Abstract
Background
Batrachochytrium dendrobatidis (Bd), the causative agent of chytridiomycosis, is decimating amphibians worldwide. Unsurprisingly, the majority of studies have therefore concentrated on documenting morbidity and mortality of susceptible species and projecting population consequences as a consequence of this emerging infectious disease. Currently, there is a paucity of studies investigating the sub-lethal costs of Bd in apparently asymptomatic species, particularly in controlled experimental conditions. Here we report the consequences of a single dose of B. dendrobatidis zoospores on captive adult palmate newts (Lissotriton helveticus) for morphological and behavioural traits that associate with reproductive success.
Results
A single exposure to ~2000 zoospores induced a subclinical Bd infection. One week after inoculation 84% of newts tested positive for Bd, and of those, 98% had apparently lost the infection by the day 30. However, exposed newts suffered significant mass loss compared with control newts, and those experimental newts removing higher levels of Bd lost most mass. We found no evidence to suggest that three secondary sexual characteristics (areas of dorsal crest and rear foot webbing, and length of tail filament) were reduced between experimental versus control newts; in fact, rear foot webbing was 26% more expansive at the end of the experiment in exposed newts. Finally, compared with unexposed controls, exposure to Bd was associated with a 50% earlier initiation of the non-reproductive terrestrial phase.
Conclusions
Our results suggest that Bd has measureable, but sub-lethal effects, on adult palmate newts, at least under the laboratory conditions presented. We conclude that the effects reported are most likely to be mediated through the initiation of costly immune responses and/or tissue repair mechanisms. Although we found no evidence of hastened secondary sexual trait regression, through reducing individual body condition and potentially, breeding season duration, we predict that Bd exposure might have negative impacts on populations of palmate newts through reducing individual reproductive success and adult recruitment.
Keywords: Body condition, Cost of immunity, Chytridiomycosis, Emerging infectious disease, Resistance, Secondary sexual traits
Background
Batrachochytrium dendrobatidis (Bd), the causative agent of chytridiomycosis, has been implicated in worldwide amphibian declines [1]. Although Bd is able to infect a wide range of species and hence displays extreme host generality [2-4], amphibian morbidity and mortality in response to infection is highly variable and host-species specific [5,6]. For example, while some studies report devastating consequences of Bd infection [7-9], others have shown different levels of resistance to Bd infection (capacity to prevent or clear Bd infection) and have provided experimental evidence showing some species are able to clear Bd infection [7,10-13]. In several species, infiltration of neutrophils, lymphocytes, macrophages and inflammation have been reported on the skin of susceptible (i.e., those which are not able to clear Bd infection or to prevent colonization of Bd) amphibians [11,14-16]. Histological examinations of resistant hosts have revealed that Bd infections can cause skin injuries similar to those observed in susceptible species, albeit distributed more patchily on the skin [10-12]. These results suggest that those amphibians that are apparently resistance to Bd infection might still experience sub-lethal costs but whether any such costs are likely to impair traits associated with reproductive success and survival is unclear.
It is well known that pathogens exert significant cost for the host [17,18]. Costs may be manifest through the host’s energetic and cellular response to the infection [19-21], through the pathogens acquisition of host resources, or through inducing greater host susceptibility to other pathogens [22-24]. As such, hosts might show clinical symptoms and visible damage [24], but even where such effects are lacking, significant negative consequences can arise through reductions in investment in traits associated with fitness (e.g., growth, survival and reproduction, [18,19]). As a consequence, in species exhibiting apparent resistance to Bd, we might also expect there to be sub-lethal costs of infection and for such costs to influence populations. In support, a number of studies in frogs and toads have reported subclinical effects of Bd on tadpole growth [25-28], as well as adult body size [29] and body condition [30]. Nevertheless, in such cases, tests of the consequences of Bd infection for traits important in reproduction (morphology, behaviour) of resistant amphibians are generally lacking.
The palmate newt, Lissotriton helveticus (Cuadata: Salamandridae; formerly Triturus helveticus), is a common, semi-aquatic amphibian of Western Europe. Palmate newts inhabit aquatic habitats with still or very slow-moving water during the breeding season, but otherwise have a terrestrial life style [31], occupying moist habitats and refugia [32]. Males develop large ornaments when they enter water for breeding, manifested as a low, straight edged crest which extends dorsally, a thread-like filament projecting from the end of the tail and conspicuous webbing between the toes of the hind feet [33]. These secondary sexual characteristics have been shown to be associated with male reproductive success [31,34-36]. They then gradually start to regress as the newts leave their aquatic habitat, but may not necessarily have fully disappeared as individuals start their migration to their terrestrial feeding [33].
Interestingly, palmate newts have been reported in several Bd infected sites across Europe and have been found to test positively for Bd (global Bd maps, see [37] for details). Despite this, no morbidity or mass mortality have been reported [37,38] and under laboratory conditions, excessive weekly exposures to more than 106 Bd zoospores per week for more than 4 weeks does not induce symptomatic chytridiomycosis even up to 40 days following a month-long exposure course (Cheatsazan, unpublished results 2011, n = 35 newts). These observations suggest that this species exhibits some level of resistance to Bd. However, the consequences of exposure to Bd, which will most likely occur during the aquatic phase as a result of increased probability of exposure to waterborne Bd zoospores, have not been investigated for this species. Here, we inoculated a group of wild-caught palmate newts experimentally with a single dose of Bd (18 controls and 50 exposed) and monitored Bd infection, survival and clinical symptoms, as well as changes in morphological and behavioural traits known to be associated with reproductive success in this species. Our specific aim was to use this experiment to test: (1) whether palmate newts become infected by exposure to low doses of Bd, whether they show external symptoms and whether they are able to tolerate or clear the infection; (2) the consequences of Bd exposure and infection load for secondary sexual characters in males; (3) the consequences of Bd exposure and infection load for mass change in both males and females; and (4) whether exposure to Bd is associated with changes in the probability of an early entry into the terrestrial phase. Throughout, we consider sex differences, where possible.
Methods
In mid May 2011, 68 newts (34 males and 34 females) in aquatic phase were captured in narrow streams on the borders of Bouconne forest, Haute Garonne, France (43° 39’ N and 1° 14’ W, 190 m altitude). Our rationale for using wild-caught rather than laboratory-derived newts was that we wished to use individuals as close as possible to their natural state. The sites used are in a forest reserve with natural and man-made streams, and are located about 100 km outside the current extent of the known Bd infection zone. In addition, our inspections for Bd in this area since 2009 have failed to detect evidence of Bd infection of any amphibian species in the area, and all animals brought back to the laboratory tested negative for Bd. Male and female newts were randomly paired and transferred into plastic tanks (205× 205 × 140 mm). Our reason for this is that wished to examine the effects of Bd exposure on male secondary sexual characteristics, which we considered to be likely influenced by the presence of a female. Each tank contained 1.0 litre of aged tap water and a hollow brick for shelter. We randomly assigned tanks to one of 2 treatment groups: controls (9 males, 9 females) and Bd-exposed (25 males and 25 females). All newts were maintained in the laboratory for 7 days prior to experimentation and were provided with live midge larvae (blood worms), daphnia and/or tubifex every two days. Food was provided by ‘sucking’ a known quantity of liquid (tube length x diameter = 55 cmm x 0.5 cm) containing roughly 75 larvae. Although the precise number of larvae was unknown, there is no reason to suppose this varied systematically between experimentally exposed and control tanks. Room temperature was maintained at 18.6 ± 1.9°C throughout the experiment. Light exposure was adjusted weekly to equal the average day length of the first and of the last days of the focal week (approximately 14 h light: 10 h dark). Water was changed 3 days after inoculation and then weekly thereafter to prevent bacterial blooms in the tank. This also means that levels of Bd loads detected were unlikely to be explained by zoospore survival in the water [39].
