Abstract
ATPase associated with various cellular activities (AAA) proteins are important regulators involved in diverse cellular functions. To date, the molecular mechanisms of AAA proteins involved in response to salt and drought stresses in plants are largely unknown. In this study, a putative SKD1 (suppressor of K + transport growth defect 1) ortholog from Zea mays (ZmSKD1), which encodes a putative AAA protein, was isolated. The transcript levels of ZmSKD1 were higher in aerial tissues and were markedly up-regulated by salt or drought stress. Over-expression of ZmSKD1 in tobacco plants enhanced their tolerances not only to salt but to drought. Moreover, reactive oxygen species accumulations in ZmSKD1 transgenic lines were relative less than those in wild-type plants during salt or PEG-induced water stress. The interaction between ZmSKD1 and NtLIP5 (Lyst-Interacting Protein 5 homolog from Nicotiana tabacum) was confirmed by both yeast two-hybrid and immuno-precipitation assays; moreover, the α-helix-rich domain in the C-terminus of ZmSKD1 was identified to be required for its interaction with NtLIP5 using truncation mutations. Collectively, these data demonstrate that ZmSKD1could be involved in salt and drought stress responses and its over-expression enhances salt or drought stress tolerance possibly through interacting with LIP5 in tobacco. This study may facilitate our understandings of the biological roles of SKD1-mediated ESCRT pathway under stress conditions in higher plants and accelerate genetic improvement of crop plants tolerant to environmental stresses.
Introduction
ATPase associated with various cellular activities (AAA) proteins constitute a family of highly conserved proteins present in all organisms. They are important regulators involved in diverse cellular functions, including vesicle trafficking, proteosome-mediated protein degradation, and chaperone-like activity [1], [2], [3].
SKD1 (suppressor of K + transport growth defect 1), a member of AAA family, has been identified from yeast, mammals and plants [4]–[8]. Mammalian SKD1 was first identified by screening mouse cDNA library for complementing the K+-uptake-defect phenotype in yeast vps4 mutant [4]. The structure of SKD1 consists of a central AAA domain capable of catalyzing ATP hydrolysis and an N-terminal MIT (microtubule interacting and trafficking) domain. Mammalian SKD1 and its yeast homolog VPS4 (vacuolar protein sorting 4) are orthologues and involved in intracellular protein trafficking [6]. SKD1 participates in the sorting of monoubiquitylated transmembrane cargo to the lysosome/vacuole by disassembling the members of the ESCRT (endosomal sorting complex required for transport) complexes from the endosomal membrane [5], [9], [10], [11]. More importantly, yeast mutants defective in VPS4 display a salt-sensitive phenotype, suggesting a strong link between ESCRT-mediated endosomal/vacuolar trafficking and salt tolerance [11].
In higher plants, SKD1 homolog from the halophyte ice plant Mesembryanthemum crystallinum (mcSKD1) was first identified as a salt-induced gene by suppression subtractive hybridization [12] and was found capable of complementing the phenotype of yeast vps4 mutant [7].The Arabidopsis SKD1 homolog (AtSKD1) has been shown to be an ortholog of SKD1/Vps4 with ATPase activity and colocalize with endosomal markers on multivescular bodies (MVBs) [8]. However, overexpression of dominant-negative AtSKD1E232Q is lethal for Arabidopsis plants [8]. Further studies have showed that AtSKD1 is involved in MVB function and contributes to vacuolar maintenance; moreover, AtSKD1 can interact with its positive regulator LYST-INTERACTING PROTEIN5 (LIP5) in yeast cells [8], [13]. Most recently, direct genetic evidence has shown that reduced expression of AtSKD1 decreases salt tolerance in Arabidopsis using RNA interference approach, suggesting AtSKD1 participates in salt tolerance in plants [14].
In spite of the progress made in understanding molecular and biological function of SKD1 in model plant, the knowledge of molecular and functional aspects of the SKD1 from higher plants is still limited. Maize (Zea mays) is an important cereal crop worldwide that is a staple food to many populations. Salt and drought stresses often adversely affect plant growth and productivity, thus they are becoming serious problems in a plenty of maize-planting regions worldwide. Unfortunately, the molecular mechanisms of AAA proteins involved in response to salt and drought stresses in plants are largely unknown, let alone in crop plants. In this study, a putative SKD1 ortholog from Zea mays (ZmSKD1), which encodes a putative AAA-type protein, was found by a salt-induced microarray analysis. We further characterized the putative maize SKD1 homolog in transgenic tobacco to investigate salt and drought tolerances and possible function mechanisms.
Results
Molecular Characterization of ZmSKD1
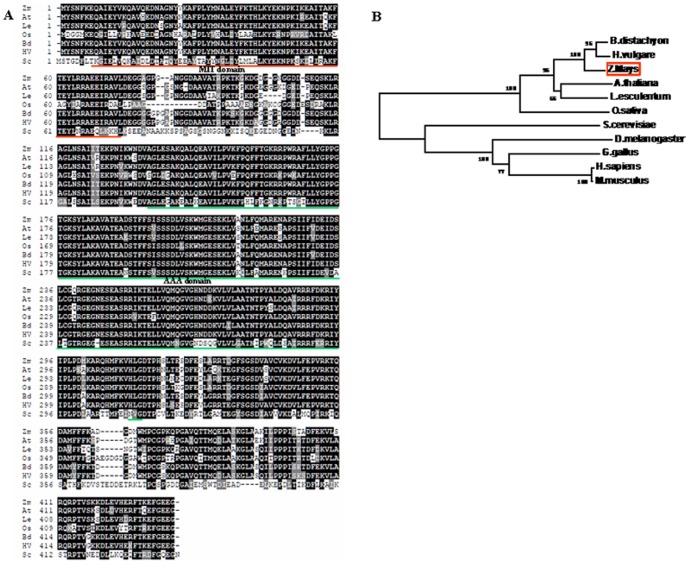
At the start of this work, total RNAs from the leaves of maize plants, which had been treated with 200 mM NaCl, were used as samples for microarray experiments. A partial cDNA fragment with about 12-fold induction was obtained (data not shown). Homology search by BLAST analysis showed that the gene is highly homologous to AtSKD1, a member of the AAA-type ATPase family in Arabidopsis, and thus is named ZmSKD1 (accession no. AY105155).The ORF of the ZmSKD1 consists of 1308 nucleotide acids and encodes a protein of 435 amino acids with a predicted molecular mass of about 48 kDa. Like other known plant SKD1s, the deduced amino acid sequence of the ZmSKD1 exhibits similar structural characteristics with two functional domains. One is a central AAA domain (residues 135 to 298); the other is an N-terminal MIT domain (residues 7 to 73) (Fig. 1A). Amino acid sequence comparisons have revealed that ZmSKD1 exhibits high identity to counterpart proteins from Arabidopsis thaliana (86% identity), Lycopersicon esculentum (87% identity), Oryza sativa (74% identity), Brachypodium distachyon (92% identity) and Hordeum vulgare (93% identity), but low identity to Saccharomyces cerevisiae (52% identity) (Fig. 1A).
Figure 1. Sequence alignment and phylogenetic analysis of SKD1 proteins from Zea Mays and other species.
