Abstract
We previously showed that pre-exposure of the cornea to Toll-like receptor 5 ligand flagellin induces profound mucosal innate protection against infections by modifying gene expression. Taking advantage of easily procurable epithelial cell population, this study is the first report to use genome-wide cDNA microarray approach to document genes associated with flagellin-induced protection against Pseudomonas aeruginosa in corneal epithelial cells (CECs). Infection altered the expression of 675 genes (497 up and 178 down), while flagellin pretreatment followed by infection resulted in a great increase in 890 gene upregulated and 37 genes downregulated. Comparing these two groups showed 209 differentially expressed genes (157 up, 52 down). Notably, among 114 genes categorized as defense related, S100A8/A9 are the two most highly induced genes by flagellin, and their expression in the corneal was confirmed by realtime PCR and immunohistochemistry. Neutralization of S100A8 and, to a less extent, A9, resulted in significantly increased bacterial burden and severe keratitis. Collectively, our study identifies many differentially expressed genes by flagellin in CECs in response to Pseudomonas. These novel gene expression signatures provide new insights and clues into the nature of protective mechanisms established by flagellin and new therapeutic targets for reducing inflammation and for controlling microbial infection.
INTRODUCTION
The protective ability of innate defense is largely dependent on germ-line-encoded pattern-recognition receptors.1 Prominent among pattern-recognition receptors are the Toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns to initiate innate immune responses, including inflammatory mediators, antimicrobial effectors, and signals inducing adaptive immune responses.2 Discovery of TLRs and other pathogen-associated molecular pattern-recognizing receptors has also put the epithelium, a barrier between internal and external environments, at center stage not only in defending the host against infection at the front line but also in influencing the development and direction of immune cell response to ensure clearance of the invading pathogen.3–8 A large body of recent literature revealed that mucosal epithelia, including the ocular surface, express almost all TLRs, rendering them able to sense and to mount the innate defense against invading pathogens.9 Conversely, the epithelial innate and inflammatory response to pathogens, if not properly controlled, can also cause tissue damage, resulting in the development of human diseases, such as corneal haze and scarring,10 airway asthma,11 and allergic rhinitis.12 Multiple regulatory mechanisms exist in epithelial cells to control the inflammatory response, including the expression of negative regulators13 and induction of hyporesponsiveness, to further and more rigorously challenge the same or different pathogens, a phenomenon similar to endotoxin tolerance.14 It is now well-known that pre-exposure of different cells, tissues, or organisms to TLR ligands dampens the expression of inflammatory cytokines, while exhibits no effect or even augments the induced expression of other functional groups of genes such as antimicrobial, anti-oxidative, and/or cytoprotective genes in response to pathogens and other adverse challenges, a phenomenon similar to endotoxin tolerance or lipopolysaccharide-reprogramed TLR response.15–18
As an immune-privileged site, the avascular cornea contains mechanisms that limit immune cell entry and has scant antigen-presenting cells.19,20 Under normal conditions, the cornea is remarkably resistant to infection. However, when the epithelial barrier is breached, which often occurs during routine contact lens wearing, or when immune function is compromised, opportunistic pathogens can gain access to the deep layers of the epithelium, causing infectious keratitis.21–24 If not treated promptly and properly, significant vision loss or even loss of the eye may occur.25–27 Among pathogens, Pseudomonas aeruginosa is a major cause of keratitis and is the most sight threatening.28 Our previous work showed that P. aeruginosa is recognized by TLR5 and that mice deficient in TLR5 are more susceptible to the pathogens.18,22,29 We have also shown that application of purified flagellin, the ligand of TLR5, before microbial inoculation induces profound protection in the cornea against infectious pathogens regardless of whether or not they express flagellin.17,18 Similar protection against P. aeruginosa was also observed in the lung by nasal application of flagellin.30 In addition, flagellin was also reported to induce protection against lethal radiation and chemicals in mice and monkeys,31,32 to restore antibiotic-impaired innate immune defenses,33 and to protect mice from acute Clostridium difficile colitis.34 Among all known TLR ligands, the flagellin–TLR5 axis, but not the flagellin–Ipaf pathway,35,36 exhibits several distinctive properties: stimulating mucosal epithelial cells more than immune cells,37 inducing unique anti-inflammatory genes (such as interleukin (IL)-1Ra, but not IL-1β),32,38 and preserving epithelial barrier function.33,34 However, to date, a genome-wide screen for genes and pathways affected by flagellin preconditioning in the mucosal epithelia and their roles in subsequent protection of mucosal tissues have not been reported.
In this study, we took advantage of the topical application of flagellin and readily isolated epithelial cells from the cornea and used genome-wide cDNA microarray to profile gene expression in P. aeruginosa–infected CECs with or without flagellin pretreatment. We identified 1261 genes with more than two fold changes (n = 6, P<0.05) in response to infection; among those, 209 genes were altered by flagellin pretreatment. Targeting the 2 most highly induced genes, S100A8 and S100A9, we found that neutralizing these two proteins increased susceptibility of the cornea to P. aeruginosa infection and abolished flagellin-induced protection in B6 mice. Based on data mining, we constructed a corneal innate defense network, activation of which promotes mucosal innate immunity and renders tissue resistance to invading pathogens.
RESULTS
Flagellin-induced protection
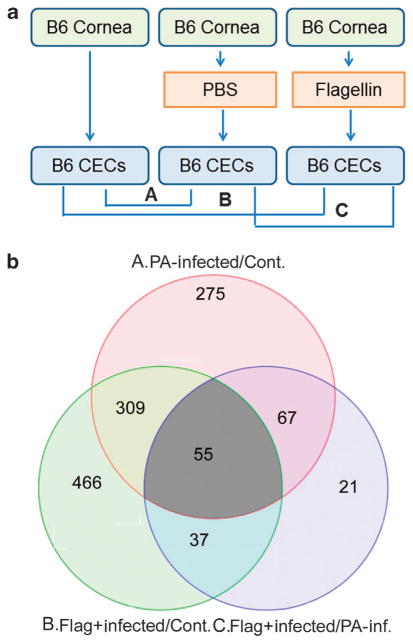
We previously showed that flagellin preconditioning resulted in the resistance to ocular and lung pathogenic microbes with or without expressing flagellin, suggesting a general, broad innate protection against infection. We reasoned that genes differentially expressed in flagellin-pretreated and P. aeruginosa–infected corneas, compared with P. aeruginosa–infected corneas, are responsible for the innate protection observed in the cornea and performed genome-wide microarray expression analysis to compare genes expressed in P. aeruginosa–infected corneal epithelial cells (CECs) with or without flagellin pretreatment at 6 hours post-infection (hpi; Figure 1). In our previous studies, we used P. aeruginosa ATCC 19660 strain, the most virulent strain, to illustrate the profound mucosal protective effects. However, as ATCC 19660 is known to possess epithelial cytotoxicity and cause epithelial erosion, we used a laboratory strain PA01 with 10-fold in inoculate dosage, 105 colony-forming units (CFU)/per eye in phosphate-buffered saline (PBS). As our focus was on differentially expressed genes in flagellin-pretreated CECs, we prepared three biological replicates of CECs obtained directly by scrapping cells off from the corneas: scratched and topical applied 5 μl PBS or 500 ng purified flagellin in 5 μl PBS, followed by 1 × 105 PA01 infection, with no injury and no infection naive CECs as the control. The six-sample (array spot) Illumina MouseWG-6 v2 BeadChip (San Diego, CA) was used. Each array spot contains a total of 45,281 different oligonucleotide gene probes (i.e., Illumina source IDs,). After the normalization of raw data for all three replicates, we selected those genes that exceeded a pre-set threshold for significant changes ≥2-fold (both increase or decrease) in expression intensity between infected/normal, pretreated-infected/normal, and pretreated-infected/infected. Of the genes present on the array, 1,261 were found to have altered expression at least in one of three paired comparisons with P<0.05 (Supplementary Information). As shown in Figure 1, there were a total of 675 genes, compared with the control, with altered expression in PA01-infected CECs at 6 hpi, 497 up- and 178 downregulated. In flagellin-pretreated CECs, 890 genes were upregulated but only 39 were downregulated. Comparing PA01-infected CECs with or without flagellin pretreatment revealed a total of 209 genes with altered expression; 157 up- and 52 down regulated.
Figure 1.
Expression profiling of genes regulated by PA01 with or without flagellin pretreatment. (a) B6 mouse corneas were needle scratched and instilled topically with 500 ng flagellin in 5 μl or 5 μl phosphate-buffered saline (PBS) as for 24 h and then re-scratched and inoculated with 1.0 × 105 CFU (colony-forming units) of PA01. At 6 hpi (hours post infection), epithelial cells scraped off from the corneas were harvested for expression profiling, with epithelial cells from naive mouse corneas as the control (Cont.). Binary comparisons were made as indicated, creating differential expression profiles and significance measures corresponding to the indicated effects, each based on the mean and variance of three independent experiments. (b) Venn diagrams indicating overlap of genes induced due to infection, flagellin pretreatment/infection. Numbers of overlapped and non-overlapped genes are in different shaded areas. The cutoff values are twofold difference with the P values <0.05. CEC, corneal epithelial cell; PA, Pseudomonas aeruginosa.
