Abstract
Background
Blunted diurnal cortisol variation has been associated with overt cardiovascular disease in adults. The relationship between the diurnal cortisol variation and subclinical atherosclerosis in youth has yet to be investigated. The objectives of this study were to: 1) determine the relationship between overnight cortisol measures and CIMT in overweight and obese, African-American and Latino children; 2) assess ethnic differences in these relationships and 3) explore whether overnight cortisol and CIMT relationships were independent of inflammatory markers, C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).
Methods
One hundred and fifty-six overweight and obese African-American and Latino children (ages 8–17, 86M/70F, 55 African-American /101 Latino) underwent measures of CIMT by B-mode ultrasound, nocturnal cortisol rise (NCR=salivary cortisol rise from 2200hrs to awakening at 0530hrs), cortisol awakening response (CAR=salivary cortisol from time of awakening to 30 min later), fasting serum cortisol and overnight urinary free cortisol.
Results
Using linear regression, salivary cortisol0530hrs and NCR were negatively associated with CIMT (βstandardized = −0.215 and −0.220, p<0.01) independent of age, height, percent body fat, ethnicity and systolic blood pressure. Nocturnal salivary cortisol2200hrs, morning serum cortisol, and overnight urinary free cortisol were not associated with CIMT. Using ANCOVA, participants with LOW NCR (NCR <0.44µg/dL, n=52) had significantly greater CIMT than those with HIGH NCR (NCR ≥0.91 µg/dL, n=52; 0.632±0.008 vs. 0.603±0.008mm p=0.01) after controlling for covariates. Ethnicity was independently associated with CIMT, whereby African-American children had greater CIMT than Latino children (−0.028±0.009, p=0.006). The relationships between cortisol measures and CIMT did not differ between the two ethnic groups (all pinteraction=0.28–0.97). CRP, IL-6 and TNF-α were not associated with CIMT (p>0.05). IL-6 was inversely related to NCR (r=−0.186, p=0.03), but it did not explain the relationship between NCR and CIMT.
Conclusions
Salivary cortisol0530hrs and NCR, but not CAR, nocturnal salivary cortisol 2200hrs, morning serum cortisol or overnight urinary free cortisol, were associated with CIMT, independent of relevant covariates, including inflammatory factors. A low awakening salivary cortisol or a blunted NCR may be related to increased atherosclerosis risk in overweight and obese minority youth. These findings support adult studies suggesting flattened daytime diurnal cortisol variation impacts cardiovascular disease risk.
Keywords: Obesity, cardiovascular risk, carotid artery, intima media thickness, cortisol
Introduction
Chronic psychosocial stress results in activation of the hypothalamic-pituitary-adrenal (HPA) axis and dysregulation of cortisol levels, and is associated with increased risk of atherosclerosis and coronary heart disease (Brotman et al., 2007; Troxel et al., 2003). However, the reported relationships between diurnal cortisol patterns and cardiovascular disease remain inconsistent (Dekker et al., 2008; Kumari et al., 2011; Vogelzangs et al., 2010). Circulating and salivary levels of cortisol are characterized by a diurnal pattern in which the cortisol concentration is at its highest in the morning, then gradually decreases from that point to its nadir in the late evening, and then rises again through the night. Rosmond and Bjorntorp suggested that a blunted diurnal cortisol pattern may serve as an indicator of “HPA exhaustion” (Rosmond and Bjorntorp, 2000). Moreover, they were the first to suggest that an “exhausted HPA axis” may lead to accelerated atherogenesis and cardiovascular disorders (Rosmond and Bjorntorp, 2000). Recent support for their hypothesis has shown that a blunted diurnal cortisol variation was associated with coronary calcification (Hajat et al., 2012; Matthews et al., 2006) and with all-cause cardiovascular mortality in older Caucasian adults (Kumari et al., 2011). The relationship between diurnal cortisol patterns and sub-clinical atherosclerosis has not been explored in any population of children.
Subclinical atherosclerosis has been shown to manifest in overweight children with cardiometabolic abnormalities. Carotid artery intima-media thickness (CIMT) is a noninvasive measure of early subclinical atherosclerosis that predicts cardiovascular disease (CVD) and has been shown to be elevated in overweight children and adolescents with metabolic abnormalities (Reinehr and Wunsch, 2011). Our laboratory has reported that overweight Latino youth with persistent high blood pressure and/or metabolic syndrome have higher CIMT when compared to their healthy overweight counterparts (Toledo-Corral et al., 2009). These reports indicate that cardiometabolic risk begins early in life and further study is warranted in pediatric cohorts.
Atherosclerosis is well known as an inflammatory disease. Cytokines, such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), have strong relationships to CIMT (Kablak-Ziembicka et al., 2011; Scuteri et al., 2011). Inflammatory processes have also been linked to the activation of the HPA axis activity (Turnbull and Rivier, 1999). Recently, a report from The Multi-Ethnic Study of Atherosclerosis showed that individuals with a flatten diurnal cortisol pattern had higher levels of IL-6 when compared to those with a non-flattened diurnal cortisol response (Desantis et al., 2012). Effectively, some investigators have suggested that an inflammatory mediating process is a plausible link between a disrupted HPA response and cardiovascular conditions, such as atherosclerosis (Nijm and Jonasson, 2009; Nijm et al., 2007).
Based on the literature in adults and on our studies of subclinical atherosclerosis in Latino youth, the overall aim of this study was to assess the relationship between overnight HPA axis activity and subclinical atherosclerosis in overweight minority children. The specific objectives of this study were to: 1) determine the relationship between overnight cortisol measures and CIMT in overweight and obese, African-American and Latino children; 2) assess ethnic differences in these relationships and 3) explore whether overnight cortisol and CIMT relationships are independent of inflammatory markers, C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).