A An alignment is shown for the deduced amino acid sequence of SKD1s from Zea Mays (Zm), Arabidopsis thaliana (At), Lycopersicon esculentum (Le), Oryza sativa (Os), Brachypodium distachyon (Bd), Hordeum vulgare (Hv) and Saccharomyces cerevisiae (Sc). The numbers on the left indicate the amino acid position. Identical residues in all these proteins are shown in a black background. Dashes indicated gaps introduced for optimal alignment. The putative MIT and AAA domains are underlined with a thick red line and a thick green line, respectively. B Phylogenetic tree based on SKD1 protein sequences from yeast, plants, and animals. The bootstrap values shown were calculated based on 500 replications. The tree was constructed using the neighbor-joining method. Z.mays, AY105155; A.thaliana, At2g27600; L.esculentum, AK324437; O.sativa, AF499028; B.distachyon, LOC100837561; H.vulgare, AK359160; D.melanogaster, NP_573258; G.gallus, AJ720732; H.sapiens, AF038960; M.musculus, NP_033216; S.cerevisiae, NP_015499.
A phylogenetic tree was established based on SKD1 protein sequences available in GenBank from 12 species including 6 plant species (Arabidopsis, tomato, rice, barley, wild wheat and maize), 5 animal species (human, mouse, drosophila, nematode, chicken), and S.cerevisiae (Fig. 1B). As shown in Fig. 1B, these SKD1s were clustered into two distinct groups, including plant SKD1 and animal SKD1 groups. In plant SKD1s, interestingly, SKD1 from rice (OsSKD1) formed a subgroup distinct from the other SKD1s subgroup, including the monocot SKD1s (HvSKD1, BdSKD1 and ZmSO) and the dicot SKD1s (AtSKD1 and LeSKD1). The ZmSKD1 showed higher identities with BdSKD1 or HvSKD1, and thus was clustered into the same isoform subgroup. The dicot SKD1s (AtSKD1 and LeSKD1) were clustered into the same isoform subgroup. On the other hand, in animal SKD1s, the S.cerevisiae SKD1 (ScSKD1) formed a subgroup distinct from the SKD1s from human (HmSKD1), mouse (MmSKD1), drosophila (DmSKD1), nematode (CeSKD1), and chicken (GgSKD1). The SKD1s from mammals (HmSKD1 and MmSKD1) were clustered into the same isoform subgroup. These results clearly demonstrated that ZmSKD1 shares basic structural feature similar to the known SKD1 proteins from yeast, Arabidopsis and mammals, thus could be identified as an ortholog of SKD1.
Transcript Levels of ZmSKD1 in Various Organs of Maize and Its Responses to Salt or PEG-induced Water Stress
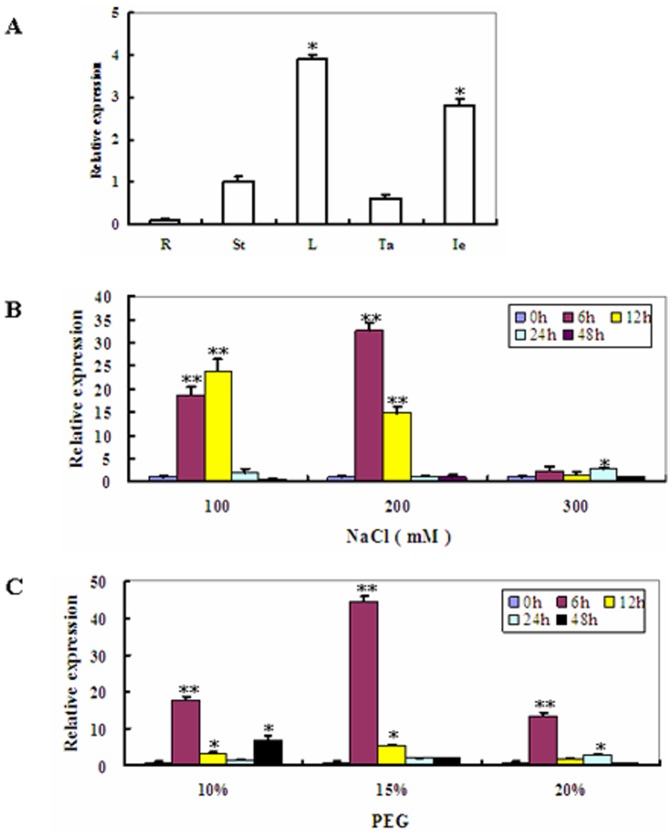
Transcriptional patterns of ZmSKD1 were examined in five organs (roots, stems, leaves, tassels, and immature ears) by qRT-PCR. As shown in Fig. 2A, ZmSKD1 mRNA was detected in roots, stems, leaves, tassels or immature ears. The ZmSKD1 transcript levels were significantly high in leaves and immature ears. In contrast, ZmSKD1 transcripts were low in roots, tassels and stems (Fig. 2A). The highest relative expression occurred in the leaves, with about 30 times as high as that of the roots (Fig. 2A).
Figure 2. Transcript profiles of ZmSKD1 in major organs of maize and its responses to salt and drought stresses.
A The transcriptional pattern of ZmSKD1 in maize root (R), stem (St), leaf (L), tassel (Ta) and immature ear (Ie) samples evaluated by real-time PCR. For each real-time PCR, the transcript levels of maize internal control gene Ubiquitin were also evaluated in various samples. For each assay, the expression level in stems was defined as 1.0, and data represented means ± SE of three technical replicates. *t-test, with P<0.05. B Time-course analysis of ZmSKD1 transcript levels under various concentrations of salt treatments by real-time PCR. Two-week-old maize seedlings were exposed to 100, 200, and 300 mM NaCl for indicated time points (0, 6, 12, 24, and 48 h), and leaf samples were used for real-time PCR analysis. C Time-course analysis of ZmSKD1 transcript levels under PEG-induced water stress by real-time PCR. Two-week-old maize seedlings were exposed to 0, 10%, 15%, and 20% PEG6000 for indicated time points (0, 6, 12, 24, and 48 h), and leaf samples were used for real-time PCR analysis. In both B and C assays, Ubiquitin was used as an internal control. For each treatment, the expression level at time point 0 was defined as 1.0, and data represented means ± SE of three technical replicates. **t-test, with P<0.01; *t-test, with P<0.05.
Time-course analysis of ZmSKD1 transcript levels in maize plants under salt or PEG-induced water stress was performed by qRT-PCR (Fig. 2B, 2C). Under salt treatment, ZmSKD1 was significantly activated by 100 or 200 mM of NaCl (Fig. 2B). This trend was clearly seen at 6 and 12 h of the treatment period (Fig. 2B). Under PEG treatment, ZmSKD1 was significantly activated by three concentrations of PEG (10%, 15%, and 20%) at 6 or 12 h of the treatment period with a peak at 6 h (17, 44, and 13-fold increases, respectively) (Fig. 2C). These results suggested that ZmSKD1 was responsive not only to salt but to drought stress, and up-regulated significantly by these stresses at the early stage.
Responses of ZmSKD1-overexpressing Tobacco Plants to Salt and Drought Stresses
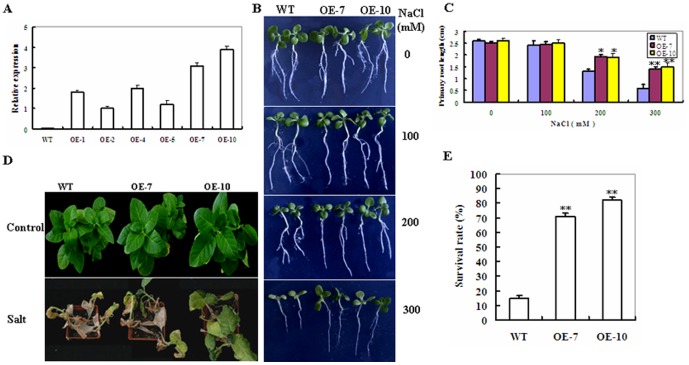
To explore the physiological function of ZmSKD1, a CaMV 35S promoter-driven binary expression construct harboring ZmSKD1 was developed and transformed into tobacco by Agrobacterium-mediated transformation. To this end, six homozygous transgenic lines (named OE-1, OE-2, OE-4, OE-5, OE-7 and OE-10) were developed, in which ZmSKD1 transcript levels were analyzed by qRT-PCR (Fig. 3A). Both lines (OE-7 and OE-10) with higher ZmSKD1 transcripts were chosen for further analysis.
