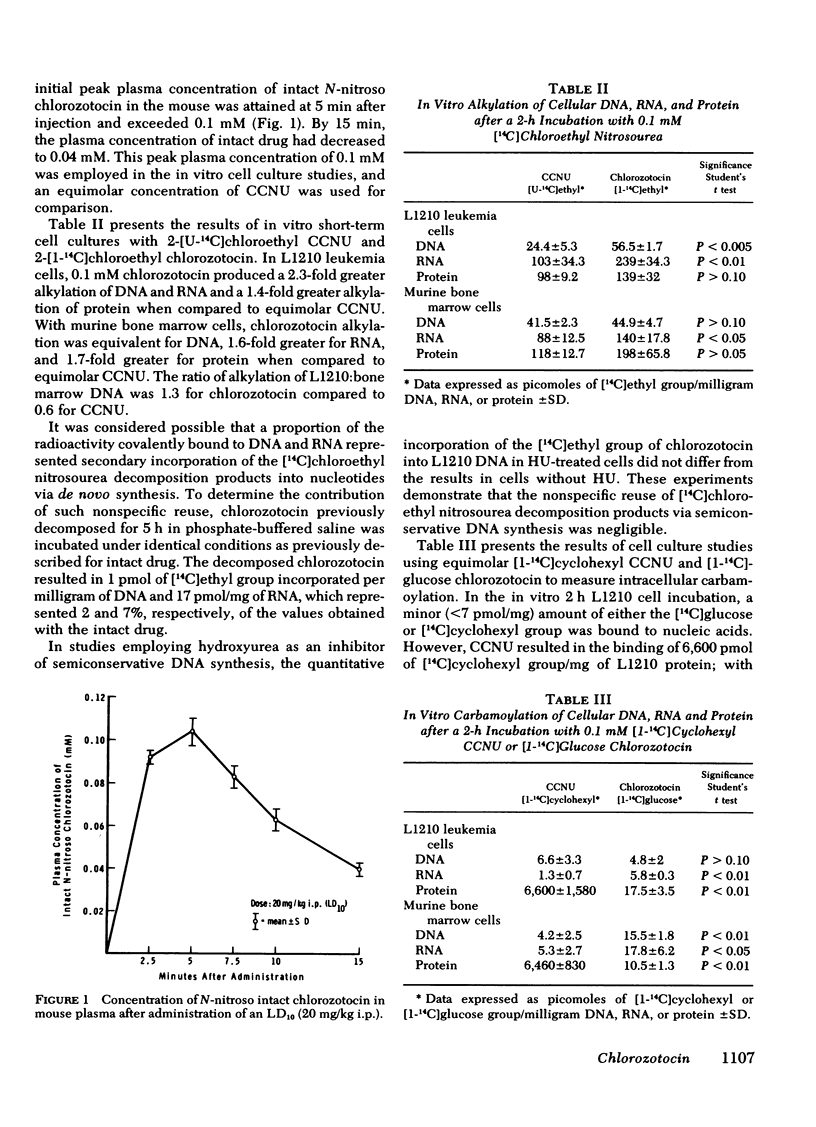
Abstract
Chlorozotocin is a chloroethyl nitrosourea with a glucose carrier that has curative activity for the murine L1210 leukemia, but is nonmyelosuppressive in mice. To determine the mechanism for this unique property of reduced bone marrow toxicity, comparative studies were conducted with chlorozotocin and CCNU, a myelotoxic chloroethyl nitrosourea.
Suspensions of L1210 leukemia and murine bone marrow cells were incubated for 2 h with 0.1 mM [14C]-chloroethyl chlorozotocin or CCNU. Chlorozotocin demonstrated a fourfold increased covalent binding of the chloroethyl group to L1210 nuclei when compared to equimolar CCNU. Chlorozotocin alkylation of L1210 cells resulted in the binding of 57 pmol of [14C]ethyl group/mg of DNA, which represented a 2.3-fold increased alkylation when compared to CCNU. In marked contrast, the binding of the chloroethyl group to bone marrow nuclei was equivalent for both drugs. In addition, chlorozotocin alkylation of murine bone marrow DNA, 45 pmol of [14C]ethyl group/mg of DNA, was equivalent to that of CCNU. The ratio of L1210:bone marrow DNA alkylation was 1.3 for chlorozotocin compared to 0.6 for CCNU. The intracellular carbamoylation of L1210 and bone marrow protein by CCNU was 400- to 600-fold greater than that produced by chlorozotocin.
