Abstract
Purpose
Radiofrequency ablation (RFA) is a minimally invasive energy delivery technique increasingly used for focal therapy to eradicate localized disease. RFA-induced tumor-cell necrosis generates an immunogenic source of tumor antigens known to induce antitumor immune responses. However, RFA-induced antitumor immunity is insufficient to control metastatic progression. We sought to characterize (a) the role of RFA dose on immunogenic modulation of tumor and generation of immune responses and (b) the potential synergy between vaccine immunotherapy and RFA aimed at local tumor control and decreased systemic progression.
Experimental Design
Murine colon carcinoma cells expressing the tumor-associated (TAA) carcinoembryonic antigen (CEA) (MC38-CEA+) were studied to examine the effect of sublethal hyperthermia in vitro on the cells’ phenotype and sensitivity to CTL-mediated killing. The effect of RFA dose was investigated in vivo impacting (a) the phenotype and growth of MC38-CEA+ tumors and (b) the induction of tumor-specific immune responses. Finally, the molecular signature was evaluated as well as the potential synergy between RFA and poxviral vaccines expressing CEA and a TRIad of COstimulatory Molecules (CEA/TRICOM).
Results
In vitro, sublethal hyperthermia of MC38-CEA+ cells (a) increased cell-surface expression of CEA, Fas, and MHC class I molecules and (b) rendered tumor cells more susceptible to CTL-mediated lysis. In vivo, RFA induced (a) immunogenic modulation on the surface of tumor cells and (b) increased T-cell responses to CEA and additional TAAs. Combination therapy with RFA and vaccine in CEA-transgenic mice induced a synergistic increase in CD4+ T-cell immune responses to CEA and eradicated both primary CEA+ and distal CEA– s.c. tumors. Sequential administration of low-dose and high-dose RFA with vaccine decreased tumor recurrence compared to RFA alone. These studies suggest a potential clinical benefit in combining RFA with vaccine in cancer patients, and augment support for this novel translational paradigm.
Introduction
Thermal ablation techniques such as microwave, cryotherapy, laser ablation, high-intensity focused ultrasound (HIFU), and radiofrequency ablation (RFA) have been increasingly used for minimally invasive treatment of local unresectable tumors [1]. RFA has proven to be safe and certain systems are cleared by the U.S. Food and Drug Administration (FDA) for ablation of soft tissue, unresectable liver tumors, and painful lytic bone metastases. RFA has been successfully used to manage kidney, liver, breast, bone, and lung malignancies [1], [2], [3].
RFA employs an alternating RF current to generate frictional heat and induce coagulative necrosis within and surrounding solid tumors. This form of cell death is accompanied by release of inflammatory cytokines, and “danger signals” that can trigger and/or amplify pre-existing antitumor immunity [4], [5], [6]. Preclinical and clinical data suggest that RFA-induced necrosis is a source of tumor antigens that can be used by the host’s immune system to generate antitumor immunity [7]. However, immune responses elicited by RFA alone are insufficient for morphologically visible responses or systemic control of tumor [8], [9].
Vaccine immunotherapy is an approach under active investigation in both preclinical and clinical settings for a wide spectrum of human cancers. Accumulating clinical evidence indicates that vaccine immunotherapy is safe and may have the greatest clinical benefit when targeted against residual disease [10]. The FDA recently approved the first therapeutic vaccine for prostate cancer (PROVENGE®; Dendreon, Seattle, WA). In this study, active vaccination was combined with RFA, to attempt to augment the immune response of each treatment. RFA was combined with active immunotherapy with tumor-associated antigen (TAA)-specific poxviral vaccine regimens to induce and potentiate T-cell responses against carcinoembryonic antigen (CEA). We hypothesized that the dose of RFA energy could be tailored to induce immunogenic modulation of the tumor or to promote an immunogenic microenvironment, to make tumor more prone to T cell-mediated killing, and transform it into an effective in situ antigen sink able to synergize with vaccine to promote effective tumor control and reduce recurrence. To test this hypothesis, we examined (a) the effect of mild hyperthermia on tumor phenotype and sensitivity to T cell-mediated lysis, (b) the effect of RFA dose on tumor burden, phenotype, and generation of immune responses to non-vaccine encoded (cascade) antigens, (c) the molecular signature induced by RFA, and (d) the potential synergy between RFA and vaccine to elicit antitumor immune responses able to promote effective tumor control of both primary and distant antigen-disparate metastases. We further sought to exploit the immune adjuvant potential of sequential delivery of low-dose (sub lethal) and high-dose (lethal) RFA to synergize with vaccine to promote effective antitumor immunity and increase durable complete responses (CRs). In clinical practice, a form of low dose RFA occurs during the ramp up to high dose RFA, and also occurs at the periphery of the ablative high dose RFA, due to spatial attenuation of thermal conduction at the periphery of a thermal lesion [11].
These findings support the therapeutic potential of combining RFA with vaccine therapy to promote both local and systemic anti-tumor effects.
Materials and Methods
Recombinant Poxviruses
The rMVA, rV and rF viruses containing the human CEA gene under control of the 40 k promoter and the murine B7.1, ICAM-1, and LFA-3 genes (designated rMVA, rV-CEA/TRICOM and rF-CEA/TRICOM, respectively) have been previously described [12], [13]. The rF virus containing the gene for murine granulocyte-macrophage colony-stimulating factor (GM-CSF) under control of the 40 k promoter has also been described [14].
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the National Cancer Institute Animal Use and Care Committee (ASP Number: LTIB-51). All efforts were made to minimize suffering. All experimental animals were monitored daily by trained animal caretakers. Animals that reached humane endpoints were euthanized by cervical dislocation. Cervical dislocation was be used to euthanize animals whose body weight reduce to 15% of normal, have difficulty breathing, or are cachectic. Mice were weighed twice a week. Any animal experiencing rapid weight loss, debilitating diarrhea, rough hair coat, hunched posture, labored breathing, lethargy, persistent recumbence, jaundice, anemia, significantly abnormal neurological signs, bleeding from any orifice, self-induced trauma, impaired mobility, becomes moribund or other wise becomes unable to obtain food or water was immediately euthanized.
Animals and Cells
Female C57BL/6 mice were obtained from the National Cancer Institute, Frederick Cancer Research Facility (Frederick, MD). CEA-transgenic (CEA-Tg) C57BL/6 mice have been previously described [15]. These studies used murine colon adenocarcinoma cells expressing human CEA (MC38-CEA+) [16]. Before transplantation to mice, tumor cells were trypsinized, dispersed through a 70-µm cell strainer (Falcon; Becton Dickinson, Franklin Lakes, NJ), and washed twice in PBS before final suspension in PBS. Colon carcinoma SW620 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in media designated by the provider for propagation and maintenance. Cells were incubated at 37°C with 5% CO2.
Peptides
The peptide CEA526–533 (EAQNTTYL), designated CEA526, is an H-2Db-binding CEA-specific CD8 epitope that has been previously characterized [17]. The H-2Kb-binding peptide p15e604–611 (KSPWFTTL), designated here as p15e604, has been described [12]. The H-2Db-restricted peptide p53232–240 (KYMCNSSCM), designated here as p53232, and the H-2Kb-restricted peptide survivin57–64 (QCFFCFKEL) have been previously described [18], [19]. The vesicular stomatitis virus peptide VSV-N52–59 (RGYVYQGL) was used as a control [20].
CEA- and gp70-specific T cells
The H-2Db-restricted, CEA-specific CD8+-CTL line, designated CAP-M8, recognizes the peptide epitope CEA526 and has been previously described [17]. The H-2Kb-restricted, gp70-specific CD8+-cytotoxic T cells, here designated gp70, recognizes the peptide p15e604 and has been previously described [12]. The HLA-A2-restricted, CEA-specific, CD8+ cytotoxic T-cell line, designated CEA CTL, recognizes the CEA peptide epitope YLSGANLNL (CAP-1) [21]. It was maintained and propagated as previously described [22].
In Vitro Studies
Cellular growth
Adherent MC38-CEA+ cells in log-growth phase were exposed in vitro to 37°C or 42°C for 1 h in incubators with 5% CO2. Tumor cells were incubated at 37C/5%CO2 additional 4 days. Cells were harvested daily and viable cells were counted by trypan blue exclusion using a Cellometer Auto T4 automated cell counter (Nexcelom Bioscience, Lawrence, MA).
Phenotypic analysis
To investigate the effects of in vitro exposure of tumor cells to hyperthermia on the expression of immune-relevant proteins, adherent MC38-CEA+ murine tumor cells were harvested 24 and 48 h after exposure to 37°C or 42°C for 1 h in 5% CO2. Tumor cell surfaces were stained using the primary labeled monoclonal antibodies (mAbs) CD95(Fas)-PE, CD54(ICAM-1)-FITC, H-2Kb-FITC, and H-2Db FITC (BD Biosciences, San Diego, CA). The appropriate isotype-matched controls were purchased from BD Biosciences. The anti-CEA mAb COL-1-FITC has been described [23]. Proteins were scored as up-regulated if detection levels or mean fluorescence intensity (MFI) increased by ≥30% or ≥50% following hyperthermia treatment, respectively. Stained cells were acquired on a FACScan flow cytometer using CellQuest software (BD Biosciences). Isotype control staining was <5% for all samples analyzed. Cell viability was >94%. Dead cells were excluded from the analysis based on side scatter profile.
Cytotoxicity assays
To evaluate the effect of hyperthermia on the sensitivity of murine tumor cells to CTL-mediated killing, MC38-CEA+ cells were exposed in vitro to 37°C or 42°C for 1 h in 5% CO2. After 24 h, MC38-CEA+ target cells were harvested and used as targets in a standard cytotoxicity assay with 111In [24], [25]. Cells were incubated at 37°C/5% CO2 at an effector:target ratio of 80∶1, using CAP-M8 and gp70 as effector cells. To examine the effect of hyperthermia and gamma radiation on sensitivity of human colon carcinoma cells to CTL-mediated lysis, SW620 cells were exposed in vitro to 37°C or 42°C for 1 h, or to gamma radiation (0 or 10 Gy). After 48 h, cells were harvested and incubated with CEA-specific effector T cells at an effector:target ratio of 30∶1. Data were averaged and depicted as percentage of lysis ± S.E.M.