METHODS
One hundred fifty-six overweight, but otherwise healthy, African-American and Latino boys and girls participated in the Diabetes Risk due to Ectopic Adiposity in Minority Youth (DREAM) study. Participants were recruited from the greater Los Angeles County through community health clinics, health fairs, and word of mouth and were required to meet the following inclusion criteria at baseline: 1) Either African-American or Latino ethnicity (all four grandparents of African-American or Latino descent); 2) age 8–17 yr; 3) BMI ≥85th percentile based on the age- and gender-based standards of the Centers for Disease Control and Prevention. Children were excluded if they had a previous major illness including Type 1 or 2 diabetes, took medications, or had any underlying condition known to influence body composition, insulin action, or insulin secretion (such as steroids or hypothyroidism). Participants and their parents provided written informed consent and assent. An additional 4 participants were diagnosed with type 2 diabetes at entry to the study, and were excluded from this analysis. The Institutional Review Board of the University of Southern California (USC)-Health Sciences Campus reviewed and approved the DREAM study.
All participants attended two visits at the Clinical Trials Unit (CTU) at the USC University Hospital. On the first visit, participants received a comprehensive medical history and physical examination by a licensed health care provider, including assessment of pubertal stage based on Tanner breast stage in girls and Tanner pubic hair stage in boys (Marshall and Tanner, 1969, 1970). Clinical staff collected vital signs, blood pressure in triplicate and performed a 2-hour oral glucose tolerance test (OGTT). Blood was sampled for glucose and insulin during the fasting state, 5 minutes prior to ingesting oral glucose solution (1.75 g of glucose/kg of body weight up to maximum of 75 g), and then sampled again 120 min after ingestion of glucose solution. Body composition measures were performed following the OGTT and included a waist circumference measurement and total body composition by dual-energy x-ray absorptiometry using a Hologic QDR 4500 W (Hologic, Bedford, MA).
CIMT was determined at the University of Southern California Atherosclerosis Research Unit Core Imaging and Reading Center as previously described (Selzer et al., 1994; Selzer et al., 2001). High-resolution B-mode ultrasound images were obtained over two successive cardiac cycles using a Siemens Acuson CV70 (13-MHz linear array; Siemens Medical Solutions Inc, Mountain View, California) imager. CIMT was measured from 70–100 computer-processed images taken along a 1-cm segment of the right distal common carotid artery, approximately 1 to 2 cm from the bifurcation into external and internal carotids. The same sonographer and reader performed all CIMT measures, and she was blinded to all participant information.
Within approximately 2 months (median: 24 days) following the outpatient visit, participants were admitted as inpatients for their second visit at the USC CTU in the late afternoon. Participants were served dinner and a snack before 2000 hrs, which marked the beginning of an overnight fast. Urine was collected from 2000 hrs to 0600 hrs the following day to determine overnight urinary free cortisol. To establish overnight salivary cortisol patterns, saliva was collected at bedtime ~2200 hrs and again the following morning when subjects were awakened at 0530 hrs by CTU nursing staff to collect saliva samples at 0530, 0545, and 0600 hrs. Samples were obtained using the Salivette system (Sarstedt, Newton, NC), which involved placing a cotton swab in the mouth for 2 minutes to passively absorb saliva at each designated time point. Saliva samples were processed immediately by centrifuging at 2500 rpm for 10 minutes and then frozen at −70° C until assayed. Measures of HPA activity included 2 traditional measures (urinary free cortisol and serum cortisol), as well as measures of diurnal patterns of salivary cortisol (Chida and Steptoe, 2009; Clow et al., 2004; Cochrane and Friesen, 1986). The nocturnal cortisol rise (NCR) was calculated as the increase in salivary cortisol2200 hrs to salivary cortisol0530 hrs, while the cortisol awakening response (CAR), was calculated as the difference in salivary cortisol0530 hrs and salivary cortisol0600 hrs. Our overnight protocol did not allow for multiple daytime cortisol collections to determine daytime cortisol slope, the more typical measure used by most neuroendocrine researchers (Edwards et al., 2001; Viardot et al., 2005), but it did offer the distinct advantage of a controlled setting for consistency of sampling. Fasting blood was drawn at ~0900 hrs to assess morning serum cortisol and lipids, followed immediately by an insulin-modified, frequentlysampled intravenous glucose tolerance test (Adam et al., 2010).
Assays
Glucose was assayed using a Yellow Springs Instruments analyzer (YSI INC., Yellow Springs, OH) that uses a membrane bound glucose oxidase technique. Lipids were assessed using Vitros Chemistry DT Slides (Johnson and Johnson Clinical Diagnostics, Inc, Rochester, New York). Insulin, salivary cortisol and serum cortisol were assayed using an automated enzyme immunoassay (Tosoh AIA 600 II analyzer, Tosoh Bioscience, Inc., South San Francisco, CA). The cortisol assay sensitivity was 0.02 µg/dl, inter-assay CV 11.2% and intra-assay CV 8.2%. In assay validation studies, we found that salivary cortisol levels from the Tosoh assay correlated strongly with simultaneously drawn total serum cortisol levels (r = 0.78, p<0.001). Assay of the same salivary samples by both the Tosoh assay and a commercial assay kit frequently used in salivary cortisol research (Salimetrics, State College, PA) showed very high correlations between both individual salivary cortisol samples (r= 0.92, p <0.001) and calculated CAR values (r=0.89, p<0.001), although absolute levels of salivary cortisol in the Tosoh assay were 2.78 ± 0.24 (β±SE) times higher than those obtained using the Salimetric kit (likely due to differences between the assays in antibody avidity, matrix, or other proprietary differences). Urinary free cortisol was assayed by Coat-A-Count RIA (Diagnostic Products, Los Angeles, CA) with correction of the total overnight urinary free cortisol (µg/total volume) for body surface area (m2)(Gomez et al., 1991). Serum blood samples from the fasting blood draw were assayed in duplicate for CRP using an ELISA kit (Chemicon International Inc.) with an inter-assay coefficient of 6.0%. Serum was also assayed in duplicate for interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) using ELISA immunoassays (Quantikine, R&D Systems, Minneapolis, MN, USA); inter-assay and intra-assay coefficients for IL-6 were 7.8% and 7.4%; inter-assay and intra-assay coefficients for TNF-α were 8.4% and 5.8%).
