Abstract
Plasma uric acid (UA) levels decrease following clinical progression and stage development of Parkinson’s disease (PD). However, the molecular mechanisms underlying decreases in plasma UA levels remain unclear, and the potential to apply mutagenesis to a PD model has not previously been discovered. We identified a unique mutant of the silkworm Bombyx mori (B.mori) op. Initially, we investigated the causality of the phenotypic “op” by microarray analysis using our constructed KAIKO functional annotation pipeline. Consequently, we found a novel UA synthesis-modulating pathway, from DJ-1 to xanthine oxidase, and established methods for large-scale analysis of gene expression in B. mori. We found that the mRNA levels of genes in this pathway were significantly lower in B. mori op mutants, indicating that downstream events in the signal transduction cascade might be prevented. Additionally, levels of B.mori tyrosine hydroxylase (TH) and DJ-1 mRNA were significantly lower in the brain of B. mori op mutants. UA content was significantly lower in the B. mori op mutant tissues and hemolymph. The possibility that the B. mori op mutant might be due to loss of DJ-1 function was supported by the observed vulnerability to oxidative stress. These results suggest that UA synthesis, transport, elimination and accumulation are decreased by environmental oxidative stress in the B. mori op mutant. In the case of B. mori op mutants, the relatively low availability of UA appears to be due both to the oxidation of DJ-1 and to its expenditure to mitigate the effects of environmental oxidative stress. Our findings are expected to provide information needed to elucidate the molecular mechanism of decreased plasma UA levels in the clinical stage progression of PD.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder with an etiology involving oxygen radicals and other oxidants that attack dopaminergic neuronal cells and which damage and deplete dopamine levels [1]. Genetic studies have identified 18 genes associated with PD at different loci based on family linkage analysis (PD; Online Mendelian Inheritance in Man (OMIM) 168600). PD-associated gene knockout animal models have been developed as familial PD models [2].
The majority of idiopathic PD cases, however, are the result of sporadic onset caused by environmental stress [3], and a molecular-based mechanism of oxidative stress has been developed. In animal models of sporadic PD, oxidative stress has been simulated using parkinsonian neurotoxins that are mitochondrial complex I inhibitors [4], namely 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxy-dopamine (6-OHDA), paraquat (PQ) and rotenone (ROT).
The final product of purine metabolism, uric acid (UA), plays an important role as a physiological antioxidant [5]. In recent years, several groups have reported the correlation between decreased plasma UA levels and neuron cell failure in the substantia nigra, clinical progression and stage of PD [6], [7], [8], [9], [10]. Conversely, high plasma UA concentrations in hyperuricemia may reduce the risk and delay the progression of PD [11]. Plasma UA might be expended to resist oxidative injury in PD, but the molecular mechanism underlying the decrease in plasma UA in advanced clinical stages of PD has not been analyzed using either of these models.
Here, we used a silkworm B. mori mutant with reduced levels of UA to examine the mechanisms involved in UA metabolism. In silkworms, UA is mainly synthesized in the fat body, from where it is transported to the integument via the hemolymph. On the other hand, UA is eliminated through the Malpighian tubules. UA accumulates as urate granules, which cause a whitening of the integument color [12]. UA is typically the ultimate metabolite in insects, but in B. mori UA is partly converted to urea by urate oxidase [13]. Mutant larvae unable to synthesize UA due to a deficiency in xanthine oxidase (XD/XO) [14], [15], [16] or failure of the UA transporter [17], cannot accumulate UA in the larval epidermis and take on a translucent appearance.
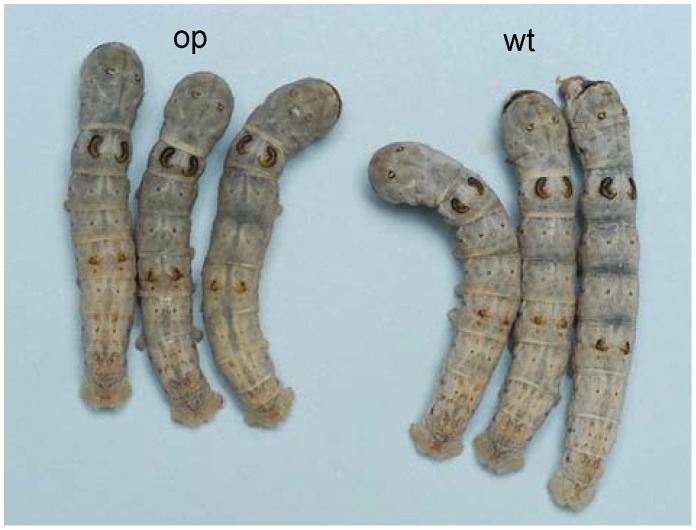
The B. mori op mutant exhibits spontaneous and pronounced translucency during the larval stage (Fig. 1) and occasional unique actions such as vibration (Video S1).
Figure 1. Phenotypes of op and wild-type larvae.
Left, op mutants (op), which have a translucent integument. Right, wild-type larvae (wt), which have a white integument.
Classical linkage analysis has demonstrated that a mutation located on chromosome 23 is responsible for the extraordinarily high phenotype mortality, particularly in the pupal stage, as well as male infertility (NBRP silkworm database: http://www.shigen.nig.ac.jp/silkwormbase/). Despite the unique phenotypes of the op mutant with reduced levels of UA, the op causative gene and position has not been clarified.
In the present study, we characterized the B. mori op mutant and identified the novel uric acid synthesis pathway using microarray analysis.
Results
Screening for Target Molecules using Microarray
We investigated the number of human homologs in the B. mori genome. We identified 8096 human homologs among 14,623 total transcripts in the B. mori consensus gene set by merging all the gene sets using GLEAN (http://sgp.dna.affrc.go.jp/pubdata/genomicsequences.html). Furthermore, enrichment analysis of the human homologs (Table S1) showed that the conserved human homologs in B. mori that showed the most statistically significant metabolic pathways were those for spliceosome (p = 1.05E-13), pyrimidine metabolism (p = 2.16E-08) and purine metabolism (p = 3.01E-08).