In Vivo Studies
Radiofrequency ablation
After animals were anesthetized with ketamine/xylazine, RFA was performed using a similar technique as previously described, using an RFA Pain Management System (Baylis, Montreal, CA) equipped with a RFA 22-gauge needle with 4 mm active tip inserted in the center of the tumor [26]. The dose of RFA was varied by exposing tumors for different lengths of time to 60–70°C in the center of the tumor. Tumors were 50–200 mm3.
Effect of ultra low-dose RFA on tumor phenotype and CEA-specific immune responses
Female C57BL/6 mice (n = 7) were injected s.c. on day 0 with 3×105 MC38-CEA+ tumor cells. Tumors were treated with low-dose RFA (7 s; 60–70°C) on day 15 and excised 2 days post-treatment. Phenotypic analysis was performed in single-cell suspensions from pooled tumors after blocking Fc receptors with anti-CD16/CD32 (2.4 G2) antibody and labeling with mAbs specific for CEA (COL-1) [27], Fas (CD95), ICAM-1 (CD54), H-2Kb, and H-2Db. Cellular immunofluorescence was analyzed as before. To evaluate CEA-specific T-cell immune responses, proliferation of pooled splenic CD4+ T cells in response to protein antigens was assessed 29 days after tumor transplant, as previously described [28]. The mean cellular proliferation of negative control responses was subtracted from proliferation in response to CEA and beta-galactosidase protein antigens. The data were averaged and depicted as delta cpm ± S.E.M.
Effect of RFA dose on tumor viability, phenotype, and T-cell immune responses
Female CEA-Tg mice (n = 8–10/group) were injected s.c. on day 0 with MC38-CEA+ tumor cells. On day 15, tumors were exposed to sham or increasing doses (30, 60, or 90 s) of RFA. For histologic studies, tumors were harvested 3 h post-RFA exposure and frozen in a liquid nitrogen bath. Tumor viability was assessed in histologic sections by tetrazolium staining [29]. Tumor dimensions in individual animals were measured using digital calipers, and tumor volumes were calculated as L 2×W/2, where L and W denote tumor length and width, respectively. On day 17, excised CEA+ tumor cells were evaluated by flow cytometry for expression of HSP70, ICAM-1, and TRAIL receptor 2 (TRAIL-R2), as described above. On day 29, purified CD4+ splenic T cells were tested for reactivity to CEA protein (25 µg/mL) in an in vitro lymphoproliferation assay as before. To evaluate CD8+ T-cell responses specific for TAAs, pooled splenocytes were stimulated with 1 ug/mL CEA526, p15e604, p53232, or survivin57. After 6 days, bulk lymphocytes were recovered by centrifugation through a Ficoll-Hypaque gradient. T cells were restimulated with CEA526, p15e604, p53232, survivin57, or the control peptide VSV-N52 for 24 h in the presence of freshly irradiated naive splenocytes. Supernatant was collected and analyzed for murine IFN-g by cytometric bead array (BD Pharmingen™; BD Biosciences) according to the manufacturer’s instructions.
RFA and Vaccine Combination Therapy Studies
Metasynchronous tumor studies
To determine whether RFA could mediate abscopal regression of antigen-disparate tumors after systemic vaccination, CEA-Tg mice (n = 5–7) received MC38-CEA+ cells (3×105) on day 0 (right flank s.c.; primary tumor) and MC38-CEA– cells (3×105) on day 5 (left flank s.c.; distal tumor). Vaccinated animals received PBS or 1×108 pfu of rV-CEA/TRICOM admixed with 1×107 pfu rF-GM-CSF on day 4 and 1×108 pfu rF-CEA/TRICOM admixed with 1×107 pfu rF-GM-CSF on days 11 and 18, alone or in combination with high-dose RFA. Untreated animals were used as controls. On day 13, only the MC38-CEA+ tumor was treated with RFA (30–300 s; 60–70°C). Volumes of both primary and distal tumors, calculated as described above, were assessed every 2–3 days using digital calipers.
Immune responses
To assess tumor-specific immune responses after combination therapy, CEA-Tg mice (n = 5–7) were transplanted with MC38-CEA+ tumor cells as before and received sham or high-dose RFA (30–300 s; 60–70°C) on day 15. Vaccinated animals received rMVA-CEA/TRICOM (1×108 pfu s.c.) on day 4 and rF-CEA/TRICOM (1×108 pfu s.c.) on days 11 and 18, alone or in combination with high-dose RFA. On day 39, an in vitro lymphoproliferation assay, as before, tested purified CD4+ splenic T cells’ reactivity to CEA protein (50 ug/mL).
Combination therapy with vaccine and sequential dosing of RFA
CEA-Tg mice (n = 25–29) received MC38-CEA+ cells (3×105 s.c.) on day 0. Tumors received sham or low-dose RFA (10–30 s; 60–70°C) on day 12, followed by curative-intent high-dose RFA (30–300 s, 60–70°C) on day 15. Vaccinated animals received rMVA-CEA/TRICOM (1×108 pfu s.c.) on day 4 and rF-CEA/TRICOM (1×108 pfu s.c.) on day 11 and every 7 days thereafter, in combination with sham or high-dose RFA. Tumor volumes were determined as before.
Molecular array studies
CEA-Tg mice (n = 3) were transplanted with MC38-CEA+ cells on day 0. Tumors were exposed to RFA sham or RFA (30 s; 60–70°C) on day 13. Vaccinated animals received rMVA-CEA/TRICOM on day 4 and rF-CEA/TRICOM on day 11, alone or in combination with RFA. On day 16, tumors were harvested and microRNA transcript analysis was performed by SABiosciences (Valencia, CA) using the murine cancer microRNA PCR array MAM-102A. The array analyzed transcript expression changes pre- and post-treatment with RFA alone or combined with vaccine. Post-treatment, transcripts were considered to be up-regulated or down-regulated if their normalized intensity ratio was ≥5 or ≥0.2 (5-fold cutoff), as previously described [24].
Statistical analysis
Significant differences between treatment groups were determined by 1-way ANOVA with Tukey’s comparison based on a confidence interval of 95% using Prism 4.0c software (GraphPad Software Inc., La Jolla, CA). Alternatively, statistical differences between 2 treatments were analyzed by unpaired Student’s t test with a 2-tailed distribution and reported as P values. Significance for the proportion of CRs was determined by contingency analysis at 95% confidence by Fisher’s exact test. Differences in the distribution of flow cytometry analysis data were considered to be significant if ≥30% (% positive) or ≥50% (MFI).
Results
In Vitro Exposure of Murine and Human Tumor Cells to Hyperthermia Modulates Phenotype and Increases Sensitivity to CTL-Mediated Killing
Certain treatment modalities have been shown to induce immunogenic modulation of tumor by altering the expression of specific immune-relevant molecules that can render the tumor more sensitive to immune-mediated attack [10]. These modalities include some chemotherapeutic regimens [25], [30] and certain forms of energy delivery such as radiation [10], [31] and HIFU [32], [33]. Delivering radiofrequency energy to tumor lesions generates heat, which exposes the lesions to a gradient of thermal stress. In this study, we first investigated whether exposing a murine colon carcinoma cell line expressing CEA to hyperthermia-induced thermal stress affected cellular survival and proliferation. These levels of sublethal hyperthermia mimic the milieu at the periphery of a RFA treatment zone [11]. MC38-CEA+ cells were exposed in vitro to 42°C for 1 h. Control cells were exposed to 37°C for 1 h. To ascertain if this dose of 42°C for 1 h hyperthermia was sublethal, the total number of viable MC38-CEA+ cells was determined daily for 4 days post-exposure by trypan blue exclusion. Results indicated that this dose of hyperthermia had a small albeit significant effect on cellular growth relative to control (Fig. S1).
Next examined was the effect of hyperthermia on cell-surface expression of CEA, Fas, ICAM-1, and MHC class I molecules, each of which has been implicated in enhancing antitumor T-cell responses through diverse mechanisms [10], [34], [35]. MC38-CEA+ cells were exposed to 37°C or 42°C, as described above, and harvested 24 and 48 h after exposure to thermal stress. Exposure of tumor cells to hyperthermia significantly increased the population of CEA+ cells and CEA expression level per cell at both time points (Table 1). Expression of Fas and MHC class I protein on the surface of tumor cells also increased after exposure to hyperthermia. No significant changes in ICAM-1 expression were observed (data not shown).
Table 1. Effect of hyperthermia on phenotype of murine colon carcinoma cells.
| Percent Positive (MFI) | |||||
| Time | Treatment | CEA | Fas | H-2 Kb | H-2 Db |
| 24 h | 37°C | 20.4% (13) | 47.9% (12) | 66.7% (12) | 18.8% (12) |
| 24 h | 42°C | 54.4% (31) | 49.0% (17) | 62.5% (18) | 18.9% (16) |
| 48 h | 37°C | 32.9% (14) | 58.7% (12) | 74.9% (14) | 35.6% (14) |
| 48 h | 42°C | 54.5% (24) | 66.3% (18) | 90.9% (30) | 65.2% (21) |
MC38-CEA+ cells were exposed in vitro to 37°C or 42°C for 1 h. At 24 or 48 h post-exposure, cells were analyzed by flow cytometry for surface expression of CEA, Fas, MHC class I H-2Kb and H-2Db. Values in italic bold denote increase in protein expression ≥30% (% positive) or ≥50% (mean MFI) relative to control cells (37°C). Data is representative of two independent experiments.