The top six genes upregulated in CECs in response to PA01 infection are matrix metallopeptidase 13 (Mmp13; 80.2-fold increase), S100A8 (36.3), Mmp10 (34.2), Krt16 (32.7), Stfna1l1 (31.2), and S100A8-binding partner S100A9 (27.5) (Table 1). Expression of these genes in flagellin-pretreated and infected CECs, compared with infected CECs, were either downregulated: MMP13 5.4-fold, MMP10 4.1-fold and Stfna1l1 2.3-fold decrease; no significant change for Krt16; or upregulated: S100A8 4.2-fold and S100A9 2.54-fold increase, bringing the total increase of these two genes over the control to 154- and 69.8-fold, respectively. These changes reflected the functions of the genes and their contribution to flagellin-induced protection, including greatly increasing bacterial clearance (S100A8/A9 also named calprotectin, a potent antimicrobial protein complex39–41), and suppression of potential harmful genes (MMP13 and -10). Interestingly, stefin A1-like 1 (an intracellular cycteine-type endopeptidase inhibitor) was also downregulated 2.3-fold. Stfna1l1 was implicated as a component of proteasomes, and its substrate and function were not reported in the literature. Taken together, the genome-wide cDNA microarray revealed that flagellin preconditioning favors the expression of a large number of mucosal innate protective genes while controlling but not totally blocking genes that are part of innate response to infection and yet can be destructive when their activities are not well-controlled.
Table 1.
Top six highly PA01-induced genes and their regulation by flagellin pretreatment
| SYMBOL | Gene title | P-value | Inf. vs. C | P-value | Flag-inf vs. C | P-value | Flag-inf. vs. inf. |
|---|---|---|---|---|---|---|---|
| Mmp13 | Matrix metallopeptidase 13 | 2.74E-11 | 80.1652 | 7.54E-09 | 14.8848 | 1.36E-06 | − 5.3857 |
| S100a8 | S100 calcium-binding protein A8 | 2.21E-08 | 36.3138 | 6.11E-10 | 154.02 | 0.00015 | 4.24136 |
| Mmp10 | Matrix metallopeptidase 10 | 7.26E-08 | 34.198 | 1.47E-05 | 8.42531 | 0.00062 | − 4.05896 |
| Krt16 | Keratin 16 | 3.82E-06 | 32.6831 | 2.44E-06 | 38.1349 | 0.7299 | 1.16681 |
| Stfna1l1 | Stefins A1-like 1 | 8.89E-07 | 31.2961 | 1.37E-05 | 13.3889 | 0.03398 | − 2.33747 |
| S100a9 | S100 calcium-binding protein A9 | 4.13E-10 | 27.4806 | 2.84E-11 | 69.8298 | 0.00013 | 2.54105 |
Flagellin-induced defense response in PA01-infected cornea epithelia
Gene ontology analyses carried out by the Genomatrix Genome Analyzer (Ann Arbor, MI) revealed that 113 genes participate in cell defense response and its regulation, starting from transcription factors in the nuclei to secreted proteins/peptides in extracellular milieu. The full names of the genes, their expression levels, and their cellular localizations are listed in Table 2. Chi3l1 has recently been shown to promote Streptococcus pneumoniae killing and to augment host tolerance to lung antibacterial responses,42 we therefore added it to the list as a defense-responsive gene to Table 2 and Figure 7. There were a total 30 nuclear proteins, many of which are the components of a transcription factor family, such as NFKBIZ, NFKB1 (precursor of p50), and NFKBIA for nuclear factor (NF)-κB, and JUN and FOS for AP-1. As expected, NF-κB components were generally downregulated by flagellin pretreatment, resulting in hyporesponsiveness of cells to bacterial challenge in terms of production of NF-κB-mediated pro-inflammatory cytokines, such as C-X-C motif chemokine ligand 1 (CXCL1) and IL-1β. On the other hand, four interferon (IFN) regulatory factors (IRFs) either remained elevated (IRF1 and 6) or further augmented (IRF 7 and 9) in flagellin-pretreated CECs. There were 33 cytosolic proteins, some of which are involved in antiviral and innate responses (ISG15, S100A14, and LTF). Twenty membrane proteins were identified, including IFN receptors and IFN-induced membrane proteins with antimicrobial activities (IFNGR1, IFNGR2, IFITM1, and IFITM2), consistent with elevated expression of multiple IRFs. Most importantly, there were a total of 30 proteins and peptides that can be potentially secreted by epithelial cells; some were inflammatory cytokines (IL-1β, CXCL1, and CXCL2) with downregulation and others were antimicrobial peptides\proteins that were upregualted by flagellin pretreatment (S100A8, A9, LCN2, DEFB1, and Chi3l1).
Table 2.
PA01-induced and flagellin-mediated defense response genes
| Gene symbol | Description | Infected vs. normal
|
Flag-infected vs. normal
|
Flag-infected vs. infected
|
Cellular location | |||
|---|---|---|---|---|---|---|---|---|
| P-value | Fold-change | P-value | Fold-change | P-value | Fold-change | |||
| IRF7a | Interferon regulatory factor 7 | 0.51536 | 1.10901 | 3.91E-07 | 5.15553 | 7.53E-07 | 4.64876 | Nucleus |
| SP100a | SP100 nuclear antigen | 0.158511 | 1.22956 | 2.13E-05 | 2.61884 | 0.000177 | 2.12989 | Nucleus |
| IRF9a | Interferon regulatory factor 9 | 0.000145 | 2.36209 | 4.47E-07 | 4.92542 | 0.000514 | 2.0852 | Nucleus |
| TRAFD1 | TRAF-type zinc finger domain containing 1 | 0.069651 | 1.31223 | 1.78E-05 | 2.64229 | 0.000306 | 2.01358 | Nucleus |
| HIST1H2BC | Histone cluster 1, H2bc | 0.422054 | 1.29191 | 0.01606 | 2.39276 | 0.069981 | 1.85212 | Nucleus |
| STAT1a | Signal transducer and activator of transcription 1, 91kDa | 0.138359 | 1.29412 | 0.000248 | 2.35606 | 0.00342 | 1.82059 | Nucleus/cyto. |
| PMLa | Promyelocytic leukemia | 0.047309 | 1.31408 | 2.20E-05 | 2.36104 | 0.000563 | 1.79672 | Nucleus |
| SAMHD1 | SAM domain and HD domain 1 | 0.001422 | 2.05283 | 2.22E-05 | 3.30108 | 0.017554 | 1.60806 | Nucleus |
| ELF3a | E74-like factor 3 (ets domain TF, epithelial-specific ) | 0.073859 | 1.59088 | 0.003721 | 2.36666 | 0.119162 | 1.48764 | Nucleus/cyto. |
| SMAD1a | SMAD family member 1 | 0.037533 | 1.53357 | 0.00179 | 2.09529 | 0.112362 | 1.36628 | Nucleus |
| PCBP2 | Poly(rC)-binding protein 2 | 0.152515 | 1.5209 | 0.02637 | 2.01211 | 0.326908 | 1.32298 | Nucleus/cyto. |
| IRF1a | Interferon regulatory factor 1 | 0.049151 | 1.60282 | 0.005609 | 2.07975 | 0.247729 | 1.29756 | Nucleus |
| HSP90AB1 | Heat shock protein 90kDaα, class B member 1 | 0.030182 | 2.27782 | 0.007928 | 2.91858 | 0.469609 | 1.2813 | Multiple |
| JAK1 | Janus kinase 1 | 0.019179 | 1.7061 | 0.003512 | 2.05573 | 0.359276 | 1.20493 | Nucleus |
| NFKBIZ | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, zeta | 0.000122 | 2.67351 | 0.00013 | 2.65498 | 0.969244 | −1.00698 | Nucleus/cyto. |