Statistical analysis
Tests of normality (histograms, Q-Q plots, and Shapiro Wilkes-test) were used to assess the distribution of the variables, and non-normally distributed variables (CRP, IL-6 and TNFα) were log transformed for analyses. CIMT was normally distributed after exclusion of one participant, who had a CIMT of 0.89 mm, who was deemed an extreme outlier (3.5 standard deviations above the mean). For descriptive purposes, independent t-tests and chi-square tests were used to determine mean physical and metabolic characteristics by ethnicity.
A priori covariates included age, height and total percent body fat, as they are well-established predictors of CIMT in children. To determine additional covariates, Pearson correlations were used to test the relationship between other known traditional correlates of CIMT in adults, including ethnicity, LDL-cholesterol, fasting glucose, fasting insulin, and systolic blood pressure. Of these, only ethnicity and systolic blood pressure were significantly associated with CIMT (p<0.05), and they were therefore included as covariates to any adjusted statistical models. Other potential predictors of CIMT, such as physical activity using acclerometry and history of smoking, were not assessed in this study.
Multivariate linear regression analysis was used for six separate models to determine the relationship between the dependent variable, CIMT, and each cortisol measure as the independent variable (salivary cortisol2200hrs, salivary cortisol0530 hrs, NCR, CAR, morning serum cortisol and overnight urinary free cortisol), while accounting for confounders of age, height, total percent body fat, ethnicity, and systolic blood pressure. Regression analysis also provided the amount of variation that each covariate and independent variable contributed to CIMT. When testing interactions, we added the appropriate interaction term to each linear regression model. For example, to test the interactive effects of NCR and ethnicity on CIMT, we added the term, NCR × ethnicity, to model 3. Standardized β coefficients (based on changes in standard deviation) were reported to allow for direct comparisons between independent variables and their influence on the dependent variable, CIMT. For interpretative purposes, for every one standard deviation change of the independent variable, the change of CIMT is the product of one standard deviation change of CIMT and the standardized β coefficient.
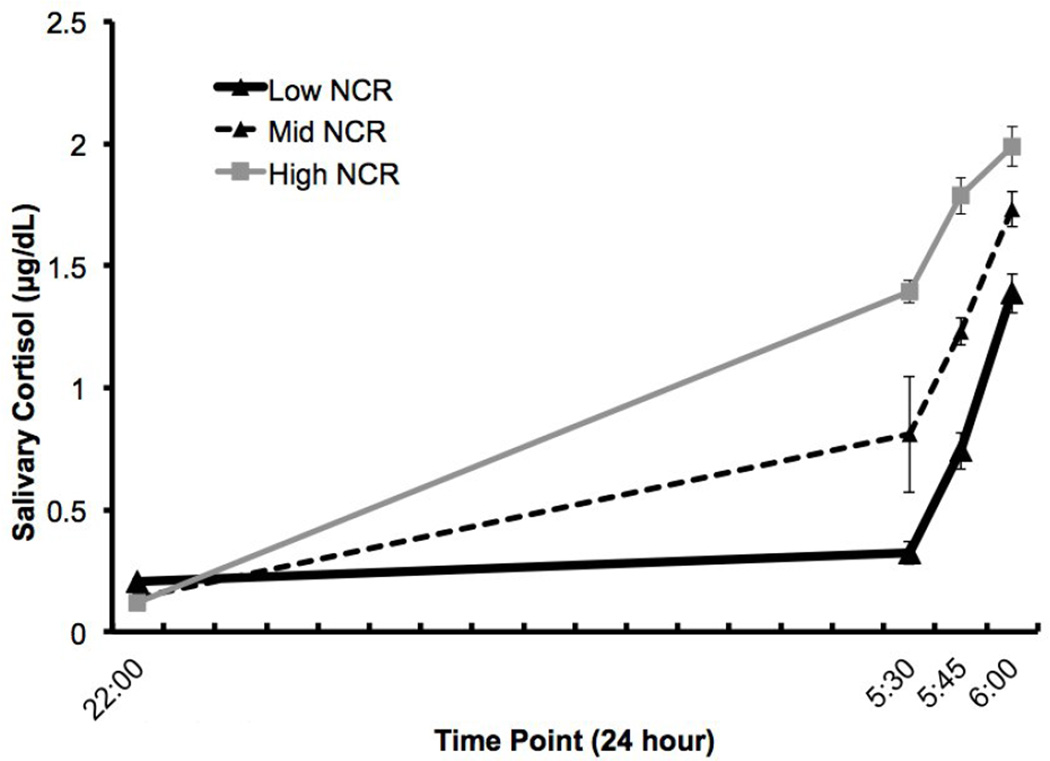
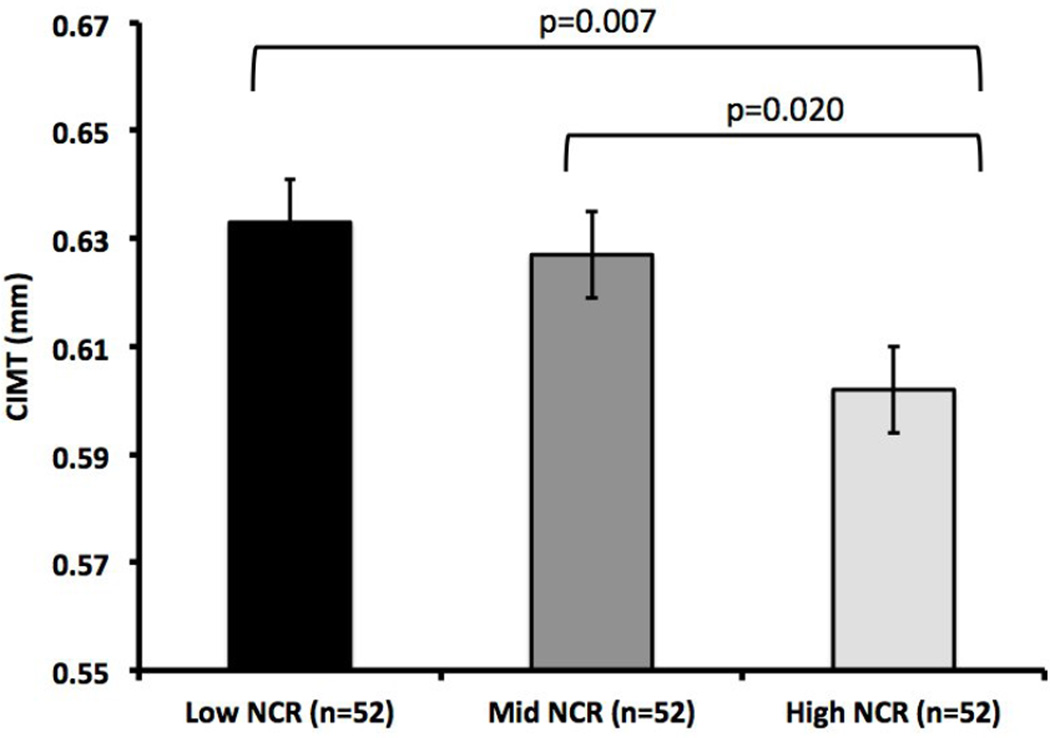
To graphically illustrate the differences in CIMT based on NCR patterns, the sample was divided into a LOW NCR group (Lowest tertile of NCR; <0.45 µg/dL, n=52), MIDDLE NCR (Middle tertile of NCR; 0.45 – 0.91µg/dL, n=52), and a HIGH NCR group (highest tertile of NCR; >0.92 µg/dL, n=52), Figure 1). To further illustrate the effects of NCR on CIMT, we used an ANCOVA to show the difference in CIMT, the dependent variable) by the three NCR groups (data shown in Figure 2), adjusting for age, height, total percent body fat, ethnicity, and systolic blood pressure.