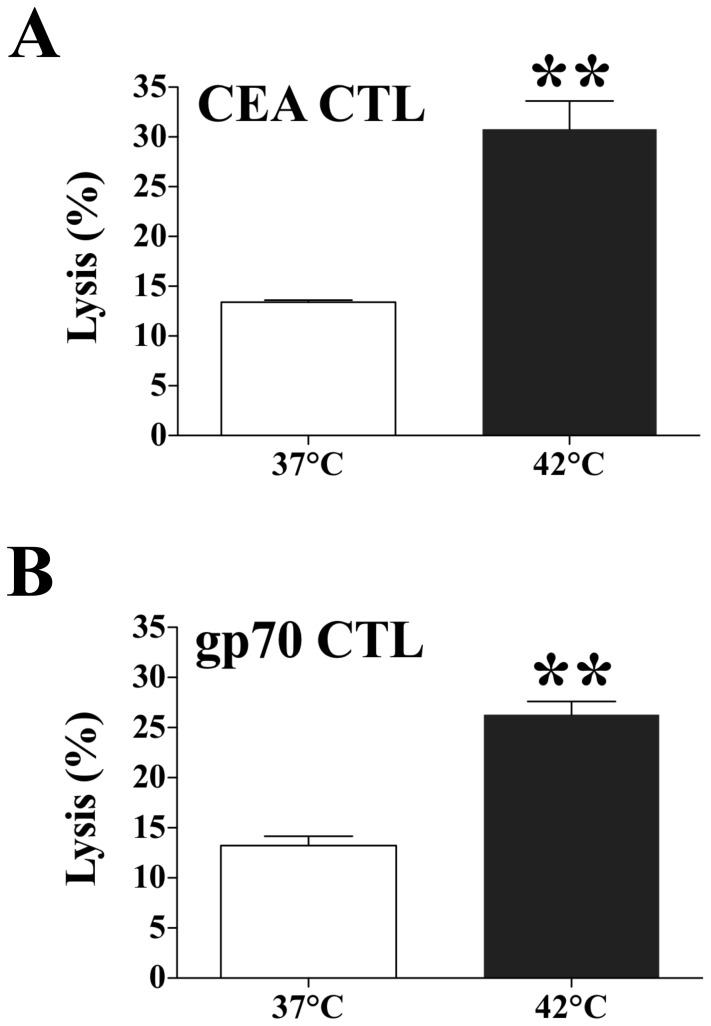
To evaluate the functional significance of phenotypic changes in tumor cells after exposure to hyperthermia, we examined the sensitivity of target MC38-CEA+ cells to CTL-mediated lysis after exposure to 37°C or 42°C. We used the CEA-specific CTL line CAP-M8, which recognizes the epitope CEA526 on carcinoma cells [17]. We also used a gp70-specific CTL line that recognizes the peptide p15e604 [12]. Murine MC38-CEA+ colon carcinoma cells positive for H-2Db, H-2Kb, and CEA were exposed to 42°C or 37°C for 1 h. After 24 h a standard 18-h assay determined the sensitivity of MC38-CEA+ target cells to CAP-M8- and gp70-mediated killing. Exposing tumor cells to 42°C significantly enhanced both CEA526- and p15e604-specific CTL-mediated lysis relative to tumor cells exposed to 37°C (P = 0.004 and P = 0.001, respectively) (Fig. 1). These data indicate that exposure of murine tumor cells to sublethal doses of hyperthermia does not markedly affect either proliferation or cellular viability, but does up-regulate immune-relevant proteins and increase tumor sensitivity to CTL-mediated killing. This sublethal hyperthermia mimics the bioheat exchange process at the periphery of an RFA treatment zone [11].
Figure 1. Effect of hyperthermia on sensitivity of murine colon carcinoma cells to CTL-mediated lysis.
MC38-CEA+ cells were exposed in vitro to 37°C or 42°C for 1 h. Twenty-four hours after exposure, cells were harvested and labeled with 111In. The sensitivity of MC38-CEA+ target cells to A. CEA– or B. GP70-specific killing was determined after cells were incubated at an effector:target ratio of 80∶1. Results are presented as mean ± S.E.M. from 3 replicate wells. Asterisks denote statistical significance (P<0.004) relative to control cells (2-tailed t test). Data is representative of two independent experiments.
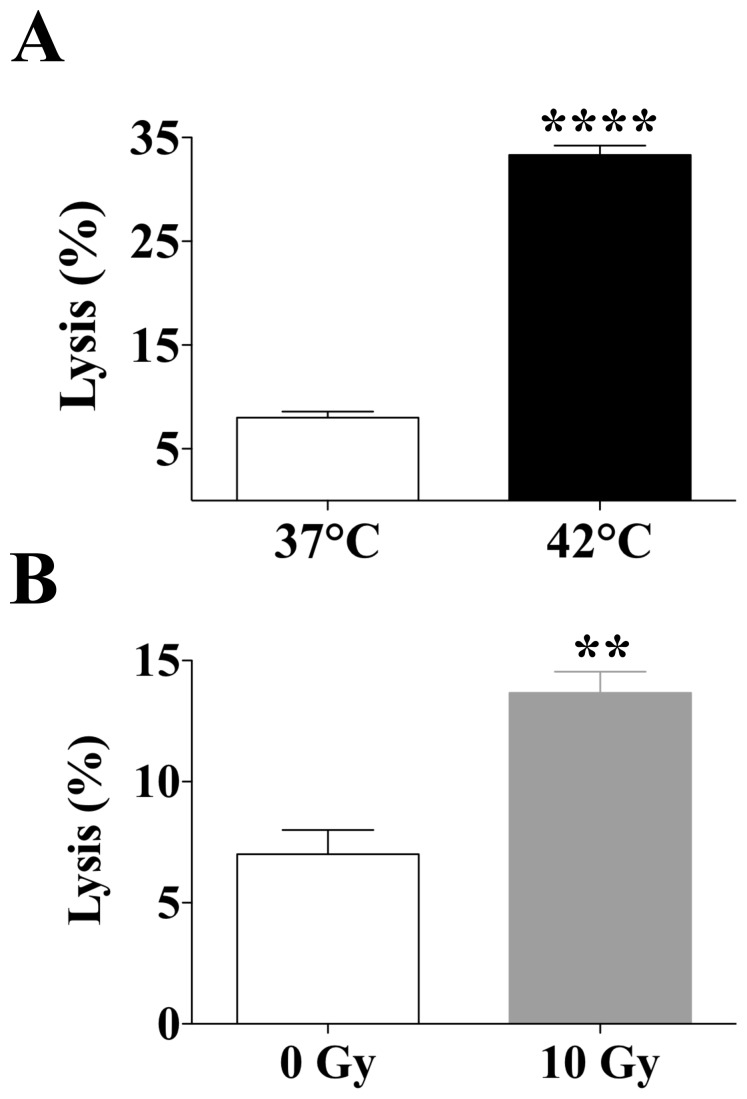
Next we sought to examine if these observations could be extended to human carcinoma cells and were comparable to the well-described enhancement in tumor sensitivity to CTL-mediated lysis mediated by another type of energy, gamma irradiation [24], [36]. SW620 cells were exposed to mild hyperthermia (37°C or 42°C), or to gamma radiation (0 or 10 Gy). After 48 h a standard 18-h assay determined the sensitivity of SW620 target cells to CEA-specific lysis by HLA-A2-restricted CD8+ cytotoxic T-cells that recognize the CEA peptide epitope CAP-1 [21]. Exposing tumor cells to 42°C significantly increased CEA-specific CTL-mediated killing relative to tumor cells exposed to 37°C (P<0.0001) (Fig. 2A). In addition, exposure of human colon carcinoma cells to radiation (10 Gy) significantly enhanced the sensitivity of SW620 cells to CEA-specific T-cell lysis (P = 0.008) (Fig. 2B). Together, these data indicate that exposure of human colon carcinoma cells to mild hyperthermia augments the sensitivity of human colon carcinoma cells to CTL-mediated killing and is comparable to that attained by gamma radiation.
Figure 2. Effect of hyperthermia and gamma radiation on sensitivity of human colon carcinoma cells to CEA-specific CTL-mediated lysis.
SW620 cells were exposed in vitro to A, 37°C or 42°C for 1 h, or to B, 0 or 10 Gy. Forty-eight hours post-exposure, cells were harvested and labeled with 111In. The sensitivity of SW620 target cells to CEA-specific killing was determined after cells were incubated with CEA-specific T cells at an effector:target ratio of 80∶1. Results are presented as mean ± S.E.M. from 3 replicate wells. Asterisks denote statistical significance (**P = 0.008; ****P<0.0001) relative to control cells (2-tailed t test). Radiation exposure data is representative of three independent experiments.
Ultra Low-Dose RFA Modulates Tumor Phenotype and CEA-Specific Immune Responses In Vivo
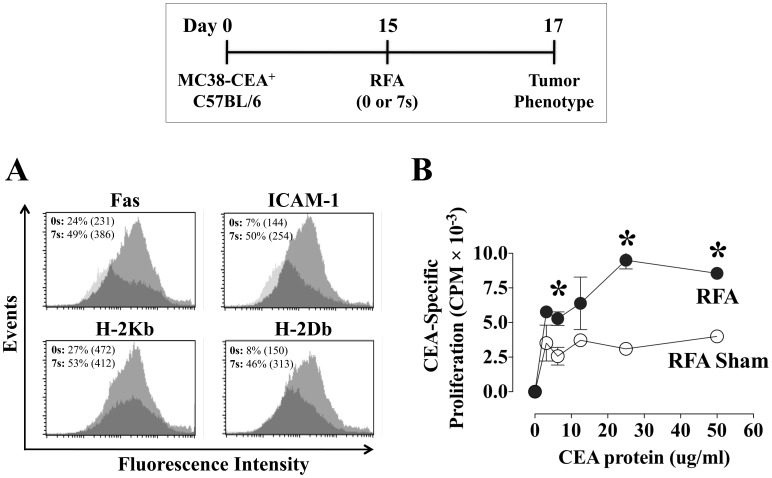
Next, we examined whether the phenotypic changes induced in vitro by exposure to sublethal hyperthermia could be captured by the gradient of thermal energy arising from treatment of MC38-CEA+ tumors with ultra-low dose RFA in vivo. On day 0, female C57BL/6 mice were transplanted with MC38-CEA+ tumor cells, and on day 15 tumors received sham or ultra low-dose RFA (7 s, 60–70°C). On day 17, the phenotype of pooled excised CEA+ tumor cells was analyzed by flow cytometry for cell-surface expression of Fas, ICAM-1, H-2Kb, and H-2Db. As shown in Fig. 3A, ultra low-dose RFA exposure induced significant up-regulation of Fas, ICAM-1, and both MHC class I molecules in MC38-CEA+ tumors. Next we sought to examine if tumor exposure to ultra low-dose RFA could induce an anti-tumor immune response. On day 29, purified CD4+ splenic T cells from animals exposed to sham or ultra low-dose RFA were tested by in vitro lymphoproliferation assay for reactivity to CEA protein. As shown in Fig. 3B, control animals exposed to RFA sham had minimal CD4+-specific proliferation to CEA. However, tumor exposure to ultra low-dose RFA induced a significant increase in CD4+ T-cell response relative to controls. Taken together, these data suggest that exposure to ultra low-dose RFA modulates tumors toward a more immunogenic phenotype and induces significant CEA-specific antitumor responses. Thus, it is feasible that clinical treatment of tumor lesions with RFA results in similar modulation at the thermal margin of the tumor [11].
