Abstract
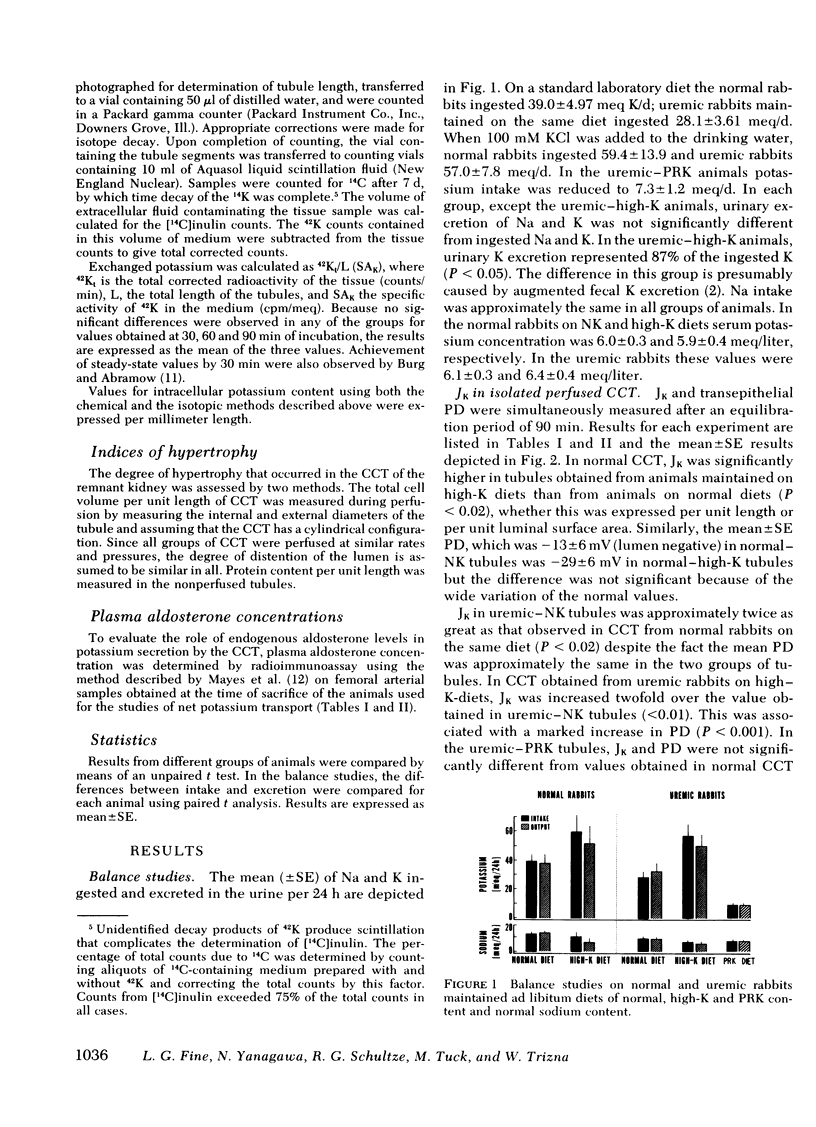
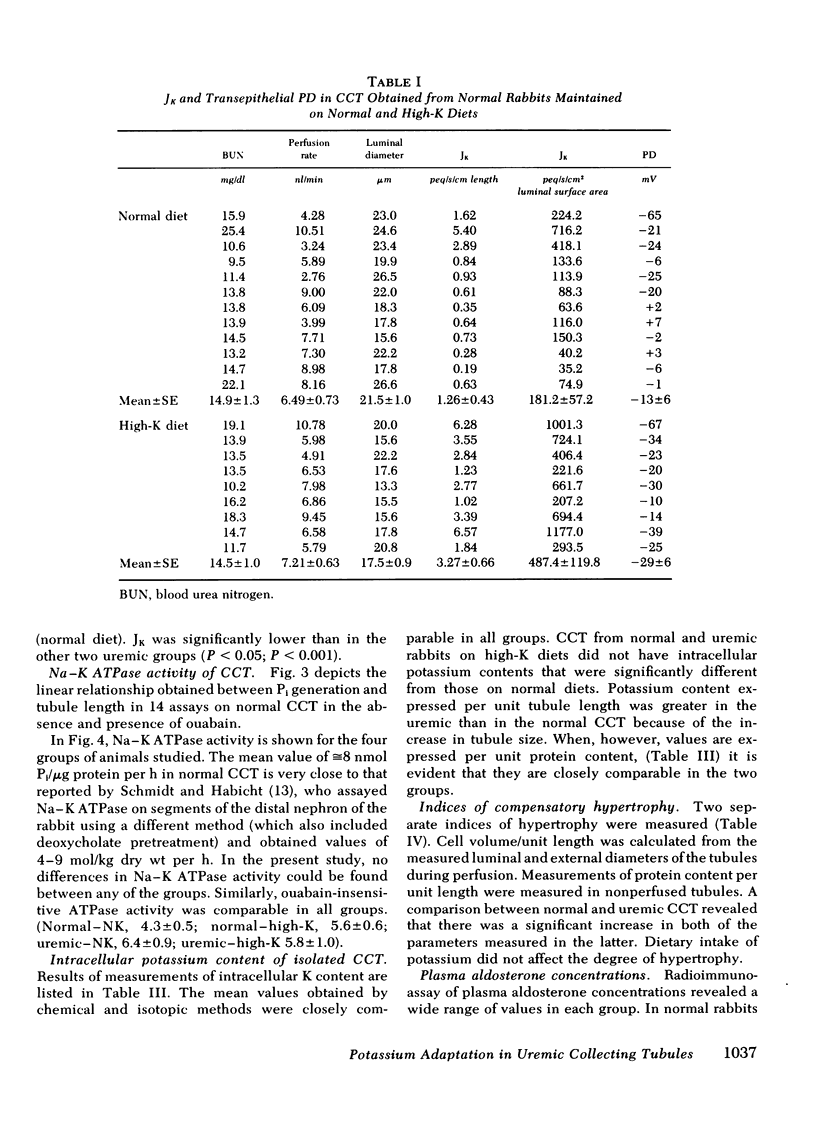
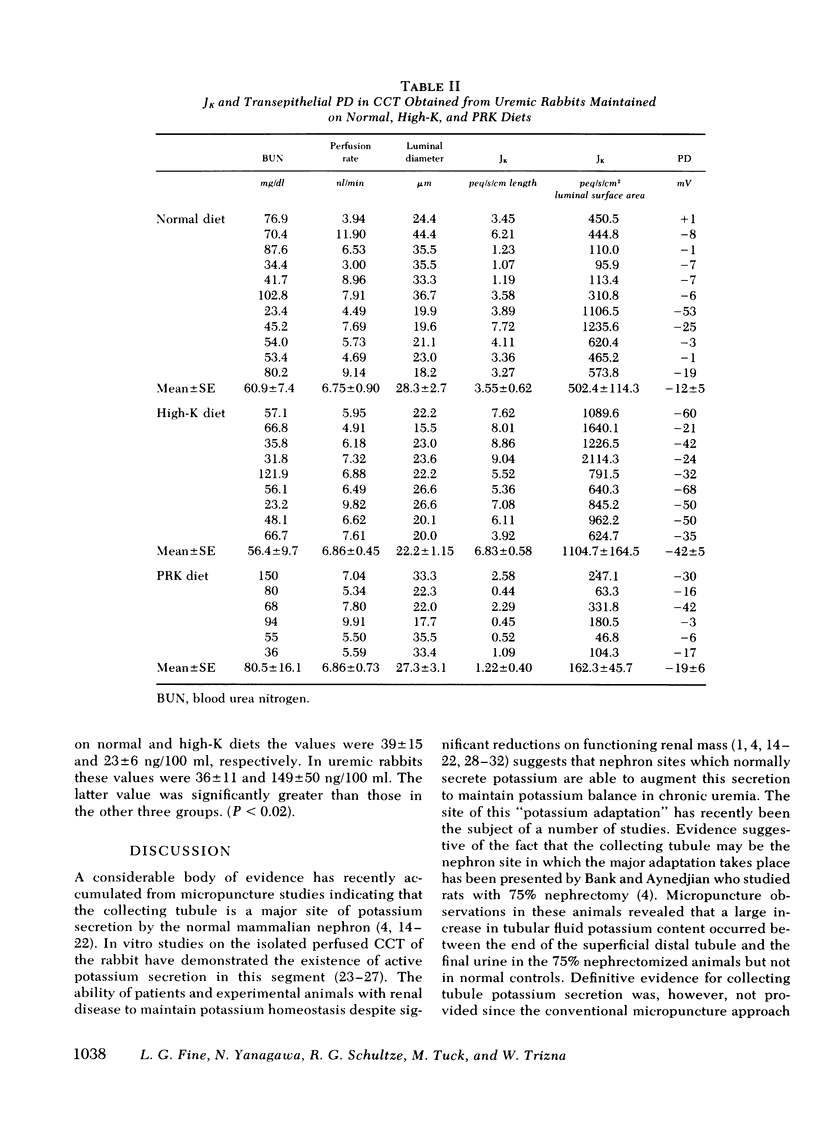
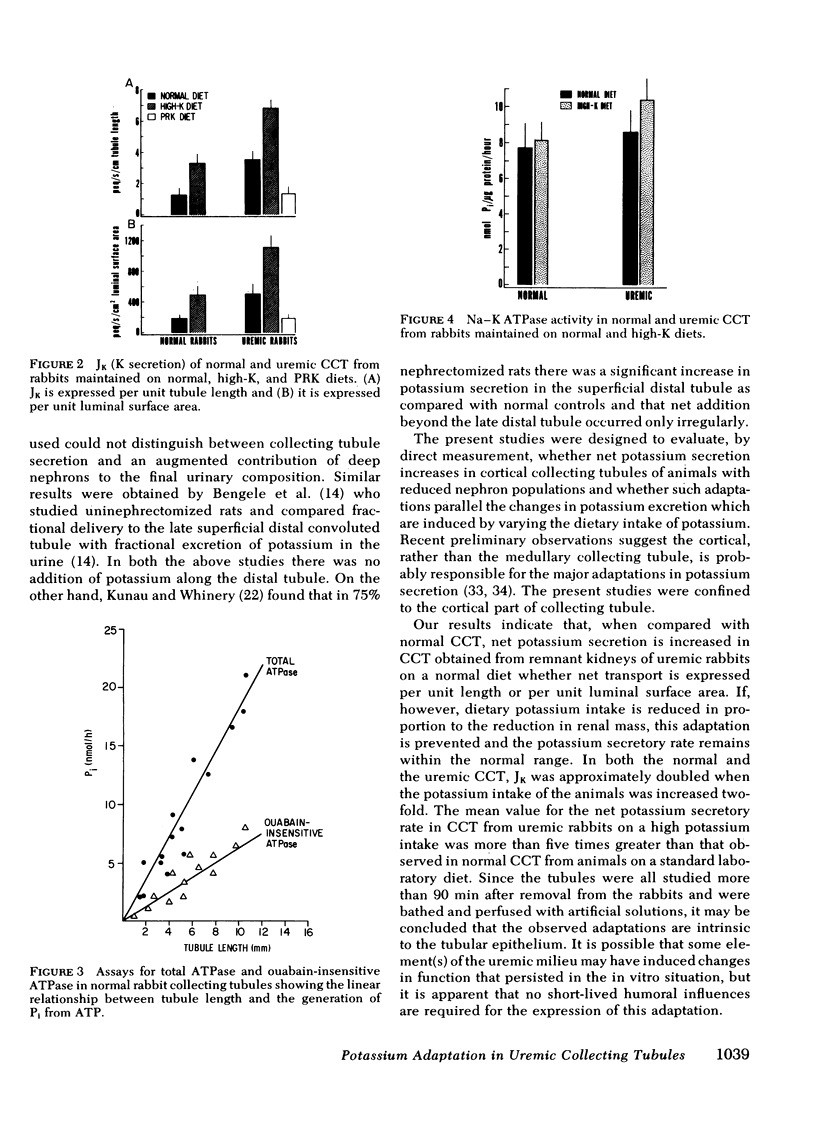
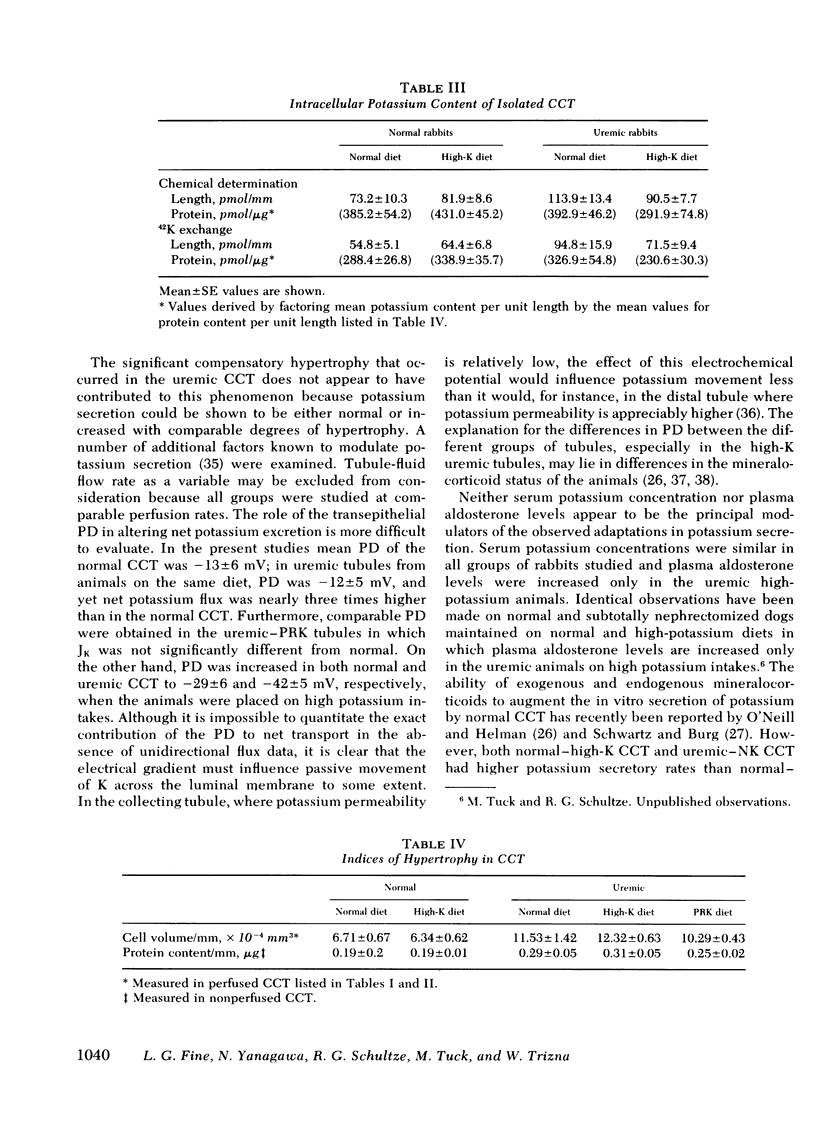
As a renal function declines in patients and experimental animals with chronic renal disease, potassium homeostasis is maintained by a progressive increase in potassium secretion by the surviving nephrons, a phenomenon known as potassium adaptation. To determine the nephron site and the underlying mechanisms responsible for this phenomenon, studies were performed on normal and 75% nephrectomized rabbits maintained on normal or high-potassium diets. Cortical collecting tubules (CCT) were dissected from the normal and remnant kidneys and perfused in vitro in an artificial solution. In normal CCT mean (+/- SE) net K secretion, JK, (peq/cm per s) was 1.26 +/- 0.43 (normal diet) and 3.27 +/- 0.66 (high-K diet). In uremic CCT, JK was 3.55 +/- 0.60 (normal diet) and 6.83 +/- 0.58 (high-K diet). By reducing the dietary intake of potassium in proportion to the reduction of renal mass in these uremic animals, the adaptation in K secretion was prevented (JK: 1.22 +/- 0.40). Transepithelial potential difference was similar in CCT from normal and uremic animals on a normal diet despite the fact that JK was significantly greater in the latter group. However, in both normal and uremic CCT, the increase in JK caused by potassium loading was associated with an increase in luminal negativity. Uremic CCT underwent significant compensatory hypertrophy regardless of the dietary intake or potassium secretory rates. Plasma aldosterone levels were elevated only in the uremic-high potassium rabbits suggesting that a mineralocorticoid effect on the CCT may be exaggerated when potassium loading is superimposed