects with sufficient follow-up after the detection of a reinfection. Triangles represent maximum viremia detected in reinfection-persistent subjects. The maximum viremia in each subject during initial infection and reinfection is connected by a line. Median maximum HCV RNA concentration of reinfections was significantly lower than that of primary infections (P < .001). B, Duration of viremia (days) during primary infections and subsequent infections in reinfection-cleared subjects. The duration of initial and reinfection viremia in each subject is connected by a line. The duration of viremia during reinfection was significantly lower than in primary infection (P = .019). Reprinted from Osburn et al [26] with permission. Abbreviation: HCV, hepatitis C virus.
PROTECTIVE IMMUNITY
Humoral Immunity
Initial studies of neutralizing antibodies found that only a minority of patients who controlled infection developed neutralizing antibodies, limiting initial support for their role in HCV control [27, 26]. However, those studies assessed neutralization of a surrogate virus that differed from the virus circulating in the infected patients. Later studies performed using specimens from single-source HCV outbreaks with a defined inoculum enabled the study of neutralizing antibodies specific for the infecting virus. In those studies, the rapid development of isolate-specific neutralizing antibodies was associated with control of initial HCV infection [29, 30]. In repeated HCV infection of PWID, neutralizing antibodies were detected in the acute phase in 60% of those who controlled the reinfecting virus, whereas cross-reactive neutralizing antibodies are rarely detected in the acute phase in patients who progress to chronic infection [26]. Neutralizing antibodies develop in those with chronic infection, but are detected later in the course of infection [29]. In the chronic phase of infection, antibodies drive HCV sequence evolution and neutralizing antibody escape in association with viral persistence [31, 32]. Thus, the timing of neutralizing antibody development seems important to control, and the existence of neutralizing antibodies prior to infection might be predicted to result in control of HCV infection.
Many successful vaccines against other pathogens are based on the generation of neutralizing antibodies prior to exposure to prevent infection. Vaccine studies with the goal of inducing neutralizing antibody to prevent infection have been carried out in chimpanzees with recombinant HCV envelope (E1E2) antigens [33]. Protection against acute infection after challenge with small quantities of homologous genotype HCV was observed in a high proportion of chimpanzees following vaccination [33]. However, the vaccine failed to uniformly prevent chronic infection or to protect against a heterologous subtype challenge [33]. Immunity induced by this candidate vaccine appeared to protect during periods in which high anti-E1E2 antibody titers were observed, but those periods were brief [33]. Another attempt at E1E2 protein immunization of chimpanzees found that this strategy induced a delay in virus replication but did not prevent chronic infection [34]. A third vaccine using plasmid DNA encoding E2 resulted in 2 vaccinated chimps controlling homologous challenge infections whereas the control chimp became chronically infected [35]. The incomplete control of HCV with vaccines designed to generate neutralizing antibody responses and data supporting a role for T cells in control of HCV prompted interest in vaccines that might induce protective T-cell immunity instead of or in addition to humoral responses.
T-Cell Immunity
CD4+ and CD8+ T-cell responses are likely required for protection against HCV infection. Rapid virologic control upon reinfection in PWID and chimpanzees has been associated with broad HCV-specific T-cell responses [22, 36, 37]. When either CD4+ T cells or CD8+ T cells were depleted in vivo prior to reinfection in chimpanzees that had controlled prior HCV infections, persistent HCV infection ensued [36, 38]. Following CD8+ T-cell depletion, HCV viremia was controlled only once CD8+ T cells reappeared in the liver [36]. This evidence supporting the necessity of T-cell–mediated immunity in control of HCV infection prompted development of candidate HCV vaccines designed to induce T-cell responses. These include vaccines using (1) DNA-based immunization, (2) DNA priming followed by recombinant virus vector or HCV protein boosting, (3) recombinant adenovirus priming and DNA boosting, (4) combinations of recombinant viruses for prime and boost, (5) recombinant baculovirus-derived virus-like particles, (6) hepatitis B virus surface antigen–HCV recombinants, and (7) peptides or peptides incorporated in lysosomes [39].