Figure 3. Phenotypes of wild-type and ZmSKD1-overexpressing tobacco plants in response to salt stress.
A Transcription levels of ZmSKD1 in wild-type (WT) tobacco plants and six homozygous over-expression (OE) lines (named OE-1, OE-2, OE-4, OE-5, OE-7 and OE-10). ZmSKD1 transcripts detected by qPCR were present in the OE lines, but not in the wild-type plants. B Growth phenotypes of 2-week-old WT and transgenic OE (OE-7 and OE-10) seedlings vertically growing on 1/2 MS medium supplemented with 0, 100, 200 and 300 mM NaCl for 10 d. C Primary root length of 10 d-salt stressed plants in Fig. B. Values are mean ± SE, n = 10. **t-test, with P<0.01; *t-test, with P<0.05. D Representative phenotype of 6-week-old WT and transgenic OE plants growing in soil pots supplied with 0 (water) or 300 mM NaCl solution every other day for 4 weeks. E Survival rates (%) under salinity stress in Fig. D were determined as the number of visibly green plants after 4 weeks. Values are mean ± SE, n = 10. **t-test, with P<0.01.
To investigate whether overexpression of ZmSKD1 in plants enhances salt tolerance, seedlings at different developmental stages from transgenic lines (OE-7 and OE-10) and WT were treated separately with different concentrations of NaCl. When 2-wk-old seedlings were transferred to medium supplemented with NaCl and grown for 10 d, the WT plants showed more chlorosis and stunted phenotypes than the OE lines under both 200 and 300 mM NaCl. This was clearly seen upon 300 mM NaCl exposure (Fig. 3B). No significant changes were observed in growth phenotypes between WT and OE plants under 0 and 100 mM of NaCl (Fig. 3B). Root length determinations showed that significant differences were observed between WT and OE plants under 200 or 300 mM of NaCl. Compared with unstressed seedlings, root length decreased by 50% for 200 mM NaCl and 77% for 300 mM NaCl, respectively in the WT plants. By contrast, root length in both OE lines only decreased by 24 and 27% for 200 mM NaCl and 44 and 42% for 300 mM NaCl, respectively (Fig. 3C). When 6-wk-old transgenic plants were exposed to a continuous stress consisting of 300 mM NaCl added to the soil every other day for 4 weeks. The growth of WT plants was severely affected and more than 80% of WT plants were dead, whereas 70–80% of transgenic plants were still alive (Fig. 3D and 3E). These results demonstrate that overexpression of ZmSKD1 in transgenic tobacco plants significantly enhances tolerance to salt stress.
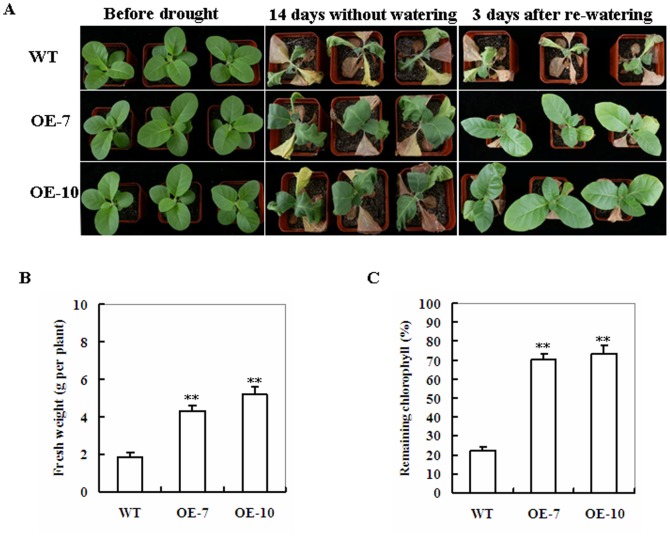
To characterize the performance of ZmSKD1 transgenic lines under watering-stress (drought) in soil, both OE lines were tested at the seedling stage (4-wk old). Under well-watered conditions, there was no obvious difference between WT and transgenic lines in leaves size and number of plants (Fig. 4A). After 14 days without watering, all WT plants displayed severe wilting (all leaves were severely curled and more than 70% leaves were turning yellow and dead), whereas ZmSKD1 transgenic lines showed signs of moderate water stress and most upper leaves of transgenic plants were still green and fully expanded (Fig. 4A). Three days after rewatering, all WT plants were nearly dead, whereas all transgenic lines survived the stress and started to grow (Fig. 4A). Accordingly, plant biomass and remaining chlorophyll content in ZmSKD1 transgenic lines were significantly higher than those in WT plants (2.5 and 3-fold on the average, respectively). (Fig. 4B, 4C). These results provide evidence that overexpression of ZmSKD1 in transgenic tobacco plants also improves tolerance to drought stress.
Figure 4. Phenotypes of wild-type and ZmSKD1-overexpressing tobacco plants in response to drought stress.
A Drought tolerance of potted plants of wild-type and ZmSKD1-overexpressing tobacco. Four-week-old WT and transgenic OE (OE-7 and OE-10) plants were grown in soil in pots for 14 d without watering, and then rewatering after 3 days. B Fresh weight of drought-stressed wild-type and ZmSKD1-overexpressing plants after 3 days recovery. Values are mean ± SE, n = 6. **t-test, with P<0.01. C Relative remaining chlorophyll (%) of drought-stressed wild-type and ZmSKD1- overexpressing plants after 3 days recovery. Values are mean ± SE, n = 6. **t-test, with P<0.01.
ROS Accumulations in ZmSKD1 Transgenic Plants During Salt or Drought Stress
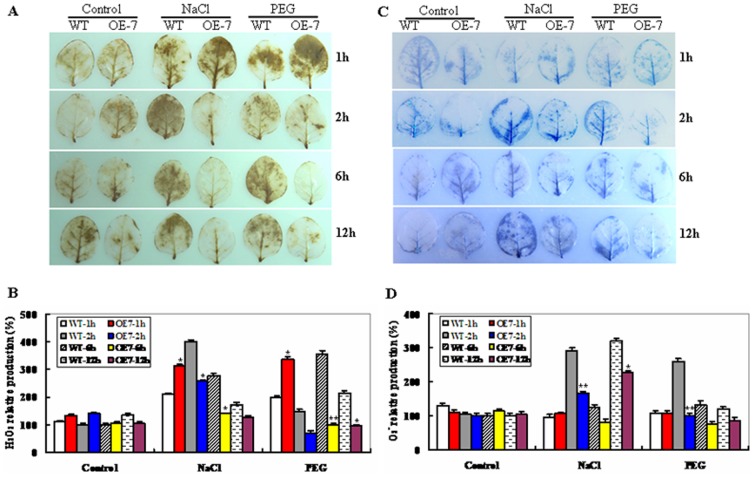
Drought or salt stress may induce production of reactive oxygen species (ROS), which cause oxidative damage to plant cells, or act as signaling molecules for regulating development and various physiological responses [15]. This promoted us to examine cellular levels of two prominent ROS (O2 − and H2O2) accumulations in OE and WT plants during salt or PEG-induced water stress. Histochemical detection of H2O2 accumulation was performed with DAB staining in NaCl or PEG-treated leaves along with controls from OE and WT plants within 12 h. As shown in Fig. 5A, both OE and WT leaves showed more DAB staining at 1 h of NaCl or PEG treatment; after 2 h, marked difference in DAB staining intensity was observed between OE and WT leaves. WT showed more DAB staining at 2 h of salt or drought stress, and remained higher levels till 12 h, whereas OE showed less staining after 2 h; moreover, the contrasting staining was much clear between the OE and WT leaves at 6 h, and till the end of the stresses (Fig. 5A). No significant differences in DAB staining intensity were observed between WT and OE lines within 12 h of distilled water-treated controls (Fig. 5A). The fluctuation in H2O2 level, especially in wild-type plants treated with PEG and stained with DAB was also noted (Fig. 5A). Quantitative determination of H2O2 accumulation at 1, 2, 6 and 12 h of salt or drought stress further demonstrated that compared to their corresponding controls, WT increased significantly in DAB staining intensity at 2, 6, or 12 h of both stresses, while the OE plants had no significant changes (Fig. 5B).