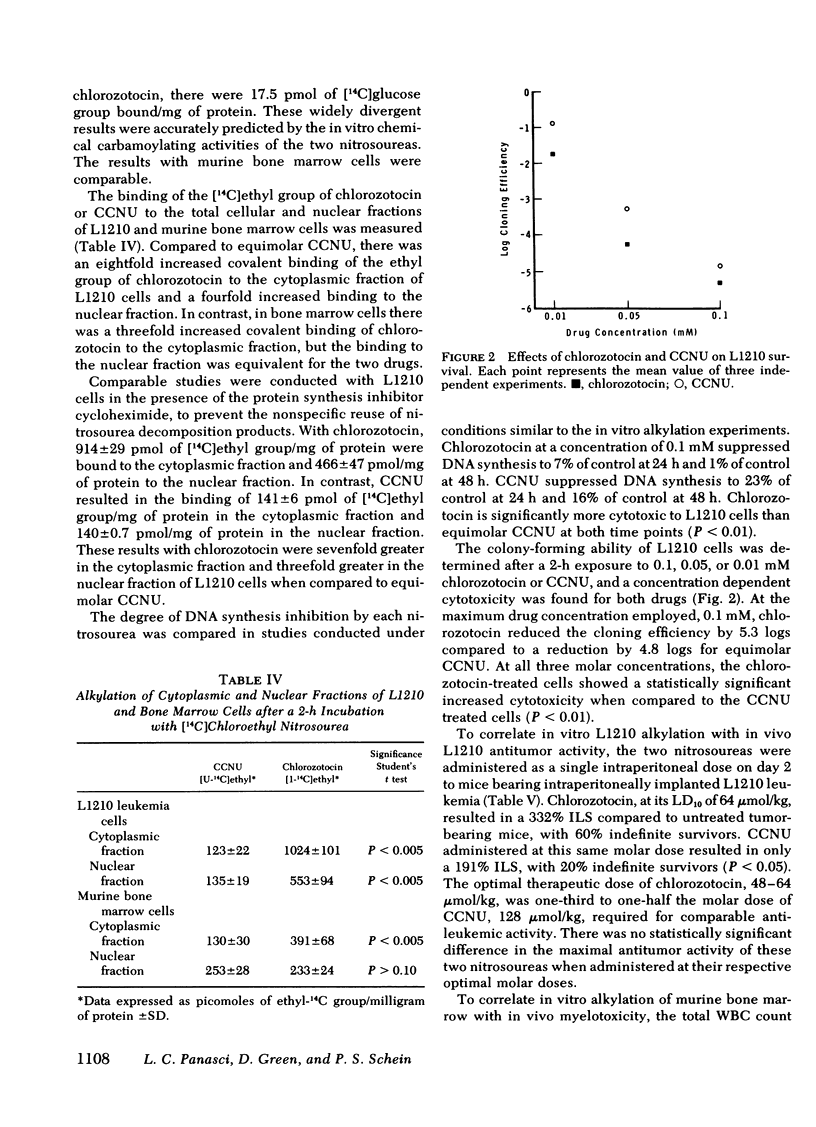
After a 2-h exposure to 0.1, 0.05, or 0.01 mM drug, both chlorozotocin and CCNU produced a reduction in the cloning efficiency of L1210 cells that was dose dependent. However, chlorozotocin was significantly more cytotoxic than CCNU at all three molar concentrations (P < 0.01). Chlorozotocin, 0.1 mM, reduced L1210 DNA synthesis to 1% of control by 48 h, in contrast to 16% with equimolar CCNU (P < 0.01).
