Abstract
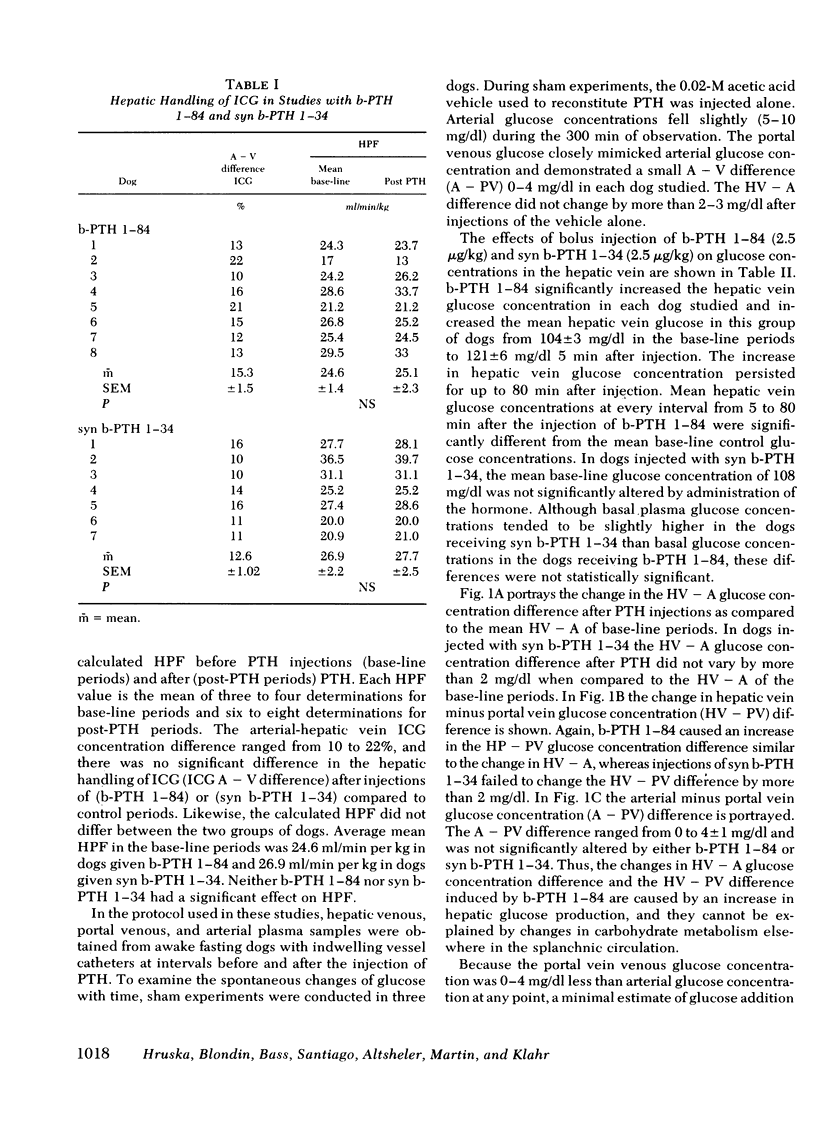
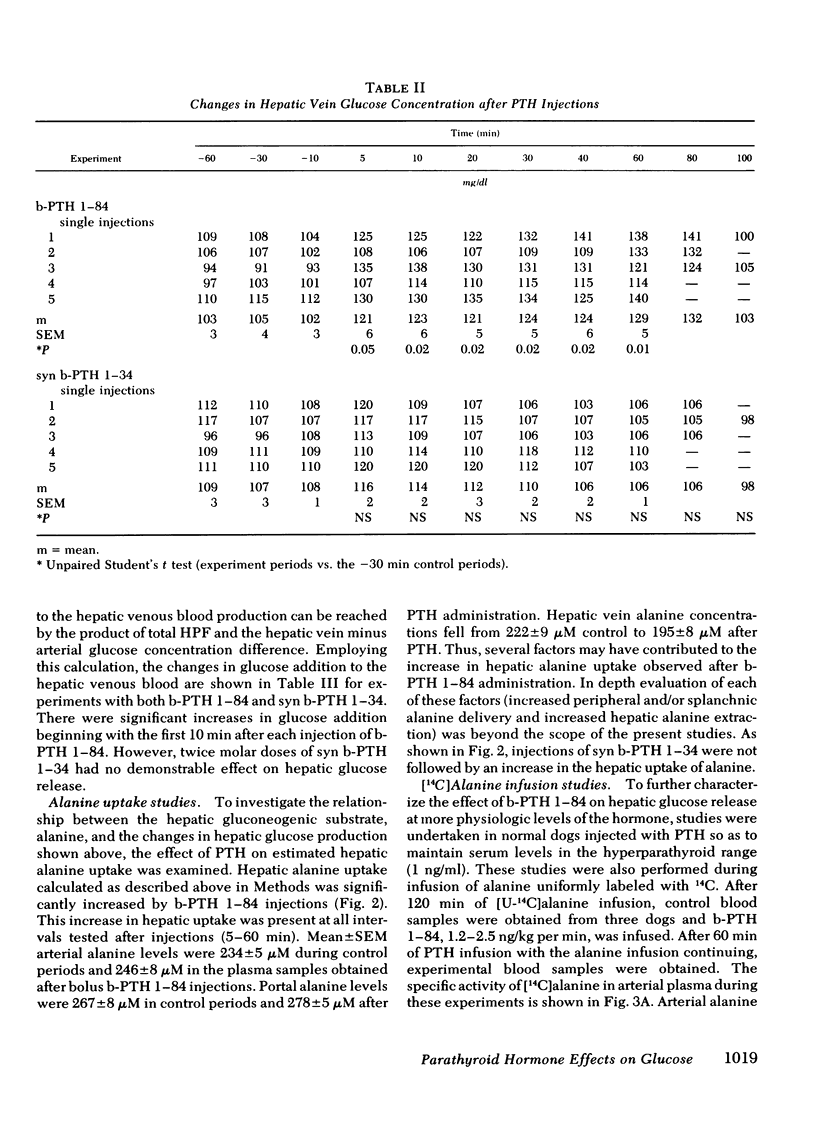
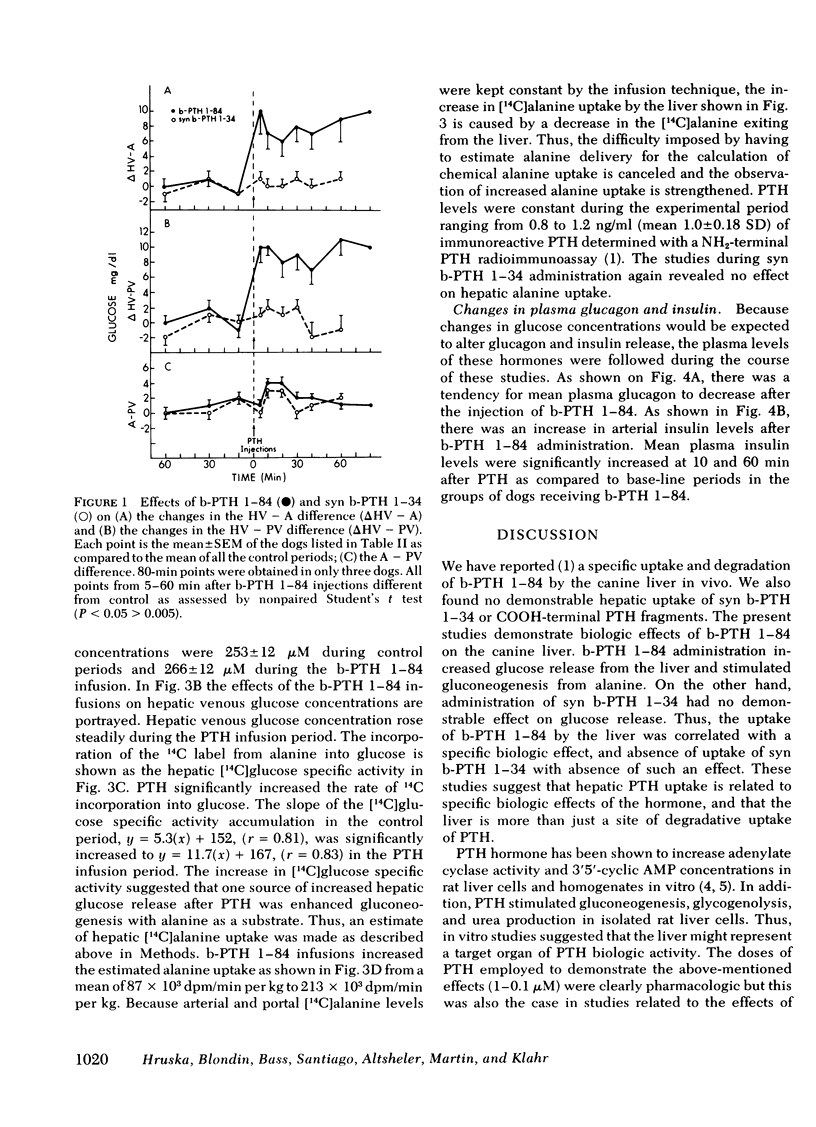
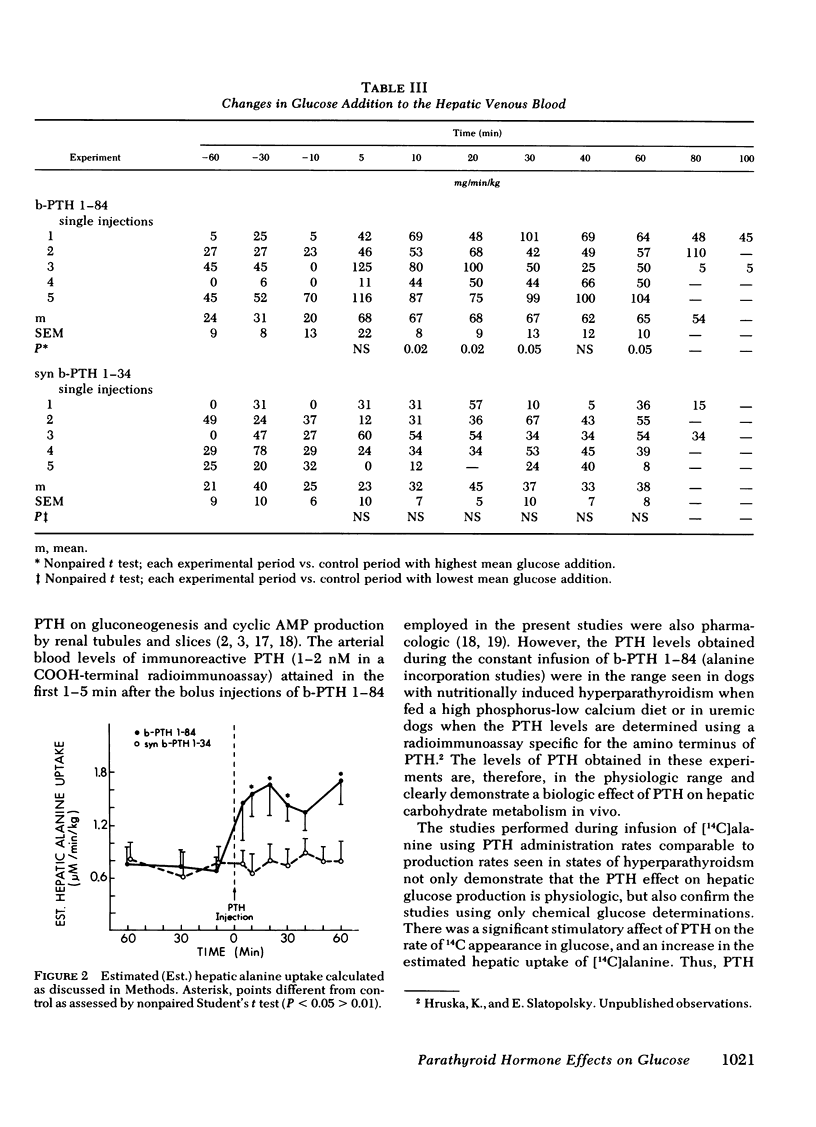
The liver has been shown to remove parathyroid hormone (PTH) from its arterial circulation by a mechanism that is selective for the intact form of the peptide (PTH 1-84). The present studies demonstrate that PTH has biologic effects on the liver in vivo. Bovine PTH 1-84 stimulated hepatic glucose release in dogs with indwelling hepatic vein catheters from basal values of 31±8 to 68±9 mg/min per kg after bolus injections of PTH. The effect on hepatic glucose release was apparent by 5 min and persisted for the 80 min of observation. The NH2-terminal PTH fragment (syn b-PTH 1-34) had no effect. Bovine PTH 1-84 administered in doses designed to produce circulating levels of immunoreactive PTH similar to the endogenous levels observed in uremic dogs also increased the incorporation of 14C from infused [14C]alanine into glucose, and increased estimated hepatic uptake of both chemical and [14C]alanine, while increasing hepatic glucose release. Thus, administration of “physiologic levels” of b-PTH 1-84 stimulated hepatic glucose release in part through increased gluconeogenesis in vivo, whereas syn b-PTH 1-34 had no demonstrable effect. Circulating levels of insulin rose after PTH administration, an increase which presumably represents a secondary response to the rise in glucose release.
