Abstract
By using cDNA microarray analysis, we identified cornulin (CRNN) gene was frequently downregulated in esophageal squamous cell carcinoma (ESCC). In the present study, we investigated the role of CRNN in ESCC development. The results showed that CRNN was frequently downregulated in primary ESCCs in both mRNA level (26/56, 46.4%) and protein level (137/249, 55%), which was significantly associated with lymph node metastases (P=0.027), advanced clinical stage (P=0.039), and overall survival rate (P<0.001). Multivariate analysis indicated that the CRNN downregulation was an independent prognostic factor for ESCC. Functional studies with both in vitro and in vivo assays demonstrated that CRNN had strong tumor suppressive ability in ESCC cells. The tumor-suppressive mechanism of CRNN was associated with its role in cell cycle arrest at G1/S checkpoint by upregulating expressions of P21WAF1/CIP1 and Rb. Silencing CRNN expression by RNA interference could effectively inhibit its tumor suppressive effect. In conclusion, our findings demonstrate that CRNN is a tumor suppressor gene that plays a critical tumor suppressive role in ESCC.
Introduction
Esophageal carcinoma (EC) is one of the most common malignancies and has been ranked as the sixth leading cause of cancer death over the world [1]. As the most common type of EC, esophageal squamous cell carcinoma (ESCC) shows high mortality and regional variation in incidence [2]. Despite advances in multimodality therapy, the prognosis of ESCC remains poor and the overall 5-year survival is less than 15% [3]. Like other types of cancers, the development of ESCC is also believed as a multiple-step process caused by the accumulation of activation of oncogenes and inactivation of tumor suppressor genes (TSG). To date, the exact cellular and molecular mechanisms leading to ESCC have not been systematically evaluated.
Systematic analysis of expression levels of thousands of genes by cDNA microarray is an effective approach to identify new genes and pathways related to the development and progression of the tested cancer. Recently, our group performed an Affymetrix cDNA microarray to compare differentially expressed genes between 10 pairs of ESCC tumors and their adjacent non-tumorous tissues (Data have been submitted to Gene Expression Omnibus under the accession number GSE33810). About 220 downregulated genes were detected including cornulin (CRNN). CRNN gene comprises three exons and encodes a protein of 495 amino acids, which contains a putative calcium-binding motif similar to S100 protein family at N-terminus [4], implying that CRNN may bind to calcium. Another study demonstrates that CRNN, which is a member of the fused gene family, might play an important role in epidermal differentiation [5].
Although CRNN has been reported to be downregulated in esophageal adenocarcinoma (EAC) or ESCC [6–9], and genetic variants of CRNN appeared to interacte with tobacco smoking that contributes to the risk for ESCC [10], the precise mechanism underlying the involvement of CRNN in ESCC remains to be elucidated.
In the present study, we studied the expression status of CRNN in clinical ESCC specimens and ESCC cell lines by quantitative and semiquantitative RT-PCR respectively. Both in vitro and in vivo functional assays were used to investigate the tumor suppressive effect of CRNN in ESCC cell lines. The results demonstrated that CRNN had strong tumor suppressive function. In addition, the tumor suppressive mechanism of CRNN and its clinical significance in ESCC were also addressed.
Materials and Methods
Cell lines and primary ESCC specimens
Chinese ESCC cell lines HKESC1, EC18 and EC109 were kindly provided by Professor Srivastava (Department of Pathology, The University of Hong Kong) [11]. Six Japanese ESCC cell lines (KYSE30, KYSE140, KYSE180, KYSE410, KYSE510, KYSE520) were obtained from DSMZ, the German Resource Center for Biological Material [12]. Primary ESCC tumors and their adjacent non-tumorous tissues were collected immediately after surgical resection at Linzhou Cancer Hospital (Henan, China). All patients did not receive preoperative treatment. Samples used in this study were approved by the Committees for Ethical Review of Research involving Human Subjects at Zhengzhou University (Henan, China). Written informed consents for the original human work that produced the tissue samples were obtained. The study was also approved by the Institutional Review Board at Cancer Center, Sun Yat-sen University.
Quantitative and semiquantitative RT-PCR
Total RNA was extracted by Trizol (Invitrogen, Carlsbad, CA) and 2µg of total RNA was used to synthesize cDNA with the Advantage RT-for-PCR Kit (Clontech, Mountain View, CA), following the standard protocols provided by the manufacturer. Semiquantitative RT-PCR was performed by using AmpliTaq (Applied Biosystems, Foster City, CA). The GAPDH or 18s were used as internal controls. For qRT-PCR, cDNA were amplified using a SYBR Green PCR Kit (Roche, Basel, Switzerland). The sequences of primers were listed in Table 1. Amplification protocol consisted of incubations at 95°C for 15sec, 60 °C for 1min for 40 cycles. Quantification was done using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). All gene expression values were normalized using the internal control and calculated using the comparative CT method (ΔΔCT method) [13]. Downregulation was determined if relative quantification (RQ) value of non-tumor tissue was more than 2-fold change than RQ of corresponding tumor tissue.
Table 1. Primers’ sequences.