| CEBPGa | CCAAT/enhancer-binding protein (C/EBP), gamma | 0.000187 | 2.22473 | 0.000209 | 2.20027 | 0.940763 | −1.01112 | Nucleus |
| IRF6a | Interferon regulatory factor 6 | 0.010491 | 2.21454 | 0.011646 | 2.18134 | 0.954401 | −1.01522 | Nucleus |
| HSPD1 | Heat shock 60kDa protein 1 (chaperonin) | 0.009322 | 2.41196 | 0.016274 | 2.21037 | 0.761036 | −1.0912 | Multipe |
| FOSa | FBJ murine osteosarcoma viral oncogene homolog | 0.009169 | 2.03559 | 0.04916 | 1.65126 | 0.379238 | −1.23275 | Nucleus |
| IRGM | Immunity-related GTPase family, M | 4.83E-06 | 4.26351 | 2.57E-05 | 3.40225 | 0.247826 | −1.25314 | Nucleus |
| JUNa | Jun proto-oncogene | 4.08E-06 | 2.21395 | 0.000184 | 1.70284 | 0.022346 | −1.30015 | Nucleus |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | 1.35E-06 | 2.71911 | 0.000176 | 1.83146 | 0.004477 | −1.48467 | Nucleus/cyto. |
| NFKB1a | NF of κ light polypeptide gene enhancer in B-cells 1 | 1.35E-06 | 2.71911 | 0.000176 | 1.83146 | 0.004477 | −1.48467 | Nucleus/cyto. |
| JAK2 | Janus kinase 2 | 1.10E-05 | 2.59087 | 0.001344 | 1.73262 | 0.010299 | −1.49535 | Nucleus |
| Jak2 | Janus kinase 2 | 1.10E-05 | 2.59087 | 0.001344 | 1.73262 | 0.010299 | −1.49535 | Nucleus |
| HIF1Aa | Hypoxia inducible factor 1, alpha subunit | 0.000271 | 2.86239 | 0.008489 | 1.89686 | 0.064399 | −1.50902 | Nucleus |
| CEBPBa | CCAAT/enhancer-binding protein (C/EBP), β | 5.69E-05 | 3.87288 | 0.002836 | 2.26818 | 0.029694 | −1.70748 | Nucleus/cyto. |
| TNFAIP3 | TNFα-induced protein 3 | 5.63E-08 | 5.68342 | 1.32E-05 | 2.81836 | 0.00045 | −2.01657 | Nucleus |
| BCL3a | B-cell CLL/lymphoma 3 | 3.32E-10 | 6.70447 | 1.32E-07 | 3.0733 | 6.24E-06 | −2.18152 | Nucleus |
| NFKBIA | IkappaBalpha | 0.000547 | 3.48829 | 0.132261 | 1.52584 | 0.008736 | −2.28615 | Nucleus/cyto. |
| EGR1a | Early growth response 1 | 0.795012 | 1.15499 | 0.090924 | −2.72613 | 0.05768 | −3.14866 | Nucleus |
| ISG15 | ISG15 ubiquitin-like modifier | 0.061282 | 1.74262 | 6.76E-07 | 14.7154 | 6.49E-06 | 8.4444 | Cytoplasm |
| OAS2 | 2′-5′-oligoadenylate synthetase 2, 69/71kDa | 0.004938 | 1.79889 | 2.75E-08 | 10.0861 | 5.57E-07 | 5.60685 | Cytoplasm |
| XAF1 | XIAP associated factor 1 | 0.000532 | 2.1492 | 6.26E-08 | 7.54388 | 7.20E-06 | 3.51009 | Cytoplasm |
| IFITM3 | Interferon-induced transmembrane protein 3 | 1.58E-07 | 4.87348 | 4.13E-10 | 16.195 | 2.51E-06 | 3.32309 | Cytoplasm |
| DHX58 | DEXH (Asp-Glu-X-His) box polypeptide 58 | 0.024163 | 1.39272 | 1.58E-07 | 4.38151 | 2.01E-06 | 3.14602 | Cytoplasm |
| USP18 | Ubiquitin specific peptidase 18 | 1.08E-05 | 5.25499 | 1.12E-07 | 13.9647 | 0.000953 | 2.65742 | Cytoplasm |
| LTF | Lactotransferrin | 0.588422 | 1.19456 | 0.005413 | 3.00677 | 0.014596 | 2.51706 | Cytoplasm |
| GCH1 | GTP cyclohydrolase 1 | 5.56E-06 | 2.6455 | 2.53E-08 | 5.27164 | 0.000125 | 1.99268 | Cytoplasm |
| APOBEC1 | Apolipoprotein B mRNA editing enzyme, catalytic polypeptide 1 | 0.546388 | 1.13101 | 0.002325 | 2.1787 | 0.006901 | 1.92633 | Cytoplasm |
| RSAD2 | Radical S-adenosyl methionine domain containing 2 | 0.000303 | 4.62087 | 2.16E-05 | 7.76524 | 0.114925 | 1.68047 | Cytoplasm |
| PSMB8 | Proteasome subunit, beta type, 8 | 0.169438 | 1.73613 | 0.016292 | 2.89413 | 0.200373 | 1.667 | Cytoplasm |
| DDX58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 0.050962 | 1.46438 | 0.000372 | 2.40996 | 0.015505 | 1.64572 | Cytoplasm |
| MAP2K1 | Mitogen-activated protein kinase kinase 1 | 0.021927 | 1.77802 | 0.00049 | 2.86454 | 0.04924 | 1.61108 | Cytoplasm |
| MX2 | Myxovirus (influenza virus) resistance 2 (mouse) | 0.049503 | 1.40197 | 0.000263 | 2.24186 | 0.01074 | 1.59907 | Cytoplasm |
| S100A14 | S100 calcium-binding protein A14 | 0.021966 | 3.17889 | 0.003959 | 4.82976 | 0.355747 | 1.51932 | Cytoplasm |
| EIF2AK2 | Eukaryotic translation initiation factor 2-alpha kinase 2 | 0.000226 | 1.95477 | 4.16E-06 | 2.84746 | 0.011772 | 1.45668 | Cytoplasm |
| ADAR | Adenosine deaminase, RNA-specific | 0.005782 | 1.55938 | 5.83E-05 | 2.27053 | 0.014778 | 1.45604 | Cytoplasm |
| YWHAZ | Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide | 0.069065 | 1.46427 | 0.002825 | 2.06232 | 0.097818 | 1.40843 | Cytoplasm |
| PRDX5 | Peroxiredoxin 5 | 0.109073 | 1.47637 | 0.007606 | 2.07156 | 0.15774 | 1.40314 | Cytoplasm |
| CDO1 | Cysteine dioxygenase, type I | 3.43E-05 | − 2.33236 | 0.001197 | −1.72982 | 0.037774 | 1.34833 | Cytoplasm |
| HSP90AB1 | Heat shock protein 90kDaα (cytosolic), class B member 1 | 0.030182 | 2.27782 | 0.007928 | 2.91858 | 0.469609 | 1.2813 | Cytoplasm |
| ube2n | Ubiquitin-conjugating enzyme E2N | 0.040128 | 1.72389 | 0.008112 | 2.12665 | 0.388974 | 1.23363 | Cytoplasm |
| PTPN1 | Protein tyrosine phosphatase, non-receptor type 1 | 0.012095 | 1.99887 | 0.003519 | 2.34843 | 0.499698 | 1.17488 | Cytoplasm |
| NLRX1 | NLR family member X1 | 0.005433 | 1.94667 | 0.0025 | 2.12138 | 0.664894 | 1.08975 | Cytoplasm |
| GSTP1 | Glutathione S-transferase pi 1 | 0.026808 | 1.89181 | 0.014856 | 2.05445 | 0.74731 | 1.08597 | Cytoplasm |
| SOCS6 | Suppressor of cytokine signaling 6 | 0.000406 | 2.09302 | 0.000358 | 2.1177 | 0.938227 | 1.01179 | Cytoplasm |
| MYD88 | Myeloid differentiation primary response gene (88) | 0.000225 | 2.27221 | 0.000475 | 2.11167 | 0.640665 | −1.07603 | Cytoplasm |
| HSPD1 | Heat shock 60kDa protein 1 (chaperonin) | 0.009322 | 2.41196 | 0.016274 | 2.21037 | 0.761036 | −1.0912 | Cytoplasm |
| BCL10 | B-cell CLL/lymphoma 10 | 3.00E-06 | 2.28395 | 1.90E-05 | 1.98742 | 0.193723 | −1.1492 | Cytoplasm |
| DUSP7 | Dual specificity phosphatase 7 | 3.44E-06 | 3.69737 | 0.000135 | 2.44088 | 0.024882 | −1.51477 | Cytoplasm |
| SBNO2 | Strawberry notch homolog 2 (Drosophila) | 8.10E-06 | 2.62494 | 0.0022 | 1.65622 | 0.004081 | −1.5849 | Cytoplasm |
| Sbno2 | Strawberry notch homolog 2 (Drosophila) | 8.10E-06 | 2.62494 | 0.0022 | 1.65622 | 0.004081 | −1.5849 | Cytoplasm |
| SOCS3 | Suppressor of cytokine signaling 3 | 0.000319 | 8.99332 | 0.048015 | 2.58296 | 0.013837 | −3.48179 | Cytoplasm |
| IFI27 | Interferon, alpha-inducible protein 27 | 0.436113 | 1.25338 | 9.38E-07 | 15.3115 | 2.20E-06 | 12.2162 | M Membrane |
| BST2 | Bone marrow stromal cell antigen 2 | 0.281809 | 1.22368 | 3.13E-06 | 4.66776 | 1.19E-05 | 3.81452 | Golgi apparatus |
| IFITM1 | Interferon-induced transmembrane protein 1 | 0.077827 | 1.52453 | 2.82E-05 | 4.40229 | 0.000479 | 2.88764 | Cell Membrane |