Figure 1.
LOW NCR (n=52), MIDDLE NCR (n=52), and HIGH NCR (n=52).
Figure 2.
CIMT estimated marginal means (EMM) are shown ± SE and are adjusted for age, height, total percent body fat, ethnicity, and systolic blood pressure.
To explore the contribution of inflammation to the relationship between cortisol measures and CIMT, we first examined the unadjusted bivariate correlations between CRP, IL-6 and TNF-α with CIMT and with cortisol. Where relevant, inflammatory markers were added as covariates to the linear regression models described above (i.e. Models 2 and 3), and interactive effects of inflammation and cortisol on CIMT were also examined. Data were analyzed using SPSS for Mac version 18.0 (IBM Inc., Chicago, IL) with an a priori significance level of p<0.05.
RESULTS
The participants’ physical and metabolic characteristics are given in Table 1. There were slightly more males than females (86 vs. 70), and the group was composed of more Latinos than African-Americans (101 vs. 55). No significant differences were observed between the two ethnic groups in body composition (height, weight, BMI, BMI percentile, total percent fat) and blood pressure. Compared to African-Americans, Latinos had higher morning serum cortisol (11.5±3.9 vs. 9.6±3.1 µg/dL, p=0.003) and lower CAR (0.68±0.52 vs. 0.97±0.65 µg/dL, p=0.008). There were no significant ethnic differences in NCR, the individual salivary cortisol2200 hrs, salivary cortisol0530 hrs, or urinary free cortisol (p=0.14–0.39). The African-American group had 5% higher CIMT than the Latino group (0.644±0.001 vs. 0.615±0.001 mm; p<0.001).
Table I.
Physical and metabolic characteristics the sample population (n=156)
| Mean ± SD or % Frequency |
Range | |
|---|---|---|
| Age (years) | 15.1 ± 2.4 | 8–18 |
| Gender (% Male/ % Female) | 55 / 45 | - |
| Ethnicity (% African-American/ % Latino) | 35 / 65 | - |
| Pubertal Stage by Tanner (%) | ||
| 1 | 11 | - |
| 2 | 8 | - |
| 3 | 1 | - |
| 4 | 16 | - |
| 5 | 64 | - |
| Height (cm) | 162.2 ± 12.5 | 126.1 – 192.9 |
| Weight (kg) | 84.0 ± 22.6 | 32.3 – 144.0 |
| BMI (kg/m2) | 31.5 ± 6.3 | 18.8 – 51.6 |
| BMI percentile | 96.5 ± 3.3 | 85.9 – 99.9 |
| BMI z-score | 1.98 ± 0.46 | 1.08 – 3.21 |
| Total Percent Fat (%) | 36.7 ± 7.4 | 16.7 – 54.2 |
| CIMT (mm) | 0.622 ± 0.058 | 0.47 – 0.76 |
| Traditional CIMT correlates | ||
| Systolic Blood Pressure (mmHg) | 117.0 ± 12.4 | 92.0 – 156.0 |
| Diastolic Blood Pressure (mmHg) | 66.3 ± 6.3 | 50.0 – 86.0 |
| Fasting glucose (mg/dL) | 86.7 ± 6.4 | 66.0 – 111.0 |
| Fasting insulin (µU/ml) | 16.6 ± 12.2 | 2.9 – 102.9 |
| LDL-cholesterol (mg/dL) | 80.8 ± 27.9 | 14.0 – 172.0 |
| HDL-cholesterol (mg/dL) | 40.7 ± 12.1 | 24.0 – 92.0 |
| Cortisol measures | ||
| Salivary Cortisol2200 hrs (µg/dL) | 0.155 ± 0.161 | 0.01 – 1.32 |
| Salivary Cortisol0530hrs (µg/dL) | 0.840 ± 0.507 | 0.01 – 2.59 |
| Nocturnal Cortisol Rise (µg/dL) | 0.685 ± 0.527 | −0.39 – 2.53 |
| Cortisol Awakening Response (µg/dL) | 0.861 ± 0.624 | −0.53 – 2.90 |
| Serum Cortisol (µg/dL) | 10.8 ± 3.8 | 2.5 – 21.2 |
| Urinary Free Cortisol (µg/m2) | 5.06 ± 5.38 | 0.02 – 36.40 |
| Inflammatory Markers | ||
| CRP (ng/mL) | 2438 ± 3069 | 6.5 – 21834.6 |
| IL-6 (pg/mL) | 2.04 ± 2.32 | 0.36 – 19.17 |
| TNF-α (pg/mL) | 1.66 ± 1.78 | 0.07 – 15.93 |
Results of linear regression modeling to determine the relationship between cortisol measures and CIMT are shown in Table 2. CIMT was significantly predicted by salivary cortisol0530 hrs (Model 2) and NCR (Model 3), but not by salivary cortisol2200 hrs (Model 1) or CAR (Model 4). In Model 3, this would be interpreted that for every 1 standard deviation decrease of NCR (0.527 µg/dL), CIMT increased by 0.013mm. NCR and salivary cortisol0530 hrs each accounted for ~4.5% of the variance in CIMT in their respective models, beyond the 10% variance accounted for by the other covariates of age, height, total percent body fat, ethnicity, and systolic blood pressure. Neither morning serum cortisol nor urinary free cortisol predicted CIMT (data not shown, p= 0.59 and 0.76). Ethnicity was a significant predictor of CIMT in all models, accounting for 5.3 – 5.7% of the variance in CIMT, with African Americans having higher CIMT than Latinos (p<0.01 for all models). However, ethnicity had no effect on the relationships between any of the cortisol measures and CIMT, indicating that the relationship between cortisol predictors of CIMT was the same in both African-American and Latino participants (pinteraction = 0.28–0.97).
