Abstract
Vitamin D deficiency is a global health problem. This study aimed to investigate the efficacy of ultraviolet (UV) B radiation for improving vitamin D3 content of eggs and meat. In a two-factorial design hens that received diets with 0 (-D3) or 3,000 IU (+D3) vitamin D3/kg were non-exposed (-UVB) or exposed to UVB radiation (+UVB) for 3 h daily over 4 weeks. Data show that UVB radiation was very effective in raising the vitamin D3 content of egg yolk and meat. Egg yolk from +UVB/−D3 hens had a higher vitamin D3 content (17.5±7.2 µg/100 g dry matter (DM)) than those from the –UVB/+D3 group (5.2±2.4 µg/100 g DM, p<0.01). Vitamin D3 content in egg yolk of vitamin D3-supplemented hens could be further increased by UVB radiation (32.4±10.9 µg/100 g DM). The content of 25-hydroxyvitamin D3 (25(OH)D3) in the egg yolk also increased in response to UVB, although less pronounced than vitamin D3. Meat revealed about 4-fold higher vitamin D3 contents in response to UVB than to dietary vitamin D3 (p<0.001). In conclusion, exposure of hens to UVB is an efficient approach to provide consumers with vitamin D3-enriched foods from animal sources.
Introduction
Vitamin D3 deficiency is a global health problem that has considerable impact on health [1], [2], [3]. It is suggested that up to 50% of young adults suffer from vitamin D insufficiency worldwide [4]. Vitamin D3 promotes calcium and phosphate absorption in the intestine, decreases the clearance of these minerals from the kidney and is needed for bone mineralization and bone growth [5], [6]. In the last years, more attention has been paid to vitamin D3 due to its multiple health benefits. More than 220 genes are identified that significantly changed in expression in response to vitamin D3 [3], particularly those that are involved in cell proliferation, cell differentiation, and immune function [5], [7], [8], and vitamin D deficiency is associated with several diseases such as cancer and autoimmune disorders [3], [7], [9]. Thus, the maintenance of an adequate vitamin D3 status seems to provide a great preventive health potential. The main source (80–90%) of vitamin D3 is the endogenous synthesis of vitamin D3 in the skin by exposure to natural sunlight, whereas nutrition contributes to only 10–20% of the vitamin D3 supply [6], [10]. Failing outdoor activities, seasonal variations, air pollution, pigmented skin, and the use of sunscreens affect the efficacy of UVB radiation for cutaneous vitamin D3 synthesis. Therefore, an increasing number of people depend on dietary sources of vitamin D3 to prevent vitamin D3 deficiency or inadequacy. With the exception of fatty fish species, such as salmon and mackerel, and fish liver oils [11], most natural foods contain very low amounts of vitamin D3 and are not capable of improving vitamin D3 status or fulfilling the recommendations for diet intake of vitamin D3. Based on a report of the U.S. Institute of Medicine, vitamin D3 is recommended in daily amounts of 15 µg for people younger than 71 years in the USA and Canada [12]. Recommendations for vitamin D3 intake in different European countries range between 5 and 20 µg daily for adult men and women [13]. In most of the European countries, the recommended amounts of vitamin D3 were not met by the intake of natural foods [14]. Therefore, food-based strategies need to be developed to improve vitamin D3 status. In the United States and in Canada, a series of industrial produced foods were fortified with vitamin D [15]. In Europe, vitamin D fortification of food is highly regulated and critically discussed. A novel approach to enrich foods with specific nutrients is the “bio-addition”; thereby, foods are fortified through the addition of nutrients to animal feed during livestock farming production, or manipulation of post-harvest food processes. Eggs are widely and regularly consumed, and offer an interesting target for vitamin D3 fortification. However, with respect to vitamin D, it is not allowed to fortify animal feed with vitamin D beyond a defined maximum. We therefore came up with the idea that UVB exposure of farm animals such as laying hens might become a promising option to further improve the vitamin D content of foods from animal origin. In an initial experiment we could show that chickens whose upper part of their body was exposed to UVB did not produce vitamin D-enriched eggs [16]. Current analysis from our research group showed that most of the 7-dehydrocholesterol (7-DHC), the pre-cursor and limiting factor for vitamin D3 synthesis, was located in the unfeathered skin of the chicken legs. Based on this finding, we hypothesized that an UVB exposure which ensured irradiation of the whole chicken body, including legs, should increase the vitamin D content of eggs and meat. In order to assess the effectiveness of a whole body irradiation of chickens in producing vitamin D-enriched eggs and meat, we analyzed the vitamin D content of eggs and meat in response to UVB treatment of chickens that were fed either a vitamin D3-deficient diet or a diet that contained the maximum permissible amount of dietary vitamin D. Besides vitamin D3 metabolites in plasma, eggs, and meat, the laying performance, and also egg shell quality and bone stability were analyzed. We further investigated the folate status of the animals to rule out pronounced side effects of the UVB treatment, since solar radiation is supposed to affect the folic acid levels [17], [18], [19].
Materials and Methods
Comparative Analysis of 7-DHC Concentrations in Different Skin Areas of Chickens
To obtain information about the amounts and distribution of cutaneous 7-DHC in chickens, we analyzed the 7-DHC concentrations in skin of comb, wattles, unfeathered and feathered legs, and wing of 8 vitamin D3-adequately supplied Lohmann layers with an age of 21 weeks. Prior to skin sample preparation feathers were plucked and the skin was dissected free from underlying muscle and fat. Skin samples (approximately 2 × 2 cm) were then snap frozen in liquid nitrogen and stored at −80°C until 7-DHC analysis. Sample treatment and analysis of the 7-DHC concentration in skin is described below.
Animals and Treatment
The experiment was conducted with 36 Lohmann layers with an initial age of 27 weeks and an average body weight of 1777 g (±141 g). Before starting the experiment, hens were fed a standard diet containing 2,500 IU vitamin D3/kg for 2 weeks. Then, the hens were randomly assigned into four groups of 9 hens each. The hens were individually housed in an environmentally controlled room at 16°C and light (30 lx) from 6∶00 a.m. to 8∶00 p.m. All hens were fed a diet that consisted of (g/kg diet) wheat (470), extracted soy bean meal (220), corn (100), barley (68.2), calcium carbonate (85), soybean oil (30), dicalcium phosphate (13), vitamin and mineral mix (10), sodium chloride (2) and DL methionine (1.8). Except vitamin D3, vitamins and minerals were added according to the recommendations of the GfE [20]. From the 36 layers, 18 hens received a diet without any vitamin D3 (0 IU vitamin D3/kg; vitamin D3-deficient diet, -D3), the other 18 hens were fed a diet supplemented with 3,000 IU vitamin D3 (Molekula, Gillingham, U.K.) per kg diet (vitamin D3-adequate diet, +D3). The diets were calculated on the basis of GfE recommendations for laying hens and contained 11.6 MJ/kg [20]. All diets were fed over a period of 4 weeks. Feed and water from nipple drinkers were available ad libitum during the whole experiment. The experimental procedure was performed according to the established guidelines for care and handling of laboratory animals and was approved by the council of Saxony-Anhalt, Germany (No. 42502-3-656 MLU). The hens were weighted once a week. Food intake, laying performance, egg weight and shell quality were monitored weekly.