| IFITM2 | Interferon-induced transmembrane protein 2 | 0.00601 | 1.68559 | 1.68E-06 | 4.12321 | 0.000117 | 2.44616 | Cell Membrane |
| GBP2 | Guanylate-binding protein 2, IFN-inducible | 0.079648 | 1.52856 | 0.000102 | 3.66391 | 0.002165 | 2.39697 | Cell Membrane |
| CD8B | CD8b molecule | 8.17E-05 | − 2.02671 | 0.220707 | −1.16967 | 0.000688 | 1.73272 | P Membrane |
| F2R | Coagulation factor II (thrombin) receptor | 8.18E-06 | − 2.6071 | 0.003663 | −1.5925 | 0.002479 | 1.63712 | P Membrane |
| OSMR | Oncostatin M receptor | 0.000903 | 1.9475 | 4.27E-05 | 2.63016 | 0.067472 | 1.35053 | M membrane |
| BCL2 | B-cell CLL/lymphoma 2 | 0.00031 | − 2.03885 | 0.006683 | −1.594 | 0.109761 | 1.27908 | M membrane |
| IFNGR2 | Interferon gamma receptor 2 | 0.02024 | 1.90289 | 0.007121 | 2.18633 | 0.570234 | 1.14896 | Membrane |
| FAIM3 | Fas apoptotic inhibitory molecule 3 | 0.000325 | 1.94655 | 0.000101 | 2.15268 | 0.453915 | 1.1059 | Membrane |
| ANXA1 | Annexin A1 | 1.13E-05 | 4.84605 | 9.21E-06 | 5.01949 | 0.869516 | 1.03579 | P Membrane |
| TFRC | Transferrin receptor (p90, CD71) | 4.37E-05 | 3.24094 | 0.000103 | 2.90335 | 0.55496 | −1.11627 | P Membrane |
| IFNGR1 | Interferon gamma receptor 1 | 1.10E-06 | 3.69699 | 6.30E-06 | 3.01819 | 0.187901 | −1.2249 | P Membrane |
| TNFRSF1A | TNF receptor superfamily, member 1A | 0.000808 | 2.13404 | 0.016552 | 1.59796 | 0.109212 | −1.33547 | P Membrane |
| LTB4R | Leukotriene B4 receptor | 1.22E-05 | 3.13629 | 0.000302 | 2.23702 | 0.057132 | −1.40199 | P Membrane |
| ADORA2B | Adenosine A2b receptor | 7.11E-05 | 2.76577 | 0.002171 | 1.92857 | 0.051597 | −1.43411 | P Membrane |
| CD44 | CD44 molecule (Indian blood group) | 1.32E-05 | 4.62088 | 0.000152 | 3.19812 | 0.101889 | −1.44487 | P Membrane |
| ITGB6 | Integrin, beta 6 | 6.34E-09 | 5.04524 | 1.29E-05 | 2.21894 | 9.56E-06 | −2.27372 | P Membrane |
| ICAM1 | Intercellular adhesion molecule 1 | 5.28E-09 | 6.78982 | 1.34E-05 | 2.521 | 6.74E-06 | −2.6933 | P Membrane |
| LGALS3BP | Lectin, galactoside-binding, soluble, 3 binding protein | 0.114266 | 1.39806 | 6.79E-08 | 11.8169 | 3.03E-07 | 8.45235 | Secreted |
| IFIT3 | IFN-induced protein with tetratricopeptide repeats 3 | 0.043905 | 2.63634 | 5.42E-05 | 14.9829 | 0.001826 | 5.68322 | Cyto/secreted |
| B2M | β-2-microglobulin | 0.003516 | 2.13397 | 1.16E-07 | 11.7143 | 4.56E-06 | 5.48946 | Secreted |
| S100A8 | S100 calcium-binding protein A8 | 2.21E-08 | 36.3138 | 6.11E-10 | 154.02 | 0.000145 | 4.24136 | Cyto/secreted |
| Chi3l1 | Chitinase 3-like 1 (cartilage glycoprotein-39) | 0.001137 | 4.40342 | 6.64E-06 | 15.1109 | 0.003982 | 3.43164 | Secreted |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | 0.708221 | 1.08622 | 6.23E-05 | 3.64897 | 0.000113 | 3.35934 | Secreted |
| LCN2 | Lipocalin 2 | 0.029482 | 2.3174 | 0.000117 | 7.05984 | 0.006901 | 3.04644 | Secreted |
| S100A9 | S100 calcium-binding protein A9 | 4.13E-10 | 27.4806 | 2.84E-11 | 69.8298 | 0.000125 | 2.54105 | cyto/secreted |
| CFB | Complement factor B | 0.766377 | 1.04103 | 1.63E-05 | 2.60608 | 2.42E-05 | 2.50337 | Secreted |
| IL15 | Interleukin 15 | 0.877282 | − 1.02468 | 0.00014 | 2.4058 | 0.000111 | 2.46517 | Secreted |
| PGLYRP4 | Peptidoglycan recognition protein 4 | 0.027166 | 1.55242 | 8.69E-06 | 3.625 | 0.000399 | 2.33507 | Secreted |
| Apol7a | Apolipoprotein L, 3 (human) | 0.795086 | − 1.05631 | 0.003141 | 2.16983 | 0.001987 | 2.29202 | Secreted |
| PGLYRP1 | Peptidoglycan recognition protein 1 | 0.164294 | − 1.14491 | 1.14E-05 | 1.92439 | 1.68E-06 | 2.20325 | Secreted |
| IFI35 | Interferon-induced protein 35 | 0.134494 | 1.30361 | 9.04E-05 | 2.67338 | 0.001106 | 2.05075 | Cyto/secreted |
| C3 | Complement component 3 | 0.039871 | 1.4769 | 3.91E-05 | 3.01314 | 0.001342 | 2.04017 | Secreted |
| LY96 | Lymphocyte antigen 96 | 0.033754 | 1.46302 | 0.000174 | 2.38787 | 0.009729 | 1.63216 | Secreted |
| DEFB1 | Defensin, beta 1 | 0.015749 | 2.00685 | 0.000803 | 3.05398 | 0.1136 | 1.52178 | Secreted |
| CST3 | cystatin C | 0.118388 | 1.38634 | 0.003283 | 2.05592 | 0.065736 | 1.48298 | Secreted |
| PPBP | Pro-platelet basic protein (chemokine (C-X-C motif) ligand 7) | 0.026004 | 2.55941 | 0.021646 | 2.65552 | 0.922327 | 1.03755 | Secreted |
| IL1RN | Interleukin 1 receptor antagonist | 5.06E-08 | 2.64206 | 2.52E-07 | 2.31599 | 0.131153 | −1.14079 | Secreted |
| TNF | Tumor necrosis factor | 1.08E-05 | 2.49795 | 9.49E-05 | 2.06984 | 0.1646 | −1.20683 | Cyto/secreted |
| IL1A | Interleukin 1, alpha | 4.71E-05 | 3.26766 | 0.000382 | 2.51774 | 0.182952 | −1.29786 | Secreted |
| CCL4 | Chemokine (C-C motif) ligand 4 | 0.012826 | 2.01984 | 0.305991 | 1.29354 | 0.088875 | −1.56149 | Secreted |
| CXCL16 | Chemokine (C-X-C motif) ligand 16 | 2.78E-10 | 4.77 | 8.55E-08 | 2.57246 | 7.74E-06 | −1.85426 | Secreted |
| FN1 | Fibronectin 1 | 0.623819 | − 1.08296 | 0.000859 | −2.04503 | 0.002001 | −1.88836 | Secreted |
| NGF | Nerve growth factor (β polypeptide) | 0.000775 | 2.67217 | 0.15406 | 1.38771 | 0.010828 | −1.9256 | Secreted |
| IL1B | Interleukin 1, beta | 0.044493 | 2.36038 | 0.692287 | 1.16788 | 0.09088 | −2.02109 | Cyto/secreted |
| CXCL2 | Chemokine (C-X-C motif) ligand 2 | 2.35E-08 | 8.4622 | 2.37E-05 | 3.03106 | 4.92E-05 | −2.79183 | Secreted |
| NPPB | Natriuretic peptide B | 1.74E-06 | 8.02694 | 0.003829 | 2.37175 | 0.000287 | −3.38439 | Secreted |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | 2.13E-08 | 18.0992 | 2.63E-05 | 4.36975 | 3.74E-05 | −4.14194 | Secreted |
Transcription factors.
Figure 7.
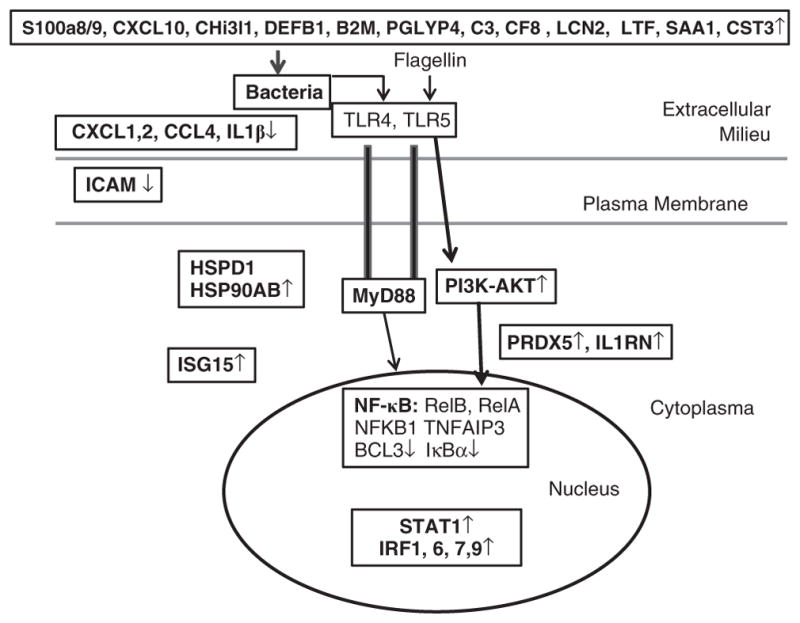
The diagram shows flagellin-induced defense response to microbial infection in the ocular surface. An innate defense network, activation of which will result in tissue resistance to infection, was constructed using the data presented in Table 2. A myriad of genes are expressed in response to infection and flagellin pretreatment may suppress (↓), have no effects, or augment (↑) the expression of these genes. Transcription factors distributed in the nuclei regulates (long arrowheads) the expression of cytoprotective (blue) and defense (green), inflammatory (red) genes that form an effective defense network against microbial infection starting from extracellular space, to plasma membrane and to cytosol without excessive inflammation as many pro-inflammatory factors are suppressed by flagellin pretreatment.