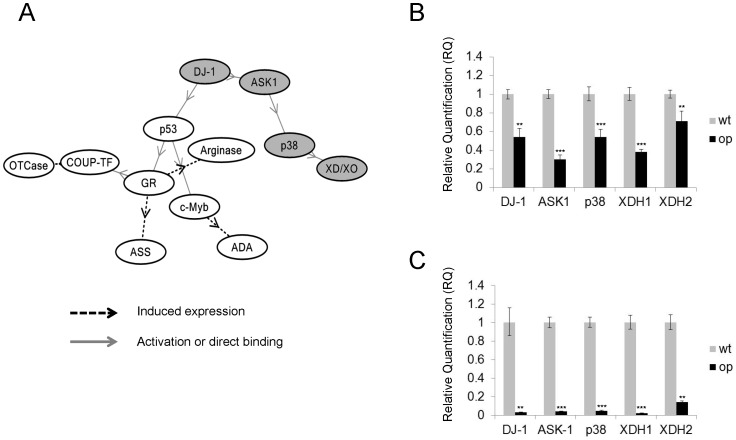
Microarray analysis was performed to investigate the causality of the phenotype of op. We used TIBCO Spotfire software (TIBCO, Palo Alto, CA, USA) for the identification of differentially expressed probe sets between op and wild-type larvae, for the calculation of correlation coefficients, and for bi-directional hierarchical clustering using Ward’s method (Figure S1). Next, we imported the list of Kaiko array IDs for these genes and their expression levels into KeyMolnet. KeyMolnet generated a molecular network, presenting a significant relationship for the pathway of purine metabolism (score = 177.109 with P-value = 4.841E-054) and transcriptional regulation by p53 (score = 78.113 P-value = 3.059E-024). In further exploration of associations between the genes involved in purine metabolism and transcriptional regulation by p53 using KeyMolnet, we detected the unique pathway (score = 14.888 with the P-value = 3.299E-005) from DJ-1 to xanthine dehydrogenase/xanthine oxidase (XD/XO) (Fig. 2A; highlighted in gray).
Figure 2. Microarray screening and quantitative RT-PCR confirmation of genes that are down-regulated in op.
(A) Molecular network generated by KeyMolnet for genes showing variation in op. Molecular relations are indicated by solid lines with arrows (direct binding or activation) or broken lines with arrows (induced expression). The pathway from DJ-1 to XD/XO is highlighted in gray. Relative mRNA expression in (B) fat body and (C) testis in wild-type and op mutant as Relative Quantification (RQ) values. RQ represents the relative expression level compared to the reference sample. Error bars represent the relative minimum/maximum expression levels about the mean RQ expression level. (B) DJ-1(p = 0.0038), ASK1(p = 0.0005), p38(p = 0.0004), XDH1(p = 0.0009), XDH2(p = 0.0011), (C) DJ-1(p = 0.0008), ASK1(p<0.001), p38(p<0.001), XDH1(p = 0.0009), XDH2(p = 0.0075).
Several target molecules related to this pathway (Fig. 2A; highlighted in gray) were validated by qRT-PCR. B. mori has two genes encoding xanthine dehydrogenase, XDH1 (Gene ID: 692751) and XDH2 (Gene ID: 692757), whichare orthologous to human XD/XO [15]. In this pathway, DJ-1, apoptosis signal-regulating kinase 1 (ASK-1), p38 mitogen-activated protein kinase (MAPK) and XDH1 and XDH2 mRNA were significantly decreased in the fat body (Fig. 2B) and testis (Fig. 2C) of the op larval mutant.
Levels of TH and DJ-1 mRNA and TH Immunohistochemistry in op Larval Brain
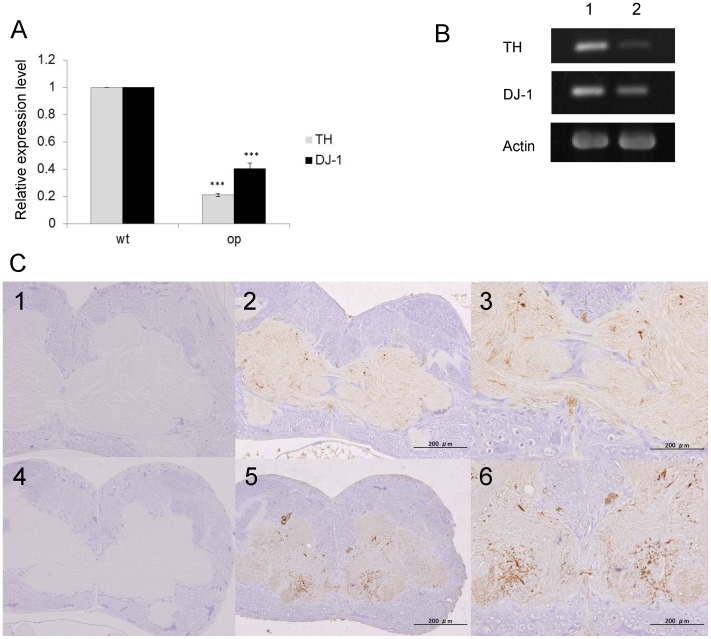
Tyrosine hydroxylase plays (TH) an important role in the biosynthesis of dopamine andproduction is reduced by a decrease in TH expression, leading to the onset of PD [18]. Thus, TH expression levels are used for diagnosis of PD. To investigate the expression levels of B. mori TH and DJ-1 mRNA in fifth instar op mutant and wild-type larval brain, we performed reverse transcription polymerase chain reaction (RT-PCR) and immunohistochemistry. B. mori TH and DJ-1 mRNA levels were found to be significantly lower in the brains of op larval mutants (Fig. 3A and B, Figure S2).
Figure 3. B. mori TH and DJ-1 mRNA expression in brain of wild-type and op mutant larvae by RT-PCR.
(A) mRNA expression levels were measured by Image J ver. 1.37 c, and plotted to compare op and wild-type mRNA levels (TH; P<0.0001, DJ-1; P = 0.0017). (B) Lane 1, wild-type; lane 2, op mutant. (C) Immunohistochemical localization of tyrosine hydroxylase (TH) in op and wild-type brain. Tissue sections were prepared from day 5 to 7 fifth instar larvae. 1 (op) and 4 (wild-type) are negative controls. TH positive regions (2 and 3:op, 5 and 6:wild-type) are stained brown. Bar: 200 µm.