UVB Treatment
Two groups of hens (-D3/+UVB and +D3/+UVB) were exposed to UVB for 3 h daily (from 8∶00 to 8∶30, from 11∶00 to 12∶00, from 14∶00 to 15∶00 and from 16∶30 to 17∶00). The 10 cm long, 23 W UVB lamps (Hobby UV Kompakt Desert 8% UVB, Dohse Aquaristik KG, Gelsdorf, Germany) with equipped heat protection (Dohse Aquaristik KG) were placed near the cage doors to ensure optimal UVB exposure of the hens’ legs. The lamps emitted UVB in ranges of 280 to 310 nm. The UVB radiation dosage at a distance of 20 cm was 76 µW/cm2 (according to the manufacturer’s specification). This UVB irradiation intensity corresponds to that of natural sunlight during summer in the Middle Europe (50° latitude) [21]. An UVB opaque board was placed between the UVB-treated and the non-exposed groups to block incidental irradiation. The UVB lamps had no influence on the temperature inside the cages.
Sample Collection
Blood samples for analysis of vitamin D metabolites, minerals, and folate from each hen were taken at the beginning and at the end of the experiment. The blood was collected in heparinized tubes (Sarstedt, Nümbrecht, Germany) and centrifuged at 1,100 g for 10 min at 4oC. Plasma samples were stored at −80°C pending analysis. To determine egg weight and shell quality, eggs from each hen were collected at the beginning and after week 1, week 2, week 3, and week 4 of the experiment. Egg yolk for analysis of vitamin D3 and 25(OH)D3 was collected from eggs of each hen at the beginning and at the end of the experiment. At the end of the experimental period, the hens were killed by decapitation. Fibularis longus muscle of each hen was removed for quantification of vitamin D3 and 25(OH)D3, tibiotarsus was excised to measure bone stability, and liver was removed for analysis of folate. All samples were stored at −80°C pending further analysis.
Analysis of 25(OH)D3 and 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in Plasma
The plasma concentration of 25(OH)D3 was determined by coupled liquid chromatography-mass spectrometry (LC-MS/MS) according to Higashi et al. [22]. In brief, plasma samples were mixed with deuterated 25(OH)D3, which was solved in acetonitrile, (Chemaphor Incorporation, Ottawa, Canada) as an internal standard and extracted with n-hexane. To the dried residue, 4-phenyl-1,2,4-triazolin-3,5-dione (solved in acetonitrile) was added for derivatization. Subsequently to the addition of ethanol and salvation in the mobile phase, the samples were analyzed by HPLC (Agilent 1100, Agilent Technologies, Waldbronn, Germany) equipped with Hypersil ODS-column 100 × 2 mm2, 5 µm (Agilent Technologies), coupled to a MS system (API 2000, Applied Biosystems, Darmstadt, Germany). The detection limit for 25(OH)D3 was 3.7 nmol/l. Between run precision data were calculated from 2 control sera. The coefficient of variation for 25(OH)D3 was 3.0%.
The plasma concentration of 1,25(OH)2D3 was determined using a commercially available ELISA kit (IDS, Boldon, U.K.) according to the manufacturer’s protocol.
Analysis of Calcium and Inorganic Phosphate in Plasma
Calcium in plasma samples was quantified by a colorimetric assay. The test system was based on the formation of a calcium-o-kresolphtalein complex (Analyticon Biotechnologies AG, Lichtenfels, Germany). Prior to analysis, plasma was diluted 1∶4 with 0.9% NaCl to avoid interferences with triglycerides in plasma.
The plasma concentration of inorganic phosphate was measured spectrophotometrically according to the manufacturer’s protocol (Analyticon Biotechnologies AG). The test system was based on the measurement of ammonium molybdate which forms a complex with inorganic phosphate.
Analysis of 7-DHC in Skin, and Vitamin D3, 25(OH)D3 in Egg Yolk and Meat
Vitamin D3, 25(OH)D3, and 7-DHC were determined by LC-MS/MS. In brief, samples were homogenized, deuterated internal standard (D3-d3, 25(OH)D3-d6, and ergosterol, Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany) was added and hydrolyzed under alkaline conditions and oxygen exclusion. Samples were extracted with n-hexane and hexane phase was washed with ultrapure water. The vitamin D metabolites were fractionated using HPLC (Agilent 1100 HPLC, Agilent Technologies) according to Mattila et al. [23]. Further analytical steps were in accordance to those described for 25(OH)D3 in plasma. The detection limit was 0.17 µg/100 g for vitamin D3, and 0.1 µg/100 g for 25(OH)D3. The coefficient of variation for vitamin D3 was 3.9%, and 4.6% for 25(OH)D3.
Analysis of Egg Shell Thickness and Stability
Thickness of each egg shell was measured by use of a micrometer screw capable of 0.01 mm accuracy. Thickness of three fragments from the equatorial region of each egg shell was averaged. Prior to analysis, shell membranes were removed. The stability of egg shells were determined by an electronically controlled breaking strength tester (Messtechnik Gutsch, Nauendorf, Germany). Values were expressed in Newton (N).
Analysis of Tibiotarsus Stability
Three-point bending tests were performed to determine the fracture loads. The specimens were tested using a Zwick Z050 electro-mechanical testing machine (Zwick GmbH & Co KG, Ulm, Germany). The loading rate was set to 80 mm/min, the span (distance between the supports) to 80 mm and the radius of the cylindrical supports and the cylindrical loading blocks to 5 mm. The specimens were carried out at 23±2°C and a relative humidity of 50%.
Analysis of Folate in Plasma and Liver
Folate in plasma and liver samples was quantified using a microbiological test kit containing Lactobacillus rhamnosus coated microtiter plates according to the manufacturer’s protocol (R-Biopharm AG, Darmstadt, Germany). Prior to analysis, liver samples were homogenized and enzymatically hydrolyzed using pancreatin (R-Biopharm AG, Darmstadt Germany).
Statistical Analysis
Values are expressed as mean ± SD. Statistical analyses were performed using SPSS 20 (IBM, Armonk, NY, USA). Two-way ANOVA was used to compare the effects of UVB irradiation (-UVB vs. +UVB), dietary vitamin D3 (vitamin D3-deficient diet vs. vitamin D3-adequate diet), and their interaction. When two-way ANOVA revealed a significant interaction between UVB and vitamin D3, a post-hoc comparison was performed. In case of variance homogeneity, means of the four groups were compared by Tukey’s test, or in case of variance heterogeneity by Games-Howell test. Significances of differences between basal and final means were tested by the paired t-test. Means were considered significantly different at p<0.05. Values under the detection limit are represented by randomly assigned values.