Bd cultures were prepared from a Bd extract of an infected tadpole from an introduced population of American bullfrog (Lithobates catesbeianus) in southern France (for extraction protocol see [40]). This extract was grown in 1.0% Trypton (SCHARLAU, cat. No 07–119) and 0.32 g × l-1 glucose (ROTH, cat. No X997) in ultrapure water. Cultures were incubated at 18.5°C to optimize growth of the fungus [5]. Approximately one hour prior to inoculation, we counted approximately 160 active zoospores × ml-1 of the 5th passage of our culture using a haemocytometer. We then added 12 ml of this culture to each Bd exposed tank (~2000 zoospores × l-1 × tank-1). The experiment was stopped 30 days post-inoculation, when about approximately half of the newts had entered or were entering the terrestrial phase of the season (t-phase). When a newt consistently remained out of the water for five consecutive days, we considered the day the newt had left the water for the first time as the start of t-phase. Animal capture was with permission of the prefecture of Haute Garonne (Permission No. 2009–12). Animal housing facility and experiments comply with the regulations of the housing organization (CNRS: National centre for scientific research) and the current rules of France.
Infection was detected by quantitative amplification of Bd-DNA content of weekly swab samples (from: forelimbs, hind limbs, abdomen and cloaca) using fine tip dry swabs (Tubed sterile dryswabTM tip, Medical wire & Equipment, cat. No MW100). Swab samples were taken at upon arrival, day 7, 14, 21, and 30 after the first inoculation from all individuals. Quantitative PCR’s were conducted as described in [41], and after incorporating the changes suggested by Kriger et al. and Hyatt et al. [42-44] except that the we reduced the final volume of reactions to 10 μl due to higher sensitivity of our q-PCR machine (Mastercycler ep realplex4, Eppendorf). Throughout this paper, results of all quantifications are presented in Bd Genome Equivalent (BdGE) per swab bout. In addition to standard duplicates and negative controls, we also included 6–12 samples of control newts in each test plates of exposed samples and utilized internal positive control reagent in all samples (TaqMan® Exogenous Internal Positive Control). To ensure the efficiency of inoculations, we also analysed swab samples of 66 individuals of the same population, after 2 weekly exposures to 106 zoospores of the same Bd strain. The average Bd load of these newts, one week after the second inoculation, was (mean ± S.E.) 442.2 ± 187.4 BdGE, showing the Bd strain and inoculation method was able to generate an infection in this population.
On days 0 and 30 post-inoculation, we measured body mass using a digital scale (±0.01 gram). Snout-to-vent length (SVL) and male secondary sexual traits were measured by ImageJ software on digital photographs of all individuals: area of tail crest, length of tail filament, and average of webbed area of hind feet (left and right feet) one day before inoculation and day 30 after inoculation, according to [36]. Photographs were taken on a single plastic board with a millimetre scaled area of 2 × 10 cm. All morphological measurements were taken twice by the same person (HC) and their average was used in statistical analyses. In all cases a strong correlation (r ≥ 0.90, p < 0.001) was observed between replicates. Finally, we recorded survival, and the start of t-phase as well as symptoms of clinical chytridiomycosis [5].
Infection patterns and symptoms
Changes in the probability of being infected during the experiment were analysed by fitting whether (1) or not (0) a newt was infected on a given day as the response term in generalized linear mixed-effects model (GLMM) with a binomial error structure and logit link function. The binomial denominator and dispersion parameters were 1. Changes to infection loads during the course of the experiment, were analysed using a GLMM with Poisson error structure and log-link function in which load size (numbers of BdGE) was fitted as the response term. In both models, individual subjects’ codes (ID’s) nested within box number were fitted as random terms to account for repeated sampling of each. The SVL and body mass of individuals on day zero were fitted as covariates, while sex, days after the first inoculations (7, 14, 21, 30) and their interaction were fitted as fixed effects. Finally, throughout the experiment we examined the animals for signs to Bd-mediated symptoms.
Secondary sexual characteristics in males
Analysis of change in male secondary sexual characters over the course of the experiment was conducting using multivariate analysis of variance, in order to account for multiple correlated terms of interest (crest area, tail filament length and rear foot webbing: Spearman’s rank correlations between the traits, rs = 0.4-0.86, p < 0.005-0.0001). The changes in each trait were fitted as the response terms, while proportional mass change was fitted as a covariate to account for changes in body condition, and treatment was fitted as a the fixed effect of interest. Because in this case we only used a single measure from a single individual from each box (i.e. the male), there was no need to conduct a mixed model in this analysis.
Mass loss in males and females
The effect of Bd on mass was investigated in two ways. First, an analysis of the effect of treatment on mass change was conducted by fitting mass change between day 0 and 30 as the response term in a general linear model with normal errors. Body length and mass at experimental onset were fitted as covariates, while sex and treatment were fitted as the primary fixed effects of interest, along with their interaction. Second, an analysis of the effect of Bd-load change during the course of the experiment on mass change was conducted in the same way, but wherein treatment was swapped for load change among exposed individuals. Because both of these analyses included a male and a female from the same box, it could be argued that such pairings are not independent units. However, initial residual maximum likelihood models fitting box number as a random term showed that the box from which individuals were obtained had a negative component of variance, indicating that the variation within boxes was indistinguishable from that between boxes. We therefore elected for the more parsimonious GLM rather than REML approach, since the results were almost identical and the former allows estimation of variance explained.
Terrestrial phase
The probability that individuals initiated t-phase within the 30-day experimental period was investigated by fitting whether (1) or not (0) individuals entered t-phase as the response term using two GLMs with binomial error structures with logit link functions. In the first case, treatment was fitted as the primary factor of interest, and in the second, treatment was replaced by Bd-load. In each case, body length (SVL) and mass at experimental onset were fitted as covariates, and sex was fitted as an additional factor. In both analyses, we investigated the effect of tank identity in GLMMs, but in both cases it represented a negative component of variance.
All statistics were conducted in Genstat Release 14 (Rothamsted Experimental Station, Harpenden, UK). The statistics are provided for all terms included in the models, and effect sizes ± standard errors are provided for terms of interest. Terms were dropped from models when their exclusion failed to generate a significant loss of model variance. All p values provided are two-tailed.