Most candidate vaccines have produced humoral and cell-mediated immune responses in the animals in which they were tested (primarily mice). In the relatively few studies in which immunization and challenge of chimpanzees was carried out, all but 1 of these studies failed to completely prevent chronic infections [40]. Challenges in the chimpanzee trials include the small number of animals vaccinated, making statistical significance difficult to achieve even with a potentially effective vaccine. One of the vaccines tested in chimpanzees prevented 4 of 5 chronic infections and produced viral kinetics similar to those seen in PWID who successfully control HCV with repeated exposure [18]. With this vaccine, all vaccinated chimpanzees showed a significantly blunted peak of viremia with the average peak >100 times lower in the HCV vaccination group than in the control group. Four of the 5 vaccinated chimpanzees had a significantly shorter duration of viremia vs the control group, and the 4 eventually cleared the virus. However, 1 vaccinated chimpanzee maintained low levels of HCV RNA during the entire observation period of the study. Variants of the same vaccine were tested in humans who were not at risk of HCV infection and found to induce very robust HCV-specific CD4+ and CD8+ T-cell responses that recognized peptides from multiple genotypes of HCV [41]. What remains is to determine efficacy in an at-risk population.
CHALLENGES TO VACCINE DEVELOPMENT
Challenges to testing a vaccine in PWID are significant and include increased risk of death due to trauma and drug overdose, high rates of incarceration, and frequent changes in contact information and social instability that make following subjects difficult. However, the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases funded a trial in at-risk PWID that began in 2012. The HCV vaccines used this study are based on the vaccine previously shown to induce robust T-cell responses in chimpanzees and healthy volunteers [18, 41]. The prime/boost strategy employed in the trial uses chimpanzee adenoviral (prime) and Modified Vaccinia virus Ankara (boost) vectors encoding the HCV nonstructural region from a genotype 1b virus with genetically inactivated RNA-dependent RNA polymerase activity (NS5b) to induce HCV-specific T-cell responses. The goals of the study are (1) to assess the safety of these new candidate HCV vaccines, (2) to determine if the vaccines will reduce the incidence in HCV-uninfected PWID of chronic HCV infection compared to placebo, and (3) to evaluate the immunogenicity of the new candidate HCV vaccines compared to placebo when administered to HCV-uninfected PWID. Thus, the trial goal is to determine if the vaccine is likely to reduce the rate of HCV disease rather than reducing the incidence of acute infection. Phase 1 of the trial completed enrollment and a planned phase 2 trial is scheduled to begin later in 2013.
If this vaccine proves to be successful in reducing the incidence of chronic HCV infection in PWID, discussion of whether the vaccine should be administered to the general population or only to those at risk is necessary given the challenges in identifying those at risk prior to infection. This vaccine trial will at minimum demonstrate whether or not it is feasible to conduct trials in PWID. The combination of effective antiviral agents to treat HCV infection and a vaccine to prevent HCV infection would make real the possibility of a global reduction in the prevalence of HCV infection. Prophylactic hepatitis B virus vaccination has been associated with a reduction in the rate of hepatocellular carcinoma in Taiwanese children [42]. Studies have also demonstrated reduced mortality in patients with successfully treated HCV vs those in whom HCV was not successfully eradicated [43–45]. Therefore, it is reasonable to anticipate that the combination of prophylactic HCV vaccination and effective therapy of chronic HCV infection will result in reduced cirrhosis, hepatocellular carcinoma, and death due to HCV infection. Given the potential power of effective prophylactic vaccine and chronic treatment combinations, ongoing research to develop a successful HCV vaccine is needed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grants R01AI077757 and U19AI088791 to A. L. C. and DA R37013806 to D. L. T.).