Figure 3. Tumor phenotype and CEA-specific immune responses after ultra low-dose RFA.
Female C57BL/6 mice (n = 7/group) were injected s.c. on day 0 with MC38-CEA+ tumor cells. On day 15, tumors were exposed to RFA sham (0 s) or ultra low-dose RFA (7 s, 60–70°C). A, on day 17, the cell-surface phenotype of pooled excised tumor cells was analyzed by flow cytometry. Histograms depict fluorescence intensity of CEA+ tumor cells expressing Fas, ICAM-1, and MHC class I H-2Kb and H-2Db before (dark histograms) and after (light histograms) RFA. Inset numbers represent % positive cells and MFI (parentheses) for each marker. B, on day 29, purified CD4+ splenic T cells from animals exposed to sham or low-dose RFA were tested by in vitro lymphoproliferation assay for reactivity to CEA protein (0–50 ug/mL). Results are depicted as mean CEA-specific CD4+ proliferation ± S.E.M. after subtraction of background CD4+ reactivity to control beta-galactosidase protein. Asterisks denote statistical significance between treatment groups (P<0.05, 2-tailed t test). Effect of RFA on tumor phenotype data was performed twice yielding similar results.
Different RFA Doses Distinctively Modulate Tumor Growth, Phenotype, and Immune Responses to Multiple TAAs
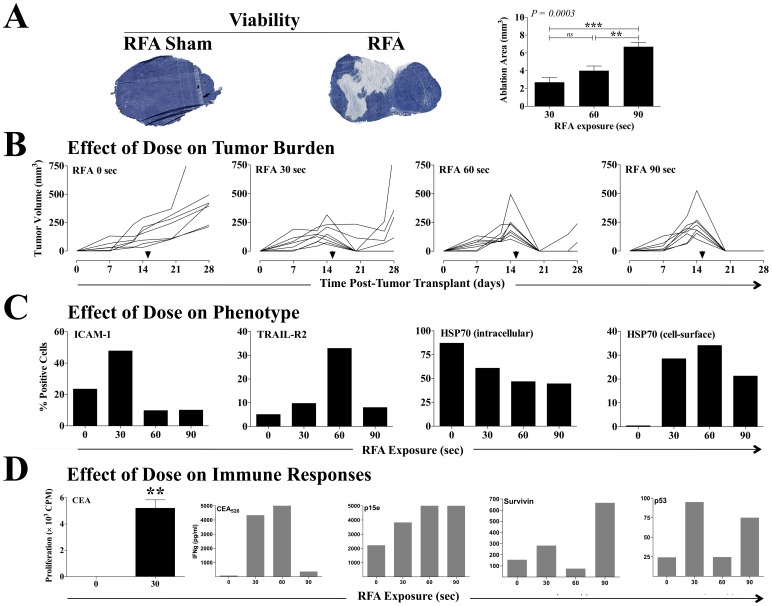
To further investigate the spectrum of immunomodulatory events resulting from the RFA-induced gradient of thermal stress, we examined the effects on tumor of exposure to higher, clinically relevant doses of RFA. Female C57BL/6 mice transplanted with MC38-CEA+ s.c. tumors were exposed to sham RFA or to low (30 s), medium (60 s), or high (90 s) doses of RFA 15 days after tumor transplant. A cohort of animals (n = 4–7/group) was sacrificed 3 h post-RFA, and cellular viability in excised tumors exposed to sham or low dose RFA was assessed in histological sections by tetrazolium staining. As shown in Fig. 4A, exposure to RFA resulted in fewer tetrazolium+ tumor cells (dark blue), indicating a loss of viability. Quantification of tetrazolium– cells (light blue) indicated that exposure to RFA induced significant loss of tumor-cell viability in a dose-dependent manner, suggesting that our RFA murine model could mimic clinical outcomes (Fig. 4A, right panel). To further examine the role of dose on treatment outcome, tumor volumes in individual animals were monitored for 28 days. As shown in Fig. 4B, RFA sham (0 s) had no effect on tumor growth and no complete response (CR) (defined as tumor eradication). Low-dose RFA (30 s) reduced tumor growth and resulted in a CR rate of 75%; however, 33% of eradicated tumors relapsed. Although 100% of tumors treated with a medium dose of RFA (60 s) attained a CR, 29% relapsed. The rate of CR also reached 100% with maximum RFA exposure (90 s), and there were no relapses. These data suggest that exposing MC38-CEA+ s.c. tumors to RFA mimics a wide spectrum of clinical outcomes, ranging from treatment failure to full treatment success, translated as CR.
Figure 4. Effect of RFA dose on tumor phenotype and antigen cascade immune responses.
Female C57BL/6 mice (n = 8–10/group) were injected s.c. on day 0 with MC38-CEA+ tumor cells. On day 15, tumors were exposed to RFA sham or to increasing doses of RFA (30, 60, or 90 s). A, 3 h post-RFA, tumor viability was assessed by tetrazolium staining (n = 4–7) in tumors exposed to sham or 30 s RFA. Histological images are representative of each treatment. Dark blue denotes viable cells. B depicts tumor volumes in individual animals. Arrows denote RFA on day 15. C, on day 17, excised CEA+ tumor cells were evaluated by flow cytometry for percentage of cells with cell-surface expression of ICAM-1, TRAIL-R2, and HSP70, and intracellular expression of HSP70. D, on day 29, purified CD4+ splenic T cells from animals exposed to RFA were tested for specific reactivity to CEA protein (25 ug/mL) in an in vitro lymphoproliferation assay. Results are depicted as mean CD4+ proliferation ± S.E.M. after subtraction of background CD4+ reactivity to control beta-galactosidase protein. Asterisks denote statistical significance (P = 0.001, 2-tailed t test). CD8+ T-cell responses specific for the peptides CEA526, p15e604, p53232, and survivin57 were evaluated in pooled splenocytes through quantification of secreted IFN-g over 24 h of in vitro restimulation. Results are depicted as IFN-g (pg/mL) after subtraction of background IFN-g secretion in response to the control peptide VSV-N52. This experiment was performed twice yielding similar results.
Next, the immunomodulatory effects of distinct RFA doses were examined. Two days post-exposure to RFA sham or low-dose (30 s), medium-dose (60 s), or high-dose (90 s) RFA, excised CEA+ tumor cells were evaluated by flow cytometry for expression of ICAM-1, TRAIL-R2, MHC class I, Fas, and HSP70 proteins. Each of these molecules has been shown to enhance antitumor T-cell responses through diverse mechanisms [10], [34], [37], [38]. As shown in Fig. 4C, exposure to low-dose RFA doubled the population of cells with cell-surface expression of ICAM-1 relative to RFA sham control tumors. However, mean fluorescence intensity (MFI) of ICAM-1+ cells decreased 38% after exposure to low-dose RFA relative to controls (data not shown), suggesting an increase in ICAM-1 translation favoring cells with low endogenous ICAM-1 expression. Similar observations were attained with TRAIL-R2. The population of CEA+ cells expressing this receptor increased 2-fold with low-dose RFA compared to tumors receiving RFA sham. However, TRAIL-R2 MFI decreased 46% after exposure to low-dose RFA relative to controls (data not shown). After exposure to medium-dose RFA, the population of cells with cell-surface expression of TRAIL-R2 increased 7-fold with MFI decreasing 9% compared to controls. No changes in MHC class I or Fas expression was observed with the RFA doses used (data not shown).
Mounting evidence indicates that tumor cells exposed to thermal stress increase the expression of total HSP70 [39], [40]. This protein is constitutively expressed intracellularly in most cancer cells, where it functions as anti-apoptotic, and has been shown to bind to TRAIL receptors, thus inhibiting TRAIL-mediated cell death [37]. Intracellular HSP70 expression has also been associated with poor prognosis and resistance to both chemotherapy and radiation therapy [41]. In contrast, HSP70 expressed on the tumor cell surface has been shown to promote innate and adaptive immune responses, including enhanced T-cell activation and promotion of CD4+ and CD8+ cytotoxic immune responses [4], [38]. Thus, to further examine the effect of RFA dose on immune modulation of tumor, we examined intracellular and cell-surface expression of HSP70. As shown in Fig. 4C, exposure to RFA induced a dose-dependent decrease in intracellular HSP70 from >90% (RFA sham) to <50% (high-dose RFA) without affecting cellular MFI (data not shown). Further, RFA treatment translated into increase in HSP70 translocation to the tumor cell surface with all RFA doses relative to sham controls. Less than 2% of tumors treated with RFA sham exhibited HSP70 on their surface. This level increased to 34% with medium-dose RFA with a concomitant 349% increase in cellular MFI. Increases in cellular cell-surface HSP70 MFI 3370% were also observed in tumors treated with both low and high dose RFA (data not shown), suggesting that RFA modulates tumor phenotype into a more immunogenic platform.
In this context, we examined the effect of different RFA doses on the generation of CD4+ and CD8+ T-cell responses. Purified CD4+ splenic T cells were tested for reactivity in vitro to CEA protein 29 days post-tumor transplant. Compared to sham RFA, exposure to low-dose RFA (30 s) significantly increased proliferation of CD4+ T cells in response to CEA (P = 0.001) (Fig. 4D, left panel). CD8+ T-cell responses specific for the peptides CEA526, p15e604, survivin57, and p53232 were evaluated in pooled splenocytes by quantifying secreted IFN-γ over 24 h of restimulation in vitro. With varying dose-response profiles and magnitudes (Fig. 4D), RFA increased CD8+ IFN-γ secretion in response to CEA, p15e, survivin, and p53. These data suggest that RFA alone can induce a more immunogenic tumor phenotype, as well as a plethora of immune responses to multiple TAAs. Further, these data suggest that the immunogenic modulation profile of tumor cells at the thermal margin of a RFA ablated zone and its resulting contribution to the overall anti-tumor immune response will have distinct patterns based on the level of thermal stress received during the RFA treatment.