Also, the TH-positive region was decreased in the brain of B. mori op larvae (Fig. 3C, 1–3) compared to wild-type larvae (Fig.3C, 4–6) by immunohistochemistry.
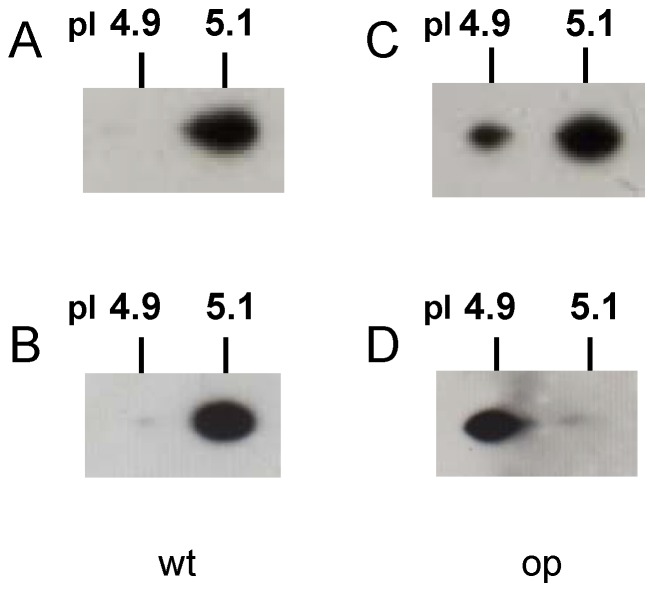
Figure 4. Analysis of oxidized DJ-1 protein in op and wild-type larvae.
(A) and (B) show the wild-type, (C) and (D) show the op mutant. Oxidized DJ-1 was determined by 2D-PAGE and immunoblotting. Upper panel, fat body; lower panel, testis.
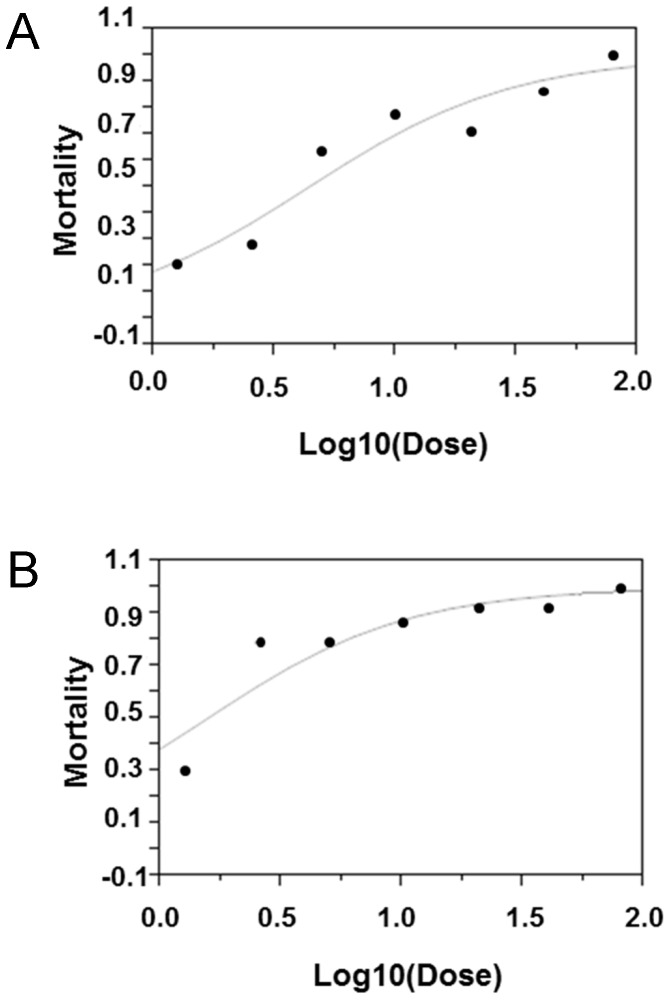
Figure 6. Dose mortality curve of op and wild-type larvae exposed to rotenone.
(A) wild-type, (B) op mutant. The vertical axis shows the mortality and the horizontal axis shows the rotenone Log10 dose. Solid circles represent observational data.
Detection of the Oxidized form of DJ-1
To determine whether the oxidized DJ-1 is present in B. mori, testis and fat body dissected from op or wild-type silkworms were examined. The fatbody (Fig. 4A) and testis (Fig. 4B) DJ-1 from wild-type larvae had a pI in the non-acidic range (pI, 5.1; non-oxidized form) by 2D-PAGE and immunoblotting. In contrast, the fat body (Fig. 4C) and testis (Fig. 4D) from op mutants produced a shift in pI to the acidic range (pI, 4.9; oxidized form) by 2D-PAGE and immunoblotting. Therefore, the oxidization of B. mori DJ-1 occurs only in the op mutant.
UA Content was Decreased in op Mutants
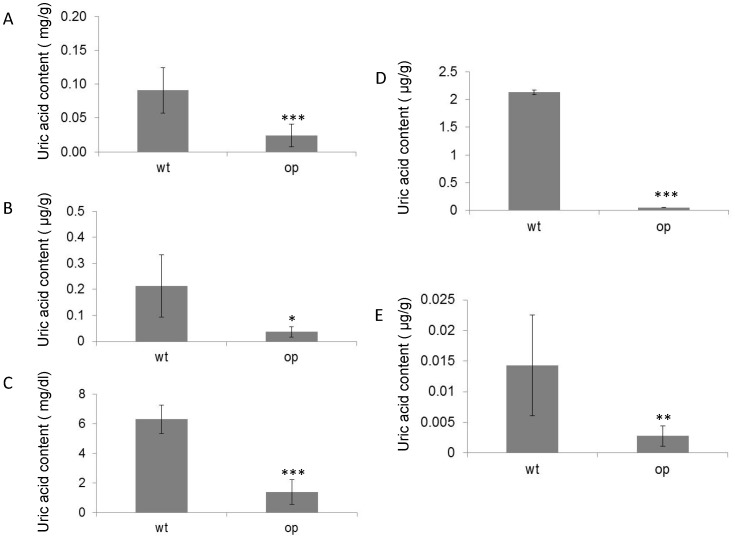
UA content was measured in the integument, hemolymph, fat body and Malpighian tubule of wild-type and op larvae. UA content was significantly decreased in all samples of op compared to wild-type larvae (Fig. 5A–E).