Results
Concentration of 7-DHC Varies Strongly in the Different Skin Areas of Hens
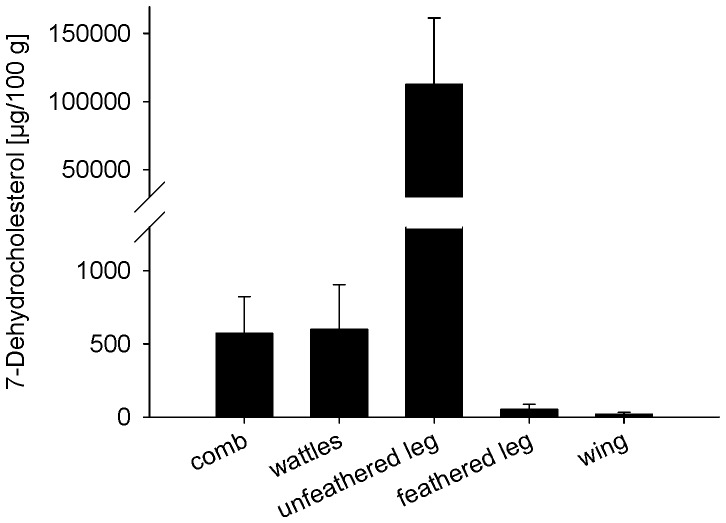
7-DHC is the limiting factor for vitamin D3 synthesis. To figure out which part of the chicken skin contains most of the vitamin D3 precursor molecule and should be inevitably exposed to UVB irradiation to increase vitamin D synthesis, we determined the concentrations of 7-DHC in 5 different skin samples of 8 laying hens by LC-MS/MS. Figure 1 shows large differences in 7-DHC contents between the chosen skin parts. The cutaneous area of the unfeathered legs contained the highest 7-DHC levels, which were on average 190-fold higher than that of the comb. The lowest 7-DHC concentrations were observed in the feathered parts of the skin such as wings and feathered legs. These findings prompted us to mount the UVB lamps in the experimental housing system in a lateral position to ensure an adequate UVB irradiation of skin in the leg area.
Figure 1. Cutaneous contents of 7-DHC to assess the capacity of different areas of chicken skin to produce vitamin D3.
Data represent mean ± SD of 7-DHC contents of comb, wattles, unfeathered legs, feathered legs, and wing (n = 8).
UVB Radiation and Dietary Vitamin D3 did not Influence Food Intake and Body Weight
None of the hens showed behavioral peculiarities or symptoms of erythema in response to UVB radiation. Two-way ANOVA did not reveal main and interactive effects of UVB exposure and dietary vitamin D3 on daily food intake (-D3/−UVB group, 114.1±12.8 g; +D3/−UVB group, 118.6±10.0 g; -D3/+UVB group, 121.9±7.3 g; +D3/+UVB group, 117.3±8.1 g; mean ± SD) and body weight (-D3/−UVB group, 1734±151 g; +D3/−UVB group, 1797±187 g; -D3/+UVB group, 1952±196 g; +D3/+UVB group, 1810±97 g; mean ± SD; Table S1).
Effects of UVB Exposure on Plasma Concentrations of 25(OH)D3 and 1,25(OH)2D3 of hens on a Vitamin D3-deficient and Vitamin D3-adequate Diet
To examine the vitamin D status of hens in response to UVB radiation and dietary vitamin D3, the plasma concentrations of 25(OH)D3 and 1,25(OH)2D3 were analyzed. Figure 2A and 2B show that the plasma concentration of 25(OH)D3 increased much more in response to UVB radiation and dietary vitamin D3 than the plasma level of 1,25(OH)2D3. Two-way ANOVA data reveal a strong interactive effect of UVB exposure and dietary vitamin D3 on the plasma concentration of 25(OH)D3 (p<0.001, Figure 2A, Table S1), and independent effects of UVB exposure (p<0.01) and dietary vitamin D3 (p<0.001) without treatment factor interaction on the circulating plasma level of 1,25(OH)2D3 (Figure 2B, Table S1). By comparison of the 25(OH)D3 plasma levels in response to the treatment factors it was noticeable that UVB treatment was capable of increasing the 25(OH)D3 plasma levels in the group of hens on a vitamin D3-deficient diet (p<0.001) but not in the hens that received the vitamin D3-adequate diet (Figure 2A). Supplementation of dietary vitamin D3 markedly increased the plasma level of 25(OH)D3 in the group which was non-exposed to UVB radiation but not in the group exposed to UVB radiation (p<0.001, Figure 2A). Figure 2B shows that hens on a vitamin D3-deficient diet that were non-exposed to UVB had the lowest plasma level of 1,25(OH)2D3 compared to the other groups. Two-way ANOVA data revealed that both treatment factors contributed to increase the 1,25(OH)2D3 plasma concentration (p<0.01, Table S1).
Figure 2. Vitamin D status of chickens in response to UVB exposure and dietary vitamin D3.
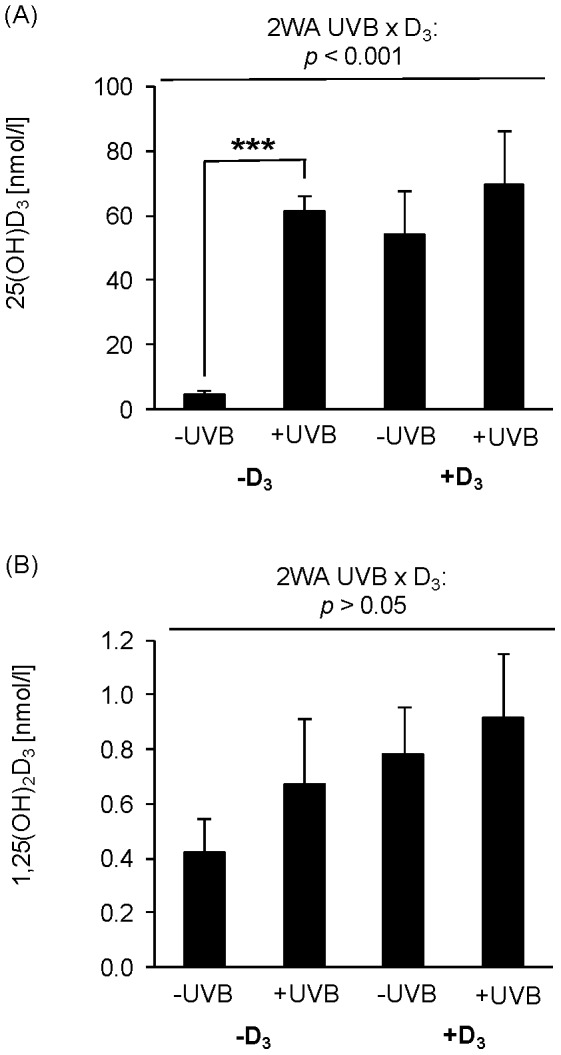
(A) Data in the top panel represent mean ± SD (n = 9) of plasma 25(OH)D3 concentrations in non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Data were analyzed by two-way ANOVA with the classification factors UVB exposure, vitamin D3 in the diet, and the interaction between both factors. Effect of UVB: p<0.001, vitamin D3: p<0.001, UVB × vitamin D3: p<0.001. Individual means of the groups were compared by post-hoc test. Asterisks within one diet (-D3 and +D3) indicate a significant difference between -UVB and +UVB groups, ***p<0.001. (B) Data in the bottom panel represent mean ± SD (n = 9) of plasma 1,25(OH)2D3 concentrations in non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Data were analyzed by two-way ANOVA with the classification factors UVB exposure, vitamin D3 in the diet, and the interaction between both factors. Effect of UVB: p<0.001, vitamin D3: p<0.001.