Supplement sponsorship. This article was published as part of a supplement entitled “Prevention and Management of Hepatitis C Virus Among People Who Inject Drugs: Moving the Agenda Forward,” sponsored by an unrestricted grant from the International Network on Hepatitis in Substance Users (INHSU), The Kirby Institute (University of New South Wales), Abbvie, Gilead Sciences, Janssen-Cilag, and Merck.
Potential conflicts of interest. Both authors: No reported conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infections among injection drug users in Baltimore. Medicine. 1995;74:212–20. doi: 10.1097/00005792-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Hoek JA, van Haastrecht HJ, Goudsmit J, de Wolf F, Coutinho RA. Prevalence, incidence, and risk factors of hepatitis C virus infection among drug users in Amsterdam. J Infect Dis. 1990;162:823–6. doi: 10.1093/infdis/162.4.823. [DOI] [PubMed] [Google Scholar]
- 4.Fairley CK, Leslie DE, Nicholson S, Gust ID. Epidemiology and hepatitis C virus in Victoria. Med J Aust. 1990;153:271–3. doi: 10.5694/j.1326-5377.1990.tb136899.x. [DOI] [PubMed] [Google Scholar]
- 5.Jittiwutikarn J, Thongsawat S, Suriyanon V, et al. Hepatitis C infection among drug users in northern Thailand. Am J Trop Med Hyg. 2006;74:1111–6. [PubMed] [Google Scholar]
- 6.Hagan H, Pouget ER, Williams IT, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201:378–85. doi: 10.1086/649783. [DOI] [PubMed] [Google Scholar]
- 7.Maher L, Li J, Jalaludin B, Chant KG, Kaldor JM. High hepatitis C incidence in new injecting drug users: a policy failure? Aust N Z J Public Health. 2007;31:30–5. doi: 10.1111/j.1753-6405.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- 8.Garfein RS, Doherty MC, Monterroso ER, Thomas DL, Nelson KE, Vlahov D. Prevalence and incidence of hepatitis C virus infection among young adult injection drug users. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(suppl 1):S11–9. doi: 10.1097/00042560-199802001-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hagan H, Pouget ER, Des Jarlais DC. A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs. J Infect Dis. 2011;204:74–83. doi: 10.1093/infdis/jir196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta SH, Astemborski J, Kirk GD, Strathdee SA, Nelson KE, Vlahov D, Thomas DL. Changes in blood-borne infection risk among injection drug users. J Infect Dis. 2011;203:587–94. doi: 10.1093/infdis/jiq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paintsil E, He H, Peters C, Lindenbach BD, Heimer R. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis. 2010;202:984–90. doi: 10.1086/656212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thibault V, Bara JL, Nefau T, Duplessy-Garson C. Hepatitis C transmission in injection drug users: could swabs be the main culprit? J Infect Dis. 2011;204:1839–42. doi: 10.1093/infdis/jir650. [DOI] [PubMed] [Google Scholar]
- 14.Doerrbecker J, Behrendt P, Mateu-Gelabert P, et al. Transmission of hepatitis C virus among people who inject drugs: viral stability and association with drug preparation equipment. J Infect Dis. 2013;207:281–7. doi: 10.1093/infdis/jis677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dore GJ, Kaldor JM, McCaughan GW. Systematic review of role of polymerase chain reaction in defining infectiousness among people infected with hepatitis C virus. Br Med J. 1997;315:333–7. doi: 10.1136/bmj.315.7104.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 17.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–83. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 18.Folgori A, Capone S, Ruggeri L, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–7. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 19.Major ME, Mihalik K, Puig M, et al. Previously infected and recovered chimpanzees exhibit rapid responses that control hepatitis C virus replication upon rechallenge. J Virol. 2002;76:6586–95. doi: 10.1128/JVI.76.13.6586-6595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett SE, Guerra B, Brasky K, et al. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–87. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 21.Lanford RE, Guerra B, Chavez D, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–81. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince AM, Brotman B, Lee DH, et al. Protection against chronic hepatitis C virus infection after rechallenge with homologous, but not heterologous, genotypes in a chimpanzee model. J Infect Dis. 2005;192:1701–9. doi: 10.1086/496889. [DOI] [PubMed] [Google Scholar]