Combination Therapy with RFA Plus Vaccine Alters Tumor Biology Toward a Less Invasive Signature through Modulation of MicroRNA Transcripts
The role of microRNAs in tumor response to energy-based therapies, including radiation, is under active investigation [42], [43]. MicroRNAs play a key role in regulating gene expression through post-transcriptional interference of messenger RNAs and can have various functions, including tumor suppression. Thus, to identify molecular signatures that could potentially be involved with RFA-induced antitumor immunity, a focused microRNA (miR) analysis of excised tumors was performed. On day 13 post-tumor transplant, CEA-Tg mice with established MC38-CEA+ tumors were exposed to RFA sham, high-dose RFA, vaccine alone, or vaccine combined with high-dose RFA. Tumors were harvested on day 16 and 88 microRNA transcripts were examined (Table S1). Transcripts were considered differentially expressed if their levels of expression differed ≥5-fold in any of the 3 treatment modalities compared with matched RFA sham controls. As shown in Table 2, 9 microRNAs were modulated by vaccine, RFA and/or RFA combined with vaccine based on this stringent criterion. Interestingly, all microRNAs differentially regulated by treatment have been shown to be tumor-suppressive [44], [45], [46], [47], [48], [49]. Vaccine alone increased the expression of miR-142-5p and miR-150, and downregulated miR-133b and miR-1. RFA alone induced up-regulation of 5 specific microRNA transcripts relative to treatment control: miR-1, -133b, -150, -203, and -205. However, in tumors exposed to RFA plus vaccine we observed a significant increase in microRNA expression of 7 of 9 transcripts relative to control tumors. Further, of the 5 microRNAs whose expression was modulated by both RFA alone and/or RFA combined with vaccine indicated that combination therapy induced a robust up-regulation of 3 of 5 tumor-suppressor transcripts. Strikingly, RFA alone increased miR-133b 13.3-fold, whereas combination therapy markedly decreased its expression (−30.1-fold) relative to control tumors. Similar results were observed with miR-1. Expression of miR-150 in MC38-CEA+ tumors increased 25.2-fold after exposure to RFA alone, and 32-fold after exposure to RFA plus vaccine. Expression of miR-363, -124, -142-5p, and -141 in tumors exposed to RFA alone did not differ from that of control tumors. However, all 4 microRNAs were markedly up-regulated after exposure to RFA plus vaccine. These data suggest that combination therapy with RFA plus vaccine modulates tumor biology toward a less invasive, less metastatic signature by synergistically increasing the expression of tumor-suppressive microRNAs.
Table 2. In vivo modulation of tumor suppressor microRNA transcripts in tumors exposed to vaccine, RFA alone, or RFA combined with vaccine.
| Accession Number | microRNA | Vaccine | RFA | Vaccine+RFA |
| MIMAT0000134 | mmu-miR-124 | 1 | 1 | 5.1 |
| MIMAT0000154 | mmu-miR-142-5p | 6.2 | 1 | 5.3 |
| MIMAT0000708 | mmu-miR-363 | 1 | 1 | 6.1 |
| MIMAT0000153 | mmu-miR-141 | 1 | 1 | 13.6 |
| MIMAT0000236 | mmu-miR-203 | 1 | 5.4 | 13.7 |
| MIMAT0000238 | mmu-miR-205 | 1 | 6.0 | 44.8 |
| MIMAT0000769 | mmu-miR-133b | −10.0 | 13.3 | −30.1 |
| MIMAT0000160 | mmu-miR-150 | 31.7 | 25.2 | 32.0 |
| MIMAT0000123 | mmu-miR-1 | −19.6 | 73.2 | −38.7 |
CEA-Tg mice (n = 3) received MC38-CEA+ cells on day 0. Tumors were exposed to RFA sham or intermediate-dose RFA (30 s; 60–70°C) on day 13. Vaccinated animals received rMVA-CEA/TRICOM on day 4, and rF-CEA/TRICOM on day 11, alone, or in combination with RFA. On day 16, tumors were harvested and microRNA array analysis was performed. Transcripts were considered differentially expressed (>1) if their levels of expression differed at least 5-fold in either treatment modality as compared with matched RFA sham control tumors. Accession numbers of microRNA transcripts refer to mirBase v14.0.
RFA Plus Vaccine Enhanced CEA-Specific T-Cell Responses and Induced Abscopal Regression of Antigen-Disparate Tumors in a Metachronous Murine Model of Colon Carcinoma
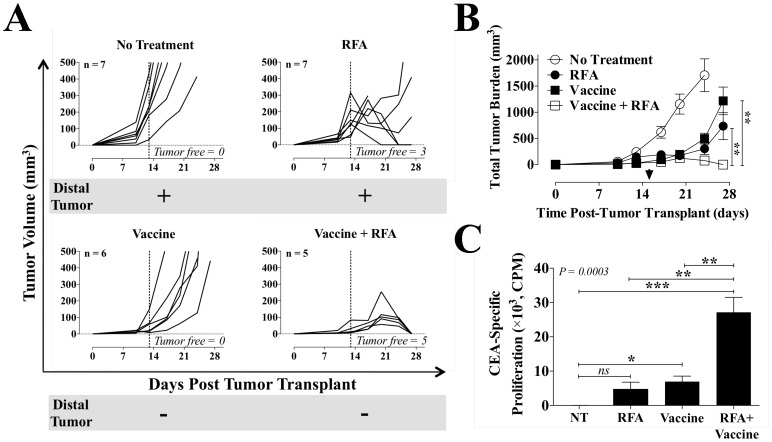
Localized energy delivery such as tumor irradiation has been shown to induce a systemic antitumor immune response synergistic with vaccine, which translates into effective abscopal regression of distant metastases [50]. We investigated whether RFA-induced systemic immunity could synergize with a vaccine encoding CEA to promote antitumor efficacy and abscopal regression of CEA– distal metastases in the setting of a self-antigen. We designed a metachronous tumor model in which mice were transplanted with 2 antigen-disparate tumors in discrete sites. CEA-Tg mice were transplanted with primary CEA+ tumors (MC38-CEA+ cells) on day 0, and distal CEA– tumors (MC38 cells) on day 5. CEA+ tumors received sham or high-dose RFA on day 13. Vaccinated animals received vaccine weekly starting on day 4, alone or in sequential combination with high-dose RFA. As shown in Fig. 5A, RFA sham had no impact on regression of either primary or distal tumors. Treatment of the primary tumor with RFA alone resulted in a 43% CR rate, with 3 of 7 animals tumor-free after ablation. However, RFA alone was not able to eradicate the CEA– distal tumor. No CR or regression of the primary tumor were observed in animals receiving vaccine alone. However, vaccine alone prevented the development of secondary tumors, which was also observed in animals receiving RFA plus vaccine, confirming previous observations in this tumor model (results not shown). Moreover, combination therapy eliminated 100% of primary CEA+ tumors, indicating that RFA synergizes with vaccine to promote effective antitumor immune responses, confirming previous observations from single-tumor models (not shown). Total tumor burden was calculated to better capture the overall antitumor effects of each treatment in both primary and distal tumors. Animals treated with vaccine, RFA, or vaccine plus RFA had a significant reduction in total tumor burden (P<0.0001) at day 24 (Fig. 5B). Further, treatment with RFA plus vaccine resulted in significant reduction of total tumor burden relative to vaccine or RFA alone (P = 0.0054).
Figure 5. Antitumor efficacy and immune response elicited by combination therapy with RFA and recombinant vaccine.
CEA-Tg mice (n = 5–7/group) received MC38-CEA+ cells on day 0 (right flank s.c.; primary tumor) and CEA– MC38 cells on day 5 (left flank s.c.; distal tumor). A, primary tumor growth. MC38-CEA+ tumors received sham or high-dose RFA (30–300 s; 60–70°C) on day 13 (dotted line). Vaccinated animals received rV-CEA/TRICOM+rF-GM-CSF on day 4 and rF-CEA/TRICOM+rF-GM-CSF on days 11 and 18, alone or in combination with RFA. Each graph denotes the number of primary tumor-free mice and the presence (+) or absence (–) of a distal tumor on day 28. B, total tumor burden (primary plus distal). Asterisks denote statistical significance among treatment groups at day 27 (P<0.0001, 1-way ANOVA with Tukey’s multiple comparison test). Arrow indicates RFA treatment on day 15. C, CEA-specific proliferation of CD4+ T cells. CEA-Tg mice were transplanted with MC38-CEA+ tumor cells as before and received sham or 30 s RFA on day 15. Vaccinated mice received rMVA-CEA/TRICOM on day 4 and rF-CEA/TRICOM on days 11 and 18, alone or in combination with 30 s RFA. On day 39, purified CD4+ splenic T cells were tested for reactivity to CEA protein (50 ug/mL) in an in vitro lymphoproliferation assay. Results are depicted as mean CEA-specific CD4+ proliferation ± S.E.M. after subtraction of background CD4+ reactivity to control beta-galactosidase protein. Asterisks denote statistical significance among treatment groups (P = 0.0003, 1-way ANOVA with Tukey’s multiple comparison test). This experiment was performed twice yielding similar results.
To better understand the mechanisms involved in the antitumor efficacy mediated by vaccine plus RFA, CEA-Tg mice were transplanted with MC38-CEA+ tumor cells as before and received sham or RFA (30 s) on day 15. Vaccinated mice received rMVA-CEA/TRICOM on day 4 and rF-CEA/TRICOM on days 11 and 18, alone or in combination with RFA. On day 39, purified CD4+ splenic T cells from animals with similar tumor burden were tested for reactivity to CEA protein in an in vitro lymphoproliferation assay. As shown in Fig. 5C, control animals exposed to RFA sham had minimal CD4+-specific proliferation to CEA. RFA alone induced a higher CD4+ response, although not significantly different from that of controls. Vaccine alone was able to break immune tolerance to CEA and elicit a significant increase in CEA-specific CD4+ responses relative to RFA sham controls. However, CEA-specific CD4+ immune responses were highest in mice that received vaccine in combination with RFA vs. RFA alone, vaccine alone, or RFA sham (P = 0.0003). Taken together, these data indicate that RFA enhances CD4+ T-cell responses generated by CEA poxviral vaccines, which translates into a synergistic eradication of both CEA+ primary tumors and distal CEA– metastases.