Infection-induced S100A8/A9 expression is augmented by flagellin pretreatment
Having ascertained flagellin preconditioning–mediated differential expression of genes by genome-wide cDNA array, we next used PCR and immunohistochemistry to confirm the expression pattern of the two most highly induced genes: S100A8 (calgranulin A) and S100A9 (calgranulin B) in PA01-infected B6 mouse corneas. Both Real time- (Figure 2a) and reverse transcriptase-PCR (Figure 2b) revealed similar expression pattern to that detected by cDNA array for both genes; infection moderately increased their expression which was greatly enhanced by flagellin pretreatment. At the protein levels revealed by western blotting (Figure 2c), there were no detectable S100A8 and A9 in normal homoestatic CECs, whereas a faint band of S100A9 was seen in PA10-infected corneas. Flagellin pretreatment resulted in the expression of the pair (Figure 2c).
Figure 2.
Infection-induced expression of S100A8 and A9 is augmented by flagellin pretreatment. B6 mice were treated as described in Figure 1. At 6 hpi (hours post infection), the scraped corneal epithelial cells from the control (CT), PA01-infected phosphate-buffered saline (PBS) + PA (Pseudomonas aeruginosa), or flagellin-pretreated and PA01-infected (Flag +PA) were subjected to (a) real-time PCR, (b) reverse transcriptase-PCR or (c) western blot analyses for S100A8 and A9 expression levels.
To assess the expression of these two genes at tissue levels, immunohistochemistry was performed. Although there was little staining for both S100A8 and A9 in normal controls as well as PA01-infected corneas at 6 hpi, the whole epithelial layer was positively stained with both S100A8 and A9 with strong staining at apical layer in flagellin-pretreated corneas. Confocal imaging confirmed apical expression of S100A8 and more apparent S100A9 (panels g and h, Figure 3). S100A8 was also found throughout the epithelial layer. There were also positive cells in the stroma, presumably the infiltrated polymorphonuclear neutrophils (PMNs) in which 45% of soluble proteins are calprotectin.43 The expression patterns of MMP13 and MMP10 were also confirmed (data not shown), while the expression pattern of IL-1β was similar to what we previously reported.17,44
Figure 3.
Immunohistochemistry of S100A8 and A9 distribution in PA01-infected and flagellin-pretreated B6 corneas. B6 mice were treated as described in Figure 1. At 6 hpi (hours post infection), corneas were OCT (optimal cutting temperature) snap frozen, followed by sectioning and immunostaining with antibodies against S100A8 (a, c, e) and S100A9 (b, d, f). DAPI (4,6-diamidino-2-phenylindole dihydrochloride) was used to stain nuclei. The images of S100A8 or A9 and DAPI were merged. The staining of PA01-infected and flagellin-pretreated B6 corneas with S100A8 (g) and S100A9 (h), or omitted first antibody as negative control, were also examined by confocal microscope. The figure is representative of three corneas per condition from two independent experiments. E, epithelium; IgG, immunoglobulin G; S, stroma.
S100A8 and A9 neutralization decreases bacterial clearance in B6 mouse cornea
Having shown that flagellin augments the expression of S100A8 and A9 in the cornea in response to infection, we then tested the function of S100A8 and A9 using neutralizing antibodies.45 At 6 hpi, there was no sign of inflammation visible by slit lamp examination in all the corneas (data not shown). However, bacterial burden determination revealed a great reduction of bacterial load in mouse corneas (Figure 4a). S100A8 and A9 depletion increased bacterial burden 2.4- and 1.9-fold in the control and 13- and 5.6-fold in flagellin-pretreated corneas, respectively. The expression of macrophage-inflammatory protein 2 (MIP-2) had a similar pattern to bacterial load (Figure 4b) but myeloperoxidase activity was undetectable at this time point.
Figure 4.
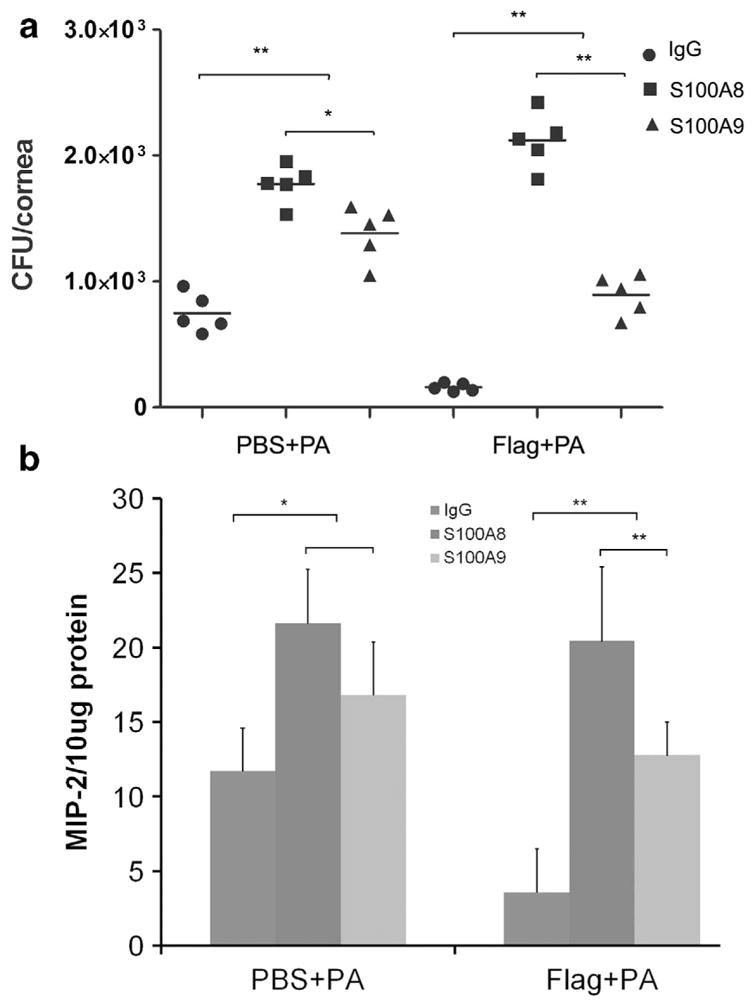
S100A8 and -A9 neutralization decreases bacterial clearance in B6 mouse cornea at 6 h post infection (hpi). B6 mice were pretreated with flagellin (500 ng per eye) or 5 μl phosphate-buffered saline (PBS) at − 24 h and then subconjunctivally injected with 100 ng rabbit immunoglobulin G (IgG; control) or anti-S100A8- or -A9-neutralizing antibodies at − 16 h. The corneas were then infected with 104 colony-forming units (CFU) ATCC 19660 (0 h). At 6 hpi, by which time no opacity or sign of infection was observed, the corneas were processed for (a) bacterial load and enzyme-linked immunosorbent assay analysis for (b) macrophage-inflammatory protein 2 (MIP-2). The figure is a representative of two independent experiments. *P≤0.05, **P≤0.01, n =5.