| Primers | sequence |
|---|---|
| CRNN- F | 5’-ACTCTTGGAGCAAGAGTTTG-3’ |
| CRNN- R | 5’-TGGAGGCTTCCAGAACTCTTG-3’ |
| p53-F | 5’-TTGCCAACTGGCCAAGACCTG-3’ |
| p53-R | 5’-ACGCAAATTTCCTTCCACTCGG-3’ |
| p21-F | 5’-TGTCCGTCAGAACCCATGC-3’ |
| p21-R | 5’-AAAGTCGAAGTTCCATCGCTC-3’ |
| GAPDH-F | 5’-CATGAGAAGTATGACAACAGCCT-3’ |
| GAPDH-R | 5’-AGTCCTTCCACGATACCAAAGT-3’ |
| 18s-F | 5’-CTCTTAGCTGAGTGTCCCGC-3’ |
| 18s-R | 5’-CTGATCGTCTTCGAACCTCC-3’ |
Tissue microarray (TMA) and immunohistochemistry (IHC)
A TMA composed of 300 ESCC tumor specimens were collected from Linzhou Cancer Hospital (Henan, China). Tissue samples used in this study were approved by the Committees for Ethical Review of Research Involving Human Subjects at Zhengzhou University. Written informed consents for the original human work that produced the tissue samples were obtained. TMA was constructed as described previously [14]. IHC staining was carried out following standard streptavidin-biotin-peroxidase complex method [15]. Briefly, TMA sections were deparaffinized, and nonspecific bindings were blocked with 10% normal goat serum for 10min. The TMA section was then incubated with anti-CRNN polyclonal antibody (1:100 dilution, Abcam, Cambridge, UK) at 4 °C overnight. Slides were then incubated with HRP-conjugated goat anti-rabbit immunoglobulin at a concentration of 1:100 at 37°C for 30min. Cytoplasmic expression of CRNN was assessed by three independent investigators. The immunoreactivity of CRNN was scored by staining intensity only (0 = negative staining; 1 = weak staining; 2 = strong staining) because no obvious difference was observed in the percentage of cells stained.
In vitro tumorigenic assays
To test tumor suppressive function of CRNN, CRNN was cloned into pcDNA3.1/V5-His TOPO TA vector (Invitrogen, Carlsbad, CA) and transfected into ESCC cell line KYSE30 and KYSE180 cells (CRNN-30 and CRNN-180, respectively). Stable CRNN-expressing clones were selected for further study. Empty vector-transfected KYSE30 and KYSE180 cells (Vec-30/Vec-180) were used as controls. Cell growth, foci formation, and soft agar assays were carried out as described previously [11]. For cell growth assay, 1×103 cells were seeded into 96-well plate and cell growth rate was detected using cell proliferation XTT kit (Dojindo, Japan) according to the manufacturer’s instructions. For foci formation assay, 1×103 cells were plated in wells of a 6-well plate. After 7 days culture, surviving colonies (> 50 cells/colony) were counted with crystal violet staining. For soft agar assay, 5×103 cells were seeded into 0.4% bactoagar on a bottom layer of solidified 0.6% bactoagar in 6-well plates. After 3 weeks, colonies consisted of more than 80 cells were counted. All above assays’ data were expressed as the means ± S.E.M. of triplicate independent experiments.
Tumor formation in nude mice
The study was approved by Institutional Animal Care and Use Committee of Cancer Cancer, Sun Yat-sen University. Animal experiments were performed in compliance with the guidelines for the Welfare of Experimental Animals in Cancer Center, Sun Yat-sen University. The in vivo tumor-suppressive ability of CRNN was investigated by tumor xenograft experiment. About 2×106 CRNN-transfected cells and empty vector-transfected cells were injected subcutaneously into the right and left sides of 4-week-old nude mice (n=9 for KYSE30 and n=6 for KYSE180), respectively. Tumor formation in nude mice was monitored by measuring the tumor volume, which was calculated by the formula, V=0.5×L (length of tumor) ×W2 (width of tumor) over a 4-week period [16]. To confirm CRNN expression in xenograft tumor, IHC staining with anti-CRNN antibody was performed in sections (5µm in thick) of paraffin-embedded xenograft tumor.
RNA interfering (RNAi)
CRNN-expressing clones CRNN-C2, C3 (KYSE30) or CRNN-C1 (KYSE180) were transfected with double-stranded siRNAs (Ambion, Carlsbad, CA) with lipofectamine 2000TM reagent (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours after transfection, the gene-silencing effect was measured by qRT-PCR and western blot analysis, respectively. Three independent experiments were performed.
Cell cycle analysis
CRNN-C2/CRNN-C1 or Vec-30/Vec-180(2×105) were fixed in 70% ethanol and stained with propidium iodide, and DNA content was analyzed by Cytomics FC (Beckman Coulter, Indianapolis, IN).
Western blot analysis
Western blotting was done according to the standard protocol with antibodies for GAPDH, CRNN (Santa Cruz Biotechnology, Santa Cruz, CA), P53, P21WAF1/CIP1, cyclin D1, CDK4, cyclin E, CDK2, Rb, tubulin (Cell Signaling Technology, Danvers, MA).
Statistical analysis
Statistical analysis was performed using SPSS standard version 13.0 software (SPSS Inc, Chicago, IL). Data were expressed as mean ± S.E.M. from at least three independent determinations. Significance of difference was analyzed using Student’s t-tests. The correlation between CRNN expression and clinicopathologic characteristics was analyzed using the Fisher’s exact test. Cum survival was calculated from the date of diagnosis to the date of cancer-related death or last follow-up. Survival curve was assessed by the Kaplan-Meier method and compared by the log-rank test. Relative risks of cancer-related death associated with CRNN expression status and other predictor variables were estimated by univariate analysis. Multivariate survival analysis was done on all parameters that were found to be significant on univariate level using the Cox regression model. Differences were considered significant for P<0.05.
Results
Downregulation of CRNN is frequently detected in ESCC
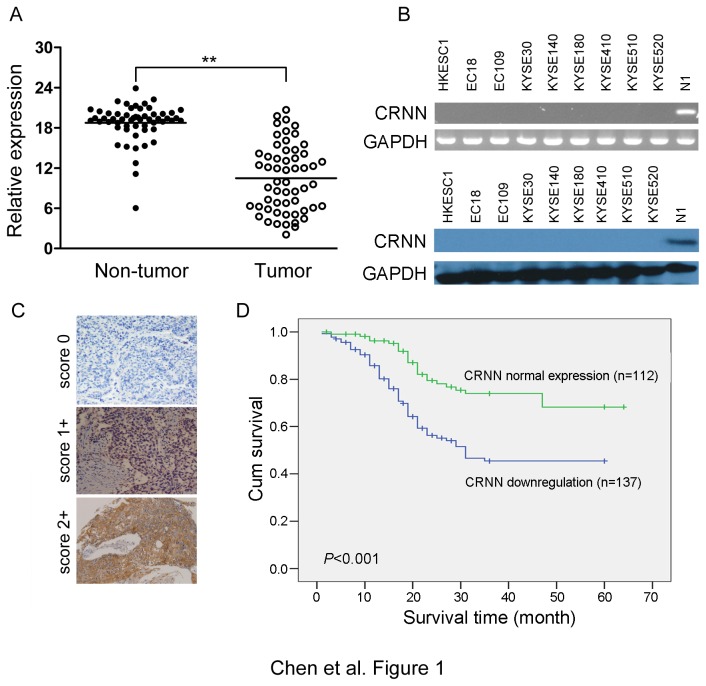
The mRNA expression of CRNN in 9 ESCC cell lines and 56 primary ESCC tumors and their paired non-tumorous tissues were detected by semiquantitative and qRT-PCR, respectively. The results showed that downregulation of CRNN was detected in 26/56 (46.4%) of primary ESCCs (Figure 1A) and 9/9 of ESCC cell lines (Figure 1B).