Plasma Concentrations of Calcium and Inorganic Phosphate were not Affected by UVB Exposure and Dietary Vitamin D3
Despite strong differences in vitamin D status, two-way ANOVA did not reveal any significant effects of UVB exposure or dietary vitamin D3 on plasma concentrations of calcium (-UVB/−D3 group, 6.79±1.66 nmol/l; -UVB/+D3 group, 8.11±1.65 nmol/l; +UVB/−D3 group, 7.50±1.96 nmol/l; +UVB/+D3 group, 8.22±1.52 nmol/l; mean ± SD) and inorganic phosphate (-UVB/−D3 group, 1.73±0.37 nmol/l; -UVB/+D3 group, 1.89±0.39 nmol/l; +UVB/−D3 group, 1.76±0.20 nmol/l; +UVB/+D3 group, 1.98±0.39 nmol/l; mean ± SD) (Table S1).
Effects of UVB Exposure on Vitamin D3 and 25(OH)D3 in Egg Yolk of Hens on a Vitamin D3-deficient and Vitamin D3-adequate Diet
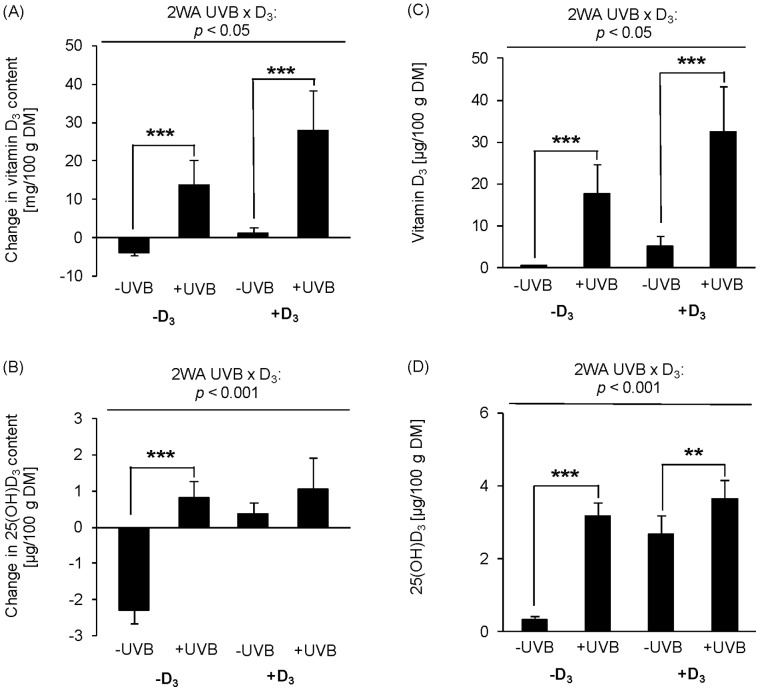
Figures 3A and 3C show the changes (final - basal) and final contents of vitamin D3 in egg yolk in response to UVB exposure of hens on a vitamin D3-deficient and vitamin D3-adequate diet. Two-way ANOVA revealed significant effects of UVB radiation (p<0.001), dietary vitamin D3 (p<0.001) and an interaction between these two factors (p<0.05) on the vitamin D3 content of the egg yolk (Table S1). The findings demonstrate that both treatment factors were capable of increasing the vitamin D3 content in eggs, even though the UVB irradiation was more effective than the dietary vitamin D3 supplementation. Importantly, we found that dietary vitamin D3 could increase the vitamin D3 content of egg yolk stronger in the group exposed to UVB than in the group non-exposed to UVB radiation. Thus, by far the highest content of vitamin D3 in eggs could be obtained with a combination of UVB exposure and dietary vitamin D3. Figures 3B and 3D show the changes (final - basal) and final 25(OH)D3 contents in egg yolk in response to the dietary vitamin D3 and UVB treatment. An interactive effect of dietary vitamin D3 and UVB exposure on 25(OH)D3 changes (p<0.001) and the final 25(OH)D3 (p<0.001) content of egg yolk was confirmed by two-way ANOVA (Table S1). Main effects for UVB exposure (p<0.001) and dietary vitamin D3 (p<0.001) were also significant (Table S1). As expected, the 25(OH)D3 content of eggs decreased compared to baseline if hens on a vitamin D3-deficient diet were non-exposed to UVB radiation (Figure 3B, p<0.001). UVB irradiation and dietary vitamin D3 improved the 25(OH)D3 content in egg yolk (two-way ANOVA, p<0.001, Figure 3D, Table S1), although the UVB irradiation was marginally more effective than the dietary vitamin D3 in increasing the 25(OH)D3 content in egg yolk. Interestingly, dietary vitamin D3 particularly increased the 25(OH)D3 contents of egg yolk in UVB-non-exposed hens on the vitamin D3-deficient diet, and to a minor extent in UVB-exposed animals (Figure 3D). Nevertheless, as shown for the vitamin D3 content of eggs, the highest 25(OH)D3 contents in eggs resulted from a combination of UVB radiation and dietary vitamin D3. In all treatment groups, egg white did not show any detectable contents of vitamin D2 (detection limit 0.17 µg/100 g) and 25(OH)D2 (0.1 µg/100 g) (data not shown).
Figure 3. UVB exposure is an effective approach to fortify egg yolk with vitamin D3.
Changes between basal and final vitamin D3 (A) and 25(OH)D3 (B) contents in egg yolk dry matter (DM) and final contents of vitamin D3 (C) and 25(OH)D3 (D) in egg yolk DM and in in non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Data represent mean ± SD (n = 9). Data were analyzed by two-way ANOVA with the classification factors were UVB exposure, vitamin D3 in the diet, and the interaction between both factors. (A) Effect of UVB: p<0.001, vitamin D3: p<0.001, UVB × vitamin D3: p<0.05. (B) Effect of UVB: p<0.001, vitamin D3: p<0.001, UVB × vitamin D3: p<0.001. When two-way ANOVA (2WA) revealed a significant interaction between UVB x vitamin D3 individual means of the groups were compared by post-hoc test. Asterisks within one diet group (-D3 and +D3) indicate a significant difference between -UVB and +UVB groups, *p<0.05, **p<0.01, ***p<0.001.
Effects of UVB Exposure on Vitamin D3 and 25(OH)D3 in Fibularis Longus Muscle of Hens on a Vitamin D3-deficient and Vitamin D3-adequate Diet
Irrespective of the vitamin D3 in the diet, hens non-exposed to UVB radiation had no detectable vitamin D3 contents in their fibularis longus muscles (Figure 4A). UVB irradiation increased the vitamin D3 content in the muscles of chickens to values that ranged between 0.16 and 0.96 µg/100 g. By comparison of both UVB-exposed groups, the vitamin D3 content of muscle was higher in the group that received the vitamin D3-adequate diet than in the group that was fed the vitamin D3-deficient diet (p<0.05). Figure 4B demonstrates the 25(OH)D3 content in the muscles in response to UVB radiation in hens on a vitamin D3-deficient and -adequate diet. Similar to the vitamin D3 data, hens of the -D3/−UVB group had undetectable contents of 25(OH)D3 in their muscles. Supplementation with dietary vitamin D3 and also UVB exposure slightly increased the muscle contents of 25(OH)D3, whereby the UVB-exposed groups reached higher contents in their muscles than the group fed the vitamin D3-adequate diet (p<0.05).