Results
Infection patterns and symptoms
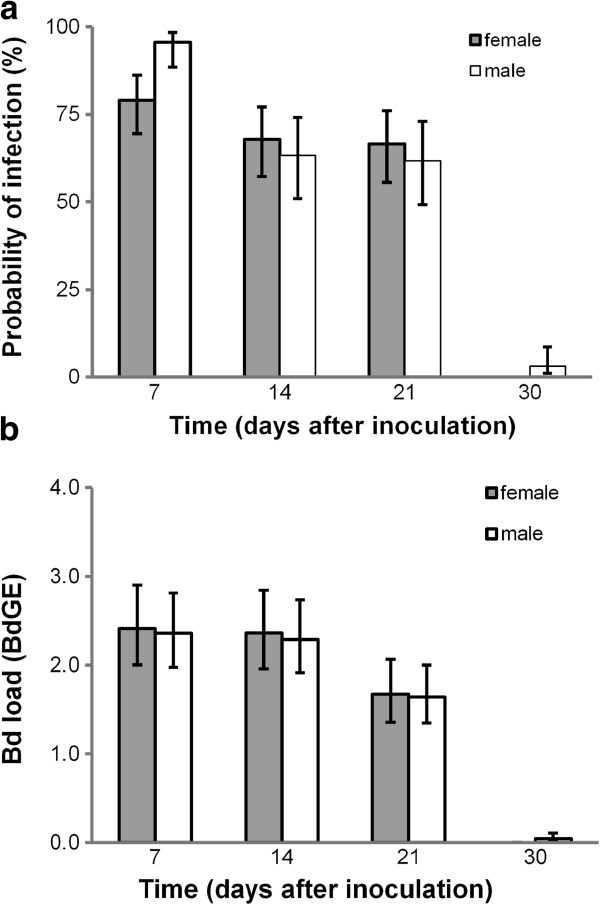
At experimental onset, all newts tested negative for Bd, but following exposure to Bd, 94% of individuals became infected. Of these, most became infected in the first week, although 4% became infected by the end of week two and 2% were found to be infected at the end of week three. Only 4% of newts remained infected 30 days after the onset of the experiment. As a consequence, the probability that individuals were infected with Bd changed substantially during the course of the month-long experiment (Figure 1a). Larger newts were less likely to be infected on a given day (effect ± S.E. = −0.20 ± 0.097; χ2 = 4.16, df = 1, p = 0.041), but there was no effect of initial body mass (effect ± S.E. = −0.73 ± 1.46 χ2 = 0.49, df = 1, p = 0.49) and no differences between the sexes (χ2 = 0.15, df = 1, p = 0.70; Figure 1a) on patterns of infection.
Figure 1.
Changes in probability and intensity of the infection during the course of the experiment. (a) The probability of infection gradually decreased after the first week and by 30 days post-inoculation, most newts tested negative for Bd (GLMM: days post-inoculation; χ2 = 30.12, df = 3, p < 0.0001; interaction sex x days post-inoculation χ2 = 2.68, df = 3, p = 0.44).(b) The average infection burden was constant until 2 weeks after inoculation; during the third week after inoculation the infection intensity started to decline and generally reached zero at day 30 post-inoculation (GLMM: days post-inoculation; χ2 = 9.20, df = 3, p < 0.001; interaction sex x days post-inoculation χ2 = 0.01, df = 3, p = 0.99). Figures show predicted means ± SE generated from the mixed model analyses. In both analyses, tank had no influence on patterns of Bd infection (tank component = 0), but there was some consistency in the prevalence of Bd infection within versus among individuals (component = 0.01 ±0.009 (S.E.)) and a significant difference between individuals in Bd loads (component = 0.12 ±0.004 (S.E.)).
Over the course of the experiment, Bd loads ranged from 0–14 BdGE per swab. Newts that were long (SVL) had reduced loads on average (GLMM with Poisson errors, effect ± S.E.; -0.075 ±0.035; χ2 = 4.70, df = 1, p = 0.030), but there was no effect of body mass at the onset of the experiment (0.42 ± 0.44; χ2 = 0.90, df = 1, p = 0.34) and no difference between the sexes (χ2 = 0.01, df = 1, p = 0.93, Figure 1b). As with the incidence data above, Bd loads varied dramatically throughout the month-long experiment: in both sexes, Bd loads were at their maximum seven days after inoculation and declined to zero after a month (Figure 1b).
The results above suggest that palmate newts are resistant to Bd. In support, we found no evidence to suggest that Bd induces mortality or visible clinical symptoms in the captive palmate newts. During the course of our 30-day study, only three newts died. Two infected newts died (1 male and 1 female) 15 days post-inoculation, and one control (a female) did so 24 days following the onset of the experiment. Prior to their death, none of the exposed newts showed symptoms of clinical chytridiomycosis, such as skin lesions, haemorrhage, or absence of righting reflex [45,46]. Bd contents of swab samples taken the day before of death of the two treated newts were 0 and 14 BdGE in female and male individual, respectively.
Secondary sexual characteristics in males
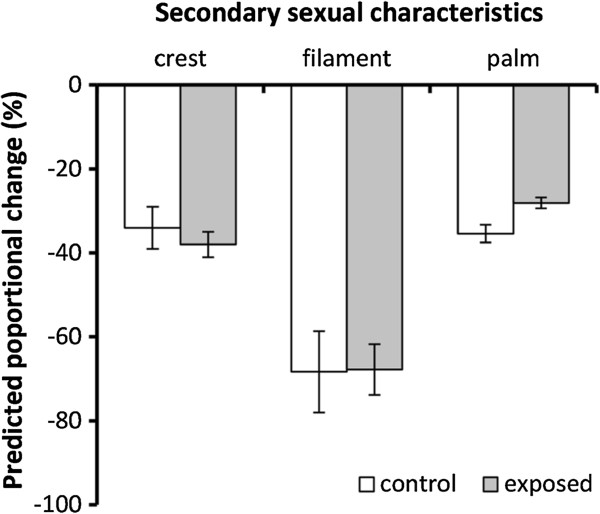
Male palmate newts show three obvious morphological characteristics during the aquatic breeding phase. At experimental onset, the mean (±SD) crest area, tail filament length and hind food webbing area were 110.4 (±12.2) mm2, 4.6 (±1.2) mm, 18.2 (±1.3) mm2, respectively. The onset of the experiment was timed to coincide with the peak breeding season and hence the maximum extent of male secondary sexual characteristic sizes. During the course of the experiment, crest and hind food web area declined by 37% and 31%, respectively, while tail filament was reduced in length by 68% (Figure 2). A multivariate analysis of variance (MANOVA) revealed that after controlling for the proportion of mass lost (F3,26 = 3.04, p = 0.047), there was an overall effect of treatment on the reduction of the extent of secondary sexual characteristics (F3,26 = 5.43, p = 0.005). However, univariate ANOVAs showed that this overall effect was driven entirely by the effect of Bd treatment on a reduction (not increase) of the loss of hind food web area (crest area, F1,28 = 0.45, p = 0.51; filament length, F1,28 = 0.01, p = 0.94; hind food web area, F1,28 = 12.37, p = 0.002).