Figure 5. Dynamic changes of ROS in wild-type and ZmSKD1-overexpressing tobacco plants in response to salt or PEG-induced water stress.
A H2O2 production in leaves of wild-type and OE (OE-7) plants treated with distilled water(control), 200 mM NaCl or 15% PEG-6000 solutions, respectively, was visualized by staining with 3, 3′-diaminobenzidine (DAB). Plants were treated for 1, 2, 6, and 12 h, and were subsequently stained with DAB as described in the experimental procedures. B Relative H2O2 levels were quantified in leaves of wild-type and OE (OE-7) plants exposed to 200 mM NaCl or 15% PEG-6000 for 1, 2, 6, and 12 h. Error bars indicate SE (n = 6). **t-test, with P<0.01; *t-test, with P<0.05. C O2 − production in leaves of wild-type and OE (OE-7) plants treated with 200 mM NaCl or 15% PEG-6000 solutions, respectively, was visualized by staining with nitroblue tetrazolium (NBT). Plants were treated for 1, 2, 6, and 12 h, and were subsequently stained with NBT as described in the experimental procedures. D Relative O2 − levels were quantified in leaves of wild-type and OE (OE-7) plants exposed to 200 mM NaCl or 15% PEG-6000 for 1, 2, 6, and 12 h. Error bars indicate SE (n = 6). **t-test, with P<0.01; *t-test, with P<0.05.
Similarly, O2 − accumulation was examined in situ with NBT staining. As shown in Fig. 5C, both OE and WT leaves showed more NBT staining at 2 h of NaCl or PEG treatment; after 2 h, marked difference in NBT staining intensity was observed between OE and WT leaves. WT showed increasing NBT staining at 2 h, and remained high levels till 12 h, whereas OE showed decreasing staining after 2 h; moreover, the contrasting staining was clear between the OE and WT leaves at 2 h, after that the difference is becoming unclear in the remaining period of the stresses (Fig. 5C). No significant differences in NBT staining intensity were observed between WT and OE lines within 12 h of distilled water-treated controls (Fig. 5C). Also, there is a fluctuation in O2 − level, especially in wild-type and the OE plants treated with salt and stained with NBT (Fig. 5C). Quantitative determination of O2 − accumulation at 1, 2, 6 and 12 h of salt or drought stress further showed that compared to their corresponding controls, WT increased significantly in DAB staining intensity at 2 h of both stresses, while the OE plants had no significant changes (Fig. 5D). These results demonstrate that ROS accumulations in ZmSKD1 transgenic lines were relative less than those in WT plants during salt or PEG-induced water stress.
ZmSKD1 Interacts with NtLIP5 in Yeast and in planta
In Arabidopsis, LIP5 (LYST-INTERACTING PROTEIN 5) has been characterized as an AtSKD1 interactor [8]. Therefore, it would be of interest to examine whether ZmSKD1 had the ability to interact with LIP5 homolog from tobacco (NtLIP5) in transgenic plants. NtLIP5 was cloned by RT-PCR from tobacco leaves, which encodes a predicted protein of 414 amino acids. This protein is 59.4% identical to AtLIP5 (At4g26750). A database search revealed that there is only one LIP5 gene in the released tobacco genome (Data not shown).
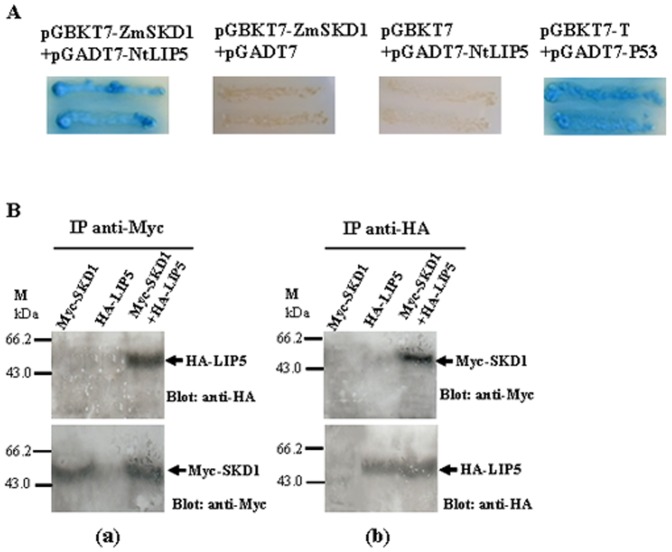
A yeast two-hybrid assay was performed to determine whether ZmSKD1 could interact with NtLIP5 in vitro. Combinations of plasmids expressing bait protein BD-ZmSKD1 and prey protein AD-NtLIP5 were transformed into AH109, respectively. It was verified that expression of BD-ZmSKD1 and AD-NtLIP5 was not toxic for AH109 yeast cells and that these fusion proteins did not bind or trans-activate the lacZ reporter gene non-specifically when expressed separately (data not shown). Co-transformation assays showed that independent yeast colonies containing pGBKT7-ZmSKD1and pGADT7-NtLIP5, as well as those containing pGADT7-T and pGBKT7-p53 (positive control), grew well on SD/−Ade/−His/−Leu/−Trp medium and turned blue in the β-galactosidase colony lift filter assay, indicating that there were strong interactions between ZmSKD1and NtLIP5. In contrast, no growth was observed for the negative controls (pGBKT7-ZmSKD1+pGADT7 or pGBKT7+pGADT7-NtLIP5) (Fig. 6A). This suggested that ZmSKD1 has the ability to interact with NtLIP5 in vitro.
Figure 6. ZmSKD1 interacts with NtLIP5 in yeast and in planta.
A ZmSKD1 interacts with NtLIP5 in YTH system. Yeast colonies containing pGBKT7-SKD1/pGADT7-LIP5 grew well on the selective medium (SD/−Ade/−His/−Leu/−Trp) and turned blue in the β-galactosidase assays as did yeast colonies containing pGBKT7-T/pGADT7-p53, which was used as the positive control, whereas yeast transformed with pGBKT7-SKD1/pGADT7 or pGBKT7/pGADT7-LIP5 used as negative controls were unable to grow. B ZmSKD1 interacts with NtLIP5 in coimmunoprecipitation assays in Nicotiana benthamiana. Different cell lysates (HA-LIP5, Myc-SKD1, or HA-LIP5 and Myc-SKD1) were immunoprecipitated with anti-Myc or anti-HA antibodies. These immunoprecipitates were then separated by SDS-PAGE and immunoblotted with anti-HA or anti-Myc antibodies. The numbers on the left show the molecular masses of marker proteins in kilodaltons. (a) Samples were immunoprecipitated with anti-Myc antibody and immunoblotted with anti-HA (upper) or anti-Myc antibody (lower). (b) Samples were immunoprecipitated with anti-HA antibody and immunoblotted with anti-Myc (upper) or anti-HA antibody (lower).