Figure 5. Uric acid content in wild-type and op larve.
Samples were collected from wild-type and op larvae and Uric acid was extracted and measured in (A) integument (P<0.001), (B) fat body (P = 0.037), (C) hemolymph (P = 0.0002), (D) Malpighian tubule (P<0.0001) and (E) testis (P = 0.004).
Op Mutant has Decreased Resistance to Rotenone-induced Oxidative Stress
ROT was used to examine the resistance to oxidizing stress in op larvae. The 50% lethal dose (LD50) of ROT for day 3 fifth instar larvae was determined to be 4.3 µg/g (95% CI, 1.5–52.5; Fig. 6A) for wild-type and 1.6 µg/g (95% CI, 0.8–13.0; Fig. 6B) for op larvae. The LD50 was significantly lower for op larvae than for wild-type larvae.
Discussion
In this study, we characterized the B. mori op mutant, one of the B. mori translucent larval skin mutants.
To investigate the causative factorsof phenotype op mutant, we employed microarray analysis using our constructed KAIKO functional annotation pipeline. Microarray analysis has shown efficacy for detecting many causative factors related to phenotypic op despite the fact that the op causative gene has yet to be clarified.
Here, we found a non-canonical pathway that is involved in the UA synthesis pathway from DJ-1 to XD/XO (Fig. 2A; highlighted in gray).
ASK-1 is a member of the mitogen-activated protein kinase family that activates the MKK3/MKK6-p38 signaling pathway [19]. P38 kinase belongs to the MAP kinase family and can be activated by a wide variety of stressors, while XD/XO is phosphorylated through a signaling pathway that involves p38 kinase [20]. XD/XO oxidizes hypoxanthine to xanthine, and then xanthine to UA. Most of the genes identified by KeyMolnet analysis were oxidative stress-responsive genes. In addition, DJ-1, ASK-1, p38 and two XD/XOs mRNAs were significantly down-regulated in op mutants.
We focused on DJ-1 for two reasons: DJ-1 is the initiator of this pathway, and the function of DJ-1 varies with the degree of oxidative stress [21].
Next, we examined TH and DJ-1 mRNA levels in the brain of B. mori op larvae. Interestingly, B. mori op larvae showed significantly lower mRNA levels of TH and DJ-1. While, the number of TH-positive neurons did not change in the DJ-1 deficient mouse model [22], [23], DJ-1 silencing by small interference RNA (siRNA) results in decreased TH expression in the human dopaminergic CHP-212 cell line [24].
In the case of expression level of mRNA between B. mori, DJ-1 and TH might share certain traits with human.
Moreover, oxidized B. mori DJ-1 was only present in the fat body and testis of the op larvae, while almost all of the B. mori DJ-1 in the testis was present in the oxidized form.
It is known that the isoelectric point (pI) of DJ-1 shifts to acidic (the oxidized form) during exposure to oxidative stress. Findings from our previous study also showed that B. mori DJ-1 in BmN4 cells changes to an oxidized form that is affected by ROT treatment, indicating its susceptibility to oxidative stress [25].
Human DJ-1 is induced by oxidative modification and is rapidly oxidized at position Cys 106 [26]; DJ-1 appears to directly scavenge free radicals from mitochondria in response to many different types of oxidative stress. DJ-1 produced by the oxidation of Cys 106 plays an important role in resistance to oxidative stress. However, under high levels of oxidative stress, DJ-1 is transformed to a more acidic form and becomes less resistant to oxidation; its subsequent degradation promotes the development of PD [21].
B.mori DJ-1 has two cysteines at residues 46 and 106 from the N-terminal methionine [25]. However, we could not verify the oxidative modification of Cys 106 in the acidic form of B.mori DJ-1. Our current study might suggest that DJ-1 in the B. mori op larvae had not only lost its function but also depressed mRNA expression due to prolonged exposure to environmental stress. Furthermore, expression of ASK1 in B. mori is decreased by exposure to strong UV irradiation [27]. We also confirmed that mRNA levels of DJ-1, ASK1, p38 and two XD/XOs were decreased by exposure to strong UV irradiation in day 3 of fifth instar larvae of wild-type B. mori (data not shown). Hence, these environmental stressors may disrupt the signal transduction cascade.
UA content was significantly decreased in the integument, fat body, hemolymph, Malpighian tubule and testis in B. mori op mutants, suggesting that UA synthesis, transport, elimination and accumulation are all decreased by the loss of BmDJ-1 function and environmental oxidative stress in B. mori op mutants.
UA directly scavenges oxygen radicals and produces allantonin and allantonic acid, which confer resistance to oxidative damage in humans [28]. Furthermore, UA plays a protective role against photooxidative stress in B. mori, as evidenced by the significantly reduced survival rate in larvae under UV irradiation with exposure to allopurinol, an inhibitor of UA synthesis [29]. It has been reported that a 6-OHDA-induced decrease of GSH (Glutathione-SH) and SOD (Superoxide dismutase) was partially reversed by addition of urate [30]. This result may suggest that urate, GSH and SOD were cooperated in protection against 6-OHDA-induced cell injury in PC12 cells.
Our results show that the op larvae are 63% less resistant to ROT-induced oxidative stress than the wild-type larvae.
Thus, in the case of the B. mori op mutants, the relatively low availability of UA is due to the oxidation of B. mori DJ-1 with this small amount being expended to mitigate the effects of environmental oxidative stress. Consequently, the phenotype of the B. mori mutant op larvae is characterized by translucent skin due to the oxidation of B. mori DJ-1 caused by a vicious cycle that has the potential to exacerbate the damage resulting from oxidative stress.
Low DJ-1 levels and the oxidized form of DJ-1 in spermatozoa are known to cause male infertility in humans [31]. In our data, the expression levels of B. mori DJ-1 protein were decreased (data not shown) and DJ-1 changed to the oxidized form in the testis of fifth instar op mutant larvae. Taken together, these observations are consistent with the phenotypic characteristics of B. mori op mutants observed in the present study.