Figure 2.
Effects of Bd exposure on male’s secondary sexual traits. Exposed and non-exposed males showed similar reductions in crest area and tail filament length, but the former showed reduced reabsorption of their rear feet webbing. Figures show predicted means ± S.E. from MANOVA.
Mass loss in males and females
At the time of capture, females were slightly longer than males (mean ± SD snout-vent length (hereafter body length) = 34.9 ± 1.9 vs. 32.6 ± 1.8 mm: t-test, t66 = 5.10, p < 0.001), but there was no difference in their respective masses (1.19 ±0.23 vs. 1.20 ±0.20 g: t63 = 0.49, p = 0.63). Individuals that were heavy at experimental onset lost significantly more mass over the 30-day experiment than those that were light (GLM: F1,63 = 30.72, p < 0.001), but there was no effect of body length (GLM: effect ± s.e. = −0.0064 ± 0.0058; F1,62 = 1.22, p = 0.27). After controlling for significant effects of the former, we found that exposed newts lost 33% more mass than control newts, with treatment explaining 7.5% of the variation in mass change during the experiment (F1,63 = 10.55, p = 0.002; Figure 3a). Males and females lost similar amounts of mass (F1,62 = 1.23, p = 0.27) and the effect of experimental treatment on mass loss did not differ between the sexes (F1,61 = 0.28, p = 0.60; Figure 3a).
Figure 3.
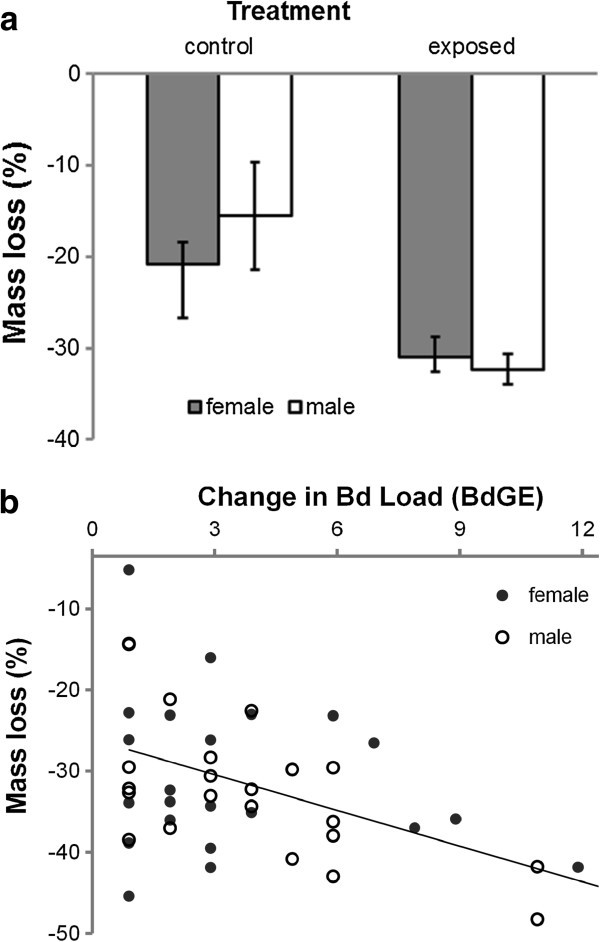
Effects of Bd exposure and load on body mass during the 30-day experiment. (a) Exposed newts of both sexes lost more mass than unexposed controls; (b) among the infected newts, the greater variation in the Bd load was correspondent with greater mass loss. Figures show (a) predicted means ± S.E. and (b) raw values and predicted line.
The results above suggest that palmate newts suffer a cost of clearing Bd from their system. However, a better test of this hypothesis is to investigate the relationship between the change in Bd load and the change in mass over the course of the experiment. The mass lost by experimental newts increased as a function of increasing body mass at the onset of the experiment (GLM: effect ± S.E. = 0.27 ±0.066; F1,44 = 17.61, p < 0.001), but was uninfluenced by SVL (effect ± S.E. = −0.0022 ± 0.0062; F1,43 = 0.13, p = 0.73). After controlling for the effects of mass at experimental onset, we found a significant negative relationship with Bd load change and mass loss, with newts losing 5% of their body mass for every four BdGE’s that they cleared (effect ± S.E. = 0.051 ± 0.012; F1,44 = 18.36, p < 0.001, R2 = 20%; Figure 3b). This effect was common to both males and females (GLM: sex*Bd change interaction; F1,42 = 0.03, p = 0.86).
Terrestrial phase
Overall, 46% of newts were judged to have entered t-phase by the end of the 30-day experiment. There were non-significant tendencies for males to enter t-phase after females (GLM, χ2 = 1.31, p = 0.25) and those in poor body condition to enter t-phase before those in good condition (body mass correcting for body length, χ2 = 2.56, p = 0.11). After controlling for these effects, we found that exposed newts were 50% more likely to enter into t-phase during the 30-day experiment that control newts (χ2 = 3.99, p = 0.046; Figure 4a).
Figure 4.
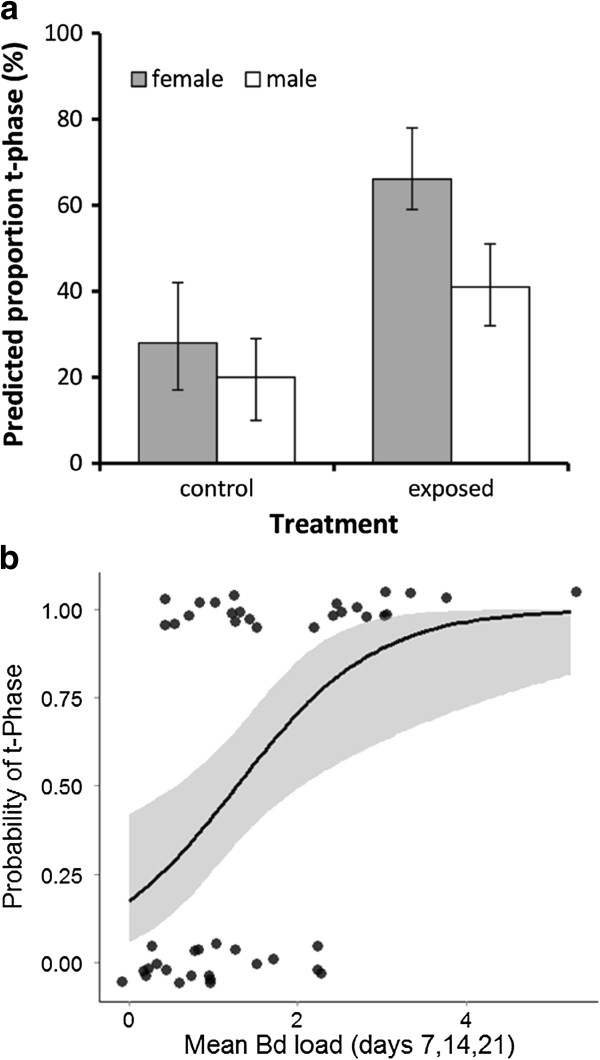
Consequences of Bd-exposure and Bd-infection for probability of termination of aquatic breeding season. (a) Exposed individuals were more likely to enter the terrestrial phase than unexposed controls; and (b) higher infection intensities were associated with increased probability of transition to terrestrial phase. Figures show GLM predicted (a) predicted percentages from a GLM ± SE and (b) shows the relationship between average Bd load and occurrence of t-phase among the Bd exposed newts. The line represents the linear predictor of the GLM (probability of t-phase) and the shaded area shows ± SE of the predictor.