upon decreased excretory capacity. The activity of Na-K ATPase was comparable in normal and uremic CCT from rabbits on either normal or high-K diets indicating that potassium adaptation may occur independently of changes in the activity of this enzyme. Intracellular potassium content measured chemically and by 42K exchange, was not significantly altered in either normal or uremic CCT when dietary potassium intake was increased, despite the fact the JK was increased under these circumstances. These data indicate that the CCT is an important site of potassium adaptation in the surviving nephrons of animals with reduced renal mass. This adaptation is an intrinsic property of the CCT and is expressed in the absence of a uremic milieu. Potassium adaptation by the uremic CCT is not fixed according to the degree of compensatory hypertrophy but varies according to the excretory requirements of the animal. Transepithelial potential difference and circulating aldosterone levels contribute to the adaptation but neither factor can entirely account for the phenomenon. Potassium adaptation by the CCT occurs in the absence of changes in Na-K ATPase activity and intracellular potassium content.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank N., Aynedjian H. S. A micropuncture study of potassium excretion by the remnant kidney. J Clin Invest. 1973 Jun;52(6):1480–1490. doi: 10.1172/JCI107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastl C., Hayslett J. P., Binder H. J. Increased large intestinal secretion of potassium in renal insufficiency. Kidney Int. 1977 Jul;12(1):9–16. doi: 10.1038/ki.1977.73. [DOI] [PubMed] [Google Scholar]
- Beeuwkes R., 3rd, Rosen S. Renal sodium-potassium adenosine triphosphatase. Optical localization and x-ray microanalysis. J Histochem Cytochem. 1975 Nov;23(11):828–839. doi: 10.1177/23.11.127810. [DOI] [PubMed] [Google Scholar]