Figure 1. Downregulation of CRNN in ESCC.
(A) qRT-PCR was used to compare CRNN mRNA levels between tumor and corresponding non-tumor tissues in 56 ESCC cases. **, P<0.01. (B) Absent expression of CRNN could be observed in all tested ESCC cell lines in both mRNA level (upper panel) and protein level (lower panel) detected by RT-PCR and western blot analysis, respectively. GAPDH was used as a loading control. N1, pool total RNA or protein from 5 non-tumor esophageal tissue specimens. (C) Representatives of immunostaining with anit-CRNN antibody in ESCC cases. Positive staining (brown) was detected in non-tumor esophageal epithelial cells but not in tumor cells. The slide was counterstained with hematoxylin. Original magnification, 200×magnification. (D) Kaplan-Meier analysis shows that downregulation of CRNN was significantly associated with poorer overall survival in 249 ESCC cases (P<0.001, Log-rank test).
CRNN downregulation correlates with poor outcome of ESCC
To investigate the clinical significance of CRNN downregulation in esophageal carcinogenesis, CRNN expression in protein level was also studied using ESCC tissue microarray. Expression of CRNN was classified into absent (scored as 0), weak-positive (scored as 1+) and strong-positive (scored as 2+) cytoplasmic staining. Informative expression of CRNN was detected in 249 ESCC cases. Noninformative samples included lost sample and sample with too few tumor cells; such cases were excluded for data complication. Normal expression of CRNN (strong/weak staining) was observed in all non-tumorous esophageal epithelial cells (Figure 1C). Downregulated expression of CRNN (absent staining) was detected in 137/249 (55.02%) of informative ESCC cases.
The correlation of CRNN expression with various clinicopathologic features was investigated and the result showed that downregulation of CRNN was significantly associated with advanced clinical stage (P=0.039) and lymph node metastases (P=0.027, Table 2). Furthermore, log-rank test showed that ESCC patients with CRNN downregulation (mean survival time: 36 months) had a significant shorter survival time than patients with CRNN normal expression (mean survival time: 51 months; P<0.001) (Figure 1D). By univariable analyses, downregulation of CRNN (P<0.001), tumor differentiation (P=0.018), tumor invasion (P=0.007), and presence of lymph node metastases (P=0.001) were significantly negative prognostic factors for cum survival in ESCC patients (Table 3). Nevertheless, multivariable analyses showed that downregulation of CRNN and lymph node metastases were independent prognostic markers for ESCC patients enrolled in this study (P<0.05, Table 3).
Table 2. Association of CRNN downregulation with clinicopathological features in 249 ESCCs.
| Clinical features | Number | CRNN downregulation | P value# |
|---|---|---|---|
| Age(yearsold) | 0.907 | ||
| ≤57 | 117 | 61 (52.1%) | |
| >57 | 132 | 76 (57.6%) | |
| Gender | 0.873 | ||
| Male | 137 | 76 (55.5%) | |
| Female | 112 | 61 (54.5%) | |
| Differentiation | 0.243 | ||
| Well | 56 | 36 (64.3%) | |
| Moderate | 162 | 88 (54.3%) | |
| Poor | 31 | 13 (41.9%) | |
| Tumorinvasion | 0.371 | ||
| T1 | 15 | 7 (46.7%) | |
| T2 | 19 | 11 (57.9%) | |
| T3 | 54 | 24 (44.4%) | |
| T4 | 161 | 95 (59.0%) | |
| Clinicalstage | 0.039 | ||
| I | 11 | 6 (54.5%) | |
| II | 155 | 76 (49.0%) | |
| III | 83 | 55 (66.3%) | |
| Lymphnodemetastasis | 0.027 | ||
| N0 | 141 | 69 (48.9%) | |
| N1 | 108 | 68 (63.0%) | |
#: 2-tailed Fisher’s exact test.
Table 3. Cox proportional hazard regression analyses for overall survival.
| Clinical features |
Univariable analysis* |
Multivariable analysis* |
||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 1.143 (0.740-1.764) | 0.547 | ||
| Age | 1.000 (0.977-1.025) | 0.981 | ||
| Differentiation | 1.565 (1.080-2.268) | 0.018 | 1.408 (0.964-2.057) | 0.077 |
| Tumor invasion | 1.526 (1.125-2.072) | 0.007 | 1.412 (0.964-2.069) | 0.077 |
| LN metastasis | 2.059 (1.338-3.168) | 0.001 | 1.980 (1.017-3.856) | 0.045 |
| CRNN | 0.375 (0.232-0.607) | <0.001 | 0.429 (0.263-0.701) | 0.001 |
HR = hazard ratio; CI = confidence interval.