Figure 4. UVB exposure increases vitamin D3 content in skeletal muscle.
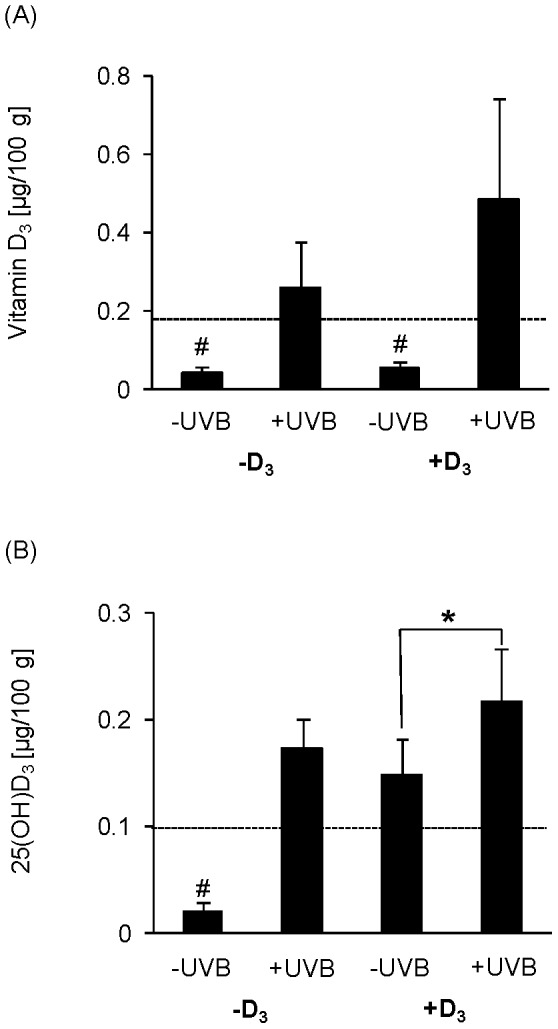
(A) Data in the top panel represent mean ± SD (n = 9) of vitamin D3 content in fibularis longus muscle of non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Values below the detection limit of 0.17 µg/100g for vitamin D3 are represented by randomly assigned values (#). The detection limit is marked by a dotted line (…). UVB exposure, but not dietary vitamin D3 was capable of increasing the vitamin D3 content in muscle to values above the detection limit. (B) Data in the bottom panel represent mean ± SD (n = 9) of 25(OH)D3 content in fibularis longus muscle of non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Values below the detection limit of 0.1 µg/100g for 25(OH)D3 are represented by randomly assigned values (#). The detection limit is marked by a dotted line (…). Individual means of the groups were compared by post-hoc test. Asterisks within one diet group (-D3 and +D3) indicate a significant difference between -UVB and +UVB groups, *p<0.05.
Effect of UVB Exposure on Laying Performance, Egg Weight and Egg Shell Quality of Hens on a Vitamin D3-deficient and Vitamin D3-adequate Diet
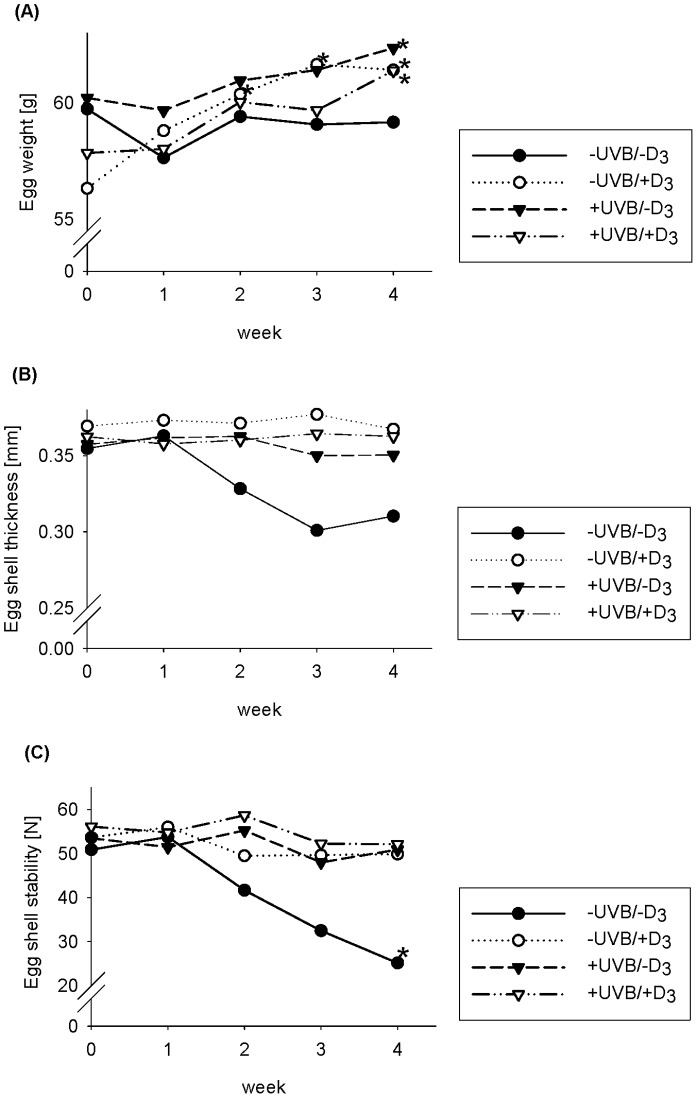
Mean egg production rate (number of eggs per hen and week) was not significantly influenced by the treatment factors, although hens from the -UVB/−D3 group showed a slight drop in egg production within the last experimental week compared to the other group (-UVB/−D3 group, 6.0±1.6 eggs/week; -UVB/+D3 group, 7.0±0.0 eggs/week; +UVB/−D3 group, 6.9±0.3 eggs/week; +UVB/+D3 group, 7.0±0.0 eggs/week) (Table S1). Two-way ANOVA data show that the mean egg weights at defined times within the 4-week period of the experiment were not significantly influenced by UVB exposure and dietary vitamin D3, respectively (Figure 5A, Table S1). Data demonstrate higher egg weights at the end of the 4-week experiment compared to baseline in the groups that were UVB exposed and/or received vitamin D3 with their diets (p<0.05, paired t-test), but not in the -UVB/−D3 group (Figure 5A).
Figure 5. Egg weight and egg shell quality in response to UVB exposure and dietary vitamin D3.