The interaction between ZmSKD1 and NtLIP5 in N.benthamiana leaves was further confirmed by an immunoprecipitation assay. The cell extracts were immunopurified with anti-Myc antibody to pull down Myc-SKD1 protein, and then the immunoprecipitated products were fractionated via SDS-PAGE and detected with anti-HA antibody. As shown in Fig. 6B-a, the HA-LIP5 protein can only be detected in the mixture of Myc-SKD1 and HA-LIP5 extracts (upper). Likewise, Myc-SKD1 protein can be detected in the Myc-SKD1 extracts or the mixture of Myc-SKD1 and HA-LIP5 extracts using anti-Myc antibody, but not in the HA-LIP5 extracts (lower). To verify the interaction, we performed the same assay, replacing the anti-Myc antibody with anti-HA antibody, and then detected the results with an anti-Myc antibody. In this assay, the Myc-SKD1 protein could also be detected in the same sample (Fig. 6B-b, upper); Also, HA-LIP5 protein can be detected in the HA-LIP5 extracts or the mixture of Myc-SKD1 and HA-LIP5 extracts using anti-HA antibody, but not in the Myc-SKD1 extracts (lower) (Fig. 6B-b, lower). Both results indicated that a direct interaction occurred between the ZmSKD1 and NtLIP5 proteins extracted from transient expression in N.benthamiana by agroinfiltration.These results, together with above results confirm that ZmSKD1 can interact with NtLIP5 in vitro as well as in planta.
Mapping of the Domain of ZmSKD1 Required for Interacting with NtLIP5
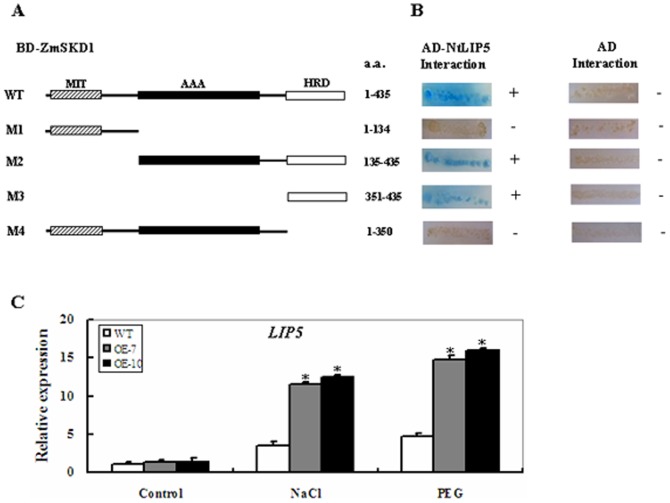
Two functional domains of ZmSKD1 were identified; one is an N-terminal MIT domain (residues 7 to 73) and the other is a central AAA domain (residues 135 to 298) (Figs. 1A, 7A). In addition, a α-helix-rich domain (HRD) in the C-terminus (residues 351 to 435) was found in the ZmSKD1 protein by META Predict Protein Server analysis (http://www.embl-heidelberg.de/predictprotein/) (Fig. 7A).
Figure 7. Mapping of the ZmSKD1 region involved in SKD1-LIP5 interaction and transcript levels of NtLIP5 in responses to salt or PEG-induced water stress in transgenic and wild-type plants.
A Schematic representation of wild type (WT) and mutant SKD1s in the study. M1 and M4 are C-terminal deletion mutants, and M2 and M3 are N-terminal deletion mutants. MIT marks microtubule-interacting and trafficking; AAA marks ATPase associated with a variety of cellular activities; HRD marks the α-helix-rich domain located at the C-terminus of SKD1. The numbers denote SKD1 amino acid positions. B The interaction between LIP5 or empty vector pGAD-T7 and SKD1 deletions in YTH assays. The ability of SKD1 truncations to interact with LIP5 or AD vector is indicated on the right (+, positive; −, negative). C NtLIP5 transcript levels under salt or PEG-induced water stress in transgenic and wild-type plants. Four-week-old transgenic and wild-type tobacco plants were exposed to distilled water (control), 15% PEG6000 and 200 mM NaCl for 12 h, respectively, and leaf samples were used for real-time PCR analysis. NtActin was used as an internal control. For each treatment, the expression level under control conditions was defined as 1.0, and data represented means ± SE of three technical replicates. *t-test, with P<0.05.
To examine the roles of these three domains in mediating the interaction between SKD1 and LIP5 and to determine further the domain necessary for SKD1-LIP5 interaction, we sequentially constructed a serial of truncation derivatives that express different structural elements of the SKD1. Homologous binding capabilities between these truncated mutants and NtLIP5 were investigated via the YTH assay. Schematic representation of the different SKD1 truncations is shown in Fig. 7A. Firstly, the interactions between NtLIP5 and M1 (1–134) (the N-terminal region of 134 residues of ZmSKD1, Fig. 7A) or M2 (135–435) (the C-terminal region of 301 residues of ZmSKD1, Fig. 7A) were examined. As for wild type SKD1, M2 (135–435) was able to interact with the NtLIP5 (Fig. 7B). However, no clear yeast colonies growth was observed for M1 (1–134) (Fig. 7B), suggesting the C-terminal region containing AAA and HRD domains is responsible for SKD1-LIP5 interaction. Then, a deletion mutant M3 (351–435) was made by amplifying the fragment containing the last 85 residues from the C-terminus of SKD1, in which only the HRD was present (Fig. 7A).YTH results also showed a strong interaction between M3 and the NtLIP5 (Fig. 7B). Finally, the M4 mutant (1–350), a deletion of this HRD domain alone in the YTH assays led to the loss of interaction between M4 and the NtLIP5 (Fig. 7B). Also, control experiments included pGADT7 only with WT, M1, M2, M3, or M4 showed no positive interactions (Fig. 7B).Collectively, these results demonstrate that the C-terminal domain (residues 351–435) of ZmSKD1 is required for its interaction with NtLIP5.
Changes in LIP5 Gene Expression in ZmSKD1 Transgenic Plants During Salt or Drought Stress
To further look at the changes in LIP5 gene expression levels during salt or drought stress, transcript levels of NtLIP5 were monitored in the wild-type and OE plants by qRT-PCR. After 12 h, the abundance of NtLIP5 transcripts displayed differential patterns between the wild-type and OE lines. Under salt or PEG-induced water stress, compared with controls, the transcript levels of NtLIP5 were significantly up-regulated in both WT and OE plants, but the magnitudes of increase in NtLIP5 transcripts were differential (Fig. 7C). Under 200 mM NaCl stress, WT showed a 2.5-fold increase, while the two OE lines exhibited 7.5 and 8-fold increases, respectively (Fig. 7C). Similarly, under 15% PEG stress, the transcription levels of NtLIP5 were significantly up-regulated in both OE lines (about 10-fold increase for both lines), while only 3.5-fold increase in the WT (Fig. 7C). In addition, no significant changes in NtLIP5 transcripts were detected between WT and OE lines under control conditions (Fig. 7C). The results further indicate that LIP5 together with ZmSKD1 responds to salt or drought stress in transgenic plants.