Due to the lack of adequate animal models, the function of these molecules in PD pathogenesis is poorly understood. In addition, lower plasma UA levels in DJ-1 mutant PD patients or in other animal models have not been reported to date. We speculate that the B. mori DJ-1 gene is located in scaffold 2995719-2998746 of chromosome 23, suggesting that the B. mori DJ-1 protein might control oxidative stress in the cell due to nitric oxide (NO) and serve as a development modulation factor in metamorphosis [25]. Human DJ-1 is a causative gene of PARK7-linked familial PD [32] and plays a role in resisting oxidative stress as well as being related to male infertility in human studies [33], [34], [35]. Hence, the gene responsible for op might be B. mori DJ-1, which would be consistent with several phenotypic characteristics of B. mori op mutants.
In future studies, we aim to investigate whether BmDJ-1 alone regulates the phenotype of the B. mori op mutant and also to further elucidate the advanced molecular mechanisms in our identified pathway using an RNAi approach in vitro and in vivo and investigate relationships with other anti-oxidant enzymes such as GSH and SOD.
In summary, characterization of the B. mori op mutant with its unique phenotype shows that it has reduced levels of UA. Using microarray analysis of the B. mori op mutant, we identified a key pathway in UA synthesis. This is the first report indicating the possible involvement of DJ-1 in the initiation of UA synthesis at the molecular level. UA appears to be present in lower quantities due to the presence of dysfunctional DJ-1, in addition to being expended to limit oxidative stress. Our findings may facilitate a better understanding of the molecular mechanisms behind decreased plasma UA levels and clinical stage progression of PD.
Methods
Ethics Statement
The study protocol for the experimental use of animals to minimize animal suffering was approved by the Ethics Committee of Meiji Pharmaceutical University, Japan (Approval ID 2302).
Insects
B. mori strain o751 (op and wild-type), maintained at the Institute of Genetic Resources, Faculty of Agriculture, Kyushu University (NBRP silkworm database: http://www.shigen.nig.ac.jp/silkwormbase/) was used in all experiments. Silkworms were reared on fresh mulberry leaves and kept at 25°C on a 12-hour light/12-hour dark cycle.
Microarray Analysis
Total RNA was isolated from testis of day 3 fifth instar wild-type and op larvae using RNeasy Mini Kits (Qiagen, Valencia, CA, USA) and quantified on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Microarrays of Cy3-labeled cRNA were prepared for op and wild type (n = 5 each). Briefly, 300 ng total RNA was processed using an Agilent Quick Amp Labeling Kit (Agilent Technologies) according to the manufacturer’s instructions followed by purification by an RNeasy Mini Kit (Qiagen) with quantification on an Agilent 2100 Bioanalyzer (Agilent Technologies) and a NanoDrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Hybridization was performed using a Gene Expression Hybridization Kit (Agilent Technologies) for which 1.65 µg of Cy3-labelled cRNA was mixed, fragmented, and hybridized to a silkworm 4×44 K custom oligo-microarray slide containing 43,864 spots of 60-mer oligonucleotides constructed from 17,615 EST silkworm sequences (Agilent Technologies) and incubated at 65°C for 17 h with shaking at 10 rpm. For screening, two wild-type and two op samples were applied to a single array slide. The array was washed in Agilent Gene Expression Wash Buffer 1 (Agilent Technologies) for 1 min at room temperature and Agilent Gene Expression Wash Buffer 2 (Agilent Technologies) for 1 min at 37°C. Intensities of the hybridized probes were detected with an Agilent G2565BA Microarray Scanner (Agilent Technologies) with 5-µm scan resolution, and the signals were extracted with G2565AA Feature Extraction Software v. 9.5 (Agilent Technologies). The raw data collected in text files were normalized ‘per spot’ and ‘per chip’ using the GENESPRING GX v. 10.3 program (Agilent Technologies). The microarray data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) database and are accessible through GEO accession numbers GSM926580, GSM926581, GSM926582 and GSM926583 (series GSE37735).
KAIKO Functional Annotation Pipeline for Molecular Network Analysis
In order to functionally annotate silkworm genes, silkworm genes homologous to human genes were identified by conducting a systematic BLAST search (tblastx) with a cut-off E-value of significant homology at 1e-10 (query: silkworm cDNA sequence; database: whole human cDNA sequence set from Ensembl database). However, because B. mori does not have a formal gene ID due to the lack of functional annotation for B. mori genes, we used the Kaiko array ID number for the assignment table. KeyMolnet could not recognize the Ensembl transcription ID, so we converted the human Ensembl transcription ID to Uniprot ID. The Kaiko array ID both with Ensemble transcription ID and Uniprot ID annotations (12,981 in Kaiko array ID, 28.7% of total) were used for further analyses (Table S3). Using the generated assignment table, conserved pathways common to silkworms and humans were reconstructed by projecting silkworm genes onto the human pathway.
Molecular Network Analysis
Molecular network analysis was performed using KeyMolnet software (version 5.6 I, IMMD Inc., Tokyo, Japan) in which known molecular data have been curated by expert biologists [36]. By importing the list of molecule IDs and gene expression values from microarray data, KeyMolnet automatically generates the corresponding molecules as nodes on a network. We converted the molecular IDs from the silkworm array to human UniProtKB IDs, utilizing the assignment table described above and then input the list of genes obtained from the microarray data into this software to yield the significant molecular networks with corresponding E-values.
Quantitative RT-PCR
To quantify RNA expression levels, total RNA was extracted from pooled testis and fat body tissue dissected from day 3 fifth instar larvae (n = 5 each) using an RNeasy Mini Kit (Qiagen) separately from the extractions for the array experiments. One-step RT-PCR was performed in 20-µl reaction volumes using 1 µg of RNA template and custom-made primers and probes (Table 1) with a TaqMan RNA-to-CT 1-Step Kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). Quantitative RT-PCR (qRT-PCR) was performed on a 7500 Fast Real-Time PCR system (Applied Biosystems) following the Delta-Delta Ct method. Actin was utilized as an endogenous reference against which RNA expression levels were standardized, and all data were calibrated against universal reference data. All assays were performed in triplicate.