Effects of UVB exposure (UVB) and dietary vitamin D3 (D3) on egg weight (A), egg shell thickness (B), and egg shell stability (C) over 4 weeks. Data in the panels represented means (n = 9). Data were analyzed by two-way ANOVA with the classification factors UVB exposure, vitamin D3 in the diet, and the interaction between both factors. (A) No significant difference. (B) Effect of vitamin D3 (week 2): p<0.05, UVB × vitamin D3 (week 2): p<0.05. Effect of vitamin D3 (week 3 and 4): p<0.01, UVB × vitamin D3 (week 3 and 4): p<0.10. (C) Effect of UVB (week 2): p<0.05. Effect of UVB (week 3): p<0.10, vitamin D3 (week 3): p<0.10. Effect of UVB (week 4): p<0.01, vitamin D3 (week 4): p<0.05, UVB × vitamin D3 (week 4): p<0.05. Data were additionally analyzed by paired t-test, *significantly different from baseline.
Figures 5B and 5C show the egg shell thickness and the egg shell stability in response to UVB radiation and dietary vitamin D3 during the 4-week experiment. From the beginning of the second experimental week, egg shell thickness was significantly influenced by dietary vitamin D3 (p<0.05, two-way ANOVA, Figure 5B), and there was a tendency of an interaction effect on egg shell thickness at the end of the experiment (p = 0.053, two-way ANOVA). Although the -UVB/−D3 group showed a trend toward lower egg shell thickness after 3 and 4 weeks of the experiment, paired t-test data did not reveal differences compared to baseline. During the experimental period an increasing influence of UVB and dietary vitamin D3 on egg shell stability became evident. At the end of the experiment, two-way ANOVA analysis revealed significant main effects of UVB radiation (p<0.01) and dietary vitamin D3 (p<0.05) and a significant interaction between these both factors (p<0.05) on egg shell stability (Figure 5C). Compared to baseline, the stability of eggs from the -UVB/−D3 group was constantly dropping during the experimental period, and reached a minimum after 4 weeks which was significantly lower compared to baseline (p<0.05, paired t-test, Figure 5C).
Effect of UVB Exposure on Tibiotarsus Stability and Folate Status of Hens on a Vitamin D3-deficient and Vitamin D3-adequate Diet
Hens non-exposed to UVB radiation that received the vitamin D3-deficient diet revealed a lower mechanical stability of tibiotarsus than hens from the other groups (Figure 6, p<0.01). Two-way ANOVA data revealed an interactive effect of dietary vitamin D3 and UVB exposure on tibiotarsus stability (p<0.01, Table S1). In order to investigate possible effects of UVB radiation on folate status, the concentrations of folate in plasma and liver of the hens were determined. Neither the concentration of folate in plasma (-UVB/−D3 group, 49.8±18.6 nmol/l; -UVB/+D3 group, 45.5±17.4 nmol/l; +UVB/−D3 group, 56.8±21.5 nmol/l; +UVB/+D3 group, 41.5±10.4 nmol/l; mean ± SD), nor that in liver (-UVB/−D3 group, 10.5±1.1 µg/g; -UVB/+D3 group, 9.8±2.3 µg/g; +UVB/−D3 group, 10.1±1.7 µg/g; +UVB/+D3 group, 10.5±1.4 µg/g; mean ± SD) was influenced by dietary vitamin D3 and UVB exposure, respectively. This was confirmed by the two-way ANOVA data (Table S1).
Figure 6. Bone stability in response to UVB exposure and dietary vitamin D3.
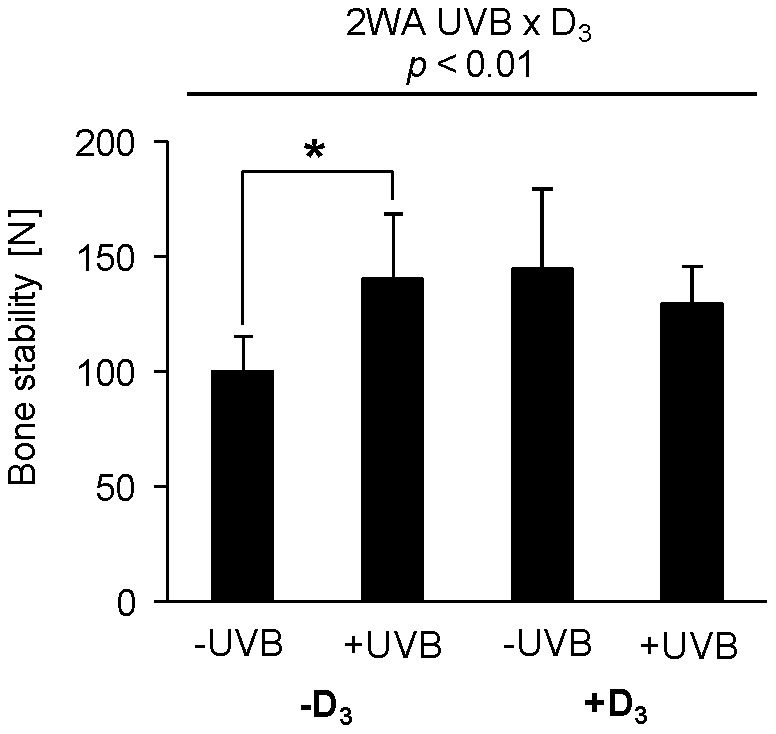
Data in represent mean ± SD (n = 9) of tibiotarsus stability of non-treated (-UVB) or UVB-treated (+UVB) hens that were fed either a vitamin D3-deficient (-D3) or vitamin D3-adequate diet (+D3), respectively. Data were analyzed by two-way ANOVA. Classification factors were UVB exposure, vitamin D3 in the diet, and the interaction between both factors. Effect of vitamin D3: p<0.10, vitamin D3× UVB: p<0.01. Individual means between two groups were compared by the unpaired Student’s t-test, **p<0.01.
Discussion
Results from the current study reveal UVB exposure of hens as an appropriate way and a highly effective approach to increase the vitamin D content mainly in eggs and also in meat. Data further show that an exposure to UVB is capable of raising the vitamin D content in egg yolk and muscle much stronger than feeding hens with diets that contain maximum permissible dosages of vitamin D3. UVB radiation was still effective in increasing the vitamin D content of eggs and meat even in the group that received 3,000 IU vitamin D3/kg feed. Previous studies that aimed to increase the vitamin D3 content in eggs used vitamin D3-enriched feeds. In these experiments the transfer of vitamin D3 from the feed into eggs proved to be very efficient and highly responsive [24], [25], [26]. In one of these studies in which hens received diets with 9,700, 17,200, 24,700 and 102,200 IU vitamin D3 per kg diet, the peak vitamin D3 contents of egg yolk were 22, 41, 60 and 870 µg/100 g (wet basis), respectively [24]. In Europe, the maximum amount of supplemented vitamin D3 specified by the Council of the European Communities (Council Directive 70/524/EEC) is set to a quantitative limit of 3,000 IU per kg feed for laying hens. This means that beyond this limit further diet-induced increases of vitamin D3 content in eggs are not feasible. Our findings show that exposure of chickens to UVB radiation or natural sunlight seems to offer a promising alternative to fortify foods with vitamin D. Assuming that an average-sized egg comprised of 7 g yolk dry matter, an egg from an UVB exposed hen on a vitamin D3-adequate diet would provide on average 2.5 µg vitamin D (vitamin D3+25(OH)D3) compared to eggs from non-exposed hens on the same diet which contained 0.55 µg. Vitamin D3 analysis reveal that meat from the +D3/+UVB group contained 0.7 µg vitamin D/100 g compared to meat of the +D3/−UVB group that contained 0.2 µg/100 g.