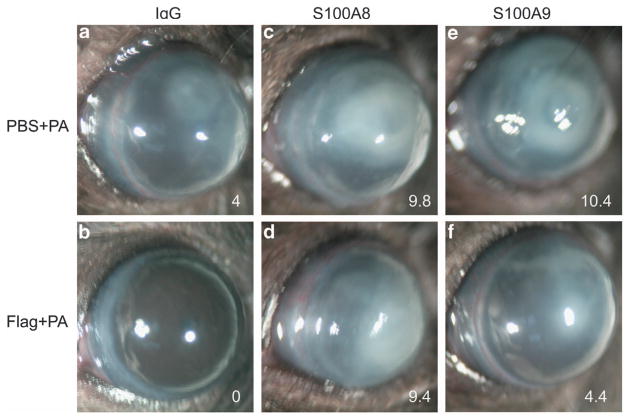
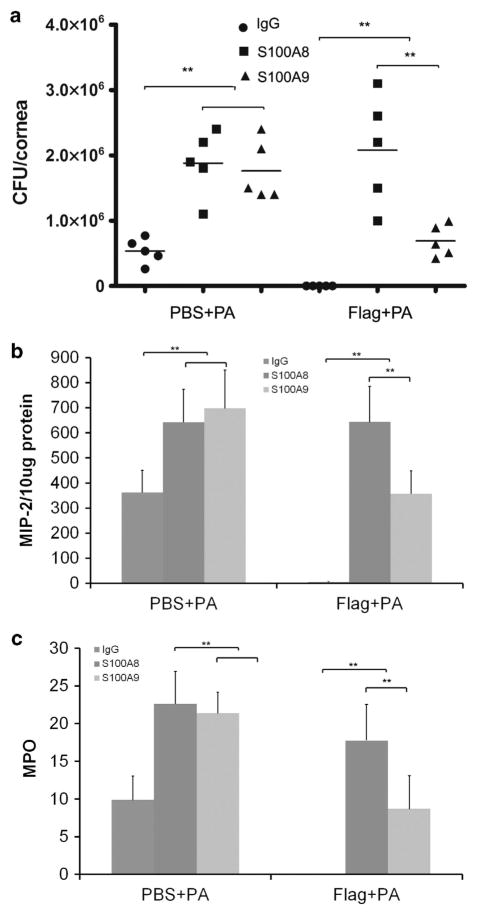
At 24 hpi, the cornea pretreated with PBS was partially opaque, whereas flagellin-pretreated corneas were clean and showed no sign of infection or inflammation (Figure 5). Opacity was greatly increased in the corneas injected with S100A8- or A9-neutralizing antibodies when compared with immunoglobulin G (IgG)-treated corneas without (c and e vs. a) or without (d and f vs. b) pretreatment. Compared with S100A9 (e and f), S100A8 neutralization resulted in more severe keratitis (c and d), and this was more apparent for flagellin-pretreated corneas (f vs. d). The different effects of S100A8 and A9 neutralizations were also observed in corneal bacterial burden, PMN infiltration, and pro-inflammatory cytokine expression. As shown in Figure 6a, 5.3 × 105 CFU of P. aeruginosa were detected in the control antibody-injected corneas while depletion of S100A8 or A9 resulted in increase of bacterial loads ~3.5-fold in the PBS-pretreated corneas. In flagellin-pretreated corneas, no recoverable bacteria were detected in control antibody-injected corneas, depletion of S100A8 and A9 resulted in 2.1 × 106 and 6.9 × 105 CFU, respectively. Similar patterns of MIP-2 expression and PMN infiltration detected by myeloperoxidase assay were also detected: in the control, PBS-pretreated corneas, S100A8 and A9 neutralization exhibited similar levels of exacerbated inflammation (elevated MIP-2 and PMN infiltration), while in flagellin-pretreated corneas, S100A8 neutralization resulted in greatly elevated inflammation comparable with that of without flagellin pretreatment and S100A9 neutralization, which, on the other hand, elevated inflammation to a level similar to PBS-pretreated corneas without neutralizing antibodies (Figure 6b).
Figure 5.
S100A8/9-neutralized mouse corneas are more susceptible than the wild type to P. aeruginosa (PA) at 1-day post infection. B6 mice were treated as described in Figure 4 except that the mice were killed at 24 hpi (hours post infection). The corneas were photographed and clinically scored with 0 (no infection) to 12 (perforated) system.18 The number on the low right cornea of each panel is the average clinical score of five corneas. Three independent experiments were performed, and a representative cornea was presented for each experimental condition. IgG, immunoglobulin G; PBS, phosphate-buffered saline.
Figure 6.
Topical flagellin is ineffective in protecting the cornea from P. aeruginosa S100A8/9-neutralized mice cornea. The corneas presented in Figure 5 were subjected to (a) bacteria counting, (b) macrophage-inflammatory protein 2 (MIP-2) expression determined by enzyme-linked immunosorbent assay, and (c) myeloperoxidase (MPO) determination (units per cornea). *P≤0.05 and **P≤0.01, n =5. CFU, colony-forming units; IgG, immunoglobulin G; PBS, phosphate-buffered saline.
DISCUSSION
In this study, we used genome-wide cDNA microarrays to identify genes expressed in CECs in response to infection, with focus on the genes differentially expressed in response to flagellin preconditioning. Our results revealed that flagellin pretreatment dramatically altered the epithelial response to infection. Contrary to the concept of tolerance or hyperresponsiveness, flagellin pretreatment augmented more than suppressed the expression of infection-induced genes at a 3:1 ratio, indicating more positive regulatory mechanisms for flagellin preconditioning–mediated gene expression in CECs. Using the data presented in the Table 2, we constructed a defense network elicited by flagellin pretreatment in response to infection (Figure 7). Although the augmented genes identified, such as CXCL10, a multifunctional chemokine with antimicrobial activity,46 might be used as therapeutic reagents, the suppressed genes, such as MMP13, might be targeted for reducing deleterious inflammation and tissue damage in keratitis cornea.
The cDNA array analyses revealed greatly altered expression of genes in CECs in response to P. aeruginosa infection and illustrated the flagellin-induced alteration in gene expression at a much larger scale than that of a functional approach. In response to P. aeruginosa, 682 genes had more than twofold changes. The highest levels of increased genes are MMPs, AMPs, and pro-inflammatory cytokines, whereas the most downregulated gene is sestrin 1, which has a critical role in antioxidant defense in tissues, such as the retina.47 Clearly, while innate defense mechanisms are activated in response to infection, the changes in some host genes, such as highly induced MMP13 and suppressed sestrin 1 may contribute to infection- and inflammation-associated tissue damages. In flagellin-pretreated and PA01-infected corneas, 946 genes exhibited more than twofold changes, with 907 increased and 39 decreased. The genes with the highest upregulation are S100A8 and A9, which can form the heterodimer calprotectin, a potent AMP.41 Krt16 and Sprr2 are structural proteins and are expressed at similar levels in PA01-infected cells with or without flagellin pretreatment. Surprisingly, five genes, ifi204, IFITM3, Rsad2, ifit3, and ifi27, are IFN-stimulated genes with antiviral activity. Ifi204 and Ifitm3 may also be involved in innate defense against other microbes by regulating monocyte differentiation to macrophages and dendritic cells48,49 and by limiting host cell proliferation to prevent spreading of pathogens,50,51 respectively.
The most interesting comparison is between infected CECs with or without flagellin pretreatment. There are a total of 209 genes with altered expression levels; 157 up- and 52 down-regulated. The top10 upregulated genes are mostly, with the exception of Apol9a and 9b, professional immune molecules involved in the IFN-α induction pathway with antimicrobial and/or anti-inflammatory activities. For example, Lgals3bp has been shown to activate naive and primed neutrophils, while Gbp3 I solicits defense proteins and protects the host from infection.52 The endogenous antioxidant gene sestrin 1 was also on the list of upregulated genes (2.7 fold increase), consistent with increased protection in the cornea. On the other hand, the top 10 downregulated genes are either inflammatory molecules (IL-24, CXCL1, EGR1, SOCS3, CXCL12), proteinases/proteinase inhibitors (MMP13, MMP10, Serpina3g), or genes with unknown functions (Nppb and Prl2c3). As the altered expression of these genes results in profound innate mucosal surface protection against pathogens, many of the genes may also be involved in flagellin uniquely induced protection against other harmful environmental challenges.31–34
The genes were subjected to gene ontology analysis of biological processes. Prominently among different processes are the defense response (P-value 1.37e-7, 83/910) and its regulation (P-value 2.18e-5, 34/310) that include 114 genes, some of which are overlapping in these two groups. Table 2 listed the genes of expression levels, cellular locations, and known or suggested functions we constructed a diagram of corneal defense system activated by flagellin pretreatment (Figure 7). In nuclei, the pro-inflammatory transcription factor, the NF-κB family, are downregulated by flagellin pretreatment. On the other hand, four IRFs are upregulated in infected corneas, whereas IRF7 and 9 are further augmented by flagellin pretreatment. The augmented expression of these IFN-stimulated transferrins indicates that many increased genes are IFN-induced and suggests that flagellin is able to induce IFN expression and signaling. Indeed, two IFN-δ receptors, IFNGR1 and R2, are upregulated in infected CECs (Table 2) and, consequentially, the expression of many IFN-induced genes such as ISG15 and IFIs are also are augmented. To date, it is not clear whether IFNs are produced directly by CECs after TLR5 activation or whether IFNs are produced by residential dendritic cells and/or other innate immune cells.
Many IFN-inducible, flagellin-augmented genes are either in cytoplasm (ISG15 and OAS2) or at the cell membrane (three IFITMs). These genes are known to be dramatically induced upon viral or bacterial infection and have antiviral and innate immune activities.50,51,53,54 The other antimicrobial proteins, LTF and S100A14, and cytoprotective PRDX5, HSP90AB1, and HSPD1 are also found in cytosol along with several ubiquitination and proteasome proteins (Table 2). Three TLR-related proteins can also be found in Table 2: MyD88, SOCS3, and SOCS6. Although MyD88 and SOCS6 remain elevated, SOCS3 expression is significantly suppressed by flagellin pretreatment. The suppressed expression of SOCS3, a suppressor of cytokine signaling,55 is inconsistent with greatly reduced inflammation in flagellin-pretreated corneas. Interestingly, overexpression of SOCS3 has been shown to be harmful to keratinocytes by exacerbating wound inflammation.56 Hence, its suppression by flagellin may have beneficial effects on CECs. Downregulation of intercellular adhesion molecule, an important pro-inflammatory factor, is also of importance in reducing inflammation and tissue damage caused by bacterial infection.
Among 113 defense response genes, 28 encode proteins that can be secreted by CECs. The flagellin pretreatment augmented the expression of 14 genes, most of which possess antimicrobial and innate defense activities, including S100A8, A9, Chi3l1, LCN2, CXCL10, IFIT3, IFI35, PGKYRP4, PGLYRP1, C3, and CFB (components of complement system). Synergetic effects of these factors can form a biological barrier to prevent and/or kill invading pathogens.