CRNN has strong tumor suppressive ability
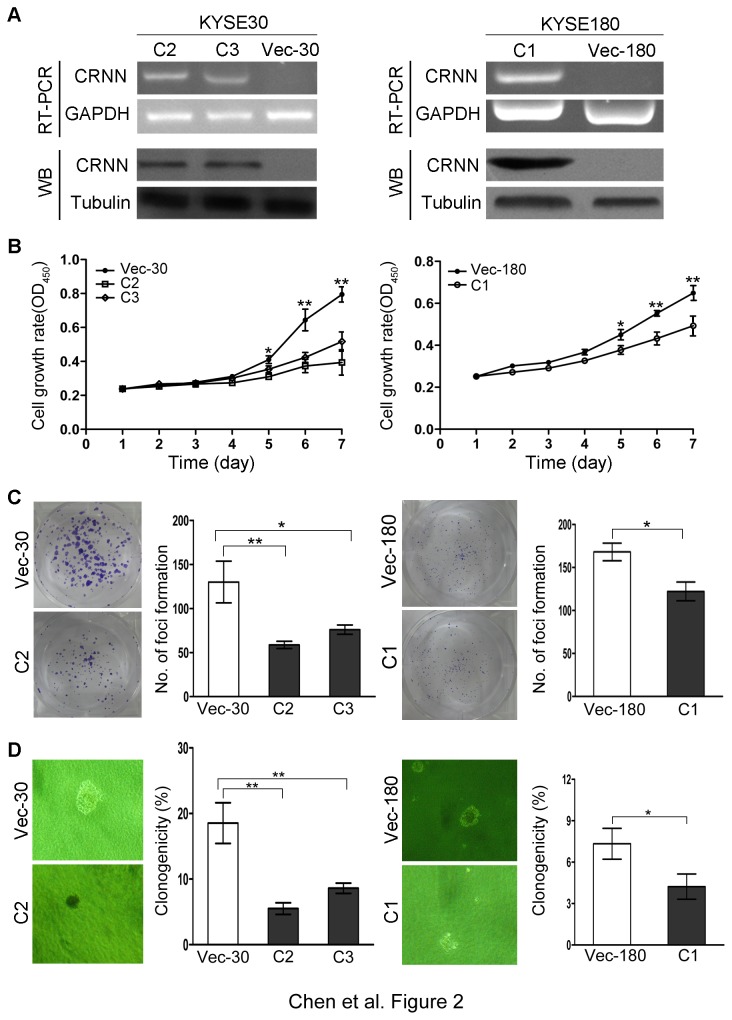
To determine if CRNN has tumor suppressive function, CRNN gene was stably transfected into ESCC cell lines KYSE30 and KYSE180 cells. Stably CRNN-expressing clones from KYSE30 (CRNN-C2 and CRNN-C3) and from KYSE180 (CRNN-C1) were selected. Empty vector-transfected cells (Vec-30 and Vec-180) were used as controls. Expression of CRNN in these clones was confirmed by RT-PCR and western blotting (Figure 2A). Tumor suppressive function of CRNN was assessed by cell growth, foci formation and soft agar assays. XTT assay showed that the cell growth rates in CRNN-expressing clones were significantly inhibited compared with control cells (P<0.01) (Figure 2B). Foci formation assay showed that the frequency of foci formation was significantly inhibited in CRNN-expressing clones compared with control cells (P<0.05) (Figure 2C). A similar result was obtained from soft agar assay, in which the colony formation in soft agar was significantly inhibited in CRNN-expressing clones compared with control cells (P<0.01 for CRNN-30 and P<0.05 for CRNN-180) (Figure 2D).
Figure 2. Tumor-suppressive function of CRNN in ESCC cell lines.
(A) Expression of CRNN in CRNN-transfected ESCC cell lines KYSE30 (clones 2 and 3) and KYSE180 (clone 1) were confirmed by RT-PCR and western blot analysis, respectively. Empty vector-transfected ESCC cells (Vec-30 and Vec-180) were used as controls. GAPDH and Tubulin were used as internal and loading controls, respectively. (B) Growth curves of CRNN-transfected cells were compared with controls by XTT assay. Results were summarized from three independent experiments. *, P<0.05; **, P<0.01. (C) Representative inhibition of foci formation in monolayer culture by CRNN. Quantitative analyses of foci numbers are summarized from three independent experiments. *, P<0.05; **, P<0.01. (D) Inhibition of colony formation in soft agar by CRNN. The results are summarized from three independent experiments. *, P<0.05; **, P<0.01.
CRNN inhibits tumor formation in nude mice
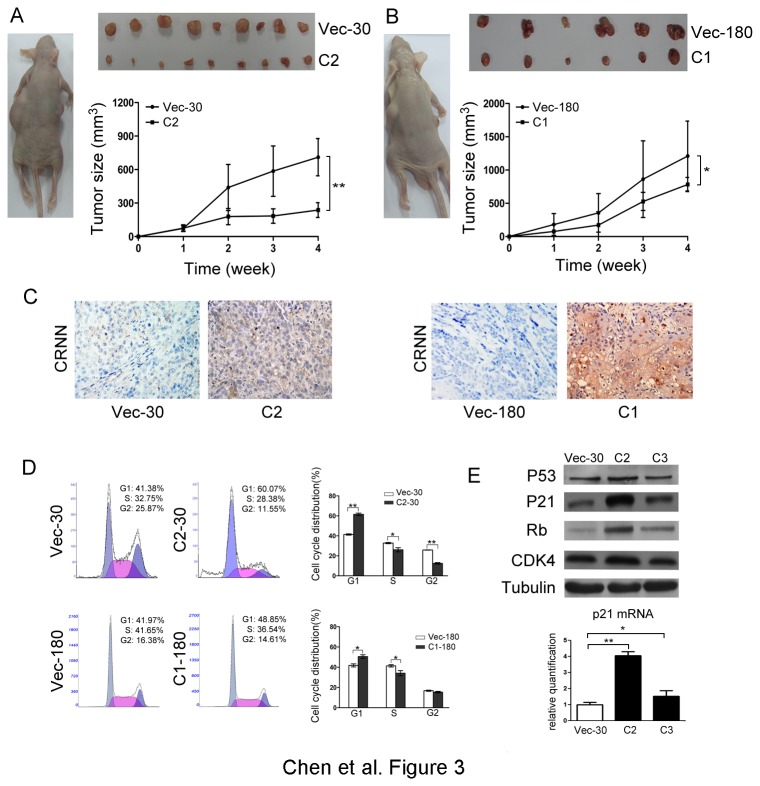
To further explore the in vivo tumor suppressive ability of CRNN, tumor formation in nude mice was carried out by the injection of CRNN-C2 (KYSE30), CRNN-C1 (KYSE180), whereas Vec-30 and Vec-180 were used as controls. The results showed that tumor formation in nude mice was significantly inhibited in CRNN-expressing cells (P<0.01 for CRNN-30 and P<0.05 for CRNN-180) (Figure 3A and 3B). With immunohistochemical staining using anti-CRNN antibody, we confirmed that CRNN expression was re-established in CRNN-C2 or CRNN-C1-induced tumors (Figure 3C).