Within the exposed individuals, males and females had a similar probability of entering into t-phase (GLM; Sex, χ2 = 1.02, p = 0.31). There was no effect of body length on the probability of entering t phase (SVL effect ± S.E. = 0.027 ± 0.15; χ2 = 0.03, p = 0.86), but those individuals in poor conditions were more likely to enter the t-phase than those in better condition (initial mass effect ± S.E.-3.46 ± 1.69; χ2 = 4.71, p = 0.030). After controlling for these effects, we found that newts were more likely to enter t-phase if they had high average Bd loads during the first three weeks of the experiment, independently of sex (Bd load effects ± S.E. = 0.73 ± 0.33; χ2 = 5.92, p = 0.015; sex x Bd load χ2 = 0.01, p = 0.99; Figure 4b).
Discussion
Our results suggest that the Bd dose administered is capable of inducing a subclinical infection in the palmate newt within one to two weeks after inoculation. We found no evidence to suggest that Bd caused signs of chytridiomycosis [46] or death, and indeed, virtually all exposed newts appeared to have cleared any infection by one month post-inoculation. These results complement circumstantial field evidence documenting that in Bd areas, palmate newts appear not to suffer mass mortality (e.g., [37,38]). Nevertheless, our evidence also suggest that such apparent resistance to Bd comes at a cost of increased mass loss during the aquatic phase and a more rapid transition to terrestrial t-phase compared to non-exposed controls. Within exposed newts, both the amount of mass lost and probability of entering t-phase increased as a function of increasing pathogen load clearance. By contrast, the rate of loss of secondary sexual characteristics were generally not influenced by Bd infection, with the exception of hind foot webbing that remained longer in exposed newts than controls. While the devastating impacts of Bd on amphibians are well publicised [7-9,47], much less is known about the extent, form and underlying causes of more subtle symptoms in apparently resistant amphibian species. Our results suggest that caution should be exercised before concluding that Bd has negligible consequences for apparently resistant species.
Bd is known to invade the host epidermis; feeding on various nutrients (e.g., keratin), causing pathological abnormalities and impairing critical cutaneous functions, such as the maintenance of osmotic balance (reviewed in [5]). Although Bd infection can have devastating consequences (see Introduction), accumulating evidence suggests that some amphibians only exhibit subclinical symptoms and might be able to effectively clear the infection through mechanisms such as antimicrobial peptides [13,48], Bd killing microbial flora on their skin [49-51], anti-Bd immunoglobulin’s [52-54], increasing body temperature during the infection [55] and improvements to dietary condition [56] (reviewed in [57]). In such circumstances, individuals might still suffer costs: (i) because pathogens impair body functioning; (ii) because mounting an immune response or repairing damaged tissues requires energy; (iii) because pathogens actually consume host energy resources; and/or (iv) because immune-associated illness-induced anorexia reduces energy intake [23,58-60]. For instance, in wild frog populations, Bd infection has found to be associated with smaller body size [29,61], although the mechanism(s) causing the reduction in body size in these frog studies was unclear. The evidence for our study suggests that increased mass loss might be mediated by a cost of immunity [22], but verification of this as a specific mechanism needs elucidating through more targeted experimentation in our and other studies. For example, we are not able to rule out a role of adaptive anorexia, but we suggest that such an effect is unlikely to explain our results fully, since newts were not fed adlib and we noticed no obvious surplus of food in experimental tanks. Indeed, that a recent study has shown experimentally that mounting an innate immune response (skin peptides) against Bd comes at cost to host body condition [62], provides some tentative support for our conclusions.
We found little evidence to suggest that the regression of secondary sexual characteristics were hastened by exposure to Bd, but we found some support for the possibility that breeding season duration might be curtailed. Both the regression of sexually selected characteristics and transition into t-phase are thought to be largely under hormonal control [63-65]. Although, we were not able to measure neuroendocrine changes of the exposed and infected newts, our results fit well with the current knowledge of amphibians’ stress responses and its impacts on reproduction. In amphibians, exposure to pathogens can cause a rapid release of anti-microbial peptides [66,67] through activation of hypothalamic-pituitary-adrenal (HPA) axis (=stress axis in mammals) [67-69]. Stimulation of this axis can result in inhibition of production and release of stress hormone (i.e. corticosterone) [70,71] which, in urodeles, inhibits the courtship behaviour, development/maintenance of male secondary sexual traits and triggers the migration toward the terrestrial habitat [63,64,72-74]. At the behavioural level, a decrease of prolactin triggers the termination of aquatic phase and migration toward terrestrial habitat while at a morphological level the decrease of prolactin induces the decline of tail crest [63,64,73,74]. However, the decline of hind feet webbing is mediated by a different mechanism which involves a synergetic effect of several hormones [73,75]. Therefore, the slowed reduction of hind feet webbings, in comparison to tail crest and tail filament, might be due to the difference of the hormonal bases which control these traits. In order to elucidate the potential mechanisms of slowed regression of foot webbing (in comparison with other secondary sexual traits, or vice versa) as well as early entry into t-phase, more studies are required. In addition, in order to understand the potential consequences, the exact role and relative importance of each sexually selected trait in female choice is required, as is the consequences of the size of each trait for survival on transition to the t-phase.
Although, for obvious ethical and conservation reasons, we were unable to measure the consequences of experimental infection for individual fitness in the wild, we suggest the consequences of Bd that we observed are likely to be significant [26,29,30,76]. In palmate newts, mating success is likely to be influenced by the duration of their aquatic phase, and is known to be condition-dependent: female fecundity and male display rate are both highly demanding energetically [77-79]. It is also highly probable that the success of terrestrial migrations are at least partly associated with having sufficient energy reserves as is the ability to survive winter hibernation, since the annual rate of survival of newts is fairly low (i.e. ≤ 50%, see [80,81]) and newts consume almost all their resources during the winter [78]. Our ability to project the population consequences of sub-lethal infections requires an understanding of whether or not individuals can acquire adaptive immunity to Bd or whether individuals with primed immune system remain susceptible to Bd. Where the former is the case, we would expect Bd to have little impact on palmate newt’s populations once resistance spreads in the populations (e.g. see [62,82]). On the other hand, if the latter is true, the sub-lethal consequences observed in this study are likely to have more significant population consequences, with possible impairment of female fecundity, juvenile recruitment and adult survival. Currently, it is unclear whether amphibian species that suffer subclinical effects of Bd are declining, as one might expect from our results. We urge that future studies are careful to monitor population sizes of all amphibian species in a given area, and attempt to determine whether Bd can also have population consequences, even for apparently resistant species. Further, in the advent of such declines being apparent, it is important to determine whether such declines are generated through biased effects on each sex or age class.