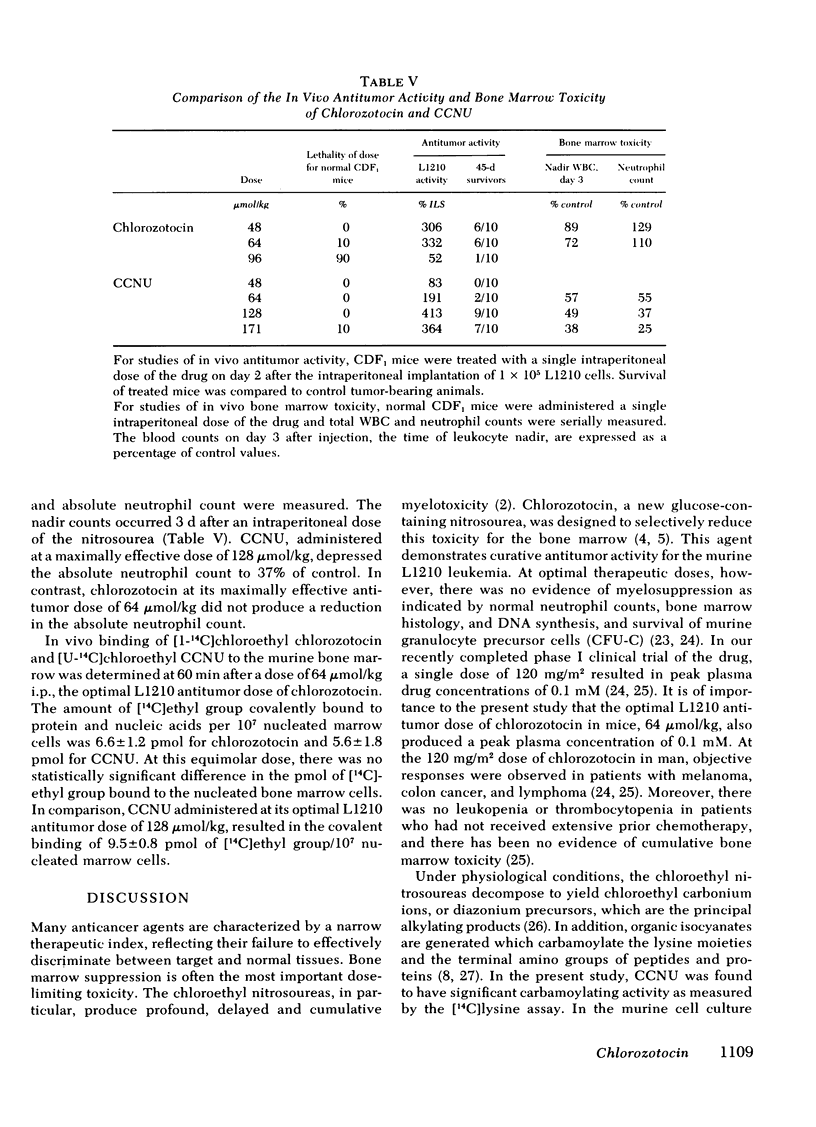
In mice bearing 105 L1210 cells, chlorozotocin produced its optimal antitumor activity (332% increased life span [ILS]) at doses of 48-64 μmol/kg, with >50% indefinite survivors. In contrast, CCNU at the same molar doses resulted in only a 191% ILS; a CCNU dose of 128 μmol/kg was required for comparable optimal L1210 antitumor activity, 413% ILS.
On a molar basis, the dose of chlorozotocin that produced optimal in vivo L1210 antitumor activity was one-third to one-half that of CCNU. Chlorozotocin, unlike CCNU, produced no murine bone marrow toxicity at its optimal therapeutic dose. This unique combination of antitumor activity without myelosuppression can be correlated with the advantageous ratio of L1210:bone marrow in vitro DNA alkylation by chlorozotocin (1.3) as compared to equimolar CCNU (0.6).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T., McMenamin M. G., Schein P. S. Chlorozotocin, 2-(3-(2-chloroethyl)-3-nitrosoureido)-D-glucopyranose, an antitumor agent with modified bone marrow toxicity. Cancer Res. 1975 Mar;35(3):761–765. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S. K. An overview of the status of the nitrosoureas in other tumors. Cancer Chemother Rep 3. 1973 May;4(3):35–46. [PubMed] [Google Scholar]
- Chu M. Y., Fischer G. A. The incorporation of 3H-cytosine arabinoside and its effect on murine leukemic cells (L5178Y). Biochem Pharmacol. 1968 May;17(5):753–767. doi: 10.1016/0006-2952(68)90012-9. [DOI] [PubMed] [Google Scholar]
- Fox P. A., Panasci L. C., Schein P. S. Biological and biochemical properties of 1-(2-chloroethyl)-3-(beta-D-glucopyranosyl)-1-nitrosourea (NSC D 254157), a nitrosourea with reduced bone marrow toxicity. Cancer Res. 1977 Mar;37(3):783–787. [PubMed] [Google Scholar]
- Hoth D., Woolley P., Green D., Macdonald J., Schein P. Phase I studies on chlorozotocin. Clin Pharmacol Ther. 1978 Jun;23(6):712–722. doi: 10.1002/cpt1978236712. [DOI] [PubMed] [Google Scholar]
- Johnston T. P., McCaleb G. S., Montgomery J. A. Synthesis of chlorozotocin, the 2-chloroethyl analog of the anticancer antibiotic streptozotocin. J Med Chem. 1975 Jan;18(1):104–106. doi: 10.1021/jm00235a023. [DOI] [PubMed] [Google Scholar]
- Kann H. E., Jr Comparison of biochemical and biological effects of four nitrosoureas with differing carbamoylating activities. Cancer Res. 1978 Aug;38(8):2363–2366. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moertel C. G. Therapy of advanced gastrointestinal cancer with the nitrosoureas. Cancer Chemother Rep 3. 1973 May;4(3):27–34. [PubMed] [Google Scholar]
- Montgomery J. A., James R., McCaleb G. S., Johnston T. P. The modes of decomposition of 1,3-bis(2-chloroethyl)-1-nitrosourea and related compounds. J Med Chem. 1967 Jul;10(4):668–674. doi: 10.1021/jm00316a033. [DOI] [PubMed] [Google Scholar]