Figure 3. CRNN inhibits tumor formation in nude mice.
(A) Representatives of tumors formed in nude mice induced by vector-transfected KYSE30 cells (left) and CRNN-transfected cells (right), respectively. Representatives of tumors derived from tested animals were shown in the upper panel. Tumor volumes were summarized in the lower panel. *, P<0.05; **, P<0.01. (B) Similar results were obtained in CRNN-transfected KYSE180 cells compared with vector controls. *, P<0.05; **, P<0.01. (C) IHC staining was performed to confirm the expression of CRNN in the tumors induced by CRNN-transfected cells but not in the tumors induced by vector-transfected cells. Original magnification, 200×magnification. (D) Representative and summary of DNA content detected by flow cytometry. The percentage of cells in S phase was significantly decreased in CRNN-transfected cells compared with control cells. *, P<0.05; **, P<0.01. (E) Western blot analysis shows that CRNN could upregulate P21WAF1/CIP1 and Rb expression significantly. P53 also increased slightly in CRNN-transfected cells. Tubulin was used as a loading control. p21 mRNA level was determined by qRT-PCR. *, P<0.05; **, P<0.01.
CRNN arrests cell cycle at G1/S transition
To elucidate the mechanism underlying growth inhibition by CRNN, flow cytometry was used to compare cell distribution in cell cycle between CRNN-transfectants and control cells. The percentage of CRNN-30 in G0/G1 phases was significantly increased (P<0.01), whereas the percentage in S-phase was significantly decreased (P<0.05), compared with that in control cells, suggesting that CRNN was able to arrest cell cycle at G1/S phase (Figure 3D). Similar results were also observed in CRNN-180 cells (Figure 3D), which is consistent with the previous report [17]. To further reveal the potential molecular mechanism of CRNN in cell cycle arrest, the effects of CRNN on key cell cycle regulators P53, P21WAF1/CIP1 and Rb were tested. The result showed that expressions of P21WAF1/CIP1, Rb were upregulated significantly in CRNN-transfected cells compared with control cells (Figure 3E). P53 was also upregulated slightly in CRNN overexpressed cells. However, no significant difference was detected for CDK4 in this study. The mRNA level of p21 also increased in CRNN overexpressed cells.
To test whether CRNN expression could induce a “differentiated state” of cells, we investigated keratin-4, an epidermal differentiation marker, in the CRNN overexpressing cells. No staining was observed in both CRNN overexpression and vector control cells (Figure S1).
Knockdown of CRNN inhibits its tumor suppressive ability
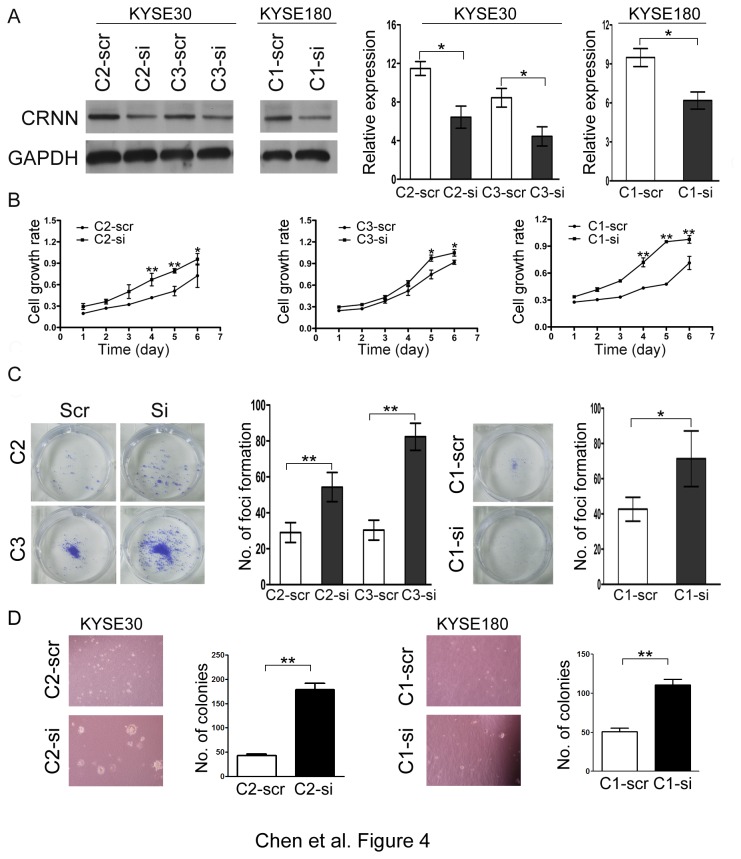
Expression of CRNN in CRNN-transfectants was silenced by RNAi with two siRNAs targeting CRNN. Western blotting result showed that CRNN expression could be effectively silenced (Figure 4A). Cell growth assay demonstrated that the cell growth rate was significantly increased in siCRNN-treated cells compared with scramble-treated cells (P<0.05) (Figure 4B). Similarly, foci formation assay revealed that the frequency of foci formation was significantly increased in siCRNN-treated cells compared with scramble-treated cells (Figure 4C). Soft-agar assay results demonstrated that the number of colonies formed in soft agar increased in CRNN knock-down cells compared with scramble control cells (Figure 4D). Furthermore, DNA content analysis by flow cytometry showed that silencing CRNN expression was able to increase the G1/S transition. The percentage of cells in the S phase was significantly increased in siCRNN-treated cells compared with scramble-treated cells (P<0.05) (Figure 5A). Western blot analysis showed that p21WAF1/CIP1 and Rb were downregulated in siCRNN-treated cells compared with scramble-treated cells (Figure 5B).