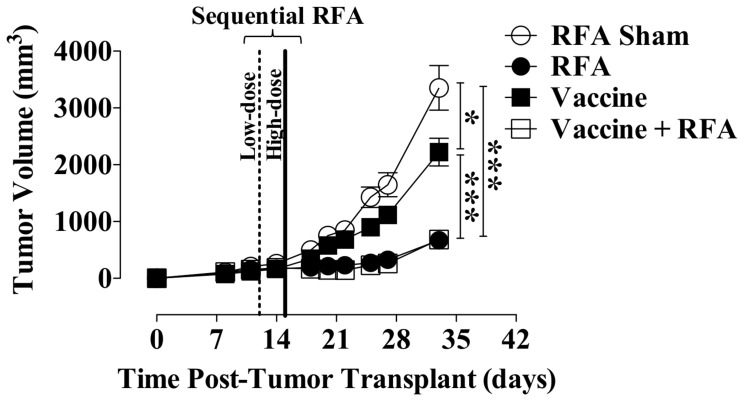
Low- and High-Dose Sequential RFA Plus Vaccine Decreases Tumor Burden and Increases Relapse-Free Survival
We hypothesized that combining vaccine with sequential RFA (low-dose immunogenic RFA followed by high-dose curative-intent RFA) would result in significant antitumor efficacy and improved relapse rates. To test this hypothesis, CEA-Tg mice received MC38-CEA+ cells on day 0. Tumors received sham or low-dose RFA on day 12, followed by ablative high-dose RFA on day 15 (defined as sequential RFA). Mice received vaccine weekly starting on day 4 in combination with sham or sequential RFA. Subsequent analysis of tumor volumes indicated significant differences among treatment groups (P<0.0001). Vaccine alone significantly decreased tumor burden relative to mice receiving RFA sham (Fig. 6) (P<0.05). Tumors exposed to sequential RFA alone or to combination therapy showed the highest decrease in tumor growth relative to controls (P<0.0001). Interestingly, sequential RFA plus vaccine did not decrease tumor volume more effectively than sequential RFA alone, confirming independent observations from two additional studies (data not shown). To better understand the antitumor effects of combination therapy relative to sequential RFA alone, we performed a focused analysis of CRs and durable CRs. In this context, any tumor eradication during the course of the study, regardless of relapse, was considered CR. Any tumor eradication without subsequent relapse during the course of the study was considered a durable CR. No CRs were observed in animals receiving RFA sham or vaccine alone (Table 3). During the course of this study, 34.5% of animals receiving sequential RFA attained a CR; 50% of these subsequently relapsed. However, animals receiving sequential RFA plus vaccine showed a higher CR rate, as well as increased relapse-free durable CR. Specifically, we observed that 52% of all animals receiving combination therapy became tumor-free. Further, 69.2% of all CRs in this cohort did not relapse. Thus, the combination therapy increased durable CRs by 19.2%. These results suggest a trend indicating that low-dose immunomodulatory RFA plus high-dose curative-intent RFA combined with vaccine may reduce the number of tumor relapses and thus prolong survival.
Figure 6. Antitumor efficacy of combination therapy with sequential RFA and recombinant vaccine.
MC38-CEA+ cells were transplanted on day 0 on the right flank of CEA-Tg mice (n = 25–29/group). MC38-CEA+ tumors received sham or low-dose RFA (10–30 s at 70°C) on day 12 (dotted line), followed by high-dose ablative RFA (30–300 s at 70°C) on day 15 (solid line). Vaccinated mice received rMVA-CEA/TRICOM s.c. on day 4 and rF-CEA/TRICOM on day 11 and every 7 days thereafter, alone or in combination with sequential RFA. A depicts tumor growth. Data represent tumor volume ± S.E.M. in individual animals. Asterisks denote statistically significant differences among treatment groups (P<0.0001, 1-way ANOVA with Tukey’s multiple comparison test).
Table 3. Complete response rates to combination therapy with sequential RFA and recombinant vaccine.
| Group | Complete Response | Durable Complete Response |
| RFA Sham | 0 | 0 |
| RFA | 34.5% (10/29) | 50% (5/10) |
| Vaccine+Sham | 0 | 0 |
| Vaccine+RFA | 52% (13/25) | 69.2% (9/13) |
| P = 0.153 | P = 0.306 |
MC38-CEA+ cells were transplanted on day 0 on the right flank of CEA-Tg mice (n = 25–29/group). MC38-CEA+ tumors received sham or low-dose RFA (10–30 s at 70°C) on day 12, followed by high-dose ablative RFA (30–300 s at 70°C) on day 15. Vaccinated mice received rMVA-CEA/TRICOM s.c. on day 4 and rF-CEA/TRICOM on day 11 and every 7 days thereafter, alone or in combination with sequential RFA. Results are depicted as percentage of mice with complete responses or durable complete responses following treatment with RFA sham, sequential RFA, vaccine combined with RFA sham, or vaccine with sequential RFA. Values in parentheses depict number of responders/number of eligible animals in each cohort. P values denote statistical analysis of response rates to sequential RFA alone or in combination with vaccine, as determined by contingency analysis (Fisher’s exact test).
Discussion
In situ tumor ablation involves destroying tumor tissue with energy sources such as microwave, laser, cryotherapy, HIFU, or RFA. Each of these techniques relies on controlled energy delivery to minimize collateral damage to adjacent structures [2]. RFA is a minimally invasive technique and perhaps the most common choice for local tumor ablation as an alternative to surgical resection [1], [2]. RFA destroys tumor by delivering a radio-wave-frequency alternating current that generates frictional hyperthermia, causing cell death through coagulative necrosis [1], [4]. Mounting preclinical and clinical evidence indicates that RFA-induced necrosis is a source of tumor antigens, to which the host’s immune system can generate antitumor immunity superior to that elicited by surgical resection [5], [7], [51]. However, despite the increased availability of antigens, RFA of nonimmunogenic tumors does not usually induce systemic immunity [52]. Moreover, clinical reports indicate that RFA is not superior to surgical resection in the management of tumor recurrence, suggesting that the immune responses elicited by RFA alone are insufficient to prevent tumor recurrence. This has also been reported with other ablative modalities, including microwave, cryotherapy, and HIFU [1], [5], [8], [9].
Several therapeutic strategies have been employed to reduce local and systemic tumor relapse in patients treated with RFA, including combination with chemotherapy and various immunotherapies [53], [54], [55]. In a randomized trial (n = 95), patients with hepatocellular carcinoma (HCC) received chemoembolization sequentially combined with ablative RFA with or without subsequent adoptive immunotherapy in the form of autologous cytokine-induced killer cells. Tumor relapse was observed in 31% of patients receiving RFA plus immunotherapy compared to 85% of patients receiving RFA alone [54]. Similar rates of HCC recurrence were observed in patients receiving ablative RFA followed by IFN-alpha2b, where patients receiving combination therapy had a median tumor-free period of 3.4 years, a significantly higher progression-free survival than the 1.4 years for patients receiving RFA alone [55].
These encouraging results suggest that RFA combined with an immunotherapy regimen could prevent tumor relapse by augmenting and/or expanding RFA-induced immune responses. Thus, we hypothesized that combination therapy with RFA plus a vaccine targeting CEA, a widely expressed TAA, could have a synergistic antitumor effect and prevent tumor relapse. To test this hypothesis, we examined whether sublethal hyperthermia could induce a more productive immunogenic modulation, i.e., alter tumor phenotype to one more susceptible to immune attack. We have previously shown that tumors can be rendered more sensitive to immune-mediated lysis by sublethal exposure to selected chemotherapies and certain forms of energy such as gamma radiation [10], [24], [56]. Here we expand these observations to other treatment modalities based on thermal stress. We report that in vitro exposure of both murine and human colon carcinoma cells to sublethal thermal stress induces productive immunogenic modulation of tumor cells rendering them more sensitive to CTL-mediated killing, which was also observed with radiation (Figs. 1 and 2), a form of energy previously demonstrated to enhance tumor sensitivity to CTL-mediated lysis [24], [36]. We then investigated whether these findings could be replicated in vivo using ultra low doses of RFA. Our results (Fig. 3) confirmed that ultra low doses of RFA induce modulation of immune-relevant molecules within the tumor, resulting in a cascade of CEA-specific CD4+ T-cell immune responses. This confirms and extends observations that RFA and other forms of thermal ablation, such as HIFU, microwave, and cryotherapy, are able to induce tumor-specific immune responses [4], [57]. Further, when tumors were exposed to higher doses of RFA, mimicking the clinical spectrum of treatment success (Fig. 4), differential effects based on dose were observed in both modulation of tumor phenotype and generation of cascade T-cell responses to multiple TAAs. Strikingly, thermal stress and RFA dose modulated each immune-relevant protein differently. Although ICAM-1 expression was not modulated following hyperthermia in vitro (Table 1), it was significantly upregulated after exposure to both ultra low (7 s, Fig. 3) and low (30 s, Fig. 4) doses of RFA in vivo, which suggests a role for the tumor microenvironment in ICAM-1 regulation, in agreement with other reports [58]. Whereas tumor exposure to hyperthermia (Table 1) and to an ultra low dose of RFA (7 s, Fig. 3) markedly increased Fas and MHC class I expression, higher RFA doses (30–90 s, Fig. 4) had no effect on the cell-surface expression of these proteins. This indicates that the energy gradient delivered by RFA differentially modulates specific elements of tumor phenotype based on the dose of thermal stress delivered to each carcinoma cell, rendering the tumor more immune-sensitive in the absence of tumor eradication, and resulting in generation of CD4+ and CD8+ T-cell responses to a variety of TAAs, a finding supported by accumulating clinical evidence [4], [5], [6]. In clinical practice, it is feasible to deliver low dose RFA, followed sequentially by the more standard high dose and lethal RFA.
Mounting evidence indicates that tumor cells exposed to sublethal levels of heat or RFA undergo heat shock, leading to increased expression of total HSP70 at both the mRNA and protein levels [39], [40]. This protein is constitutively expressed intracellularly in most cancer cells, where it functions as an anti-apoptotic and has been shown to bind to TRAIL receptors, thus inhibiting TRAIL-mediated cell death [37]. Intracellular HSP70 expression has also been associated with poor prognosis and resistance to both chemotherapy and radiation therapy [41]. Importantly, we have observed that RFA reduces intracellular levels of HSP70 by promoting its translocation and that of TRAIL-R2 to the cell surface in a dose-response manner (Fig. 4C), which may make the tumor a better target for TRAIL-mediated immune attack. Moreover, membrane-bound or secreted HSP70 promotes increased innate and adaptive immune responses, cross-presents TAAs to antigen-presenting cells, enhances T-cell activation, and promotes CD4+ and CD8+ cytotoxic immune responses [59], [60]. We have also observed increased cell-surface expression of the T-cell adhesion molecule ICAM-1, as well as cascade CD4+ T-cell immune responses to CEA and CD8+ T-cell responses to CEA, p15e, survivin, and p53, indicating that RFA may induce a multitude of immune-relevant events in response to different doses that could potentially be exploited to promote effective antitumor immunity in combination with vaccine. These data are supported by clinical observations that development of strong CD8+ T-cell responses against TAAs prolongs recurrence-free survival after RFA in patients with HCC [61].