- Bengele H. H., Evan A., McNamara E. R., Alexander E. A. Tubular sites of potassium regulation in the normal and uninephrectomized rat. Am J Physiol. 1978 Feb;234(2):F146–F153. doi: 10.1152/ajprenal.1978.234.2.F146. [DOI] [PubMed] [Google Scholar]
- Bilbrey G. L., Carter N. W., White M. G., Schilling J. F., Knochel J. P. Potassium deficiency in chronic renal failure. Kidney Int. 1973 Dec;4(6):423–430. doi: 10.1038/ki.1973.138. [DOI] [PubMed] [Google Scholar]
- Boudry J. F., Stoner L. C., Burg M. B. Effect of acid lumen pH on potassium transport in renal cortical collecting tubules. Am J Physiol. 1976 Jan;230(1):239–244. doi: 10.1152/ajplegacy.1976.230.1.239. [DOI] [PubMed] [Google Scholar]
- Burg M. B., Abramow M. Localization of tissue sodium and potassium compartments in rabbit renal cortex. Am J Physiol. 1966 Oct;211(4):1011–1017. doi: 10.1152/ajplegacy.1966.211.4.1011. [DOI] [PubMed] [Google Scholar]
- Cortas N., Walser M. (Na + -K + )-activated ATPase in isolated mucosal cells of toad bladder. Biochim Biophys Acta. 1971 Oct 12;249(1):181–187. doi: 10.1016/0005-2736(71)90095-2. [DOI] [PubMed] [Google Scholar]
- Diezi J., Michoud P., Aceves J., Giebisch G. Micropuncture study of electrolyte transport across papillary collecting duct of the rat. Am J Physiol. 1973 Mar;224(3):623–634. doi: 10.1152/ajplegacy.1973.224.3.623. [DOI] [PubMed] [Google Scholar]
- Ernst S. A. Transport ATPase cytochemistry: ultrastructural localization of potassium-dependent and potassium-independent phosphatase activities in rat kidney cortex. J Cell Biol. 1975 Sep;66(3):586–608. doi: 10.1083/jcb.66.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Bourgoignie J. J., Hwang K. H., Bricker N. S. On the influence of the natriuretic factor from patients with chronic uremia on the bioelectric properties and sodium transport of the isolated mammalian collecting tubule. J Clin Invest. 