Discussion
Environmental stresses such as drought and high salinity have drastic effects on both biomass and grain yields of crops worldwide. However, the molecular mechanisms underlying AAA-type proteins-mediated ESCRT pathway involved in response to salt or drought stress in plants are largely unknown. In this study, we investigated physiological roles of a putative AAA-type ATPase SKD1 from maize and its possible function mechanisms using several complementary approaches. Our genetic evidence has demonstrated that ZmSKD1 can interact with NtLIP5 and its over-expression enhances not only salt but drought stress tolerance in transgenic plants. To the best of our knowledge, this is the first SKD1 gene from monocot plants to be functionally characterized, in which the novel function for SKD1 in drought has never been reported before in higher plants.
SKD1 is Conserved in Yeast, Plants and Mammals
Like known yeast, plant and mammal SKD1s, ZmSKD1 has higher sequence identities (52–93%) and two typical structural domains (Fig. 1A). From this, it can be reasonably concluded that the ZmSKD1 cDNA clone encodes maize SKD1 isoform. Both Arabidopsis and rice (Oryza sativa) contain one copy of the SKD1gene in their genomes [8]. A database search revealed that ZmSKD1 is also encoded by a single-copy gene and located on chromosome 3 in maize genome. This indicates that SKD1 is evolutionarily conserved and may be functionally important even essential in plant species. This view can be supported by the fact that over-expression of ATPase-deficient SKD1 caused a lethal phenotype in Arabidopsis, but not in yeast or mammalian cells [8].
The Effects of ZmSKD1 on Salt or Drought Stress Tolerance Might Be Specific in Plants
ZmSKD1 was found to be highly expressed in aerial tissues of maize, and its highest expression level occurred in leaves (Fig. 2A), indicating this gene may be constitutively expressed during both vegetative and reproductive growth. This is consistent with the expression potential of AtSKD1 as determined in Arabidopsis by transcript and protein levels analyses [8]. Previous studies have shown that SKD1 orthologs from Arabidopsis (AtSKD1) and ice plant (McSKD1) were induced by salt stress [16], [17], but no drought-induced expression of both genes was reported. Compared to this expression pattern, ZmSKD1 is significantly affected not only by salt, but by drought stress in maize (Fig. 2B, 2C). Genetic evidence further proved that ZmSKD1 OE lines showed enhanced salt and drought tolerance in tobacco (Figs. 3, 4). These results indicate that function divergence of the SKD1 orthologs may occur in the genomes of Arabidopsis and maize, although these genes have high similarities in sequences. In addition, the effects of ZmSKD1 on heat or cold stress were tested using transgenic tobacco lines, but no significant enhancing effects have been found at present (data not shown); demonstrating that the ZmSKD1's effect on tobacco stress tolerance could be specific. Based on these results, it can be speculated that ZmSKD1 might participate in salt and drought stress responses and plays a positive role in both stresses.
The Possible Involvement of ZmSKD1 in ROS Levels Modulation in Transgenic Plants During Salt and Drought Stresses
Proper ROS levels are critical for tolerance to abiotic stress in plants. In this study, both OE and WT leaves showed increased DAB staining within 1 h of NaCl or PEG stress (Fig. 5A, 5B); showing that ZmSKD1 transgenic plants accumulated more H2O2 than the WT at early stage of stresses. After 2 h, OE plants showed less staining than the WT in DAB or NBT staining; moreover, the contrasting DAB staining was much clear between OE and WT leaves in the remaining of the period; the ZmSKD1 transgenic plants accumulated less ROS than WT (Fig. 5). This observation indicated that the more H2O2 in transgenic plants at early stage might act as early signaling molecules to activate downstream ROS-scavenging or stress-responsive gene expression promptly, resulting in less toxic levels of ROS accumulations in subsequent stress period. In addition, there are fluctuations in both H2O2 and O2 − levels, especially in PEG-treated wild-type plants stained with DAB, and in salt-treated wild-type and OE plants stained with NBT (Fig. 5A, 5C). The fluctuations in ROS levels may indicate the complex regulating mechanisms of ROS's involvement during ZmSKD1-mediated drought or salt tolerance in plants. Thus, it could be speculated that ZmSKD1 probably modulates ROS levels by more efficient removal or a delay in sensing or transducing the stress signals. Further work is needed to dissect the involvement of ZmSKD1 in the ROS signaling network using microarray analysis in ZmSKD1-modified plants under drought or salt stress.
ZmSKD1 might Interact with LIP5 in Transgenic Plants During Salt or Drought Stress
Database search has revealed that one LIP5 homolog exists in the maize genome. It encodes a predicted protein of 477 amino acids, which is 51.7% and 49.3% identical to NtLIP5 and AtLIP5, respectively (Data not shown). This indicates that LIP5 homologues have high similarities in sequences. The AtSKD1 can interact with AtLIP5 in yeast and plants [8]; also, the interaction between ZmSKD1 and NtLIP5 was confirmed by Y2H and immuno-precipitation (in this study); indicating that the LIP5 homologs are conserved in function. Thus it could be speculated that ZmSKD1 might interact with maize LIP5.
The Arabidopsis mutant atlip5 plants are viable and show no phenotypic alterations under normal growth conditions, suggesting that basal SKD1 ATPase activity is sufficient for plant development and growth [8]. However, no further investigations have been reported for plant LIP5 in response to salt or drought stress. In our study, we have found that ZmSKD1 is capable of interacting with NtLIP5 by its α-helix-rich domain in the C-terminus (Fig. 7A); moreover, the induced expression pattern of NtLIP5 showed a resemblance to that of ZmSKD1 under salt and drought stresses (Figs. 2A, 2B; 7C). More importantly, the transcript levels of LIP5 in transgenic plants had greater magnitudes of increase than those in WT plants under salt or drought stress; suggesting that the increased LIP5 abundance may contribute to ZmSKD1 activity enhancement, resulting in increased endurance of transgenic plants under stress conditions. This notion could be proved by the evidence that Arabidopsis LIP5 acts as a positive regulator of SKD1 by increasing its ATPase activity by 4∼5 folds in vitro [8]. Although the roles of LIP5 in plant salt or drought tolerance have not been established, the expression patterns indicate that LIP5 might play a positive role in salt or drought tolerance by interacting with SKD1. The true roles of the LIP5 gene under salt or drought stress need to be fully explored using transgenic plants in future studies.
Besides LIP5, AtSKD1 has been characterized to interact with ESCRT-III components and putative ESCRT-III-associated proteins on MVBs in Arabidopsis [13], [18], [19], [20]. Therefore, it will be interesting to compare possible mechanisms of SKD1's involvement in the ESCRT pathway under stress conditions between the model plant Arabidopsis and crop plants.
SKD1-mediated ESCRT Machinery may Be Involved in Cellular Responses to Environmental Stress
The AAA ATPase SKD1 exploits the energy from ATP hydrolysis to induce conformational changes in other proteins or protein complexes causing unfolding or dissociation of substrate proteins [3]. It has been reported that the yeast vps4/skd1 mutant showed cadmium sensitivity; suggesting that the ESCRT machinery is specifically involved in targeting of vacuolar proteins responsible for cadmium detoxification [21]. A microarray analysis on AtSKD1-knockdown Arabidopsis mutant under salt stress has shown that expressions of one stress-related kinase, several salt-induced transcription factors and one auxin efflux transporter were altered [14]. Moreover, the SKD1-knockdown plants experienced a reduced salinity response and altered root development; indicating the importance of intracellular protein trafficking in both auxin-mediated plant growth and in maintaining ion homeostasis under salt stress [14]. These lines of evidence could demonstrate that SKD1-mediated ESCRT machinery may be involved in cellular responses to environmental stress.
