Abstract
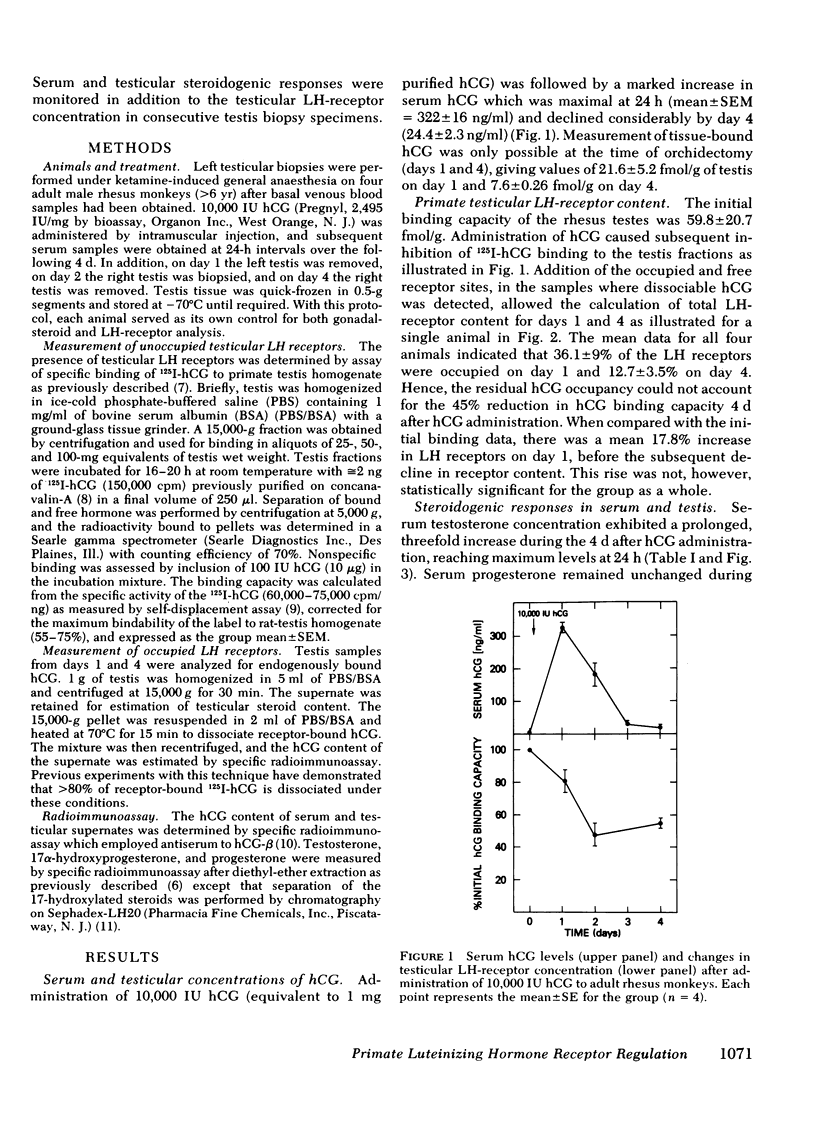
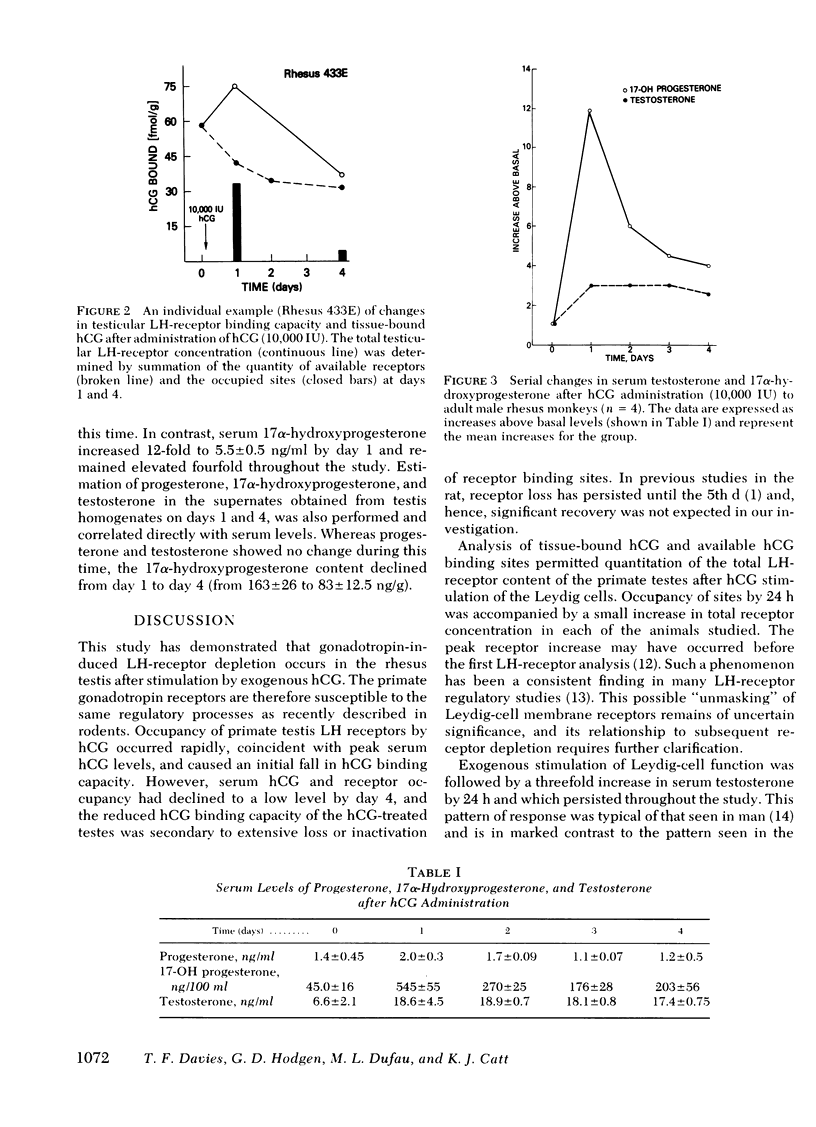
The testicular luteinizing hormone (LH) receptors of the rhesus monkey and human have many features in common, including high equilibrium association constant, marked species specificity, and relatively low binding capacity. We have, therefore, used rhesus monkeys as models for human LH-receptor regulation in vivo during gonadotropin treatment. In four adult male monkeys, treated with 10,000 IU human chorionic gonadotropin (hCG), serum and testicular steroidogenic responses were monitored at 24-h intervals during the following 4 d, and LH-receptor concentrations were measured by 125I-hCG binding to 15,000-g particles prepared from testis biopsy specimens. In treated animals, serum hCG was maximal on day 1 at 322±16 ng/ml and declined to 24.4±2.3 ng/ml by day 4. Serum testosterone was increased threefold during the subsequent 4 d (from 6.5±2.0 to 18.6±4.4 ng/ml) but serum progesterone remained unchanged. In contrast, serum 17α-hydroxyprogesterone increased 12-fold to 5.5±0.5 ng/ml at day 1 and was increased fourfold during the subsequent 3 d. The LH-receptor binding capacity of the primate testis was reduced by 18.3±6.0% on day 1, 51.7±7.4% on day 2, and 45.3±2.4% on day 4. Occupancy of the LH receptors by endogenously bound hCG was significant on day 1 but was negligible by day 4. These data demonstrate that gonadotropin-induced LH-receptor depletion occurs in the rhesus testis and indicate that primate gonadotropin receptors are susceptible to the regulatory processes recently described in the rat. In addition, the simultaneous and disproportionate accumulation of 17α-hydroxyprogesterone indicates that 17,20-desmolase was rate-limiting under these conditions in the primate testis Leydig cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auclair C., Kelly P. A., Coy D. H., Schally A. V., Labrie F. Potent inhibitory activity of [D-Leu6, Des-Gly-NH2(10)]LHRH ethylamide on LH/hCG and PRL testicular receptor levels in the rat. Endocrinology. 1977 Dec;101(6):1890–1893. doi: 10.1210/endo-101-6-1890. [DOI] [PubMed] [Google Scholar]