1976 Sep;58(3):590–597. doi: 10.1172/JCI108505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Schlondorff D., Trizna W., Gilbert R. M., Bricker N. S. Functional profile of the isolated uremic nephron. Impaired water permeability and adenylate cyclase responsiveness of the cortical collecting tubule to vasopressin. J Clin Invest. 1978 Jun;61(6):1519–1527. doi: 10.1172/JCI109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. G., Trizna W., Bourgoignie J. J., Bricker N. S. Functional profile of the isolated uremic nephron. Role of compensatory hypertrophy in the control of fluid reabsorption by the proximal straight tubule. J Clin Invest. 1978 Jun;61(6):1508–1518. doi: 10.1172/JCI109071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein F. O., Hayslett J. P. Role of medullary structures in the functional adaptation of renal insufficiency. Kidney Int. 1974 Dec;6(6):419–425. doi: 10.1038/ki.1974.127. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- Giebisch G. Some reflections on the mechanism of renal tubular potassium transport. Yale J Biol Med. 1975 Sep;48(4):315–336. [PMC free article] [PubMed] [Google Scholar]
- Gonick H. C., Kleeman C. R., Rubini M. E., Maxwell M. H. Functional impairment in chronic renal disease. 3. Studies of potassium excretion. Am J Med Sci. 1971 May;261(5):281–290. doi: 10.1097/00000441-197105000-00007. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Kurg M. B., Obloff J. The nature of transtubular Na and K transport in isolated rabbit renal collecting tubules. J Clin Invest. 1970 Oct;49(10):1815–1826. doi: 10.1172/JCI106399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham J. J., Lowe C. M., Dellasega M., Cole B. R. Effect of hypotonic medium on K and Na content of proximal renal tubules. Am J Physiol. 1977 Jan;232(1):F42–F49. doi: 10.1152/ajprenal.1977.232.1.F42. [DOI] [PubMed] [Google Scholar]