In summary, these data have demonstrated that the monocot ZmSKD1 could be involved in salt and drought responses and its over-expression enhances not only salt but drought stress tolerance. This study may facilitate our understanding of the biological roles of SKD1-mediated ESCRT pathway under stress conditions in higher plants and accelerate genetic improvement of crop plants tolerant to environmental stresses.
Materials and Methods
Plant Material and Stress Treatments
A maize inbred line, Zea mays cv. Zheng58, was used throughout this study. Tobacco plants (Nicotiana tabacum cv. Xanthi) were used for gene transformation. Plants were grown in a growth room as described previously [22]. Drought or salt stress in two-week-old seedlings was realized by replacing the water with PEG 6000 (10%, 15%, or 20%) and NaCl (100, 200, or 300 mM), respectively, and leaves were sampled at 0, 6, 12, 24, or 48 h for expression analysis as described by us [23].
Cloning of ZmSKD1 and Sequence Analysis
The salt-induced EST encoding a putative AAA protein was used to do BLAST (http://www.ncbi.nlm.nih.gov/) and mRNA sequences containing such an EST were downloaded for gene prediction. The gene is highly homologous to AtSKD1, and thus is named ZmSKD1. Two primers SKD1-F and SKD1-R (Table S1) were designed for amplifying the open reading frame (ORF) of ZmSKD1. The 1308 bp PCR product was verified by sequencing.
The primary structural analysis was performed using InterProScan (http: //www. ebi.ac.uk/InterProScan). The alignment of the deduced protein sequences and phylogenetic tree analyses were done by DNASTAR and MEGA 5.1, respectively, using standard parameters [24].
Real-time PCR Analysis
Real-time PCR was used to determine the expression patterns of ZmSKD1 in different organs and under stress conditions. The qRT-PCR was performed in triplicate with an IQ5 light cycler system (Bio-Rad) using SYBR Premix ExTaq II (Takara, Japan) with gene-specific primers SKD1-F1 and SKD1-R1 (Table S1), which produces a141-bp product. The maize Ubiquitin transcript was used as an internal control to quantify the relative transcript levels [23]. The relative level of gene expression was detected using the 2−ΔΔC T method [25].
The expression of NtLIP5 in transgenic plants was analyzed by real-time PCR with RNA samples isolated from four-week-old transgenic plants harvested after 12 h of distilled water (control), 15% PEG6000 and 200 mM NaCl treatments. PCR was performed with specific primers NtLIP5-F3 and NtLIP5-R3 (Table S1), which produces a 138-bp product. Amplification of tobacco Actin was used as an internal control as described by us previously [22]. For the entire qRT-PCR assay, three technical replicates were performed for each experiment and the expression of each gene was investigated in three biological replicates.
Construction of Plant Expression Vectors and Development of Transgenic Tobacco Lines
The ZmSKD1 coding sequence was amplified and introduced into the pART7 plasmid using primers SKD1-F2 with EcoRI restriction site (underlined) and SKD1-R2 with BamHI restriction site (underlined) (Table S1) and was subsequently inserted downstream of the 35S promoter in the plasmid vector pART7. The resulting expression cassette containing the 35S promoter and ZmSKD1 coding sequence was cut and inserted into the binary vector pART27 [22], producing the transformation construct pART27-35S-ZmSKD1. The binary construct was introduced into Agrobacterium tumefaciens strain LBA4404 and then transformed into tobacco via the leaf-disc method as described by us previously [22].
The kanamycin-resistant transgenic plantlets were confirmed by PCR with primers 35SP-F and SKD1-R3 (the forward primer 35SP-F is from CaMV 35S promoter sequence) (Table S1). The PCR-positive plantlets were transplanted into soil for growing in the growth room. The transgenic tobacco progenies were selected using antibiotics and maintained growth to set seeds until T2 generation. Six independent homozygous ZmSKD1 over-expression (OE) transgenic lines (named OE-1, OE-2, OE-4, OE-5, OE-7, and OE-10) were developed. The expression of the transgene in the six lines was evaluated by qRT-PCR with gene-specific primers SKD1-F4 and SKD1-R4 (Table S1), which produces a 159-bp product.
Analysis of ZmSKD1-overexpressing Tobacco for Salt and Drought Stress Tolerance
For salt stress tolerance analysis, two sets of experiments were designed. One is assayed for seedlings in the MS medium plates. Two-week-old wild-type (WT) and homozygous T2 transgenic seedlings were transferred to 1/2 MS medium supplemented with 0, 100, 200 or 300 mM NaCl for growing vertically, in the growth chamber. The phenotype of seedlings was photographed after 10 d of growth and the root length and fresh weight of seedlings were measured. The other is treated with the potted adult plants. Six-week-old WT and T2 transgenic seedlings grown in soil were watered with 0 (water) or 300 mM NaCl solution every other day for 4 weeks. The experiment was repeated at least three times. Survival rates (%) under salinity stress were determined as the number of visibly green plants after 4 weeks.
For drought tolerance analysis, four-week-old plants were subjected to progressive drought by withholding water until a nearly lethal effect of dehydration (about 2 weeks) was observed on wild-type plants. The drought stress experiment was performed at least three times. Three days after rewatering, the fresh weight and total chlorophyll content of treated plants were measured as described previously [26]. The remaining chlorophyll content in WT or OE plants after drought was expressed as a percentage, in which the chlorophyll content of each treated genotype was compared to its own control plants with the same age without drought stress.
Histochemical Detection and Quantitative Determination of H2O2 and O2 − Production
Histochemical detection of H2O2 accumulation with 3, 3′- diaminobenzidine (DAB) staining was performed as described by us previously [21]. Detection of O2 − with nitro blue tetrazolium (NBT) was performed according to the method of Overmyer et al. [27]. Four-week-old WT and ZmSKD1-overexpressing tobacco plants were treated with distilled water (control), 200 mM NaCl or 15% PEG-6000 solutions, respectively, for 1, 2, 6, and 12 h, and incubated with 100 µg mL−1 DAB or NBT solution. Stained leaf samples were boiled in 97% (v/v) ethanol to remove chlorophyll and photographed directly using a Canon camera.
Wild-type and OE (OE-7) plants were exposed to 200 mM NaCl or 15% PEG-6000 for 1, 2, 6, and 12 h; after that, relative H2O2 contents in leaves were assayed according to the method of Xia et al [22], and relative O2 − levels in leaves were quantified as described previously [28].
Yeast two-hybrid Assays
Using the yeast two-hybrid (YTH) system, wild-type and three truncated SKD1 constructs were tested for their interactions with NtLIP5. WT was designed for expressing full-length SKD1 (1–435), whereas four mutants M1–M4 were constructed for expressing truncated SKD1s. M1 (1–134), M2 (135–435) were constructed by deleting 301 and 134 residues from the C-terminal and N-terminal ends of wild type SKD1, respectively. M3 (351–435) was made by amplifying the fragment containing the last 85 residues from the C-terminal end of SKD1, in which only the α-helix-rich domain (HRD) was present. M4 (1–350) was constructed by deleting 85 residues from the C-terminal end of wild type SKD1. Coding sequences of the WT SKD1 and four deletion mutants were amplified as EcoRI/BamHI fragments using the primers listed in Table S1, and then individually cloned into the yeast expression vector pGBKT7. The recombinant plasmid pGADT7-LIP5 was created as NdeI/BamHI fragment into the yeast expression vector pGADT7 using a similar strategy. All clones derived from PCR products were verified by sequencing and the recombinant plasmids w ere confirmed by restriction analyses. YTH assays were performed using the Matchmaker GAL4 Two-Hybrid System 3 (Clontech) as described by us [29]. All treatments were repeated at least three times.