- Belanger A., Auclair C., Seguin C., Kelly P. A., Labrie F. Down-regulation of testicular androgen biosynthesis and LH receptor levels by an LHRH agonist: role of prolactin. Mol Cell Endocrinol. 1979 Jan;13(1):47–53. doi: 10.1016/0303-7207(79)90075-3. [DOI] [PubMed] [Google Scholar]
- Braunstein G. D., Vaitukaitis J. L., Ross G. T. The in vivo behavior of human chorionic gonadotropin after dissociation into subunits. Endocrinology. 1972 Oct;91(4):1030–1036. doi: 10.1210/endo-91-4-1030. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Mikhail G., Flickinger G. L. Column chromatography of steroids on Sephadex LH-20. J Clin Endocrinol Metab. 1971 Aug;33(2):358–360. doi: 10.1210/jcem-33-2-358. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Baukal A. J., Davies T. F., Dufau M. L. Luteinizing hormone-releasing hormone-induced regulation of gonadotropin and prolactin receptors in the rat testis. Endocrinology. 1979 Jan;104(1):17–25. doi: 10.1210/endo-104-1-17. [DOI] [PubMed] [Google Scholar]
- Cigorraga S. B., Dufau M. L., Catt K. J. Regulation of luteinizing hormone receptors and steroidogenesis in gonadotropin-desensitized leydig cells. J Biol Chem. 1978 Jun 25;253(12):4297–4304. [PubMed] [Google Scholar]
- Davies T. F., Walsh P. C., Hodgen G. D., Dufau M. L., Catt K. J. Characterization of the primate luteinizing hormone receptor in testis homogenates and Leydig cells. J Clin Endocrinol Metab. 1979 Apr;48(4):680–685. doi: 10.1210/jcem-48-4-680. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Tsuruhara T., Catt K. J. Interaction of glycoprotein hormones with agarose-concanavalin A. Biochim Biophys Acta. 1972 Sep 29;278(2):281–292. doi: 10.1016/0005-2795(72)90233-4. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Conti M., Conn P. M., Dufau M. L., Catt K. J. Receptor regulation and target cell responses: studies in the ovarian luteal cell. Mol Cell Endocrinol. 