Table 2.
Multiple regression analysis of salivary cortisol measures on CIMT.
| Dependent Variable: CIMT | R2 | Standardized β |
p-value |
|---|---|---|---|
| Model 1: Salivary Cortisol2200 hrs | |||
| Age | 0.000 | −0.098 | 0.398 |
| Height | 0.023 | 0.062 | 0.613 |
| Total Percent Fat | 0.028 | −0.067 | 0.423 |
| Systolic Blood Pressure | 0.045 | 0.177 | 0.052 |
| Ethnicity | 0.100 | −0.232 | 0.004 |
| Single Salivary Cortisol2200 hrs | 0.101 | 0.035 | 0.661 |
| Model 2: Salivary Cortisol0530 hrs | |||
| Age | 0.000 | −0.122 | 0.274 |
| Height | 0.023 | 0.063 | 0.612 |
| Total Percent Fat | 0.028 | −0.074 | 0.366 |
| Systolic Blood Pressure | 0.045 | 0.161 | 0.069 |
| Ethnicity | 0.100 | −0.208 | 0.008 |
| Single Salivary Cortisol0530 hrs | 0.144 | −0.215 | 0.006 |
| Model 3: Nocturnal Cortisol Rise | |||
| Age | 0.000 | −0.140 | 0.211 |
| Height | 0.023 | 0.084 | 0.499 |
| Total Percent Fat | 0.028 | −0.067 | 0.413 |
| Systolic Blood Pressure | 0.045 | 0.156 | 0.079 |
| Ethnicity | 0.100 | −0.204 | 0.009 |
| Nocturnal Cortisol Rise | 0.146 | −0.220 | 0.005 |
| Model 4: Cortisol Awakening Response | |||
| Age | 0.000 | −0.072 | 0.553 |
| Height | 0.025 | 0.074 | 0.595 |
| Total Percent Fat | 0.033 | −0.088 | 0.316 |
| Systolic Blood Pressure | 0.044 | 0.143 | 0.133 |
| Ethnicity | 0.097 | −0.237 | 0.005 |
| Cortisol Awakening Response | 0.097 | 0.029 | 0.736 |
Figure 2 demonstrates that when compared to the HIGH NCR group, the LOW NCR group had significantly higher CIMT (0.633±0.008 vs. 0.602±0.008 mm, p=0.007) after adjusting for age, height, total percent body fat, ethnicity, and systolic blood pressure. Likewise, the MIDDLE NCR group had significantly higher CIMT when compared to the HIGH NCR group (0.627±0.008 vs. 0.602±0.008 mm, p=0.02) after adjusting for covariates. The overall model had a significant negative trend (p=0.014).
There were no significant relationships between CIMT and any of the inflammatory markers (all p>0.05). Bivariate correlations revealed that only salivary cortisol 0530hrs and NCR had a relationship with IL-6 (p<0.05) where a lower salivary cortisol 0530hrs and NCR was associated with higher IL-6. None of the other cortisol measures were significantly related to IL-6, CRP, or TNF-α (p>0.05). When we added IL-6 to our linear regression models 2 and 3, our results remained significant whereby both salivary cortisol 0530hrs and NCR remained significant predictors of CIMT (model 2: βstandardized=−0.19, p=0.017 and model 3: βstandardized=−0.202, p=0.014, respectively). Additionally, the interactive effects of NCR and IL-6 with CIMT were not significant (pinteraction=0.67). The interactions of CRP and TNF-α with NCR were also not significant predictors of CIMT (CRP: pinteraction =0.19 and TNF-α: pinteraction =0.83).
DISCUSSION
The primary aim of this study was to examine the relationship between overnight salivary cortisol patterns and CIMT in overweight African-American and Latino children. We found that NCR was negatively associated with CIMT, independent of age, height, percent body fat, ethnicity, and systolic blood pressure. As a secondary finding, ethnicity was also an independent predictor of CIMT, whereby African-American children had significantly higher CIMT than Latino children. However there were no differences in the relationship between NCR and CIMT by ethnic group. To our knowledge, this is the first study to show a unique relationship between a blunted NCR and CIMT in minority children. We also discovered that while the inflammatory factor IL-6 was higher in subjects with LOW NCR, the association between NCR and CIMT was independent of IL-6.