Figure 4. Silencing CRNN expression increases tumorigenicity.
(A) Expression of CRNN was decreased by siRNA against CRNN compared with scramble control cells detected by western blot analysis (left panel). Fold change of CRNN expression was calculated from siRNA-CRNN relative to the scramble control (right panel). *, P<0.05. (B) Growth curve of CRNN-transfected cells treated with siRNA-CRNN were compared with scramble control cells by XTT assay. The results are summarized from three independent experiments. *, P<0.05; **, P<0.01. (C) Representatives of foci formation induced by cells treated with siRNA-CRNN, compared with scramble control treated cells. The number of foci was calculated and summarized in bar chart. The results are summarized from three independent experiments *, P<0.05; **, P<0.01. (D) Representatives of colonies formed in soft-agar in cells treated with siRNA and scramble control. The results are summarized from three independent experiments. **, P<0.01.
Figure 5. Silencing CRNN expression increases improper G1/S transition.
(A) Cell distribution in cell cycle between siRNA-CRNN treated and scramble control cells. The results are summarized from three independent experiments (lower panel). *, P<0.05; **, P<0.01. (B) Expressions of P21WAF1/CIP1 and Rb expression in siRNA treated and scramble control cells were compared by western blot analysis. Tubulin was used as a loading control.
Discussion
CRNN has been proposed as a squamous-cell specific gene because of its expression in esophageal tissue, cervical squamous epithelium, and murine skin, and absence of expression in another 15 glandular tissues [18,19]. Downregulation of CRNN was reported in some cancers, including esophageal cancer [6–10], oral squamous cell carcinoma [17], and head and neck squamous cell carcinoma [20]. However, the molecular mechanism of CRNN in tumor remains unclear. In the present study, we found that CRNN was downregulated in 55.02% of primary ESCC tumors, which was significantly associated with advanced clinical stage (P=0.039), lymph node metastases (P=0.027) and poor survival of patients with ESCC (P<0.001). Multivariable analyses showed that the downregulation of CRNN could be used as an independent prognostic predictor for ESCC patients. Furthermore, we investigated the mechanisms underlying CRNN downregulation in ESCC cells. However, neither hypermethylation nor histone modification was associated with CRNN downregulation in ESCC (data not shown).
Loss of heterozygosity (LOH) at 1q21 region has been frequently detected in various solid tumors, including esophageal squamous cell carcinoma [21], breast cancer [22], insulinoma [23] and esophageal adenocarcinoma [24]. Interestingly, loss of 1q21 has been associated with tumor malignancy [23] and shorter overall survival [24]. To explore whether CRNN inactivation is correlated with single nucleotide variation in the promoter region of CRNN, a fragment (-2,000 to -1) from promoter region of CRNN was sequenced in 10 ESCC cases. Four known SNPs were found in this promoter region. After systematical analysis, none of them was significantly associated with CRNN downregulation (data not shown). Another possible mechanism might be involved in the CRNN inactivation, micro-RNA regulation [25], was not investigated in the present study.
CRNN gene is located on the chromosome 1q21, where thirteen S100 family members are tightly clustered [26,27]. The functions of S100 genes are very complex and they play different roles in cancer development and progression [28]. For example, S100A4, S100A6, S100A7 and S100B play oncogenic roles in cancer development [29,30], whereasS100B, S100A2, and S100A11 are believed as TSGs [31–33]. In the present study, tumor suppressive function of CRNN was characterized by both in vitro and in vivo assays including cell growth, foci formation and soft agar assays, and tumor formation in nude mice. The results demonstrated that CRNN could effectively suppress cell growth, foci formation and colony formation in soft agar, and inhibit tumor formation in nude mice. A further study revealed that CRNN was able to inhibit G1/S transition through the upregulation of P21WAF1/CIP1 and Rb. G1/S phase transition is a major checkpoint for cell cycle progression and P21WAF1/CIP1 is one of the critical negative regulators during this transition [34,35]. Rb is another important tumor suppressor in the cancer development. When it is mutated or deleted, E2F transcription factor would be released and induce the expression of genes that stimulate cell growth [36,37]. We further tested P53 protein level and found that P53 increased slightly in CRNN overexpressed cells compared with vector control cells. It was reported that p53 could be activated by DNA damage in KYSE30 with mutant p53 as other ESCC cell line with wide-type p53 [38]. qRT-PCR results also indicated that p21 mRNA level increased in CRNN overexpressed cells. Taken together, we hypothesize that overexpression of CRNN could upregulate P53, and it subsequently increases P21 and Rb and inhibit G1/S transition.
In the present study, the tumor suppressive function of CRNN has been clearly demonstrated, however, the molecular mechanism of CRNN in ESCC development remains unclear. CRNN has been reported to allow cells to tolerate normally lethal levels of deoxycholic acid and protect from the toxic effect of bile acid as a survival factor [39]. Further study identified CRNN as a potential component of epithelial immunity based on its strong signature of adaptive evolution on DNA sequence of a type that is commonly associated with a coevolutionary arms race with a pathogen [40]. Therefore, the expression of CRNN protein will presumably help maintain the barrier function in squamous epithelium in response to injury and function as a tumor suppressor [10]. In conclusion, we demonstrate that CRNN is a potential tumor suppressor in ESCC via arresting cell cycle progression at G1/S checkpoint by upregulating P21WAF1/CIP1 and Rb. A better understanding of the tumor suppressive role of CRNN will significantly improve our knowledge in the development of ESCC, and may lead to a more effective management of ESCC patients with the inactivation of CRNN.
Supporting Information
IHC staining with CK4 and CK (pan) in CRNN overexpression cells and vector control cells. Original magnification: 200× magnification.