Plasmids Construction, Agroinfiltration and Immunoprecipitation in N. benthamiana Plants
For the 35S::Myc-SKD1 construct, a PCR-amplified fragment of ZmSKD1 was inserted into XbaI/BamHI-digested plant expression vector pSPY1-35S with a 6xc-Myc tag to give the construct 35S::Myc-SKD1. For the 35S::HA-LIP5 construct, the NtLIP5 fragment with XbaI/BamHI sites was cloned into plant expression vector pSPY2-35S with a hemagglutinin (HA) tag to give the construct 35S::HA-LIP5. The primers used in the experiment were listed in Table S1. Both constructs were introduced into Agrobacterium tumefaciens strains GV3101 and recombinant clonies were cultured for agroinfiltration.
Co-infiltration in N.benthamiana leaves were performed as described by us [30]. For immunoprecipitation assays, the infiltrated parts of N. benthamiana leaves were harvested and extracted. Corresponding antibodies (HA or Myc) were added to the cell lysates. Immunoblot analysis was conducted using anti-Myc (Santa Cruz, 1∶1000) or anti-HA antibody (Santa Cruz, 1∶1000) as described previously [22], [31].
Supporting Information
PCR primers used in this study.
(DOC)
Acknowledgments
We are grateful to academic editor and anonymous reviewers for constructive suggestions on manuscript revisions.
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (30971548) and the subject of national “973 project” (2009CB118402) partially. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ogura T, Wilkinson AJ (2001) AAA+ superfamily ATPases: common structure-diverse function. Genes Cells 6: 575–597. [DOI] [PubMed] [Google Scholar]
- 2. Lupas AN, Martin J (2002) AAA proteins. Curr Opin Struct Biol 12: 746–53. [DOI] [PubMed] [Google Scholar]
- 3. Hanson PI, Whiteheart SW (2005) AAA proteins: have engine, will work. Nat Rev Mol Cell Biol 6: 519–529. [DOI] [PubMed] [Google Scholar]
- 4. Pe'rier F, Coulter KL, Liang H, Radeke CM, Garber RF, et al. (1994) Identification of a novel mammalian member of the NSF/CDC48p/Pas1p/TBP-1 family through heterologous expression in yeast. FEBS Lett 351: 286–290. [DOI] [PubMed] [Google Scholar]
- 5. Babst M, Wendland B, Estepa EJ, Emr SD (1998) The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J 17: 2982–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheuring S, Bodor O, Rohricht RA, Muller S, Beyer A, et al. (1999) Cloning, characterisation, and functional expression of the Mus musculus SKD1 gene in yeast demonstrates that the mouse SKD1 and the yeast VPS4 genes are orthologues and involved in intracellular protein trafficking. Gene 234: 149–159. [DOI] [PubMed] [Google Scholar]
- 7. Jou Y, Chou PH, He M, Hung Y, Yen HE (2004) Tissue-specific expression and functional complementation of a yeast potassium-uptake mutant by a salt-induced ice plant gene mcSKD1 . Plant Mol Biol 54: 881–893. [DOI] [PubMed] [Google Scholar]
- 8. Haas TJ, Sliwinski MK, Martinez DE, Preuss M, Ebine K, et al. (2007) The Arabidopsis AAA ATPase SKD1 is involved in multivesicular endosome function and interacts with its positive regulator LYSTINTERACTING PROTEIN5. Plant Cell 19: 1295–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshimori T, Yamagata F, Yamamoto A, Mizushima N, Kabeya Y, et al. (2000) The mouse SKD1, a homologue of yeast Vps4p, is required for normal endosomal trafficking and morphology in mammalian cells. Mol Cell Biol 11: 747–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winter V, Hauser MT (2006) Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci 11: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Logg K, Warringer J, Hashemi SH, Käll M, Blomberg A (2008) The sodium pump Ena1p provides mechanistic insight into the salt sensitivity of vacuolar protein sorting mutants. Biochim Biophys Acta 1783: 974–984. [DOI] [PubMed] [Google Scholar]
- 12. Yen HE, Wu SM, Hung YH, Yen SK (2000) Isolation of 3 salt-induced low- abundance cDNAs from light-grown calli of Mesembryanthemum crystallinum by suppression subtractive hybridzation. Physiol Plant 110: 402–409. [Google Scholar]
- 13. Shahriari M, Keshavaiah C, Scheuring D, Sabovljevic A, Pimpl P, et al. (2010) The AAA-ATPase AtSKD1 contributes to vacuolar maintenance of A.thaliana . Plant J 64: 71–85. [DOI] [PubMed] [Google Scholar]
- 14. Ho LW, Yang TT, Shieh SS, Edwards GE, Yen HE (2010) Reduced expression of a vesicle trafficking-related ATPase SKD1 decreases salt tolerance in Arabidopsis. Funct Plant Biol 37: 962–973. [Google Scholar]
- 15. Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–99. [DOI] [PubMed] [Google Scholar]
- 16. Gong Z, Koiwa H, Cushman MA, Ray A, Bufford D, et al. (2001) Genes that are uniquely stress regulated in salt overly sensitive (sos) mutants. Plant Physiol 126: 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jou Y, Chiang CP, Jauh GY, Yen HE (2006) Functional characterization of ice plant SKD1, an AAA-Type ATPase associated with the endoplasmic reticulum–Golgi network, and its role in adaptation to salt stress. Plant Physiol 141: 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, et al. (2009) The ESCRT- related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21: 749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katsiarimpa A, Anzenberger F, Schlager N, Neubert S, Hauser MT, et al. (2011) The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell 23: 3026–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson LGL, Howard ASM, Khuu N, Gidda SK, McCartney A, et al. (2011) Protein–protein interaction network and subcellular localization of the Arabidopsis thaliana ESCRT machinery. Front Plant Sci 2: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruotolo R, Marchini G, Ottonello S (2008) Membrane transporters and protein traffic networks differentially affecting metal tolerance: a genomic phenotyping study in yeast. Genome Biol 9: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia Z, Sun K, Wang M, Wu K, Zhang H (2012) Overexpression of a maize sulfite oxidase gene in tobacco enhances tolerance to sulfite stress via sulfite oxidation and CAT-mediated H2O2 scavenging. PLoS One 7: e37383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia Z, Liu Q, Wu J, Ding J (2012) ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2 E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene 495: 146–53. [DOI] [PubMed] [Google Scholar]
- 24. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livaka KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real- time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26. Jia X, Xu C, Jing R, Li R, Mao X, et al. (2008) Molecular cloning and characterization of wheat calreticulin (CRT) gene involved in drought-stressed responses. J Exp Bot 59: 739–751. [DOI] [PubMed] [Google Scholar]
- 27. Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, et al. (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide– dependent cell death. Plant Cell 12: 1849–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53: 2401–2410. [DOI] [PubMed] [Google Scholar]
- 29. Xia Z, Cao R, Sun K, Zhang H (2012) The movement protein of Barley yellow dwarf virus-GAV self-interacts and forms homodimers in vitro and in vivo . Arch Virol 157: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 30. Xia Z, Su X, Wu J, Wu K, Zhang H (2012) Molecular cloning and functional characterization of a putative sulfite oxidase (SO) ortholog from Nicotiana benthamiana . Mol Biol Rep 39: 2429–2437. [DOI] [PubMed] [Google Scholar]
- 31. Liu L, Zhang Y, Tang S, Zhao Q, Zhang Z, et al. (2010) An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana . Plant J 61: 893–903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR primers used in this study.
(DOC)