A blunted or flattened diurnal cortisol pattern from morning to evening has been associated with negative health outcomes in adult populations, including type 2 diabetes, cardiovascular disease, and stroke (Dekker et al., 2008; Kumari et al., 2011; Rosmond and Bjorntorp, 2000). In a recent population study it is reported that in Caucasian adults, a bedtime cortisol and a flatter slope in cortisol across the day, but not morning cortisol or CAR, was associated with increased risk of cardiovascular disease mortality (Kumari et al., 2011). Although the exact causes of a flattened diurnal cortisol curve have not been elucidated, Rosmond and Bjornthrop and others hypothesized that this might emanate from HPA exhaustion, possibly due to excessive psychosocial stress causing changes to circadian rhythms (Heim et al., 2000). While the diurnal cortisol pattern is classically measured by taking cortisol readings throughout the day beginning in the morning and ending in the late evening (Kirschbaum and Hellhammer, 1989; Weitzman et al., 1971), we measured salivary cortisol changes from nighttime nadir to morning. It is logistically more practical to collect at these times, requires fewer overall samples, and avoids the potentially confounding effects of daytime variability in meals and acute stressors that could affect cortisol levels at any given individual time point. In addition, the NCR would be expected to similarly capture the overall pattern of variation in daily cortisol levels, since intra-individual day-to-day cortisol pattern variability is fairly consistent (Edwards et al., 2001; Kirschbaum and Hellhammer, 1989; Ranjit et al., 2009; Weitzman et al., 1971). In the present study, the association between a flattened NCR and higher CIMT thus supports findings in adults linking a blunted diurnal cortisol slope and overt cardiovascular disease.
Flattened diurnal cortisol patterns have been found to be more common in certain populations, such as people who work night shifts and certain ethnic minority groups (Hajat et al., 2010). It has been reported that African-Americans and Latinos have flatter diurnal cortisol slopes than their Caucasian counterparts (Cohen et al., 2006). The same ethnic difference in diurnal cortisol patterns was also observed in a multi-ethnic community sample of adolescents (DeSantis et al., 2007). DeSantis et al. theorized that exposure to psychosocial stress (such as racial discrimination) and the social environment of African-American and Latino children could well be contributing to these blunted cortisol responses (DeSantis et al., 2007). Together with the literature, our findings suggest that minority populations with blunted diurnal cortisol patterns may be at higher risk for subclinical atherosclerosis. Lack of an interaction between ethnicity and cortisol patterns in our subjects suggest the risk of blunted overnight cortisol pattern is the same in African American and Latino youth in terms of subclinical atherosclerotic disease. However, it is interesting to note that although ethnicity did not have an interactive role in NCR and CIMT, ethnicity was an independent predictor of CIMT, whereby African-Americans had higher CIMT than Latino children. Although this was not surprising since it is well known that African-Americans adults have the highest CIMT compared to all other ethnicities or races, our paper is one of few (Li et al., 2007; Whincup et al., 2012) to confirm this in children.
Some investigators have shown relationships between other markers of HPA axis activity and CIMT, such as CAR in adult women (Hurwitz Eller et al., 2001), serum cortisol in Caucasian children (Soriano-Rodriguez et al., 2010), and urinary free cortisol in children with type 1 diabetes (Peppa-Patrikiou et al., 1998). In contrast, we found that morning salivary cortisol and a blunted nocturnal cortisol rise were the only HPA axis markers among those we assessed that were associated with elevated CIMT. Our finding that the morning salivary cortisol measure, and not the nocturnal salivary measure, was a correlate of CIMT, implies that it is the early morning cortisol that is driving the NCR-CIMT relationship. A single salivary cortisol measure at awakening may therefore be sufficient to predict CIMT levels, which would be useful in future large scale studies where it may not be possible to obtain controlled overnight measures. Although we did not show any relationships between CIMT and other HPA measures in this study, we have previously shown that serum cortisol might also be a marker of altered HPA axis activity associated with other metabolic disease risk, such as metabolic syndrome (Weigensberg et al., 2008) and longitudinal declines in insulin sensitivity (Adam et al., 2010). Our finding that, salivary cortisol0530hrs and NCR were the only HPA measures associated with CIMT is consistent with Kumari’s findings in adults, as well as with the general literature showing “inconsistent” links between various HPA biomarkers and disease outcomes (Dekker et al., 2008; Kumari et al., 2011; Vogelzangs et al., 2010). Such apparent inconsistencies are likely the result of the innate complexities of the HPA axis, as well as variations in measurement protocols and study populations.
High blood pressure has been shown to be related to both chronic psychosocial stress and elevated CIMT in adults and children and therefore could be an important confounder of HPA activity and cardiovascular risk. In a study of adolescents, systolic blood pressure reactivity was related to increased chronic stress and elevated CIMT (Low et al., 2009; Roemmich et al., 2011). Holt-Lunstad and Steffen showed that a decreased diurnal cortisol variation is associated with decreased diurnal variation of blood pressure (Holt-Lunstad and Steffen, 2007), the latter of which is also associated with an increase in cardiovascular morbidity in adults (Ben-Dov et al., 2007). It is unknown whether variations of nocturnal blood pressures patterns are relevant to health outcomes in children, but it has been suggested that a lack of nocturnal blood pressure decrease (or dipping) in young adults may indicate future cardiovascular risk (Parati et al., 1992). While we did not find morning systolic blood pressure to be a predictor of CIMT in the present study, the study design did not include either stress-induced cardiovascular reactivity or nocturnal blood pressure tracking. Future studies should include these measures to fully elucidate the relationship between HPA axis activity, blood pressure, and cardiovascular risk in minority children.
Our exploratory aim was to examine the interrelationships of cortisol, inflammatory factors and CIMT. CRP and TNF-alpha did not have any associations with cortisol measures or CIMT. However, the LOW NCR group had significantly higher IL-6 levels than did the HIGH NCR Group (2.5±0.4 vs 1.7±0.2ug/dL, p=0.04), but IL-6 was not related to CIMT (p>0.05). Although we did not have a large enough sample to do a full mediation analyses, adding IL-6 to our ANCOVA model did not alter the relationship between the blunted NCR and elevated CIMT. This does not preclude the notion of an inflammatory pathway mediating the observed relationship, since other inflammatory factors could play a role in this process, particularly cytokines related to vascular function, such as matrix metalloproteinase-9 (Szymanowski et al., 2011). Given the role of cortisol in modulating immune system function (Desantis et al., 2012), further study of obesity-related inflammatory factors in the context of HPA activity and their contribution to sub-clinical atherosclerosis risk are warranted.