Table 1. Primers and probes used for quantitative RT-PCR.
| Gene | Probe | Forward primer | Reverse primer |
| ASK1 | ACGACAAATCCGGCGGCCAC | 5′-TTGACGGACGTCGGAACA-3′ | 5′-GCAGAGTCTTCCCCTTTGAATTT-3′ |
| p38 | CACCTGTAGGTTCCGGCGCTTACG | 5′-AGTCCCGGAGCGATATCAGA-3′ | 5′-TGGGCATCTATTGCAGAACAAA-3′ |
| XDH1 | AAGAATCCGGACGGGAA | 5′-AATGAAGCGCCTAAAACCGTATA-3′ | 5′-TAGGCAGCTGATACCCAATTTTC-3′ |
| XDH2 | AAGCAATAATGGCCGCTCGCTCG | 5′-GCTTCAGTATTCTTCGCCATCA-3′ | 5′-CACGGGCACCCCACTGT-3′ |
| Actin | AAGGTTACGCTCTGCCCCACGCC | 5′-CTCCCACACCGTACCCATCT-3′ | 5′-AAGTCGCGACCAGCCAAGT-3′ |
| DJ-1 | TTGCTGCCATTTGTGCTGCTCCC | 5′-CCACGAGGATAATGGGAAAATC-3′ | 5′-CCGTGGGCTGCAAACG-3′ |
Probes and primer sets were custom designed with 5′ labeled 6 FAM™ and 3′ labeled TAMRA.
Determination of UA Content
Methods for determining UA content were performed using a modification of the method of Tamura et al. [14] The integument (n = 25 each), Malpighian tubules (n = 25 each), testis (wild-type, n = 90; op, n = 107) and fat body (n = 25 each) dissected from day 3 fifth instar larvae were pooled to extract UA, and intact hemolymph (n = 12 each) was collected from day 3 fifth instar larvae and stored at -80°C until use. Individual integument (2 mg) was homogenized in 1 ml distilled water (1∶500 volume of water). Pooled tissues were divided into four samples (70 mg each) for fat body, four samples (80 mg each) for Malpighian tubules, and seven samples (70–90 mg each) for testis and samples were homogenized in 0.21 to 0.27 ml distilled water (1∶3 v:v of water). Samples were individually boiled for 10 min and allowed to cool to room temperature. The homogenate was then centrifuged at 10,000×g for 15 min and the collected supernatants were used to measure UA content with a UA Test Kit (Wako, Tokyo, Japan) according to the manufacturer’s instructions.
Determination of Rotenone LD50 in Op Larvae
To determine the LD 50 of day 3 fifth instar larvae (n = 10 each group) by rotenone (ROT; Sigma) stimulation in wild-type and op mutant larvae, we injected 20 µl prepared ROT/g body weight intrahemocoelically to larvae weighing 2.5 to 3.0 g using a disposable syringe (Terumo, Tokyo, Japan) with a 30 G needle. ROT was dissolved in DMSO (prepared immediately before use and stored in the dark) at 0, 1.25, 2.5, 5.0, 10, 20, 40, and 80 µg/g. The number of dead silkworms after 24 h was counted and the mortality rate (%) = (X/Y) × 100 was calculated, where X = dead larvae in the group and Y = total larvae in the group. The mortality rates were analyzed using the JMP 9.0 software (SAS Institute Japan Ltd., Tokyo, Japan) to calculate the LD50.
Two-dimensional (2D) Gel Electrophoresis, and Detection of Oxidized DJ-1
Fat body and testis were dissected from day 3 fifth instar wild-type and op larvae for detection of oxidized DJ-1. To prepare total protein extracts for two-dimensional (2D) gel electrophoretic analysis, the tissues were sonicated in rehydration buffer comprising 8 M urea, 2% CHAPS, 0.5% carrier ampholytes at pH 4 to 7, 20 mM dithiothreitol, 0.002% bromophenol blue, and a cocktail of protease inhibitors. Urea-soluble 400 µg proteins were separated by isoelectric focusing (IEF) using the ZOOM IPGRunner system loaded with an immobilized pH 4 to 7 gradient strip (Invitrogen). After the first dimension of IEF, the protein was separated in the second dimension on a 4 to 12% NuPAGE polyacrylamide gel (Invitrogen). For detection of BmDJ-1, the gel was transferred to a polyvinylidene difluoride (PVDF) membrane for immunoblotting using rabbit anti-BmDJ-1 antibody and goat anti-rabbit IgG-conjugated horseradish peroxidase (HRP). The membranes were developed using a chemiluminescent substrate (Pierce, Rockford, IL, USA). Developing time was different between wt and op mutant.
Protein Assay
Protein concentrations were determined using a Bradford assay kit (Pierce, Rockford, IL, USA), according to the manufacturer’s instructions.
RT-PCR
Total RNA from 500 brains of day 3–5 fifth instar larvae were treated with DNase and processed for cDNA synthesis using primers of 12 to 18 oligo(dT) and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). cDNA was amplified by PCR using Taq DNA polymerase (Qiagen) with the primers for tyrosine hydroxylase (TH; 5′-ACACAACAGTGGTTCAGTCG-3′ and 5′-AGGAGTCTCGCAATGTACAC-3′), actin (5′-TATCGCCGACAGGATGCAGAAGGA-3′ and 5′-TAGAAGCACTTGCGGTGAACGATG-3′) and DJ-1 (5′-CATTTGTGCTGCTTCCATAGCGTT-3′ and 5′-CATTCCCTTTTCGACTTGATCGGC-3′), as described previously [25]. RT-PCR for B. mori actin was used as a positive loading control. Amplification was carried out with 28 cycles of denaturation for 40 s at 94°C, annealing for 40 s at 55°C, and extension for 90 s at 72°C. Amplified PCR products were separated by agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. The oligonucleotides employed in this study were designed based on the B. mori TH nucleotide sequence (Gene ID:100270767), B.mori DJ-1 sequence (Gene ID: 100422789)and actin nucleotide sequence (Gene ID: 100145913). Each experiment was performed in triplicate.