- Nagourney R. A., Fox P., Schein P. S. A comparison of the biological and biochemical properties of 1-(4-amino-2-methylpyrimidin-5-yl)methyl-3-(2-chloroethyl)-3-nitrosourea and 2-[3-(2-chloroethyl)-3-nitrosoureido]-D-glucopyranose. Cancer Res. 1978 Jan;38(1):65–68. [PubMed] [Google Scholar]
- Panasci L. C., Fox P. A., Schein P. S. Structure-activity studies of methylnitrosourea antitumor agents with reduced murine bone marrow toxicity. Cancer Res. 1977 Sep;37(9):3321–3328. [PubMed] [Google Scholar]
- Panasci L. C., Green D. C., Fox P. A., Schein P. S. A phenol technique for extraction of alkylated DNA, RNA, and protein from a single tissue sample. Anal Biochem. 1977 Dec;83(2):677–688. doi: 10.1016/0003-2697(77)90072-0. [DOI] [PubMed] [Google Scholar]
- Panasci L. C., Green D., Nagourney R., Fox P., Schein P. S. A structure-activity analysis of chemical and biological parameters of chloroethylnitrosoureas in mice. Cancer Res. 1977 Aug;37(8 Pt 1):2615–2618. [PubMed] [Google Scholar]
- Schein P. S. 1-methyl-1-nitrosourea and dialkylnitrosamine depression of nicotinamide adenine dinucleotide. Cancer Res. 1969 Jun;29(6):1226–1232. [PubMed] [Google Scholar]
- Schein P. S., Bull J. M., Doukas D., Hoth D. Sensitivity of human and murine hematopoietic precursor cells to 2-[3-(2-chloroethyl)-3-nitrosoureido]-D-glucopyranose and 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1978 Feb;38(2):257–260. [PubMed] [Google Scholar]
- Schein P. S., Panasci L., Woolley P. V., Anderson T. Pharmacology of chlorozotocin Nsc-178248), a new nitrosourea antitumor agent. Cancer Treat Rep. 1976 Jun;60(6):801–805. [PubMed] [Google Scholar]
- Schmall B., Cheng C. J., Fujimura S., Gersten N., Grunberger D., Weinstein I. B. Modification of proteins by 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (nsc 79037) in vitro. Cancer Res. 1973 Aug;33(8):1921–1924. [PubMed] [Google Scholar]
- Snyder A. L., Kann H. E., Jr, Kohn K. W. Inhibition of the processing of ribosomal precursor RNA by intercalating agents. J Mol Biol. 1971 Jun 14;58(2):555–565. doi: 10.1016/0022-2836(71)90371-8. [DOI] [PubMed] [Google Scholar]
- Sun L., Singer B. The specificity of different classes of ethylating agents toward various sites of HeLa cell DNA in vitro and in vivo. Biochemistry. 1975 Apr 22;14(8):1795–1802. doi: 10.1021/bi00679a036. [DOI] [PubMed] [Google Scholar]
- Wheeler G. P., Bowdon B. J., Grimsley J. A., Lloyd H. H. Interrelationships of some chemical, physicochemical, and biological activities of several 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1974 Jan;34(1):194–200. [PubMed] [Google Scholar]
- Wheeler G. P., Bowdon B. J., Struck R. F. Carbamoylation of amino acid, peptides, and proteins by nitrosoureas. Cancer Res. 1975 Nov;35(11 Pt 1):2974–2984. [PubMed] [Google Scholar]