The altered expression of several representative genes, including S100A8, A9 (Figure 2), CXCL10, MMP13, -10, and Chi3l1, was confirmed by real-time or reverse transcriptase-PCR, by western blotting, and/or by immunohistochemistry. Although cDNA array revealed higher flagellin-mediated induction of S100A8 than A9, Figure 2, particularly western blotting appeared to suggest more pronounced upregulation of S100A9. If indeed there was more S100A9, there should have less S100A8 dimmers, which are shown to be stronger neutrophil chemoattractant.57 Reduced S100A8 is consistent with reduced PMN infiltration observed in flagellin-pretreated corneas in response to P. aeruginosa infection.17
As S100A8 and A9 are the two most highly induced genes by flagellin pretreatment, we explored functional relevance of these two genes. S100A8 and A9 exist as noncovalently bound homodimers but also as heterodimers (S100A8/A9) that inhibit bacterial adhesion to mucosal epithelium and bacterial growth through zinc chelation.41,43,58–60 We observed that functional blocking of both peptides significantly decreases corneal bacterial clearance at 6 and 24 hpi in the control and flagellin-pretreated corneas, providing direct evidence for bactericidal activity of these calcium-binding proteins. Interestingly, functions of S100A8 and A9 are not equal as neutralizing S100A8 appears to result in more severe keratitis than S100A9. This is more apparent in flagellin-pretreated corneas in which neutralizing S100A8 totally lost flagellin-induced protection, whereas S100A9 only partially, suggesting additional roles of S100A8. It is important to mention that while inactivation of the S100A8 gene is embryonically lethal,61 the mice with depleted S100A9 are viable, albeit in these mice S100A8 expression is not detectable,62 suggesting non-redundant function of a member of the S100 gene family. Interestingly, while S100A8/A9 are inducible genes in epithelial cells, they are constitutively expressed in neutrophils where they comprise 45% of total cytoplasmic protein.63,64 The abundant expression of both S100A8 and A9 in infiltrated cells of flagellin-pretreated corneas was confirmed by immunohistochemistry (Figure 3). Calprotectin are known to be released from neutrophils upon stimulation to form extracellular traps, which kill extracellular pathogens while minimizing damage to the host cells.64 Hence, neutralizing S100A8 and A9 are likely to affect both epithelial-expressed and neutrophil-released S100A8/A9. Hence, our study identifies calprotectin as an important protective mediator in the innate immune response to bacterial keratitis caused by P. aeruginosa.
In summary, genome-wide cDNA array showed dramatic changes in the expression of many genes and revealed flagellin-elicited changes in gene expression pattern on a large scale, which would not be possible to envision by conventional approaches. The data allow us to construct an innate defense network, activation of which would result in tissue resistance to infection (Figure 7). Among these altered genes, the epithelially expressed S100A8 and A9 are shown to have a key role in corneal defense against the opportunistic but devastating ocular pathogen, P. aeruginosa. The network we constructed is of value for developing therapeutic strategies to control infection and to reduce tissue damage caused by infection-associated host inflammation.
MATERIALS AND METHODS
Bacterial strains and flagellin purification
A cytotoxic strain of P. aeruginosa (ATCC 19660), which provides a reproducible inflammatory response in the B6 mouse cornea,44,65 was used to test the protective effects of flagellin and S100A8/A9. However, as cytotoxicity of strain 19660 often causes epithelial damages, we choose strain PAO1, a wound isolate and the most widely used P. aeruginosa laboratory strain with completely sequenced genome66 for cDNA array study. Flagellin was prepared from PA01 by ammonium sulfate precipitation as described earlier.22
Mice
Wild-type C57BL6 (B6) mice (age, 8 weeks; female; weight, 20–24 g) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animals were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The Institutional Animal Care and Use Committee of Wayne State University approved all animal procedures.
Flagellin pretreatment and infection procedure
Mice were anesthetized with ketamine/xylazine and placed beneath a stereoscopic microscope at a magnification of × 40 for needle scratching (three 1-mm incisions using a sterile 25-gauge needle). The injured corneas of wild type (n =6 for cDNA array, n =5/group for other experiments) were pretreated with 500 ng purified flagellin in 5 μl of PBS or PBS (the control) by topical application. For infection, the treated corneas were re-scratched and inoculated by topical application of bacterial suspension (5 μl) containing 1 ×104 CFU of strain ATCC 19660 or 1 × 105 CFU of strain PA01. Eyes were examined daily to monitor the disease progression.
RNA extraction and real-time PCR
For RNA isolation, epithelial cells were scraped off six corneas, two pooled into one tube as one sample, of naive (the Control), infected, and flagellin-pretreated/infected mice and frozen in liquid nitrogen immediately. RNA was extracted from the collected epithelial cells using RNeasy Mini Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. cDNA was generated with an oligo(dT) primer (Invitrogen, Life Technologies, Grand Island, NY) followed by analysis using real-time PCR with the Power SYBR Green PCR Master Mix (AB Applied Biosystems, University Park, IL) based on the expression of β-actin. The following primer pairs were used: 5′-GACGGCCAGGTCATCACTATTG-3,′ 5′-AGGAAGGCTGGAAAAGAGCC-3′ for β-Actin, 5′-TGC GAT GGT GAT AAA AGT GG-3′, 5′-GGC CAG AAG CTC TGC TAC TC-3′ for S100A8 and 5′-CAC AGT TGG CAA CCT TTA TG-3′, 5′-CAG CTG ATT GTC CTG GTT TG-3′ for S100A9.
Gene array and functional analysis
Epithelial cells of normal control, PA01-infected, and flagellin-pretreated and PA01-infected B6 mouse corneas were scrapped off with dulled thin blade, scooped epithelial cells were frozen immediately in liquid nitrogen, removed from the blade with a forceps into an 1.5-ml Eppendorf tube placed on dry ice with two corneas pooled in one tube. Total RNA from pooled epithelial cells was isolated using RNeasy (Qiagen). RNA quality was verified using the Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNAs were synthesized and hybridized to an Illumina mouse WG-6v2.0 expression bead chip containing 45,200 50-mer transcripts. Data were collected at Applied Genomics Technology Center, Wayne State Univerisity School of Medicine and evaluated by Genomic Core Lab of National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA. The analysis of variance analysis for the RMA-normalized data sets was done by the commercial microarray data analysis software Partek Genomic Suite http://www.partek.com/partekgs/. Signaling pathway and functional network analyses were performed using Genomatrix Pathways System software.
Immunohistochemistry of mouse corneas
Mouse eyes were enucleated and embedded in Tissue-Tek optimal cutting temperature compound and frozen in liquid nitrogen. Six-micrometer thick sections were cut and mounted to polylysine-coated glass slides. After a 10-min fixation in 4% paraformaldehyde, slides were blocked with 10 mM sodium phosphate buffer containing 2% bovine serum albumin for 1 h at room temperature. Sections were then incubated with mouse primary antibody (S100A8 1:200 and S100A9 1:100, R&D Systems, Minneapolis, MN). This was followed by a secondary antibody, FITC anti-rat or anti-goat IgG (Jackson ImmunoResearch Laboratories; 1:100), and slides were mounted with Vectashield mounting medium containing 4, 6-diamidino-2-phenylindole dihydrochloride (DAPI) mounting media and examined under a Carl Zeiss fluorescence microscope Axioplan 2 equipped with an ApoTome digital camera or using confocal microscopy (TCSSP2; Leica). Controls were similarly treated, but the primary antibody was replaced with rat or rabbit IgG.
S100A8/9 neutralization and infection
Purified flagellin (500 ng in 5 μl of PBS) or PBS (control) was topically applied on the cornea before P. aeruginosa challenge. Sixteen hours before P. aeruginosa inoculation, B6 mice were subconjunctivally injected 5 μl PBS containing 0.5 mg ml−1 of rabbit anti-mouse S100A8/9 (kindly provided by Dr Philippe Tessier). The control mice received the same dose of rabbit IgG. The eyes were inoculated with 1 × 104 CFU of strain ATCC 19660.
Bacterial burden determination, cytokine ELISA, and myeloperoxidase measurement
Bacterial load, ELISA determination of MIP-2, and myeloperoxidase measurements were performed as described, and the same mouse cornea was used in all the three assays.18
Statistical analyses
Data were presented as means±s.d. Statistical differences among three or more groups were identified using one-way analysis of variance. Differences were considered statistically significant at P<0.05.