These results suggest that the liver is a target organ of PTH, and that PTH might potentially alter carbohydrate metabolism during hypersecretion. They also suggest that hepatic uptake of PTH may be related in part to production of a specific biologic effect rather than just simple peptide degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canterbury J. M., Levy G., Ruiz E., Reiss E. Parathyroid hormone activation of adenylate cyclase in liver. Proc Soc Exp Biol Med. 1974 Nov;147(2):366–370. doi: 10.3181/00379727-147-38343. [DOI] [PubMed] [Google Scholar]
- Charbon G. A., Hulstaert P. F. Augmentation of arterial hepatic and renal flow by extracted and synthetic parathyroid hormone. Endocrinology. 1974 Aug;95(2):621–626. doi: 10.1210/endo-95-2-621. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Andres R., Edgar P., Walker W. G. Carbohydrate metabolism in uremia: a review. Medicine (Baltimore) 1973 Sep;52(5):469–481. doi: 10.1097/00005792-197309000-00009. [DOI] [PubMed] [Google Scholar]
- Garber A. J., Cryer P. E., Santiago J. V., Haymond M. W., Pagliara A. S., Kipnis D. M. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J Clin Invest. 1976 Jul;58(1):7–15. doi: 10.1172/JCI108460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A. J. Skeletal muscle protein and amino acid metabolism in experimental chronic uremia in the rat: accelerated alanine and glutamine formation and release. J Clin Invest. 1978 Sep;62(3):623–632. doi: 10.1172/JCI109169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H., Olefsky J. M., Reaven G. M. Evaluation of insulin resistance in patients with primary hyperparathyroidism. Proc Soc Exp Biol Med. 1975 Mar;148(3):942–945. doi: 10.3181/00379727-148-38665. [DOI] [PubMed] [Google Scholar]
- Guder W. G., Wieland O. H. Metabolism of isolated kidney tubules. Additive effects of parathyroid hormone and free-fatty acids on renal gluconeogenesis. Eur J Biochem. 1972 Nov 21;31(1):69–79. doi: 10.1111/j.1432-1033.1972.tb02502.x. [DOI] [PubMed] [Google Scholar]
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Harmon C. S., Whitton P. D. Inhibition by parathyroid hormone of glycogen synthesis in the perfused rat liver. FEBS Lett. 1975 Oct 15;58(1):167–169. doi: 10.1016/0014-5793(75)80250-x. [DOI] [PubMed] [Google Scholar]
- Hruska K. A., Kopelman R., Rutherford W. E., Klahr S., Slatopolsky E., Greenwalt A., Bascom T., Markham J. Metabolism in immunoreactive parathyroid hormone in the dog. The role of the kidney and the effects of chronic renal disease. J Clin Invest. 1975 Jul;56(1):39–48. doi: 10.1172/JCI108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KETTERER S. G., WIEGAND B. D., RAPAPORT E. Hepatic uptake and biliary excretion of indocyanine green and its use in estimation of hepatic blood flow in dogs. Am J Physiol. 1960 Sep;199:481–484. doi: 10.1152/ajplegacy.1960.199.3.481. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Kim H., Kalkhoff R. K., Costrini N. V., Cerletty J. M., Jacobson M. Plasma insulin disturbances in primary hyperparathyroidism. J Clin Invest. 1971 Dec;50(12):2596–2605. doi: 10.1172/JCI106760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K., Ohno T., Rasmussen H. Ionic control of renal gluconeogenesis. II. The effects of Ca2+ and H+ upon the response to parathyroid hormone and cyclic AMP. Biochim Biophys Acta. 1973 Jun 20;313(1):32–41. doi: 10.1016/0304-4165(73)90186-4. [DOI] [PubMed] [Google Scholar]
- Leichter S. B., Pagliara A. S., Grieder M. H., Pohl S., Rosai J., Kipnis D. M. Uncontrolled diabetes mellitus and hyperglucagonemia associated with an islet cell carcinoma. Am J Med. 1975 Feb;58(2):285–293. doi: 10.1016/0002-9343(75)90579-3. [DOI] [PubMed] [Google Scholar]
- Martin K., Hruska K., Greenwalt A., Klahr S., Slatopolsky E. Selective uptake of intact parathyroid hormone by the liver: differences between hepatic and renal uptake. J Clin Invest. 1976 Oct;58(4):781–788. doi: 10.1172/JCI108529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxley M. A., Bell N. H., Wagle S. R., Allen D. O., Ashmore J. Parathyroid hormone stimulation of glucose and urea production in isolated liver cells. Am J Physiol. 1974 Nov;227(5):1058–1061. doi: 10.1152/ajplegacy.1974.227.5.1058. [DOI] [PubMed] [Google Scholar]
- Nagata N., Rasmussen H. Renal gluconeogenesis: effects of Ca2+ and H+. Biochim Biophys Acta. 1970 Jul 21;215(1):1–16. doi: 10.1016/0304-4165(70)90382-x. [DOI] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- SHOEMAKER W. C., WALKER W. F., VAN ITALLIE T. B., MOORE F. D. A method for simultaneous catheterization of major hepatic vessels in a chronic canine preparation. Am J Physiol. 1959 Feb;196(2):311–314. doi: 10.1152/ajplegacy.1959.196.2.311. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]