- HIERHOLZER K. Secretion of potassium and acidification in collecting ducts of mammalian kidney. Am J Physiol. 1961 Aug;201:318–324. doi: 10.1152/ajplegacy.1961.201.2.318. [DOI] [PubMed] [Google Scholar]
- Hayes C. P., Jr, McLeod M. E., Robinson R. R. An extravenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans Assoc Am Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- Kahn T., Kaji D. M., Nicolis G., Krakoff L. R., Stein R. M. Factors related to potassium transport in chronic stable renal disease in man. Clin Sci Mol Med. 1978 Jun;54(6):661–666. doi: 10.1042/cs0540661. [DOI] [PubMed] [Google Scholar]
- Kleeman C. R., Okun R., Heller R. J. The renal regulation of sodium and potassium in patients with chronic renal failure (CRF) and the effect of diuretics on the excretion of these ions. Ann N Y Acad Sci. 1966 Nov 22;139(2):520–539. doi: 10.1111/j.1749-6632.1966.tb41226.x. [DOI] [PubMed] [Google Scholar]
- Kunau R. T., Jr, Whinnery M. A. Potassium transfer in distal tubule of normal and remnant kidneys. Am J Physiol. 1978 Sep;235(3):F186–F191. doi: 10.1152/ajprenal.1978.235.3.F186. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Potassium transport across renal distal tubules during acid-base disturbances. Am J Physiol. 1971 Oct;221(4):1192–1208. doi: 10.1152/ajplegacy.1971.221.4.1192. [DOI] [PubMed] [Google Scholar]
- Mayes D., Furuyama S., Kem D. C., Nugent C. A. A radioimmunoassay for plasma aldosterone. J Clin Endocrinol Metab. 1970 May;30(5):682–685. doi: 10.1210/jcem-30-5-682. [DOI] [PubMed] [Google Scholar]