The novelty and strength of this study is that no literature currently exists showing a relationship between a blunted nocturnal salivary cortisol rise and CIMT in any pediatric population. Our study population is composed of a homogenous group of understudied, high disease risk children that allows us to examine the degree of risk imparted by alterations in cortisol measures above and beyond body fat in those youth most at risk for cardiometabolic disease. Several limitations must also be discussed. First, it is important to point out that the study’s cross-sectional design limits our ability to discern any causative effects of nocturnal cortisol patterns and sub-clinical atherosclerosis. Next, the pediatric cohort used for this analysis was composed of overweight and obese African-American and Latino children that were recruited primarily for the purpose of studying ectopic fat deposition and type 2 diabetes risk in minority youth (manuscript in review). This study design imposed many limitations for the current analysis addressing the study of HPA activity. First, the participants were from the inner city of Los Angeles, representative of communities with increased exposure to stressful situations, such as low SES and higher crime rates compared to the rest of Los Angeles County. Hence, our results cannot be generalized to other SES populations. Second, our sample was composed only of overweight and obese, minority children and adolescents. Future investigations would benefit from a broader sampling approach to include adolescents across all BMI percentiles and to include other ethnic groups for comparison of cortisol patterns and sub-clinical atherosclerosis risk. Thirdly, our sample is composed solely of two minority populations, African-American and Latino, precluding ethnic comparisons to non-minority populations. Another limitation of the study design was that it only allowed for salivary cortisol measures during one night to morning, 8-hr cycle. Salivary cortisol data are typically collected over 24-hour periods for multiple days to assess an average diurnal cortisol slope (Viardot et al., 2005). Measurement of CAR is particularly subject to situational changes in stress state, and measurement on at least 2 separate days is generally used to determine trait status (Hellhammer et al., 2007). However, our protocol involving very standardized procedures and sampling techniques during a controlled overnight environment, may have eliminated many of the confounding variables that lead to state changes in cortisol patterns found during outpatient collection protocols (Hellhammer et al., 2007). It may be that determination of NCR may be adequate to capture the overall degree of variation in daily cortisol levels, since intra-individual daily cortisol patterns are fairly consistent (Edwards et al., 2001; Kirschbaum and Hellhammer, 1989; Viardot et al., 2005; Weitzman et al., 1971). Future studies investigating HPA activity and metabolic status in youth should ideally include cortisol collections over the full 24-hour period on several different days to allow direct comparison between NCR and daytime cortisol slope as measures of HPA activity.
In conclusion, we have found that overnight rise in salivary cortisol and morning salivary cortisols were negatively related to CIMT, a measure of subclinical atherosclerosis, in African-American and Latino youth who are overweight and obese. This relationship was independent of traditional risk factors including age, height, total percent body fat, ethnicity, systolic blood pressure, as well as inflammatory cytokines. As a secondary finding, while African-American ethnicity was an independent predictor of CIMT, ethnicity did not alter the relationship between NCR and CIMT. Our data are consistent with the theory that chronic psychosocial stress may result in a blunted nocturnal cortisol rise and increased risk for atherosclerosis in overweight and obese minority youth. Future studies should attempt to further clarify the relationships between subjective stress, diurnal cortisol patterns, and cardiovascular and metabolic risk in adolescents, which would validate development of stress-reduction interventions aimed at decreasing cardiovascular disease risk in overweight and obese youth.
Acknowledgements
C.M. Toledo-Corral and M.J. Weigensberg had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This study is supported by NCMHD grant P60MD002254 (M.I. Goran and M.J. Weigensberg). C.M. Toledo-Corral is supported by an American Diabetes Association (ADA) Postdoctoral Fellowship (7-10-MI-04). Quintilia Ávila, MPH and Michelle Muñevar, MPH, contributed significantly to our clinical data collection and management. We would also like to thank the participants and their families.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Contributors
Claudia Toledo-Corral was the primary contributor to the conception of the research question, statistical analysis, interpretation of results and manuscript writing. She also contributed to data management and organization.
Samantha Myers contributed to the statistical analysis, interpretation of results and review of manuscript.
Yanjie Li contributed significantly to data collection and interpretation, as well as review of manuscript.
Howard Hodis and Michael Goran contributed to the study design, interpretation of results and review of manuscript.
Marc Weigensberg contributed to conception of the research question and study design, interpretation of results and manuscript writing/revision.
References
- Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le KA, Mahurkar S, Lane CJ, Weigensberg MJ, Goran MI. Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. J. Clin. Endocrinol. Metab. 2010;95:4729–4735. doi: 10.1210/jc.2010-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49:1235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262. [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cochrane G, Friesen J. Hypnotherapy in weight loss treatment. J.Consult Clin.Psychol. 1986;54:489. doi: 10.1037//0022-006x.54.4.489. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom. Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Dekker MJ, Koper JW, van Aken MO, Pols HA, Hofman A, de Jong FH, Kirschbaum C, Witteman JC, Lamberts SW, Tiemeier H. Salivary cortisol is related to atherosclerosis of carotid arteries. J. Clin. Endocrinol. Metab. 2008;93:3741–3747. doi: 10.1210/jc.2008-0496. [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J. Adolesc. Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Desantis AS, Diezroux AV, Hajat A, Aiello AE, Golden SH, Jenny NS, Seeman TE, Shea S. Associations of salivary cortisol levels with inflammatory markers: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012;37:1009–1018. doi: 10.1016/j.psyneuen.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Clow A, Evans P, Hucklebridge F. Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity. Life Sci. 2001;68:2093–2103. doi: 10.1016/s0024-3205(01)00996-1. [DOI] [PubMed] [Google Scholar]