Analysis of microRNA transcripts (Table 2) supports and expands these observations. The role of microRNAs in tumor response to energy-based therapies, including radiation, is under active investigation [42], [43]. MicroRNAs play a key role in regulating gene expression through post-transcriptional interference of messenger RNAs and can have various functions, including tumor suppression. Here we report that tumor ablation with radiofrequency energy strongly increased the expression of several tumor-suppressor microRNAs, including miR-133b, which has been shown to inhibit cell growth in lung cancer [44]. Importantly, low expression of miR-133b in colorectal cancer has been correlated with metastases and lower rates of survival [62]. Although its significance is not yet clear, combination therapy with RFA and vaccine markedly decreased miR-133. Vaccine, RFA alone, and RFA combined with vaccine induced a robust increase in expression of miR-150, a tumor suppressor shown to inhibit cancer stem cells in pancreatic cancer [46]. Compared to RFA alone, the combination of RFA and vaccine further augmented and amplified the expression of shared and novel tumor-suppressor microRNA transcripts, including miR-141, a microRNA recently shown to inhibit migration and invasion in colorectal carcinoma cells [48], and miR-205, a tumor suppressor implicated in preventing epithelial-to-mesenchymal transition and whose repression has been associated with poor prognosis [45], [63]. The novel observation from these studies is that RFA plus vaccine significantly increases the expression of multiple tumor-suppressive microRNAs relative to RFA alone. This finding suggests that RFA and vaccine work synergistically to modulate tumor biology to make tumors more indolent and less metastatic. These results may provide a mechanistic rationale that supports clinical observations that combining RFA with immunotherapy delays tumor recurrence [55], [61]. However, further research is needed to confirm and determine whether this mechanism can be exploited and expanded to the combination of RFA and non-vaccine immunotherapy platforms.
Clinical reports have indicated that RFA can induce systemic immunity and control distant metastases following treatment of the primary tumor through a process known as the abscopal effect [26]. However, immune responses elicited by RFA alone are insufficient for both local and systemic control of tumors [8], [64], as indicated by frequent recurrence. Here we show (Fig. 5) that treatment with high-dose curative-intent RFA followed by administration of a vaccine targeting CEA results in antitumor efficacy. Although vaccine alone did not impact the growth of the primary tumor it had a marked abscopal effect on the development of the distal tumor, confirming previous observations in this tumor model (results not shown). This differential effect may be a consequence of study design as distal tumors were implanted 5 days after the primary tumor. In addition, vaccine prime was given at day 4, a time where the primary tumor is fully established. Our data further indicate that RFA enhances CD4+ T-cell responses generated by CEA viral vaccines (Fig. 5C). The result is a synergistic eradication of CEA+ primary tumors and a significant abscopal effect that mediates the elimination of distal CEA– metastases through immune responses to non-vaccine encoded antigens, expanding similar findings observed with combination of vaccine and radiation [50].
We sought to further explore the antitumor synergy between RFA and vaccine by examining the immunogenic modulation of tumor induced by a regimen of low-dose RFA followed by high-dose ablative RFA plus a viral vaccine targeting CEA. Although the combination of sequential RFA with vaccine did not significantly decreased tumor burden relative to RFA alone (Fig. 6), the presence of vaccine increased the number of CRs and, more importantly, the number of durable CRs (Table 3). This suggests that while the combination of vaccine and this specific RFA regimen may not increase the rate of tumor eradication relative to RFA alone, it may work synergistically to prevent relapse. Several immune mechanisms may be associated with this outcome. Pretreatment with low-dose RFA not only makes the tumor a better target for CTL-mediated and, potentially, TRAIL-mediated immune attack (Figs. 3 and 4), but may also alter the tumor to become an in situ vaccine prime through HSP70 immune stimulation and initiation of significant CD4+ and CD8+ T-cell cascade immune responses to several TAAs not encoded by the vaccine. Low dose RFA when used in combination with vaccine should be further studied in vivo, or in the clinic, to assess whether the RFA algorithm could be modified to allow for a slower treatment, where high dose standard lethal RFA is preceded by low dose RFA. Tumor ablation with high-dose RFA has been shown to create an antigen sink for the generation of antitumor immunity [7]. Thus, sequential exposure to high-dose ablative RFA may act as a vaccine boost by increasing tumor necrosis, resulting in heightened release of antigens, including HSP70. Radiofrequency energy is currently delivered in a standard fashion, fairly rapidly, and without a slow ramping (like low dose RFA). RFA algorithms should be further studied to clarify what is the optimal fashion to ramp energy when RFA is used in combination with vaccine in order to maximize immune responses.
Our results show that RFA induces a plethora of immune events based on dose, including immunogenic modulation of tumor and CD4+ and CD8+ T-cell responses. Although the immune responses elicited by RFA alone may not be sufficient to prevent tumor recurrence, exploiting these immune events by combining RFA with vaccine has shown the potential to improve CR rates and prevent tumor recurrence. RFA can be applied to a wide range of malignancies that express CEA, including HCC, metastatic liver cancer, and renal cell, lung, and prostate carcinomas. We thus envision a wide array of potential clinical applications for the combined use of RFA with viral vaccines targeting CEA [65].
Supporting Information
Effect of hyperthermia on cellular growth of murine colon carcinoma cells. MC38-CEA+ cells were exposed in vitro to 37°C or 42°C for 1 h. Tumor cells were incubated at 37°C/5%CO2 for 4 additional days. Cells were harvested daily and viable cells were counted by trypan blue exclusion. Results are presented as fold increase in cell number relative to day zero ± S.E.M. from 2 replicate flasks. Asterisks denote statistical significance (P<0.05) relative to control cells (2-tailed t test). This experiment was performed once.
(TIF)
(DOC)
Acknowledgments
The authors thank Dr. Sergio Dromi for assistance with radiofrequency ablation experiments. We also thank Dr. Jeffrey Schlom for his helpful suggestions and Marion Taylor for his excellent technical assistance. Thank you to Bonnie L. Casey for editorial assistance in the preparation of this manuscript.
Funding Statement
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, et al. (2011) Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13: e252–265. [DOI] [PubMed] [Google Scholar]
- 2. Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, et al. (2009) Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 20: S342–347. [DOI] [PubMed] [Google Scholar]
- 3. Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK (2002) Percutaneous tumor ablation with radiofrequency. Cancer 94: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C (2011) More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011: 160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, et al. (2006) Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 66: 1139–1146. [DOI] [PubMed] [Google Scholar]
- 6. Widenmeyer M, Shebzukhov Y, Haen SP, Schmidt D, Clasen S, et al. (2011) Analysis of tumor antigen-specific T cells and antibodies in cancer patients treated with radiofrequency ablation. Int J Cancer 128: 2653–2662. [DOI] [PubMed] [Google Scholar]
- 7. den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, et al. (2004) In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 64: 4024–4029. [DOI] [PubMed] [Google Scholar]
- 8. Otto G, Duber C, Hoppe-Lotichius M, Konig J, Heise M, et al. (2010) Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 251: 796–803. [DOI] [PubMed] [Google Scholar]
- 9. Mulier S, Ni Y, Jamart J, Michel L, Marchal G, et al. (2008) Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? Ann Surg Oncol 15: 144–157. [DOI] [PubMed] [Google Scholar]
- 10. Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR (2012) The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 39: 323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rempp H, Hoffmann R, Roland J, Buck A, Kickhefel A, et al. (2012) Threshold-based prediction of the coagulation zone in sequential temperature mapping in MR-guided radiofrequency ablation of liver tumours. European radiology 22: 1091–1100. [DOI] [PubMed] [Google Scholar]
- 12. Kudo-Saito C, Schlom J, Hodge JW (2005) Induction of an antigen cascade by diversified subcutaneous/intratumoral vaccination is associated with antitumor responses. Clin Cancer Res 11: 2416–2426. [DOI] [PubMed] [Google Scholar]
- 13. Hodge JW, Poole DJ, Aarts WM, Gomez Yafal A, Gritz L, et al. (2003) Modified vaccinia virus ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res 63: 7942–7949. [PubMed] [Google Scholar]
- 14. Kass E, Panicali DL, Mazzara G, Schlom J, Greiner JW (2001) Granulocyte/macrophage-colony stimulating factor produced by recombinant avian poxviruses enriches the regional lymph nodes with antigen-presenting cells and acts as an immunoadjuvant. Cancer Res 61: 206–214. [PubMed] [Google Scholar]
- 15. Eades-Perner AM, van der Putten H, Hirth A, Thompson J, Neumaier M, et al. (1994) Mice transgenic for the human carcinoembryonic antigen gene maintain its spatiotemporal expression pattern. Cancer Res 54: 4169–4176. [PubMed] [Google Scholar]
- 16. Robbins PF, Kantor JA, Salgaller M, Hand PH, Fernsten PD, et al. (1991) Transduction and expression of the human carcinoembryonic antigen gene in a murine colon carcinoma cell line. Cancer Res 51: 3657–3662. [PubMed] [Google Scholar]
- 17. Schmitz J, Reali E, Hodge JW, Patel A, Davis G, et al. (2002) Identification of an interferon-gamma-inducible carcinoembryonic antigen (CEA) CD8(+) T-cell epitope, which mediates tumor killing in CEA transgenic mice. Cancer Res 62: 5058–5064. [PubMed] [Google Scholar]