- 23.Grebely J, Conway B, Raffa JD, Lai C, Krajden M, Tyndall MW. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–45. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 24.Grebely J, Thomas DL, Dore GJ. HCV reinfection studies and the door to vaccine development. J Hepatol. 2009;51:628–31. doi: 10.1016/j.jhep.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Vickerman P, Grebely J, Dore GJ, et al. The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. J Infect Dis. 2012;205:1342–50. doi: 10.1093/infdis/jis213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–24. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netski DM, Mosbruger T, Depla E, et al. Humoral immune response in acute hepatitis C virus infection. Clin Infect Dis. 2005;41:667–75. doi: 10.1086/432478. [DOI] [PubMed] [Google Scholar]
- 28.Logvinoff C, Major ME, Oldach D, et al. Neutralizing antibody response during acute and chronic hepatitis C virus infection. Proc Natl Acad Sci U S A. 2004;101:10149–54. doi: 10.1073/pnas.0403519101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pestka JM, Zeisel MB, Blaser E, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single-source outbreak of hepatitis C. Proc Natl Acad Sci U S A. 2007;104:6025–30. doi: 10.1073/pnas.0607026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavillette D, Morice Y, Germanidis G, et al. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J Virol. 2005;79:6023–34. doi: 10.1128/JVI.79.10.6023-6034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–78. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Dowd KA, Netski DM, Wang XH, Cox AL, Ray SC. Selection pressure from neutralizing antibodies drives sequence evolution during acute infection with hepatitis C virus. Gastroenterology. 2009;136:2377–86. doi: 10.1053/j.gastro.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choo QL, Kuo G, Ralston R, et al. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc Natl Acad Sci U S A. 1994;91:1294–8. doi: 10.1073/pnas.91.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puig M, Major ME, Mihalik K, Feinstone SM. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine. 2004;22:991–1000. doi: 10.1016/j.vaccine.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Forns X, Payette PJ, Ma X, et al. Vaccination of chimpanzees with plasmid DNA encoding the hepatitis C virus (HCV) envelope E2 protein modified the infection after challenge with homologous monoclonal HCV. Hepatology. 2000;32:618–25. doi: 10.1053/jhep.2000.9877. [DOI] [PubMed] [Google Scholar]
- 36.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimbeni M, Mizukoshi E, Bosmann M, et al. Kinetics of CD4+ and CD8+ memory T-cell responses during hepatitis C virus rechallenge of previously recovered chimpanzees. J Virol. 2003;77:4781–93. doi: 10.1128/JVI.77.8.4781-4793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 39.Youn JW, Hu YW, Tricoche N, et al. Evidence for protection against chronic hepatitis C virus infection in chimpanzees by immunization with replicating recombinant vaccinia virus. J Virol. 2008;82:10896–905. doi: 10.1128/JVI.01179-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmowalid GA, Qiao M, Jeong SH, et al. Immunization with hepatitis C virus-like particles results in control of hepatitis C virus infection in chimpanzees. Proc Natl Acad Sci U S A. 2007;104:8427–32. doi: 10.1073/pnas.0702162104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barnes E, Folgori A, Capone S, et al. Novel adenovirus-based vaccines induce broad and sustained T cell responses to HCV in man. Sci Transl Med. 2012;4:115ra1. doi: 10.1126/scitranslmed.3003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang M-H, Chen CJ, Lai M-S, et al. Universal hepatitis B vaccination in Taiwan and incidence of hepatocellular carcinoma in children. N Engl J Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 43.Veldt BJ, Heathcote EJ, Wedemeyer H, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677–84. doi: 10.7326/0003-4819-147-10-200711200-00003. [DOI] [PubMed] [Google Scholar]
- 44.Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–7. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 45.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–16. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.