In 2008, Ko et al. already established UVB radiation as a method to increase the vitamin D2 content in sliced shiitake and white button mushrooms [27]. In that study, an UVB radiation dose of 75 kJ/m2 increased the vitamin D2 contents in gill of shiitake mushrooms from less than 500 µg/100 g in the non-exposed mushrooms up to 6,000 µg/100 g in the radiated mushrooms. Sliced button mushrooms exposed to UVB doses of 30 kJ/m2 revealed a vitamin D2 content of 3,500 µg/100 g. Although irradiation of mushrooms seems to be highly efficient in vitamin D2 fortification, it should be taken into consideration that the final vitamin D contents per gram food were extremely high and probably of hazardous nature. In contrast to mushrooms, the efficiency of vitamin D3 synthesis in the skin of animals is not only influenced by UV intensity but also by skin pigmentation and the thickness of hair coat, feathers or horny scales. Currently, there are only few published data that investigated the effectiveness of solar and UV irradiation in raising the levels of vitamin D3 in animals and animal products (see review [28]). For example, Ferguson et al. have shown that vitamin D content of eggs from chameleons increased in a dose-dependent manner in response to UVB exposure, and Kurmann & Indyk demonstrated lower vitamin D concentrations in bovine milk in winter (2.4 µg per g milk fat) than in summer (9.2 µg per g milk fat) [29], [30]. We assume that the body part which is exposed to UVB is an essential factor for the efficacy of vitamin D fortification. Data of the current study reveal strong variations of 7-DHC concentrations in the different skin areas of hens, with remarkably high 7-DHC levels in the unfeathered skin of legs that were on average 190 times higher than that of comb skin. The important role of the chicken legs for synthesis of vitamin D3 has been already reported by Tian and co-workers who found 30 times higher 7-DHC concentrations in skin of the legs and feet of chickens than in body skin [31]. Thus, we assume that “bio-addition” of vitamin D in eggs via UVB radiation only works if the location of the UVB lamps guarantees an irradiation of the feet skin.
An interesting finding of this study was that UVB exposure could increase vitamin D3 and 25(OH)D3 concentrations in egg yolk and muscle, whilst an oral administration of vitamin D3 mainly increased 25(OH)D3, but had minor impact on vitamin D3 in egg yolk and muscle. 25(OH)D3 is primarily synthesized in liver by 25-hydroxylation of vitamin D3 from endogenous synthesis or diet. The hepatic 25-hydroxylation is not strictly feedback regulated and therefore mainly reflects vitamin D3 status [32]. This relationship was confirmed by the observation that the concentrations of 25(OH)D3 in plasma, egg yolk and muscle increased significantly with dietary vitamin D3 and also UVB exposure. Domestic fowls synthesize two vitamin D3-binding proteins, one that binds 25(OH)D3, and the other which mainly binds vitamin D3 [19]. It is suggested that the selective mechanism that incorporates vitamin D3 into yolk gives the chick embryo the opportunity to control its own 25(OH)D3 supply [33]. UVB irradiation of farm animals seems to provide a safe approach to increase vitamin D3 without running the risk of vitamin D3 overdose. In the case of intense UV irradiation or if animals are exposed to excessive or prolonged exposure to sun, previtamin D3 and vitamin D3 photoisomerizes to biologically inactive tachysterol and lumisterol, which are desquamated with keratinocytes during normal skin turnover [6], [33], [34]. Health and performance data further indicate no symptoms of erythema, behavioral disorders or an impaired folate status in consequence of the applied UV treatment. The folate concentrations in plasma and liver of the hens were analyzed since UV irradiation is known to be capable of degrading folate in human blood and skin [35], [36]. UVA radiation (315–400 nm) is suggested to be mainly responsible for this effect because it has a greater dermal penetration depth, and can degrade the biological form of folate, 5-methyltetrahydrofolate (5MTHF), in dermal circulation by generation of reactive oxygen species [37], [38], [39], [40]. Other mechanisms such as the direct degradation of folate in the blood by UVA may also contribute to impact folate status [35], [37]. In contrast, UVB radiation (280–315 nm) is unable to penetrate into the dermal circulation and has therefore presumably a lower potential to impact blood levels of folate [35], [38], [39]. Plasma and liver folate data of the current experiment confirm no adverse effect of UVB radiation on folate status, although it should be considered that the period of UVB exposure was relatively short.
This study further reveals that UVB irradiation is capable of optimizing laying performance, egg shell quality, and bone stability in hens that received no vitamin D3 with their diet. Although hens from the +D3/+UVB group had significantly higher plasma levels of 25(OH)D3 and 1,25(OH)2D3 then hens from the +D3/−UVB group, laying performance, egg weight, egg shell thickness, and egg shell stability could not be further improved by the additional treatment with UVB radiation. This is in accordance with previous data that did not show any additional effect of UV radiation on laying performance and egg shell quality in breeders supplemented with sufficient amount of vitamin D3 [41].
Conclusions
In conclusion, the current study shows that UVB exposure of chickens that ensures irradiation of the whole body, including legs, is highly effective in increasing the vitamin D concentration in eggs, and also meat. We therefore consider UVB treatment of farmed animals as an effective and novel approach for “bio-addition” of foods with vitamin D. Considering the option that free-ranged chickens are still exposed to natural sun light, free-range husbandry could become a cheap alternative to the artificial UVB irradiation to produce vitamin D3 fortified eggs.
Supporting Information
Two-way analysis of variance table for the chicken and egg data.
(DOCX)
Funding Statement
Vitamin D analysis of this study was supported by a grant 0315668A from the Federal Ministry of Education and Research of Gemany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for the remaining parts of this study.
References
- 1. Chiu KC, Chu A, Go VL, Saad MF (2004) Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79: 820–825. [DOI] [PubMed] [Google Scholar]
- 2. Pilz S, Tomaschitz A, Drechsler C, Dekker JM, Marz W (2010) Vitamin D deficiency and myocardial diseases. Mol Nutr Food Res 54: 1103–1113. [DOI] [PubMed] [Google Scholar]
- 3. Ramagopalan SV, Heger A, Berlanga AJ, Maugeri NJ, Lincoln MR, et al. (2010) A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res 20: 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferder M, Inserra F, Manucha W, Ferder L (2013) The world pandemic of vitamin D deficit could possibly be explained by cellular inflammatory response activity induced by the renin angiotensin system. Am J Physiol Cell Physiol: in press. [DOI] [PMC free article] [PubMed]
- 5. Verstuyf A, Carmeliet G, Bouillon R, Mathieu C (2010) Vitamin D: a pleiotropic hormone. Kidney Int 78: 140–145. [DOI] [PubMed] [Google Scholar]
- 6. Holick MF (2007) Vitamin D deficiency. N Engl J Med 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 7. Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, et al. (2007) Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 32: 210–216. [DOI] [PubMed] [Google Scholar]
- 8. Lamprecht SA, Lipkin M (2003) Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 3: 601–614. [DOI] [PubMed] [Google Scholar]
- 9. Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79: 362–371. [DOI] [PubMed] [Google Scholar]
- 10. Tremezaygues L, Seifert M, Tilgen W, Reichrath J (2009) 1,25-dihydroxyvitamin D3 protects human keratinocytes against UV-B-induced damage: In vitro analysis of cell viability/proliferation, DNA-damage and -repair. Dermatoendocrinol 1: 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture ARS (2011) USDA National Nutrient Database for Standard Reference. Nutrient Data Laboratory Home Page.