- O'Neil R. G., Helman S. I. Transport characteristics of renal collecting tubules: influences of DOCA and diet. Am J Physiol. 1977 Dec;233(6):F544–F558. doi: 10.1152/ajprenal.1977.233.6.F544. [DOI] [PubMed] [Google Scholar]
- Patrick J., Jones N. F. Cell sodium, potassium and water in uraemia and the effects of regular dialysis as studied in the leucocyte. Clin Sci Mol Med. 1974 May;46(5):583–590. doi: 10.1042/cs0460583. [DOI] [PubMed] [Google Scholar]
- Peterson L. N., Wright F. S. Effect of sodium intake on renal potassium excretion. Am J Physiol. 1977 Sep;233(3):F225–F234. doi: 10.1152/ajprenal.1977.233.3.F225. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Dubach U. C. Activity of (Na+K+)-stimulated adenosintriphosphatase in the rat nephron. Pflugers Arch. 1969;306(3):219–226. doi: 10.1007/BF00592433. [DOI] [PubMed] [Google Scholar]
- Schmidt U., Dubach U. C. Na K stimulated adenosinetriphosphatase: intracellular localisation within the proximal tubule of the rat nephron. Pflugers Arch. 1971;330(3):265–270. doi: 10.1007/BF00588617. [DOI] [PubMed] [Google Scholar]
- Schon D. A., Silva P., Hayslett J. P. Mechanism of potassium excretion in renal insufficiency. Am J Physiol. 1974 Dec;227(6):1323–1330. doi: 10.1152/ajplegacy.1974.227.6.1323. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Regal E. M. Influence of aldosterone on sodium, water and potassium metabolism in chronic renal disease. Kidney Int. 1972 Mar;1(3):156–168. doi: 10.1038/ki.1972.23. [DOI] [PubMed] [Google Scholar]
- Schultze R. G., Taggart D. D., Shapiro H., Pennell J. P., Caglar S., Bricker N. S. On the adaptation in potassium excretion associated with nephron reduction in the dog. J Clin Invest. 1971 May;50(5):1061–1068. doi: 10.1172/JCI106577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J., Burg M. B. Mineralocorticoid effects on cation transport by cortical collecting tubules in vitro. Am J Physiol. 1978 Dec;235(6):F576–F585. doi: 10.1152/ajprenal.1978.235.6.F576. [DOI] [PubMed] [Google Scholar]