- Gomez MT, Malozowski S, Winterer J, Vamvakopoulos NC, Chrousos GP. Urinary free cortisol values in normal children and adolescents. J. Pediatr. 1991;118:256–258. doi: 10.1016/s0022-3476(05)80496-2. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N, Castro C, Watson K, Sanchez B, Kirschbaum C. Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: the multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2010;35:932–943. doi: 10.1016/j.psyneuen.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Sanchez BN, Holvoet P, Lima JA, Merkin SS, Polak JF, Seeman TE, Wu M. Examining the association between salivary cortisol levels and subclinical measures of atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.10.007. Nov 9 2012 Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: state- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom. Med. 2007;69:339–343. doi: 10.1097/PSY.0b013e318050d6cc. [DOI] [PubMed] [Google Scholar]
- Hurwitz Eller N, Netterstrom B, Hansen AM. Cortisol in urine and saliva: relations to the intima media thickness, IMT. Atherosclerosis. 2001;159:175–185. doi: 10.1016/s0021-9150(01)00487-7. [DOI] [PubMed] [Google Scholar]
- Kablak-Ziembicka A, Przewlocki T, Sokolowski A, Tracz W, Podolec P. Carotid intima-media thickness, hs-CRP and TNF-alpha are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis. 2011;214:185–190. doi: 10.1016/j.atherosclerosis.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kumari M, Shipley M, Stafford M, Kivimaki M. Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: findings from the Whitehall II study. J. Clin. Endocrinol. Metab. 2011;96:1478–1485. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chen W, Srinivasan SR, Tang R, Bond MG, Berenson GS. Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima-media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis. 2007;194:421–425. doi: 10.1016/j.atherosclerosis.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Low CA, Salomon K, Matthews KA. Chronic life stress, cardiovascular reactivity, and subclinical cardiovascular disease in adolescents. Psychosom. Med. 2009;71:927–931. doi: 10.1097/PSY.0b013e3181ba18ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K, Schwartz J, Cohen S, Seeman T. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom. Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- Nijm J, Jonasson L. Inflammation and cortisol response in coronary artery disease. Ann. Med. 2009;41:224–233. doi: 10.1080/07853890802508934. [DOI] [PubMed] [Google Scholar]
- Nijm J, Kristenson M, Olsson AG, Jonasson L. Impaired cortisol response to acute stressors in patients with coronary disease. Implications for inflammatory activity. J. Intern. Med. 2007;262:375–384. doi: 10.1111/j.1365-2796.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- Parati G, Ravogli A, Giannattasio C, Mutti E, Trazzi S, Villani A, Mancia G. Changes in 24 hour blood pressure and in cardiac and vascular structure in normotensive subjects with parental hypertension. Clin. Exp. Hypertens. A. 1992;14:67–83. doi: 10.3109/10641969209036172. [DOI] [PubMed] [Google Scholar]
- Peppa-Patrikiou M, Scordili M, Antoniou A, Giannaki M, Dracopoulou M, Dacou-Voutetakis C. Carotid atherosclerosis in adolescents and young adults with IDDM. Relation to urinary endothelin, albumin, free cortisol, and other factors. Diabetes Care. 1998;21:1004–1007. doi: 10.2337/diacare.21.6.1004. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Sanchez B, Seeman T, Shea S, Shrager S, Watson K. Association of salivary cortisol circadian pattern with cynical hostility: multi-ethnic study of atherosclerosis. Psychosom. Med. 2009;71:748–755. doi: 10.1097/PSY.0b013e3181ad23e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, Wunsch R. Intima media thickness-related risk factors in childhood obesity. Int. J. Pediatr. Obes. 2011;6(Suppl 1):46–52. doi: 10.3109/17477166.2011.590199. [DOI] [PubMed] [Google Scholar]
- Roemmich JN, Feda DM, Seelbinder AM, Lambiase MJ, Kala GK, Dorn J. Stress-induced cardiovascular reactivity and atherogenesis in adolescents. Atherosclerosis. 2011;215:465–470. doi: 10.1016/j.atherosclerosis.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Orru M, Morrell C, Piras MG, Taub D, Schlessinger D, Uda M, Lakatta EG. Independent and additive effects of cytokine patterns and the metabolic syndrome on arterial aging in the SardiNIA Study. Atherosclerosis. 2011;215:459–464. doi: 10.1016/j.atherosclerosis.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer RH, Hodis HN, Kwong-Fu H, Mack WJ, Lee PL, Liu CR, Liu CH. Evaluation of computerized edge tracking for quantifying intima-media thickness of the common carotid artery from B-mode ultrasound images. Atherosclerosis. 1994;111:1–11. doi: 10.1016/0021-9150(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Selzer RH, Mack WJ, Lee PL, Kwong-Fu H, Hodis HN. Improved common carotid elasticity and intima-media thickness measurements from computer analysis of sequential ultrasound frames. Atherosclerosis. 2001;154:185–193. doi: 10.1016/s0021-9150(00)00461-5. [DOI] [PubMed] [Google Scholar]
- Soriano-Rodriguez P, Osiniri I, Grau-Cabrera P, Riera-Perez E, Prats-Puig A, Carbonell-Alferez M, Schneider S, Mora-Maruny C, De Zegher F, Ibanez L, Bassols J, Lopez-Bermejo A. Physiological concentrations of serum cortisol are related to vascular risk markers in prepubertal children. Pediatr. Res. 2010;68:452–455. doi: 10.1203/PDR.0b013e3181efc310. [DOI] [PubMed] [Google Scholar]
- Szymanowski A, Nijm J, Kristenson M, Jonasson L. Elevated levels of circulating matrix metalloproteinase-9 are associated with a dysregulated cortisol rhythm-A case-control study of coronary artery disease. Psychoneuroendocrinology. 2011;36:139–143. doi: 10.1016/j.psyneuen.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Toledo-Corral CM, Ventura EE, Hodis HN, Weigensberg MJ, Lane CJ, Li Y, Goran MI. Persistence of the metabolic syndrome and its influence on carotid artery intima media thickness in overweight Latino children. Atherosclerosis. 2009;206:594–598. doi: 10.1016/j.atherosclerosis.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 2003;22:300–309. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Muller B. Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J. Clin. Endocrinol. Metab. 2005;90:5730–5736. doi: 10.1210/jc.2004-2264. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BW. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J. Clin. Endocrinol. Metab. 2010;95:4959–4964. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Whincup PH, Nightingale CM, Owen CG, Rapala A, Bhowruth DJ, Prescott MH, Ellins EA, Donin AS, Masi S, Rudnicka AR, Sattar N, Cook DG, Deanfield JE. Ethnic differences in carotid intima-media thickness between UK children of black African-Caribbean and white European origin. Stroke. 2012;43:1747–1754. doi: 10.1161/STROKEAHA.111.644955. [DOI] [PMC free article] [PubMed] [Google Scholar]