- 12.Ross AC (2011) Dietary reference intakes: Calcium, vitamin D. Washington, DC: National Academies Press. [PubMed]
- 13. Doets EL, de Wit LS, Dhonukshe-Rutten RA, Cavelaars AE, Raats MM, et al. (2008) Current micronutrient recommendations in Europe: towards understanding their differences and similarities. Eur J Nutr 47 Suppl 117–40. [DOI] [PubMed] [Google Scholar]
- 14. Vinas BR, Barba LR, Ngo J, Gurinovic M, Novakovic R, et al. (2011) Projected prevalence of inadequate nutrient intakes in Europe. Ann Nutr Metab 59: 84–95. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine FaNB (2010) Dietary reference intakes for calcium and vitamin D. National Academy Press Washington DC.
- 16. Lietzow J, Kluge H, Brandsch C, Seeburg N, Hirche F, et al. (2012) Effect of short-term UVB exposure on vitamin D concentration of eggs and vitamin D status of laying hens. J Agric Food Chem 60: 799–804. [DOI] [PubMed] [Google Scholar]
- 17. Duthie SJ (1999) Folic acid deficiency and cancer: mechanisms of DNA instability. Br Med Bull 55: 578–592. [DOI] [PubMed] [Google Scholar]
- 18. Stanger O (2002) Physiology of folic acid in health and disease. Curr Drug Metab 3: 211–223. [DOI] [PubMed] [Google Scholar]
- 19. Lewis P, Gous R (2009) Responses of poultry to ultraviolet radiation. World’s Poultry Science Journal 65: 499–510. [Google Scholar]
- 20.Gesellschaft für Ernährungsphysiologie (1999) Empfehlungen zur Energie- und Nährstoffversorgung der Legehennen und Masthühner (Broiler). DLG-Verlag Frankfurt.
- 21.Bundesamt für Strahlenschutz (2013) Solare bodennahe UV-Strahlung in Deutschland.
- 22. Higashi T, Shibayama Y, Fuji M, Shimada K (2008) Liquid chromatography-tandem mass spectrometric method for the determination of salivary 25-hydroxyvitamin D3: a noninvasive tool for the assessment of vitamin D status. Anal Bioanal Chem 391: 229–238. [DOI] [PubMed] [Google Scholar]
- 23. Mattila PH, Piironen VI, Uusi-Rauva EJ, Koivistoinen PE (1995) Contents of cholecalciferol, ergocalciferol, and their 25-hydroxylated metabolites in milk products and raw meat and liver as determined by HPLC. J Agric Food Chem 43: 2394–2399. [Google Scholar]
- 24. Yao L, Wang T, Persia M, Horst RL, Higgins M (2013) Effects of vitamin D3 -enriched diet on egg yolk vitamin D3 content and yolk quality. J Food Sci 78: C178–183. [DOI] [PubMed] [Google Scholar]
- 25. Mattila P, Lehikoinen K, Kiiskinen T, Piironen V (1999) Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J Agric Food Chem 47: 4089–4092. [DOI] [PubMed] [Google Scholar]
- 26. Leeson S, Caston LJ (2003) Vitamin enrichment of eggs. J Appl Poult Res 12: 24–26. [Google Scholar]
- 27. Ko JA, Lee BH, Lee JS, Park HJ (2008) Effect of UV-B exposure on the concentration of vitamin D2 in sliced shiitake mushroom (Lentinus edodes) and white button mushroom (Agaricus bisporus). J Agric Food Chem 56: 3671–3674. [DOI] [PubMed] [Google Scholar]
- 28. Dittmer KE, Thompson KG (2011) Vitamin D metabolism and rickets in domestic animals: a review. Vet Pathol 48: 389–407. [DOI] [PubMed] [Google Scholar]
- 29. Ferguson GW, Gehrmann WH, Chen TC, Holick MF (2005) Vitamin D-content of the eggs of the panther chameleon Furcifer pardalis: its relationship to UVB exposure/vitamin D-condition of mother, incubation and hatching success. Journal of Herpetological Medicine and Surgery 15: 9–13. [Google Scholar]
- 30. Kurmann A, Indyk H (1994) The endogenous vitamin D content of bovine milk: influence of season. Food Chemistry 50: 75–81. [Google Scholar]
- 31. Tian XQ, Chen TC, Lu Z, Shao Q, Holick MF (1994) Characterization of the translocation process of vitamin D3 from the skin into the circulation. Endocrinology 135: 655–661. [DOI] [PubMed] [Google Scholar]
- 32. Zhu J, DeLuca HF (2012) Vitamin D 25-hydroxylase - Four decades of searching, are we there yet? Arch Biochem Biophys 523: 30–36. [DOI] [PubMed] [Google Scholar]
- 33. Fraser DR, Emtage JS (1976) Vitamin D in the avian egg. Its molecular identity and mechanism of incorporation into yolk. Biochem J 160: 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holick MF (2003) Vitamin D: A millenium perspective. J Cell Biochem 88: 296–307. [DOI] [PubMed] [Google Scholar]
- 35. Williams JD, Jacobson MK (2010) Photobiological implications of folate depletion and repletion in cultured human keratinocytes. J Photochem Photobiol B 99: 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lucock M (2011) Folic acid: Beyond metabolism. Journal of Evidence-Based Complementary & Alternative Medicine 16: 102–113. [Google Scholar]
- 37. Off MK, Steindal AE, Porojnicu AC, Juzeniene A, Vorobey A, et al. (2005) Ultraviolet photodegradation of folic acid. J Photochem Photobiol B 80: 47–55. [DOI] [PubMed] [Google Scholar]
- 38. Steindal AH, Tam TT, Lu XY, Juzeniene A, Moan J (2008) 5-Methyltetrahydrofolate is photosensitive in the presence of riboflavin. Photochem Photobiol Sci 7: 814–818. [DOI] [PubMed] [Google Scholar]
- 39. Steindal AH, Juzeniene A, Johnsson A, Moan J (2006) Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem Photobiol 82: 1651–1655. [DOI] [PubMed] [Google Scholar]
- 40. Tam TT, Juzeniene A, Steindal AH, Iani V, Moan J (2009) Photodegradation of 5-methyltetrahydrofolate in the presence of uroporphyrin. J Photochem Photobiol B 94: 201–204. [DOI] [PubMed] [Google Scholar]
- 41. Carson JR, Beall G (1955) Absence of response by breeder hens to ultraviolet energy. Poultry Science 34: 256–262. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-way analysis of variance table for the chicken and egg data.
(DOCX)