Conclusion
The costs of pathogen infection are common and include a range of sub-lethal symptoms including reduced growth, increased mass loss, increased metabolic rate and/or a readjustment of life history strategies [22,83,84]. Our results suggest that subclinical costs of Bd infection can exert significant costs on an apparently resistant host amphibian, with potential threatening consequences on long term population viability. Indeed, Bd infected palmate newts suffered from a decrease in body condition relative to controls and had delayed absorption of one temporary adaptations for aquatic life; both of which might impede the success of terrestrial migration. In addition, they showed earlier initiation of t-phase, which might reduce the duration of a breeding season, and hence the number of offspring produced by a given population. Furthermore, in addition to the general inhibitory effects of stress on amphibian reproduction, chronic exposure to Bd zoospores in the aquatic habitat may trigger a chronic activation of the stress (HPA) axis which might increase an individual’s susceptibility to other pathogens as well as other environmental stressors. Further studies are required in this species and other apparently asymptomatic species, in order to further appreciate the extent, form and mechanism of sub-lethal costs of Bd infection in amphibian species.
Competing interests
The authors confirm that this work is not a subject of financial and non-financial competing interests.
Authors’ contributions
HC conceived and conducted the experiments with help from AP. The analyses were performed and the paper was written by HC, AFR and CB. All authors read and approved the final manuscript.
Contributor Information
Hamed Cheatsazan, Email: h.cheatsazan@gmail.com.
Ana P Lugon Gavinho de Almedia, Email: aplugon@gmail.com.
Andrew F Russell, Email: A.Russell@exeter.ac.uk.
Camille Bonneaud, Email: C.Bonneaud@exeter.ac.uk.
Acknowledgements
We are grateful to Jean Clobert and Michel Baguette for logistical support and helpful comments, Jeremie Cornuau for his assistance in the field and Michele Huet for her help in molecular analyses. We thank the following for funding: Marie Curie grant (CB; FP7-PEOPLE-IRG-2008, #239257). AP conducted this work in part fulfilment of a Master’s Degree at the University of Dijon.
References
- Kilpatrick AM, Briggs CJ, Daszak P. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol Evol. 2010;25(2):109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Bonaccorso E, Guayasamin JM, Mendez D, Speare R. Chytridiomycosis as a possible cause of population declines in Atelopus cruciger (Anura: Bufonidae) Herp Rev. 2003;34:331–334. [Google Scholar]
- Green DE, Sherman CK. Diagnostic histological findings in Yosemite toads (Bufo canorus) from a die-off in the. J Herpetol. 1970;2001:92–103. [Google Scholar]
- Kriger KM, Hero JM, Ashton KJ. Cost efficiency in the detection of chytridiomycosis using PCR assay. Dis Aquat Organ. 2006;71(2):149–154. doi: 10.3354/dao071149. [DOI] [PubMed] [Google Scholar]
- Voyles J, Rosenblum EB, Berger L. Interactions between Batrachochytrium dendrobatidis and its amphibian hosts: a review of pathogenesis and immunity. Microb Infect. 2011;13(2):25–32. doi: 10.1016/j.micinf.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Gervasi S, Gondhalekar C, Olson D, Blaustein A. Host Identity Matters in the Amphibian-Batrachochytrium dendrobatidis System: Fine-Scale Patterns of Variation in Responses to a Multi-Host Pathogen. PLoS One. 2013;8(1):e54490. doi: 10.1371/journal.pone.0054490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retallick RWR, McCallum H, Speare R. Endemic infection of the amphibian chytrid fungus in a frog community post-decline. PLoS Biol. 2004;2(11):e351. doi: 10.1371/journal.pbio.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P. The decline of the sharp-snouted day frog (Taudactylus acutirostris): the first documented case of extinction by infection in a free-ranging wildlife species? Ecohealth. 2006;3(1):35–40. doi: 10.1007/s10393-005-0012-6. [DOI] [Google Scholar]
- Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth. 2007;4(2):125–134. doi: 10.1007/s10393-007-0093-5. [DOI] [Google Scholar]
- Daszak P, Strieby A, Cunningham AA, Longcore JE, Brown CC, Porter D. Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herp J. 2004;14:201–208. [Google Scholar]
- Davidson EW, Parris M, Collins JP, Longcore JE, Pessier AP, Brunner J. Pathogenicity and transmission of chytridiomycosis in tiger salamanders (Ambystoma tigrinum) Copeia. 2003;2003(3):601–607. doi: 10.1643/CP-02-120R1. [DOI] [Google Scholar]
- Reeder NMM, Pessier AP, Vredenburg VT. A Reservoir Species for the Emerging Amphibian Pathogen Batrachochytrium dendrobatidis Thrives in a Landscape Decimated by Disease. PLoS One. 2012;7(3):e33567. doi: 10.1371/journal.pone.0033567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta Biomembranes. 2009;1788(8):1593–1599. doi: 10.1016/j.bbamem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Organ. 2005;68(1):51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- Nichols DK, Lamirande EW, Pessier AP, Longcore JE. Experimental transmission of cutaneous chytridiomycosis in dendrobatid frogs. J Wildl Dis. 2001;37(1):1–11. doi: 10.7589/0090-3558-37.1.1. [DOI] [PubMed] [Google Scholar]
- Pessier AP, Nichols DK, Longcore JE, Fuller MS. Cutaneous chytridiomycosis in poison dart frogs (Dendrobates spp.) and White’s tree frogs (Litoria caerulea) J Vet Diagn Invest. 1999;11(2):194–199. doi: 10.1177/104063879901100219. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88(1):87–98. doi: 10.1034/j.1600-0706.2000.880110.x. [DOI] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11(8):317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am Nat. 2003;161(3):367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290(5494):1166–1168. doi: 10.1126/science.290.5494.1166. [DOI] [PubMed] [Google Scholar]
- Råberg L, Nilsson JÅ, Ilmonen P, Stjernman M, Hasselquist D. The cost of an immune response: vaccination reduces parental effort. Ecol Lett. 2000;3(5):382–386. doi: 10.1046/j.1461-0248.2000.00154.x. [DOI] [Google Scholar]
- Bonneaud C, Balenger SL, Hill GE, Russell AF. Experimental evidence for distinct costs of pathogenesis and immunity against a natural pathogen in a wild bird. Mol Ecol. 2012;21(19):4787–4796. doi: 10.1111/j.1365-294X.2012.05736.x. [DOI] [PubMed] [Google Scholar]
- Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002;3(11):1033–1040. doi: 10.1038/ni1102-1033. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Pirofski L. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol. 2003;1(1):17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Benard MF, Shaffer HB, Parker JM, O’Leary C, Conlon JM, Rollins-Smith LA. Effects of chytrid and carbaryl exposure on survival, growth and skin peptide defenses in foothill yellow-legged frogs. Environ Sci Technol. 2007;41(5):1771–1776. doi: 10.1021/es0611947. [DOI] [PubMed] [Google Scholar]
- Garner TWJ, Walker SF, Bosch J, Leech S, Marcus Rowcliffe J, Cunningham AA, Fisher MC. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos. 2009;118(5):783–791. doi: 10.1111/j.1600-0706.2008.17202.x. [DOI] [Google Scholar]
- Venesky MD, Parris MJ, Altig R. Pathogenecity of Batrachochytrium dendrobatidis in larval Ambystomatid Salamanders. Herp Cons Biol. 2010;5(2):174–182. [Google Scholar]
- Venesky MD, Parris MJ, Storfer A. Impacts of Batrachochytrium dendrobatidis infection on tadpole foraging performance. Ecohealth. 2009;6(4):565–575. doi: 10.1007/s10393-009-0272-7. [DOI] [PubMed] [Google Scholar]
- Burrowes PA, Longo AV, Rodríguez CA. Potential fitness cost of Batrachochytrium dendrobatidis in eleutherodactylus coqui, and comments on environment-related risk of infection. Herpetotropicos. 2007;4(2):51–57. [Google Scholar]
- Richards-Zawacki C. Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proc R Soc Lond, Ser B: Biol Sci. 2010;277(1681):519–528. doi: 10.1098/rspb.2009.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secondi J, Hinot E, Djalout Z, Sourice S, Jadas-Hécart A. Realistic nitrate concentration alters the expression of sexual traits and olfactory male attractiveness in newts. Funct Ecol. 2009;23(4):800–808. doi: 10.1111/j.1365-2435.2009.01558.x. [DOI] [Google Scholar]
- Nöllert A, Nöllert C. Guide des amphibiens d’Europe. Paris: Delachaux et Niestlé; 2003. [Google Scholar]
- Griffiths RA, Mylotte VJ. Observations on the development of the secondary sexual characters of male newts. Triturus vulgaris and T. helveticus. J Herpetol. 1988;22(4):476–480. doi: 10.2307/1564344. [DOI] [Google Scholar]
- Baker JMR. Body condition and tail height in great crested newts, Triturus cristatus. Anim Behav. 1992;43:157–159. doi: 10.1016/S0003-3472(05)80081-8. [DOI] [Google Scholar]
- Green AJ. Large male crests, an honest indicator of condition, are preferred by female smooth newts, Triturus vulgaris(Salamandridae) at the spermatophore transfer stage. Anim Behav. 1991;41(2):367–369. doi: 10.1016/S0003-3472(05)80489-0. [DOI] [Google Scholar]
- Cornuau JH, Rat M, Schmeller DS, Loyau A. Multiple signals in the palmate newt: ornaments help when courting. Behav Ecol Sociobiol. 2012;66:1045–1055. doi: 10.1007/s00265-012-1355-y. [DOI] [Google Scholar]
- Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Fisher MC. The Bd Mapping G. Mapping the Global Emergence of Batrachochytrium dendrobatidis, the Amphibian Chytrid Fungus. PLoS ONE. 2013;8(2):e56802. doi: 10.1371/journal.pone.0056802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood LR, Griffiths RA, Schley L. Amphibian chytridiomycosis in Luxembourg. Bull Soc Nat Luxemb. 2009;110:109–114. [Google Scholar]
- Johnson ML, Speare R. Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerging Infect Dis. 2003;9(8):922. doi: 10.3201/eid0908.030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcore JE. Proceedings of "Getting the Jump! on Amphibian Diseases Conference/Workshop. Queensland, Australia: James Cook University; 2000. Recognizing, isolating, and culturing Batrachochytrium dendrobatidis from amphibians; pp. 52–54. August. [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JA, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Organ. 2004;60(2):141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Bielby J, Bovero S, Sotgiu G, Tessa G, Favelli M, Angelini C, Doglio S, Clare F, Gazzaniga E, Lapietra F. et al. Fatal chytridiomycosis in the tyrrhenian painted frog. Ecohealth. 2009;6(1):27–32. doi: 10.1007/s10393-009-0232-2. [DOI] [PubMed] [Google Scholar]
- Walker SF, Salas MB, Jenkins D, Garner TW, Cunningham AA, Hyatt AD, Bosch J, Fisher MC. Environmental detection of Batrachochytrium dendrobatidis in a temperate climate. Dis Aquat Organ. 2007;77(2):105–112. doi: 10.3354/dao01850. [DOI] [PubMed] [Google Scholar]
- Kriger KM, Hines HB, Hyatt AD, Boyle DG, Hero JM. Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Dis Aquat Organ. 2006;71(2):141–148. doi: 10.3354/dao071141. [DOI] [PubMed] [Google Scholar]
- Berger L, Longcore JE, Speare R, Hyatt AD, Skerratt LF. Amphibian Biology, Volume 8. Australia: Surrey Beatty & Sons; 2009. [Google Scholar]
- Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF. et al. Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science. 2009;326(5952):582–585. doi: 10.1126/science.1176765. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Garner TW, Walker SF. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol. 2009;63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Conlon JM. Antimicrobial peptide defenses against chytridiomycosis, an emerging infectious disease of amphibian populations. Dev Comp Immunol. 2005;29(7):589–598. doi: 10.1016/j.dci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT. et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009;3(7):818–824. doi: 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- Harris RN, Lauer A, Simon MA, Banning JL, Alford RA. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis Aquat Organ. 2009;83(1):11–16. doi: 10.3354/dao02004. [DOI] [PubMed] [Google Scholar]
- Lam BA, Walke JB, Vredenburg VT, Harris RN. Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biol Conserv. 2010;143(2):529–531. doi: 10.1016/j.biocon.2009.11.015. [DOI] [Google Scholar]
- Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog. Xenopus laevis. Infect Immun. 2010;78(9):3981. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins-Smith LA, Ramsey J, Pask J, Reinert L, Woodhams D. Amphibian immune defenses against chytridiomycosis: impacts of changing environments. Integr Comp Biol. 2011;51(4):552–562. doi: 10.1093/icb/icr095. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Ramsey JP, Reinert LK, Woodhams DC, Livo LJ, Carey C. Immune defenses of Xenopus laevis against Batrachochytrium dendrobatidis. Front Biosci (Schol Ed) 2009;1:68–91. doi: 10.2741/S8. [DOI] [PubMed] [Google Scholar]
- Rowley J, Alford R. Hot bodies protect amphibians against chytrid infection in nature. Sci Rep. 2013;3:1515. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venesky MD, Wilcoxen TE, Rensel MA, Rollins-Smith L, Kerby JL, Parris MJ. Dietary protein restriction impairs growth, immunity, and disease resistance in southern leopard frog tadpoles. Oecologia. 2012;169(1):23–31. doi: 10.1007/s00442-011-2171-1. [DOI] [PubMed] [Google Scholar]
- Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, Muths E, Puschendorf R, Schmidt BR, Sheafor B. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Frontiers in Zoology. 2011;8(1):8. doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ, Killick SC. Evidence for a cost of immunity when the crustacean Daphnia magna is exposed to the bacterial pathogen Pasteuria ramosa. J Anim Ecol. 2007;76(6):1202–1207. doi: 10.1111/j.1365-2656.2007.01290.x. [DOI] [PubMed] [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond Ser B: Biol Sci. 2003;270(1511):153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Murray AB. Anorexia of infection as a mechanism of host defense. Am J Clin Nutrit. 1979;32(3):593–596. doi: 10.1093/ajcn/32.3.593. [DOI] [PubMed] [Google Scholar]
- Kriger K, Pereoglou F, Hero J-M. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conservation biology: the journal of the Society for Conservation Biology. 2007;21(5):1280–1290. doi: 10.1111/j.1523-1739.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- Woodhams D, Bigler L, Marschang R. Tolerance of fungal infection in European water frogs exposed to Batrachochytrium dendrobatidis after experimental reduction of innate immune defenses. BMC Vet Res. 2012;8:197. doi: 10.1186/1746-6148-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuyama S, Yazawa T, Abe S, Yamamoto K, Iwata T, Hoshi K, Hasunuma I, Mosconi G, Polzonetti-Magni A. Newt prolactin and its involvement in reproduction. Can J Physiol Pharm. 2000;78(12):984–993. doi: 10.1139/y00-099. [DOI] [PubMed] [Google Scholar]
- Mazzi V, Vellano C. In: Hormones and reproduction in fishes, amphibians, and reptiles. vol. 1. Norris DO, Jones RE, editor. New York and London: Plenum Press; 1987. Prolactin and reproduction; pp. 87–115. [Google Scholar]
- Tassava RA, KUENZLI C. The effects of Prolactin and Thyroxine on tailk fin height, habitat chioce, and forelimbe regeneration in the adult newt (Notophthalmus viridescens) Ohio J Sci. 1979;79(2):32–37. [Google Scholar]
- Miele R, Ponti D, Boman H, Barra D, Simmaco M. Molecular cloning of a bombinin gene from Bombina orientalis: detection of NF-kappaB and NF-IL6 binding sites in its promoter. FEBS Lett. 1998;431(1):23–28. doi: 10.1016/S0014-5793(98)00718-2. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith L. Neuroendocrine-immune system interactions in amphibians: implications for understanding global amphibian declines. Immunol Res. 2001;23(2–3):273–280. doi: 10.1385/IR:23:2-3:273. [DOI] [PubMed] [Google Scholar]
- Kapcala L, Chautard T, Eskay R. The protective role of the hypothalamic-pituitary-adrenal axis against lethality produced by immune, infectious, and inflammatory stress. Ann N Y Acad Sci. 1995;771:419–437. doi: 10.1111/j.1749-6632.1995.tb44699.x. [DOI] [PubMed] [Google Scholar]
- Gabor C, Fisher M, Bosch J. A Non-Invasive Stress Assay Shows That Tadpole Populations Infected with Batrachochytrium dendrobatidis Have Elevated Corticosterone Levels. PLoS One. 2013;8(2):e56054. doi: 10.1371/journal.pone.0056054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloas W, Hanke W. Neurohypophysial hormones and steroidogenesis in the interrenals of Xenopus laevis. Gen Comp Endocrinol. 1990;80(2):321–330. doi: 10.1016/0016-6480(90)90176-M. [DOI] [PubMed] [Google Scholar]
- Malagón M, Ruiz-Navarro A, Torronteras R, Gracia-Navarro F. Effects of ovine CRF on amphibian pituitary ACTH and TSH cells in vivo: a quantitative ultrastructural study. Gen Comp Endocrinol. 1991;83(3):487–497. doi: 10.1016/0016-6480(91)90157-2. [DOI] [PubMed] [Google Scholar]
- Brown SC, Stocking Brown P, Yamamoto K, Matsuda K, Kikuyama S. Amphibian prolactins: Activity in the eft skin transepithelial potential bioassay. Gen Comp Endocrinol. 1991;82(1):1–7. doi: 10.1016/0016-6480(91)90289-I. [DOI] [PubMed] [Google Scholar]
- Singhas CA, Dent JN. Hormonal control of the tail fin and of the nuptial pads in the male red-spotted newt. Gen Comp Endocrinol. 1975;26(3):382–393. doi: 10.1016/0016-6480(75)90092-1. [DOI] [Google Scholar]
- Toyoda F, Tanaka S, Matsuda K, Kikuyama S. Hormonal control of response to and secretion of sex attractants in Japanese newts. Physiol Behav. 1994;55(3):569–576. doi: 10.1016/0031-9384(94)90118-X. [DOI] [PubMed] [Google Scholar]
- Sever DM, Staub NL. In: Hormones and Reproduction in Vertebrates Volume 2: Amphibians. In Norris DOe, vol. 2, editor. Maryland Heights, Missouri: Elsevier Inc; 2010. Hormones, Sex Accessory Structures, and Secondary Sexual Characteristics in Amphibians. [Google Scholar]
- May S, Zeisset I, Beebee T. J. C. Larval fitness and immunogenetic diversity in chytrid-infected and uninfected natterjack toad (Bufo calamita) populations. Conserv Genet. 2011;12:805–811. doi: 10.1007/s10592-011-0187-z. [DOI] [Google Scholar]
- Harrison JD, Gittins SP, Slater FM. The breeding migration of Smooth and Palmate newts (Triturus vulgaris and T. helveticus) at a pond in mid Wales. J Zool. 1983;199(2):249–258. [Google Scholar]
- Verrell PA, Halliday TR, Griffiths ML. The annual reproductive cycle of the smooth newt (Triturus vulgaris) in England. J Zool. 1986;210(1):101–119. [Google Scholar]
- Wells KD. The ecology and behavior of amphibians. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Jehle R, Arntzen JW. Post-breeding migrations of newts (Triturus cristatus and T. marmoratus) with contrasting ecological requirements. J Zool. 2000;251(3):297–306. doi: 10.1111/j.1469-7998.2000.tb01080.x. [DOI] [Google Scholar]
- Bell G. The life of the smooth newt (Triturus vulgaris) after metamorphosis. Ecol Monogr. 1977;47(3):279–299. doi: 10.2307/1942518. [DOI] [Google Scholar]
- Bonneaud C, Balenger SL, Russell AF, Zhang J, Hill GE, Edwards SV. Rapid evolution of disease resistance is accompanied by functional changes in gene expression in a wild bird. Proc Natl Acad Sci USA. 2011;108(19):7866–7871. doi: 10.1073/pnas.1018580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraud C, Duriez O, Chastel O, Faivre B. The energetic cost of humoral immunity in the Collared Dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade-offs? Funct Ecol. 2005;19(1):110–118. doi: 10.1111/j.0269-8463.2005.00934.x. [DOI] [Google Scholar]
- Bosch J, Carrascal LM, Duran L, Walker S, Fisher MC. Climate change and outbreaks of amphibian chytridiomycosis in a montane area of Central Spain; is there a link? Proc Biol Sci. 2007;274(1607):253–260. doi: 10.1098/rspb.2006.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]