(JPG)
Funding Statement
This work was supported by Grants from the National Natural Science Foundation of China (81272416, 81172338 and 81000863); the General Research Fund (HKU 7668/11M); Sun Yat-Sen University “Hundred Talents Program” (85000-3171311) and Sun Yat-sen University Young Talent Teachers Plan (11ykpy58). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pisani P, Parkin DM, Ferlay J (1993) Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer 55: 891-903. doi:10.1002/ijc.2910550604. PubMed: 8253525. [DOI] [PubMed] [Google Scholar]
- 2. Offner FA (2000) [Etiology, molecular biology and pathology of squamous cell carcinoma of the esophagus]. Pathologe 21: 349-357. doi:10.1007/s002920000414. PubMed: 11092007. [DOI] [PubMed] [Google Scholar]
- 3. Jemal A, Murray T, Ward E, Samuels A, Tiwari RC et al. (2005) Cancer statistics, 2005. CA Cancer J Clin 55: 10-30. doi:10.3322/canjclin.55.1.10. PubMed: 15661684. [DOI] [PubMed] [Google Scholar]
- 4. Kligman D, Hilt DC (1988) The S100 protein family. Trends Biochem Sci 13: 437-443. doi:10.1016/0968-0004(88)90218-6. PubMed: 3075365. [DOI] [PubMed] [Google Scholar]
- 5. Contzler R, Favre B, Huber M, Hohl D (2005) Cornulin, a new member of the "fused gene" family, is expressed during epidermal differentiation. J Invest Dermatol 124: 990-997. doi:10.1111/j.0022-202X.2005.23694.x. PubMed: 15854041. [DOI] [PubMed] [Google Scholar]
- 6. Luthra MG, Ajani JA, Izzo J, Ensor J, Wu TT et al. (2007) Decreased expression of gene cluster at chromosome 1q21 defines molecular subgroups of chemoradiotherapy response in esophageal cancers. Clin Cancer Res 13: 912-919. doi:10.1158/1078-0432.CCR-06-1577. PubMed: 17289885. [DOI] [PubMed] [Google Scholar]
- 7. Luo A, Kong J, Hu G, Liew CC, Xiong M et al. (2004) Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene 23: 1291-1299. doi:10.1038/sj.onc.1207218. PubMed: 14647409. [DOI] [PubMed] [Google Scholar]
- 8. Kashyap MK, Marimuthu A, Kishore CJ, Peri S, Keerthikumar S et al. (2009) Genomewide mRNA profiling of esophageal squamous cell carcinoma for identification of cancer biomarkers. Cancer Biol Ther 8: 36-46. doi:10.4161/cbt.8.1.7090. PubMed: 18981721. [DOI] [PubMed] [Google Scholar]
- 9. Pawar H, Maharudraiah J, Kashyap MK, Sharma J, Srikanth SM et al. (2013) Downregulation of cornulin in esophageal squamous cell carcinoma. Acta Histochem 115: 89-99. doi:10.1016/j.acthis.2012.04.003. PubMed: 22560086. [DOI] [PubMed] [Google Scholar]
- 10. Zhang W, Chen X, Luo A, Lin D, Tan W et al. (2009) Genetic variants of C1orf10 and risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Sci 100: 1695-1700. doi:10.1111/j.1349-7006.2009.01240.x. PubMed: 19558548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu L, Qin YR, Xie D, Hu L, Kwong DL et al. (2007) Characterization of a novel tumor-suppressor gene PLC delta 1 at 3p22 in esophageal squamous cell carcinoma. Cancer Res 67: 10720-10726. doi:10.1158/0008-5472.CAN-07-2411. PubMed: 18006814. [DOI] [PubMed] [Google Scholar]
- 12. Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T (1992) Characterization of 21 newly established esophageal cancer cell lines. Cancer 69: 277-284. [DOI] [PubMed] [Google Scholar]
- 13. Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101-1108. doi:10.1038/nprot.2008.73. PubMed: 18546601. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Nie CJ, Hu L, Qin Y, Liu HB et al. (2010) Characterization of a novel mechanism of genomic instability involving the SEI1/SET/NM23H1 pathway in esophageal cancers. Cancer Res 70: 5695-5705. doi:10.1158/1538-7445.AM10-5695. PubMed: 20570897. [DOI] [PubMed] [Google Scholar]
- 15. Xie D, Sham JS, Zeng WF, Lin HL, Che LH et al. (2003) Heterogeneous expression and association of beta-catenin, p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int J Cancer 107: 896-902. doi:10.1002/ijc.11514. PubMed: 14601048. [DOI] [PubMed] [Google Scholar]
- 16. Fu L, Qin YR, Xie D, Chow HY, Ngai SM et al. (2007) Identification of alpha-actinin 4 and 67 kDa laminin receptor as stage-specific markers in esophageal cancer via proteomic approaches. Cancer 110: 2672-2681. doi:10.1002/cncr.23110. PubMed: 17960614. [DOI] [PubMed] [Google Scholar]
- 17. Imai FL, Uzawa K, Nimura Y, Moriya T, Imai MA et al. (2005) Chromosome 1 open reading frame 10 (C1orf10) gene is frequently down-regulated and inhibits cell proliferation in oral squamous cell carcinoma. Int J Biochem Cell Biol 37: 1641-1655. doi:10.1016/j.biocel.2005.02.005. PubMed: 15896671. [DOI] [PubMed] [Google Scholar]
- 18. Xu Z, Wang MR, Xu X, Cai Y, Han YL et al. (2000) Novel human esophagus-specific gene c1orf10: cDNA cloning, gene structure, and frequent loss of expression in esophageal cancer. Genomics 69: 322-330. doi:10.1006/geno.2000.6344. PubMed: 11056050. [DOI] [PubMed] [Google Scholar]
- 19. Yagui-Beltran A, Craig AL, Lawrie L, Thompson D, Pospisilova S et al. (2001) The human oesophageal squamous epithelium exhibits a novel type of heat shock protein response. Eur J Biochem 268: 5343-5355. doi:10.1046/j.0014-2956.2001.02468.x. PubMed: 11606197. [DOI] [PubMed] [Google Scholar]