- 18. Hilburger Ryan M, Abrams SI (2001) Characterization of CD8+ cytotoxic T lymphocyte/tumor cell interactions reflecting recognition of an endogenously expressed murine wild-type p53 determinant. Cancer Immunol Immunother 49: 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ciesielski MJ, Kozbor D, Castanaro CA, Barone TA, Fenstermaker RA (2008) Therapeutic effect of a T helper cell supported CTL response induced by a survivin peptide vaccine against murine cerebral glioma. Cancer Immunol Immunother 57: 1827–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, et al. (1999) MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol 29: 3295–3301. [DOI] [PubMed] [Google Scholar]
- 21. Tsang KY, Zaremba S, Nieroda CA, Zhu MZ, Hamilton JM, et al. (1995) Generation of human cytotoxic T cells specific for human carcinoembryonic antigen epitopes from patients immunized with recombinant vaccinia-CEA vaccine. J Natl Cancer Inst 87: 982–990. [DOI] [PubMed] [Google Scholar]
- 22. Tsang KY, Zhu M, Nieroda CA, Correale P, Zaremba S, et al. (1997) Phenotypic stability of a cytotoxic T-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res 3: 2439–2449. [PubMed] [Google Scholar]
- 23. Muraro R, Wunderlich D, Thor A, Lundy J, Noguchi P, et al. (1985) Definition by monoclonal antibodies of a repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinomas versus normal adult tissues. Cancer Res 45: 5769–5780. [PubMed] [Google Scholar]
- 24. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, et al. (2004) Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res 64: 7985–7994. [DOI] [PubMed] [Google Scholar]
- 25. Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, et al. (2006) Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res 12: 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, et al. (2009) Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 251: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siler K, Eggensperger D, Hand PH, Milenic DE, Miller LS, et al. (1993) Therapeutic efficacy of a high-affinity anticarcinoembryonic antigen monoclonal antibody (COL-1). Biotechnol Ther 4: 163–181. [PubMed] [Google Scholar]
- 28. Wansley EK, Chakraborty M, Hance KW, Bernstein MB, Boehm AL, et al. (2008) Vaccination with a recombinant Saccharomyces cerevisiae expressing a tumor antigen breaks immune tolerance and elicits therapeutic antitumor responses. Clin Cancer Res 14: 4316–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thaker AA, Razjouyan F, Woods DL, Haemmerich D, Sekhar K, et al. (2012) Combination therapy of radiofrequency ablation and bevacizumab monitored with power Doppler ultrasound in a murine model of hepatocellular carcinoma. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 28: 766–775. [DOI] [PubMed] [Google Scholar]
- 30. Law KS, Chen HC, Liao SK (2007) Non-cytotoxic and sublethal paclitaxel treatment potentiates the sensitivity of cultured ovarian tumor SKOV-3 cells to lysis by lymphokine-activated killer cells. Anticancer Res 27: 841–850. [PubMed] [Google Scholar]
- 31. Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, et al. (2004) External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 64: 4328–4337. [DOI] [PubMed] [Google Scholar]
- 32. Hundt W, O’Connell-Rodwell CE, Bednarski MD, Steinbach S, Guccione S (2007) In vitro effect of focused ultrasound or thermal stress on HSP70 expression and cell viability in three tumor cell lines. Acad Radiol 14: 859–870. [DOI] [PubMed] [Google Scholar]
- 33. Gravante G, Sconocchia G, Ong SL, Dennison AR, Lloyd DM (2009) Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int 29: 18–24. [DOI] [PubMed] [Google Scholar]
- 34. Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, et al. (1994) Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity 1: 357–364. [DOI] [PubMed] [Google Scholar]
- 35. Modrak DE, Gold DV, Goldenberg DM, Blumenthal RD (2003) Colonic tumor CEA, CSAp and MUC-1 expression following radioimmunotherapy or chemotherapy. Tumour Biol 24: 32–39. [DOI] [PubMed] [Google Scholar]
- 36. Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, et al. (2008) The use of chelated radionuclide (samarium-153-ethylenediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell-mediated killing. Clin Cancer Res 14: 4241–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, et al. (1997) Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 277: 818–821. [DOI] [PubMed] [Google Scholar]
- 38. Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, et al. (2009) Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood 113: 3008–3016. [DOI] [PubMed] [Google Scholar]
- 39. Yang WL, Nair DG, Makizumi R, Gallos G, Ye X, et al. (2004) Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation. Ann Surg Oncol 11: 399–406. [DOI] [PubMed] [Google Scholar]
- 40. Teng LS, Jin KT, Han N, Cao J (2010) Radiofrequency ablation, heat shock protein 70 and potential anti-tumor immunity in hepatic and pancreatic cancers: a minireview. Hepatobiliary Pancreat Dis Int 9: 361–365. [PubMed] [Google Scholar]
- 41. Schmid TE, Multhoff G (2012) Radiation-induced stress proteins - the role of heat shock proteins (HSP) in anti- tumor responses. Curr Med Chem 19: 1765–1770. [DOI] [PubMed] [Google Scholar]
- 42. Makinde AY, John-Aryankalayil M, Palayoor ST, Cerna D, Coleman CN (2013) Radiation survivors: understanding and exploiting the phenotype following fractionated radiation therapy. Mol Cancer Res 11: 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB 2nd, et al (2012) Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat Res 178: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu L, Shao X, Gao W, Zhang Z, Liu P, et al. (2012) MicroRNA-133b inhibits the growth of non-small-cell lung cancer by targeting the epidermal growth factor receptor. FEBS J 279: 3800–3812. [DOI] [PubMed] [Google Scholar]
- 45. Tucci P, Agostini M, Grespi F, Markert EK, Terrinoni A, et al. (2012) Loss of p63 and its microRNA-205 target results in enhanced cell migration and metastasis in prostate cancer. Proc Natl Acad Sci U S A 109: 15312–15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava SK, Bhardwaj A, Singh S, Arora S, Wang B, et al. (2011) MicroRNA-150 directly targets MUC4 and suppresses growth and malignant behavior of pancreatic cancer cells. Carcinogenesis 32: 1832–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang C, Zheng X, Shen C, Shi Y (2012) MicroRNA-203 suppresses cell proliferation and migration by targeting BIRC5 and LASP1 in human triple-negative breast cancer cells. J Exp Clin Cancer Res 31: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hu M, Xia M, Chen X, Lin Z, Xu Y, et al. (2010) MicroRNA-141 regulates Smad interacting protein 1 (SIP1) and inhibits migration and invasion of colorectal cancer cells. Dig Dis Sci 55: 2365–2372. [DOI] [PubMed] [Google Scholar]
- 49. Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, et al. (2013) MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 32: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hodge JW, Sharp HJ, Gameiro SR (2012) Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm 27: 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Napoletano C, Taurino F, Biffoni M, De Majo A, Coscarella G, et al. (2008) RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol 32: 481–490. [PubMed] [Google Scholar]
- 52. Waitz R, Solomon SB (2009) Can local radiofrequency ablation of tumors generate systemic immunity against metastatic disease? Radiology 251: 1–2. [DOI] [PubMed] [Google Scholar]
- 53.Ruers T, Punt C, van Coevorden F, Borel Rinkes I, Ledermann J, et al. (2010) Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM). J Clin Oncol 28 (15s): abstr 3526.
- 54. Weng DS, Zhou J, Zhou QM, Zhao M, Wang QJ, et al. (2008) Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J Immunother 31: 63–71. [DOI] [PubMed] [Google Scholar]
- 55. Sakaguchi Y, Kudo M, Fukunaga T, Minami Y, Chung H, et al. (2005) Low-dose, long-term, intermittent interferon-alpha-2b therapy after radical treatment by radiofrequency ablation delays clinical recurrence in patients with hepatitis C virus-related hepatocellular carcinoma. Intervirology 48: 64–70. [DOI] [PubMed] [Google Scholar]
- 56.Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, et al. (2013) Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer: Jan 30 Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 57. Xia JZ, Xie FL, Ran LF, Xie XP, Fan YM, et al. (2012) High-intensity focused ultrasound tumor ablation activates autologous tumor-specific cytotoxic T lymphocytes. Ultrasound Med Biol 38: 1363–1371. [DOI] [PubMed] [Google Scholar]
- 58.Park KH, Lee TH, Kim CW, Kim J (2013) Enhancement of CCL15 Expression and Monocyte Adhesion to Endothelial Cells (ECs) after Hypoxia/Reoxygenation and Induction of ICAM-1 Expression by CCL15 via the JAK2/STAT3 Pathway in ECs. Journal of immunology. [DOI] [PubMed]
- 59. Bendz H, Ruhland SC, Pandya MJ, Hainzl O, Riegelsberger S, et al. (2007) Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem 282: 31688–31702. [DOI] [PubMed] [Google Scholar]
- 60. Goloudina AR, Demidov ON, Garrido C (2012) Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 325: 117–124. [DOI] [PubMed] [Google Scholar]
- 61. Hiroishi K, Eguchi J, Baba T, Shimazaki T, Ishii S, et al. (2010) Strong CD8(+) T-cell responses against tumor-associated antigens prolong the recurrence-free interval after tumor treatment in patients with hepatocellular carcinoma. J Gastroenterol 45: 451–458. [DOI] [PubMed] [Google Scholar]
- 62. Akcakaya P, Ekelund S, Kolosenko I, Caramuta S, Ozata DM, et al. (2011) miR-185 and miR-133b deregulation is associated with overall survival and metastasis in colorectal cancer. Int J Oncol 39: 311–318. [DOI] [PubMed] [Google Scholar]
- 63.Hulf T, Sibbritt T, Wiklund ED, Patterson K, Song JZ, et al. (2012) Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene: Aug 6 Epub ahead of print. [DOI] [PubMed]
- 64. Mulier S, Ruers T, Jamart J, Michel L, Marchal G, et al. (2008) Radiofrequency ablation versus resection for resectable colorectal liver metastases: time for a randomized trial? An update. Dig Surg 25: 445–460. [DOI] [PubMed] [Google Scholar]
- 65. Madan RA, Bilusic M, Heery C, Schlom J, Gulley JL (2012) Clinical evaluation of TRICOM vector therapeutic cancer vaccines. Semin Oncol 39: 296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of hyperthermia on cellular growth of murine colon carcinoma cells. MC38-CEA+ cells were exposed in vitro to 37°C or 42°C for 1 h. Tumor cells were incubated at 37°C/5%CO2 for 4 additional days. Cells were harvested daily and viable cells were counted by trypan blue exclusion. Results are presented as fold increase in cell number relative to day zero ± S.E.M. from 2 replicate flasks. Asterisks denote statistical significance (P<0.05) relative to control cells (2-tailed t test). This experiment was performed once.
(TIF)
(DOC)