Immunohistochemistry
Serial sections (6-mm thick) were prepared from tissues fixed with 4% paraformaldehyde and embedded in paraffin. After deparaffination, tissue sections were incubated with 3% hydrogen peroxide-methanol for 40 min to block endogenous peroxidase activity. The tissue sections were incubated in a moist chamber at 4°C for 24 hours with anti-tyrosine hydroxylase polyclonal antibody (AB152, Millipore, Billerica, MA, USA) at 1∶1000, followed by incubation with Histofine simple stain MAX-PO (R) for human tissue (Nichirei Biosciences Inc, Tokyo, Japan). The stained slides were developed with ImmPACTTMDAB reagent (Vector Laboratories, Inc, Burlingame, CA USA), Mayer’s Hematoxylin solution for nuclear staining (Wako Tokyo, Japan), and examined under the Olympus DP71 universal microscope (Olympus Inc, Tokyo, Japan). Normal rabbit IgG was applied for the negative control.
Statistics
Mortality was analyzed using probit analysis to calculate LD50 levels. Two-tailed Student’s t-test was performed in other experiments. All statistical analyses were carried out using the JMP 9.0 software package (SAS Institute Japan Ltd., Tokyo, Japan). For all tests, P<0.05 was considered statistically significant. Data are presented as mean ± SD. Statistical significance was set at *P<0.05, **P<0.01, and ***P<0.001 for comparisons between wild-type and op mutants.
Supporting Information
Expression profile of each sample of wild-type and op was compared using hierarchical clustering by Ward’s method. 12,981 probe sets were re-annotated via our annotation pipeline and were used in the analyses.
(TIFF)
B. mori TH and DJ-1 mRNA expression in the brain of wild-type and op mutant larvae by qRT-PCR. Relative mRNA expression in the brain of wild-type and op mutants are given as Relative Quantification (RQ) values. RQ represents the relative expression level compared to the reference sample. Error bars represent the relative minimum/maximum expression levels about the mean RQ expression level.
(TIFF)
Gene Set Enrichment Analysis of human homologs in B. mori . Count is the number of genes associated with the term. The p-values associated for each annotated term inside each cluster have exactly the same meaning/value as the p-values (Fisher Exact/EASE Score) shown in the regular chart report for the same terms [37].
(DOC)
Primers and probes used for quantitative RT-PCR. Probes and primer sets were custom designed with 5′ labeled 6 FAM™ and 3′ labeled TAMRA.
(DOC)
Complete lists of the re-annotation of Kaiko array probe sets. Expression values were shown as the log2 ratio between wild type and op samples (average). Gene descriptions are also shown. The annotated Ensembl transcript ID, the UniProt/SwissProt accession number and gene description for each gene was extracted from Ensembl (release 69) annotations.
(XLS)
Supplementary methods.
(DOC)
Movie for occasional unique actions such as vibration of B.mori op mutant.
(MOV)
Acknowledgments
We thank Dr. Natsuo Kômoto, Dr. Kazuei Mita and Dr. Hiroaki Noda at the National Institute of Agrobiological Sciences for their advice on conducting the experiments, Dr. Rie Taniguchi at the Institute of Medical Molecular Design Inc. for her advice on pathway analysis using KeyMolnet, and Ms. Yukiko Senoo, Ms. Hiroko Nakano, Ms. Ohta Midori and Ms. Mai Ikeda for technical assistance.
Additional information
Accession number. Microarray data were deposited in the NCBI Gene Expression Omnibus Database under accession number GSE37735.
Funding Statement
Silkworms (o751) were provided by the National Bio-Resource Project (NBRP) of the Japanese Ministry of Education, Science, Sports and Culture. This work was supported by a Grant-in-Aid for Young Scientists B (18890212) to HT. This work was partly supported by grants from the High-Tech Research Center Project to JS. Support for this work was provided by the National Bioscience Database Center (NBDC) of the Japan Science and the Technology Agency (JST) to HB. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Devine MJ, Plun-Favreau H, Wood NW (2011) Parkinson’s disease and cancer: two wars, one front. Nat Rev Cancer 11: 812–823. [DOI] [PubMed] [Google Scholar]
- 2. Hatano T, Kubo S, Sato S, Hattori N (2009) Pathogenesis of familial Parkinson’s disease: new insights based on monogenic forms of Parkinson’s disease. J. Neurochem. 111: 1075–1093. [DOI] [PubMed] [Google Scholar]
- 3. Montgomery EB Jr, Baker KB, Lyons K, Koller WC (1999) Abnormal performance on the PD test battery by asymptomatic first-degree relatives. Neurology 52: 757–762. [DOI] [PubMed] [Google Scholar]
- 4. Bové J, Prou D, Perier C, Przedborski S (2005) Toxin-induced models of Parkinson’s disease. NeuroRx 2: 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical- caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 78: 6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Church WH, Ward VL (1994) Uric acid is reduced in the substantia nigra in Parkinson’s Diseases: effect on dopamine oxidation. Brain Research Bulletin 33: 419–425. [DOI] [PubMed] [Google Scholar]
- 7. Bogdanov M, Matson WR, Wang L, Matson T, Saunders-Pullman R, et al. (2008) Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain 131: 389–396. [DOI] [PubMed] [Google Scholar]
- 8. Andreadou E, Nikolaou C, Gournaras F, Rentzos M, Boufidou F, et al. (2009) Serum uric acid levels in patients with Parkinson’s disease: Their relationship to treatment and disease duration. Clin. Neurol. Neurosurg. 111: 724–728. [DOI] [PubMed] [Google Scholar]
- 9. Johansen KK, Wang L, Aasly JO, White LR, Matson WR, et al. (2009) Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS One. 4: e7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cipriani S, Chen X, Schwarzschild MA (2010) Urate: a novel biomarker of Parkinson’s disease risk, diagnosis and prognosis. Biomark Med. 4: 701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisskopf MG, Reilly EO, Chen H, Schwarzschild MA, Ascherio A (2007) Plasma urate and risk of Parkinson’s Disease. Am. J. Epidemiol. 166: 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi Y (1960) Xanthine dehydrogenase in the silkworm Bombyx mori L. Nature. 186: 1053–1054. [DOI] [PubMed] [Google Scholar]
- 13. Yamada M, Inokuchi T (1986) Conversion of uric acid to urea in the silkworm, Bombyx mori. J. Seric. Sci. Jpn. 55: 428–433. [Google Scholar]