- 20. Schaaij-Visser TB, Graveland AP, Gauci S, Braakhuis BJ, Buijze M et al. (2009) Differential Proteomics Identifies Protein Biomarkers That Predict Local Relapse of Head and Neck Squamous Cell Carcinomas. Clin Cancer Res 15: 7666-7675. doi:10.1158/1078-0432.CCR-09-2134. PubMed: 19996216. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Liu Z, Wang Y, Yu Z, Wang M et al. (2005) Allelic imbalance of chromosome 1q in esophageal squamous cell carcinomas from China: a novel region of allelic loss and significant association with differentiation. Cancer Lett 220: 221-230. doi:10.1016/j.canlet.2004.07.032. PubMed: 15766597. [DOI] [PubMed] [Google Scholar]
- 22. Gaki V, Tsopanomichalou M, Sourvinos G, Tsiftsis D, Spandidos DA (2000) Allelic loss in chromosomal region 1q21-23 in breast cancer is associated with peritumoral angiolymphatic invasion and extensive intraductal component. Eur J Surg Oncol 26: 455-460. doi:10.1053/ejso.1999.0921. PubMed: 11016465. [DOI] [PubMed] [Google Scholar]
- 23. Yang YM, Liu TH, Chen YJ, Jiang WJ, Qian JM et al. (2005) Chromosome 1q loss of heterozygosity frequently occurs in sporadic insulinomas and is associated with tumor malignancy. Int J Cancer 117: 234-240. doi:10.1002/ijc.21175. PubMed: 15900598. [DOI] [PubMed] [Google Scholar]
- 24. Maru DM, Luthra R, Correa AM, White-Cross J, Anandasabapathy S et al. (2009) Frequent loss of heterozygosity of chromosome 1q in esophageal adenocarcinoma: loss of chromosome 1q21.3 is associated with shorter overall survival. Cancer 115: 1576-1585. doi:10.1002/cncr.24122. PubMed: 19156915. [DOI] [PubMed] [Google Scholar]
- 25. Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281-297. doi:10.1016/S0092-8674(04)00045-5. PubMed: 14744438. [DOI] [PubMed] [Google Scholar]
- 26. Ridinger K, Ilg EC, Niggli FK, Heizmann CW, Schäfer BW (1998) Clustered organization of S100 genes in human and mouse. Biochim Biophys Acta 1448: 254-263. doi:10.1016/S0167-4889(98)00137-2. PubMed: 9920416. [DOI] [PubMed] [Google Scholar]
- 27. Schäfer BW, Wicki R, Engelkamp D, Mattei MG, Heizmann CW (1995) Isolation of a YAC clone covering a cluster of nine S100 genes on human chromosome 1q21: rationale for a new nomenclature of the S100 calcium-binding protein family. Genomics 25: 638-643. doi:10.1016/0888-7543(95)80005-7. PubMed: 7759097. [DOI] [PubMed] [Google Scholar]
- 28. Donato R (1999) Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1450: 191-231. doi:10.1016/S0167-4889(99)00058-0. PubMed: 10395934. [DOI] [PubMed] [Google Scholar]
- 29. Ilg EC, Schäfer BW, Heizmann CW (1996) Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer 68: 325-332. doi:10.1002/(SICI)1097-0215(19961104)68:3. PubMed: 8903474. [DOI] [PubMed] [Google Scholar]
- 30. Maelandsmo GM, Flørenes VA, Mellingsaeter T, Hovig E, Kerbel RS et al. (1997) Differential expression patterns of S100A2, S100A4 and S100A6 during progression of human malignant melanoma. Int J Cancer 74: 464-469. doi:10.1002/(SICI)1097-0215(19970822)74:4. PubMed: 9291441. [DOI] [PubMed] [Google Scholar]
- 31. Lee SW, Tomasetto C, Swisshelm K, Keyomarsi K, Sager R (1992) Down-regulation of a member of the S100 gene family in mammary carcinoma cells and reexpression by azadeoxycytidine treatment. Proc Natl Acad Sci U S A 89: 2504-2508. doi:10.1073/pnas.89.6.2504. PubMed: 1372446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakaguchi M, Miyazaki M, Inoue Y, Tsuji T, Kouchi H et al. (2000) Relationship between contact inhibition and intranuclear S100C of normal human fibroblasts. J Cell Biol 149: 1193-1206. doi:10.1083/jcb.149.6.1193. PubMed: 10851017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scotto C, Deloulme JC, Rousseau D, Chambaz E, Baudier J (1998) Calcium and S100B regulation of p53-dependent cell growth arrest and apoptosis. Mol Cell Biol 18: 4272-4281. PubMed: 9632811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parry D, Mahony D, Wills K, Lees E (1999) Cyclin D-CDK subunit arrangement is dependent on the availability of competing INK4 and p21 class inhibitors. Mol Cell Biol 19: 1775-1783. PubMed: 10022865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malumbres M, Barbacid M (2001) To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1: 222-231. doi:10.1038/35106065. PubMed: 11902577. [DOI] [PubMed] [Google Scholar]
- 36. Lu Z, Ghosh S, Wang Z, Hunter T (2003) Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell 4: 499-515. doi:10.1016/S1535-6108(03)00304-0. PubMed: 14706341. [DOI] [PubMed] [Google Scholar]
- 37. Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13: 1501-1512. doi:10.1101/gad.13.12.1501. PubMed: 10385618. [DOI] [PubMed] [Google Scholar]
- 38. Ji J, Wu K, Wu M. (2010) Zhan Q p53 functional activation is independent of its genotype in five esophageal squamous cell carcinoma cell lines. Front Med China 4: 412-418. doi:10.1007/s11684-010-0260-x. PubMed: 21191746. [DOI] [PubMed] [Google Scholar]
- 39. Darragh J, Hunter M, Pohler E, Nelson L, Dillon JF et al. (2006) The calcium-binding domain of the stress protein SEP53 is required for survival in response to deoxycholic acid-mediated injury. FEBS J 273: 1930-1947. doi:10.1111/j.1742-4658.2006.05206.x. PubMed: 16640557. [DOI] [PubMed] [Google Scholar]
- 40. Little TJ, Nelson L, Hupp T (2007) Adaptive evolution of a stress response protein. PLOS ONE 2: e1003. doi:10.1371/journal.pone.0001003. PubMed: 17925851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IHC staining with CK4 and CK (pan) in CRNN overexpression cells and vector control cells. Original magnification: 200× magnification.
(JPG)