- Silva P., Brown R. S., Epstein F. H. Adaptation to potassium. Kidney Int. 1977 Jun;11(6):466–475. doi: 10.1038/ki.1977.64. [DOI] [PubMed] [Google Scholar]
- Silva P., Hayslett J. P., Epstein F. H. The role of Na-K-activated adenosine triphosphatase in potassium adaptation. Stimulation of enzymatic activity by potassium loading. J Clin Invest. 1973 Nov;52(11):2665–2671. doi: 10.1172/JCI107460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg H. Renal response to blood volume expansion: distal tubular function and urinary excretion. Am J Physiol. 1972 Oct;223(4):916–924. doi: 10.1152/ajplegacy.1972.223.4.916. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner L. C., Burg M. B., Orloff J. Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol. 1974 Aug;227(2):453–459. doi: 10.1152/ajplegacy.1974.227.2.453. [DOI] [PubMed] [Google Scholar]
- WELT L. G., SACHS J. R., MCMANUS T. J. AN ION TRANSPORT DEFECT IN ERYTHROCYTES FROM UREMIC PATIENTS. Trans Assoc Am Physicians. 1964;77:169–181. [PubMed] [Google Scholar]
- Weidmann P., Maxwell M. H., Rowe P., Winer R., Massry S. G. Role of the renin-angiotensin-aldosterone system in the regulation of plasma potassium in chronic renal disease. Nephron. 1975;15(1):35–49. doi: 10.1159/000180491. [DOI] [PubMed] [Google Scholar]
- Wright F. S. Sites and mechanisms of potassium transport along the renal tubule. Kidney Int. 1977 Jun;11(6):415–432. doi: 10.1038/ki.1977.60. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Strieder N., Fowler N. B., Giebisch G. Potassium secretion by distal tubule after potassium adaptation. Am J Physiol. 1971 Aug;221(2):437–448. doi: 10.1152/ajplegacy.1971.221.2.437. [DOI] [PubMed] [Google Scholar]