- 14. Tamura T, Kanada T, Kômoto N, Yukuhiro K, Hasegawa T, et al. (1999) Rescue of oq, og and ogt mutants by injection of xanthine oxidase. J. Seric. Sci. Jpn. 68: 111–117. [Google Scholar]
- 15. Kômoto N (2002) A deleted portion of one of the two xanthine dehydrogenase genes causes translucent larval skin in the oq mutant of the silkworm (Bombyx mori). Insect Biochem. Mol. Biol. 32: 591–597. [DOI] [PubMed] [Google Scholar]
- 16. Kômoto N, Sezutsu H, Yukuhiro K, Banno Y, Fujii H (2003) Mutations of the silkworm molybdenum cofactor sulfurase gene, og, cause translucent larval skin. Insect Biochem. Mol. Biol. 33: 417–427. [DOI] [PubMed] [Google Scholar]
- 17. Kiuchi T, Banno Y, Katsuma S, Shimada T (2011) Mutations in an amino acid transporter gene are responsible for sex-linked translucent larval skin of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 41: 680–687. [DOI] [PubMed] [Google Scholar]
- 18. Lüdecke B, Knappskog PM, Clayton PT, Surtees RA, Clelland JD, et al. (1996) Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation (L205P) in the tyrosine hydroxylase gene. Hum Mol Genet. 7: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 19. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, et al. (1997) Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275: 90–94. [DOI] [PubMed] [Google Scholar]
- 20. Kayyali US, Donaldson C, Huang H, Abdelnour R, Hassoun PM (2001) Phosphorylation of xanthine dehydrogenase/oxidase in hypoxia. J. Biol. Chem. 276: 14359–14365. [DOI] [PubMed] [Google Scholar]
- 21. Wilson MA (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 15: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, et al. (2005) Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 45: 489–496. [DOI] [PubMed] [Google Scholar]
- 23. Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, et al. (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 102: 5215–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhong N, Kim CY, Rizzu P, Geula C, Porter DR, et al. (2006) DJ-1 transcriptionally up-regulates the human tyrosine hydroxylase by inhibiting the sumoylation of pyrimidine tract-binding protein-associated splicing factor. J. Biol. Chem. 281: 20940–20948. [DOI] [PubMed] [Google Scholar]
- 25. Tabunoki H, Ode H, Banno Y, Katsuma S, Shimada T, et al. (2011) Bm DJ-1 is a key regulator of oxidative modification in the development of the silkworm, Bombyx mori. PLoS One. 6: e17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kinumi T, Kimata J, Taira T, Ariga H, Niki E (2004) Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 317: 722–728. [DOI] [PubMed] [Google Scholar]
- 27. Zhang JY, Pan MH, Sun ZY, Huang SJ, Yu ZS, et al. (2010) The genomic underpinnings of apoptosis in the silkworm, Bombyx mori. BMC Genomics. 11: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tolun AA, Zhang H, Il’yasova D, Sztáray J, Young SP, et al. (2010) Allantoin in human urine quantified by ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Biochem. 402: 191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuo T, Ishikawa Y (1999) Protective role of uric acid against photooxidative stress in the silkworm, Bombyx mori (Lepidoptera: Bombycidae). Appl. Entomol. Zool. 34: 481–484. [Google Scholar]
- 30. Zhu TG, Wang XX, Luo WF, Zhang QL, Huang TT, et al. (2012) Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett. 506: 175–179. [DOI] [PubMed] [Google Scholar]
- 31. An CN, Jiang H, Wang Q, Yuan RP, Liu JM, et al. (2011) Down-regulation of DJ-1 protein in the ejaculated spermatozoa from Chinese asthenozoospermia patients. Fertil. Steril. 96: 19–23.e2. [DOI] [PubMed] [Google Scholar]
- 32. Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256–259. [DOI] [PubMed] [Google Scholar]
- 33. Lev N, Roncevich D, Icowicz D, Melamed E, Offen D (2006) Role of DJ-1 in Parkinson’s disease. J. Mol. Neurosci. 29: 215–25. [DOI] [PubMed] [Google Scholar]
- 34. Okada M, Matsumoto K, Niki T, Taira T, Iguchi-Ariga SMM, et al. (2002) DJ-1, a target protein for an endocrine disrupter, participates in the fertilization in mice. Biol. Pharm. Bull. 25: 853–856. [DOI] [PubMed] [Google Scholar]
- 35. Yoshida K, Sato Y, Yoshiike M, Nozawa S, Ariga H, et al. (2003) Immunocytochemical localization of DJ-1 in human male reproductive tissue. Mol Reprod Dev. 4: 391–397. [DOI] [PubMed] [Google Scholar]
- 36. Sato H, Ishida S, Toda K, Matsuda R, Hayashi Y, et al. (2005) New approaches to mechanism analysis for drug discovery using DNA microarray data combined with KeyMolnet. Curr. Drug. Discov. Technol. 2: 89–98. [DOI] [PubMed] [Google Scholar]
- 37. Huang DW, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 4: 44–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression profile of each sample of wild-type and op was compared using hierarchical clustering by Ward’s method. 12,981 probe sets were re-annotated via our annotation pipeline and were used in the analyses.
(TIFF)
B. mori TH and DJ-1 mRNA expression in the brain of wild-type and op mutant larvae by qRT-PCR. Relative mRNA expression in the brain of wild-type and op mutants are given as Relative Quantification (RQ) values. RQ represents the relative expression level compared to the reference sample. Error bars represent the relative minimum/maximum expression levels about the mean RQ expression level.
(TIFF)
Gene Set Enrichment Analysis of human homologs in B. mori . Count is the number of genes associated with the term. The p-values associated for each annotated term inside each cluster have exactly the same meaning/value as the p-values (Fisher Exact/EASE Score) shown in the regular chart report for the same terms [37].
(DOC)
Primers and probes used for quantitative RT-PCR. Probes and primer sets were custom designed with 5′ labeled 6 FAM™ and 3′ labeled TAMRA.
(DOC)
Complete lists of the re-annotation of Kaiko array probe sets. Expression values were shown as the log2 ratio between wild type and op samples (average). Gene descriptions are also shown. The annotated Ensembl transcript ID, the UniProt/SwissProt accession number and gene description for each gene was extracted from Ensembl (release 69) annotations.
(XLS)
Supplementary methods.
(DOC)
Movie for occasional unique actions such as vibration of B.mori op mutant.
(MOV)