1978 Jul-Aug;11(2):121–135. doi: 10.1016/0303-7207(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Dufau M. L., Catt K. J. Gonadotropin-induced regulation of luteinizing hormone receptors and desensitization of testicular 3':5'-cyclic AMP and testosterone responses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):592–595. doi: 10.1073/pnas.74.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh A. J., Dufau M. L., Catt K. J. Regulation of luteinizing hormone receptors in testicular interstitial cells by gonadotropin. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1145–1152. doi: 10.1016/s0006-291x(76)80251-3. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I., Martikainen H., Tikkala L. HCG-induced changes in the number of rat testis LH/HCG receptors. Mol Cell Endocrinol. 1978 Jun;11(1):43–50. doi: 10.1016/0303-7207(78)90031-x. [DOI] [PubMed] [Google Scholar]
- Jones T. M., Fang V. S., Landau R. L., Rosenfield R. Direct inhibition of Leydig cell function by estradiol. J Clin Endocrinol Metab. 1978 Dec;47(6):1368–1373. doi: 10.1210/jcem-47-6-1368. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Ross G. T., Rayford P. L. Radioimmunoassay for luteinizing hormone in human plasma or serum: physiological studies. J Clin Invest. 1967 Feb;46(2):248–255. doi: 10.1172/JCI105527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizkallah T., Gurpide E., Vande Wiele R. L. Metabolism of HCG in man. J Clin Endocrinol Metab. 1969 Jan;29(1):92–100. doi: 10.1210/jcem-29-1-92. [DOI] [PubMed] [Google Scholar]
- Ruder H. J., Loriaux D. L., Sherins R. J., Lipsett M. B. Leydig cell function in men with disorders of spermatogenesis. J Clin Endocrinol Metab. 1974 Feb;38(2):244–247. doi: 10.1210/jcem-38-2-244. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M. hCG-induced decrease in availability of rat testis receptors. Nature. 1976 Dec 16;264(5587):644–646. doi: 10.1038/264644a0. [DOI] [PubMed] [Google Scholar]
- Strott C. A., Yoshimi T., Lipsett M. B. Plasma progesterone and 17-hydroxyprogesterone in normal men and children with congenital adrenal hyperplasia. J Clin Invest. 1969 May;48(5):930–939. doi: 10.1172/JCI106052. [DOI] [PMC free article] [PubMed] [Google Scholar]