Supplementary Material
Acknowledgments
This study was supported by grants NIH/NEI R01-EY010869, R01-EY017960, P30-EY004068, R01-HL097564, Midwest Eye Bank, Research to Prevent Blindness.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/mi
References
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Nish S, Medzhitov R. Host defense pathways: role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34:629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weindl G, Wagener J, Schaller M. Epithelial cells and innate antifungal defense. J Dent Res. 2010;89:666–675. doi: 10.1177/0022034510368784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueta M, Kinoshita S. Innate immunity of the ocular surface. Brain Res Bull. 2010;81:219–228. doi: 10.1016/j.brainresbull.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Muller CA, Autenrieth IB, Peschel A. Innate defenses of the intestinal epithelial barrier. Cell Mol Life Sci. 2005;62:1297–1307. doi: 10.1007/s00018-005-5034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben Mkaddem S, Chassin C, Vandewalle A. Contribution of renal tubule epithelial cells in the innate immune response during renal bacterial infections and ischemia-reperfusion injury. Chang Gung Med J. 2010;33:225–240. [PubMed] [Google Scholar]
- 8.Marques R, Boneca IG. Expression and functional importance of innate immune receptors by intestinal epithelial cells. Cell Mol Life Sci. 2011;68:3661–3673. doi: 10.1007/s00018-011-0829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swamy M, Jamora C, Havran W, Hayday A. Epithelial decision makers: in search of the ‘epimmunome’. Nat Immunol. 2010;11:656–665. doi: 10.1038/ni.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botturi K, et al. Preventing asthma exacerbations: what are the targets? Pharmacol Ther. 2011;131:114–129. doi: 10.1016/j.pharmthera.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Bai C, Li K, Adler KB, Wang X. Role of airway epithelial cells in development of asthma and allergic rhinitis. Respir Med. 2008;102:949–955. doi: 10.1016/j.rmed.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Lang T, Mansell A. The negative regulation of Toll-like receptor and associated pathways. Immunol Cell Biol. 2007;85:425–434. doi: 10.1038/sj.icb.7100094. [DOI] [PubMed] [Google Scholar]
- 14.West MA, Heagy W. Endotoxin tolerance: A review. Crit Care Med. 2002;30:S64–S73. [PubMed] [Google Scholar]
- 15.Vartanian K, Stenzel-Poore M. Toll-like receptor tolerance as a mechanism for neuroprotection. Transl Stroke Res. 2010;1:252–260. doi: 10.1007/s12975-010-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Kumar A, Gao N, Standiford TJ, Gallo RL, Yu FS. Topical flagellin protects the injured corneas from Pseudomonas aeruginosa infection. Microbes Infect. 2010;12:978–989. doi: 10.1016/j.micinf.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao N, Kumar A, Guo H, Wu X, Wheater M, Yu FS. Topical flagellin-mediated innate defense against Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2011;52:3074–3082. doi: 10.1167/iovs.10-5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50–57. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah P, Dana MR. Corneal antigen-presenting cells. Chem Immunol Allergy. 2007;92:58–70. doi: 10.1159/000099254. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Yu FS. Toll-like receptors and corneal innate immunity. Curr Mol Med. 2006;6:327–337. doi: 10.2174/156652406776894572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 23.Fleiszig SM, Evans DJ. Contact lens infections: can they ever be eradicated? Eye Contact Lens. 2003;29:S67–S71. doi: 10.1097/00140068-200301001-00019. discussion S83-64, S192–194. [DOI] [PubMed] [Google Scholar]
- 24.Alarcon I, Kwan L, Yu C, Evans DJ, Fleiszig SM. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009;77:3264–3271. doi: 10.1128/IAI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleiszig SM, Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci. 2010;87:225–232. doi: 10.1097/OPX.0b013e3181d408ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczotka-Flynn LB, Pearlman E, Ghannoum M. Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens. 2010;36:116–129. doi: 10.1097/ICL.0b013e3181d20cae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–1324. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 28.Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa) Chem Immunol Allergy. 2007;92:185–194. doi: 10.1159/000099269. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A, Singh CN, Glybina IV, Mahmoud TH, Yu FS. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J Infect Dis. 2010;201:255–263. doi: 10.1086/649589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu FS, et al. Flagellin stimulates protective lung mucosal immunity: role of cathelicidin-related antimicrobial peptide. J Immunol. 2010;185:1142–1149. doi: 10.4049/jimmunol.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burdelya LG, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijay-Kumar M, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol. 2008;180:8280–8285. doi: 10.4049/jimmunol.180.12.8280. [DOI] [PubMed] [Google Scholar]
- 33.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201:534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarchum I, Liu M, Lipuma L, Pamer EG. Toll-like receptor-5 stimulation protects mice from acute Clostridium difficile colitis. Infect Immun. 2011;79:1498–1503. doi: 10.1128/IAI.01196-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 36.Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 37.Uematsu S, Akira S. Immune responses of TLR5( +) lamina propria dendritic cells in enterobacterial infection. J Gastroenterol. 2009;44:803–811. doi: 10.1007/s00535-009-0094-y. [DOI] [PubMed] [Google Scholar]
- 38.Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol. 2010;4:102–111. doi: 10.1038/mi.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu K, et al. Anti-Infective protective properties of S100 calgranulins. Anti-inflammatory Anti-allergy Agents Med Chem. 2009;8:290–305. doi: 10.2174/187152309789838975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandtzaeg P, Gabrielsen TO, Dale I, Muller F, Steinbakk M, Fagerhol MK. The leucocyte protein L1 (calprotectin): a putative nonspecific defence factor at epithelial surfaces. Adv Exp Med Biol. 1995;371A:201–206. doi: 10.1007/978-1-4615-1941-6_41. [DOI] [PubMed] [Google Scholar]
- 41.Liu JZ, et al. Zinc sequestration by the neutrophil protein calprotectin enhances Salmonella growth in the inflamed gut. Cell Host Microbe. 2012;11:227–239. doi: 10.1016/j.chom.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dela Cruz CS, et al. Chitinase 3-like-1 Promotes Streptococcus pneumoniae Killing and Augments Host Tolerance to Lung Antibacterial Responses. Cell Host Microbe. 2012;12:34–46. doi: 10.1016/j.chom.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lusitani D, Malawista SE, Montgomery RR. Calprotectin, an abundant cytosolic protein from human polymorphonuclear leukocytes, inhibits the growth of Borrelia burgdorferi. Infect Immun. 2003;71:4711–4716. doi: 10.1128/IAI.71.8.4711-4716.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raquil MA, Anceriz N, Rouleau P, Tessier PA. Blockade of antimicrobial proteins S100A8 and S100A9 inhibits phagocyte migration to the alveoli in streptococcal pneumonia. J Immunol. 2008;180:3366–3374. doi: 10.4049/jimmunol.180.5.3366. [DOI] [PubMed] [Google Scholar]
- 46.Crawford MA, et al. Antimicrobial effects of interferon-inducible CXC chemokines against Bacillus anthracis spores and bacilli. Infect Immun. 2009;77:1664–1678. doi: 10.1128/IAI.01208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doonan F, Wallace DM, O’Driscoll C, Cotter TG. Rosiglitazone acts as a neuroprotectant in retinal cells via up-regulation of sestrin-1 and SOD-2. J Neurochem. 2009;109:631–643. doi: 10.1111/j.1471-4159.2009.05995.x. [DOI] [PubMed] [Google Scholar]
- 48.Bourette RP, Mouchiroud G. The biological role of interferon-inducible P204 protein in the development of the mononuclear phagocyte system. Front Biosci. 2008;13:879–886. doi: 10.2741/2728. [DOI] [PubMed] [Google Scholar]
- 49.Rouabhia M, Ross G, Page N, Chakir J. Interleukin-18 and gamma interferon production by oral epithelial cells in response to exposure to Candida albicans or lipopolysaccharide stimulation. Infect Immun. 2002;70:7073–7080. doi: 10.1128/IAI.70.12.7073-7080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegrist F, Ebeling M, Certa U. The small interferon-induced transmembrane genes and proteins. J Interferon Cytokine Res. 2011;31:183–197. doi: 10.1089/jir.2010.0112. [DOI] [PubMed] [Google Scholar]
- 51.Hofman VJ, et al. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori. Mod Pathol. 2007;20:974–989. doi: 10.1038/modpathol.3800930. [DOI] [PubMed] [Google Scholar]
- 52.Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science. 2011;332:717–721. doi: 10.1126/science.1201711. [DOI] [PubMed] [Google Scholar]
- 53.Kim KI, Malakhova OA, Hoebe K, Yan M, Beutler B, Zhang DE. Enhanced antibacterial potential in UBP43-deficient mice against Salmonella typhimurium infection by up-regulating type I IFN signaling. J Immunol. 2005;175:847–854. doi: 10.4049/jimmunol.175.2.847. [DOI] [PubMed] [Google Scholar]
- 54.Liu CS, Sun Y, Zhang M, Sun L. Identification and analysis of a Sciaenops ocellatus ISG15 homologue that is involved in host immune defense against bacterial infection. Fish Shellfish Immunol. 2010;29:167–174. doi: 10.1016/j.fsi.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Caruso R, et al. Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood. 2009;113:3512–3519. doi: 10.1182/blood-2008-08-172767. [DOI] [PubMed] [Google Scholar]
- 56.Linke A, Goren I, Bosl MR, Pfeilschifter J, Frank S. Epithelial overexpression of SOCS-3 in transgenic mice exacerbates wound inflammation in the presence of elevated TGF-beta1. J Invest Dermatol. 2010;130:866–875. doi: 10.1038/jid.2009.345. [DOI] [PubMed] [Google Scholar]
- 57.Yano J, Lilly E, Barousse M, Fidel PL., Jr Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun. 2010;78:5126–5137. doi: 10.1128/IAI.00388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression in vitro by oral epithelial cells confers resistance to infection by Porphyromonas gingivalis. Infect Immun. 2001;69:4242–4247. doi: 10.1128/IAI.69.7.4242-4247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun. 2001;69:3692–3696. doi: 10.1128/IAI.69.6.3692-3696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murthy AR, Lehrer RI, Harwig SS, Miyasaki KT. In vitro candidastatic properties of the human neutrophil calprotectin complex. J Immunol. 1993;151:6291–6301. [PubMed] [Google Scholar]
- 61.Passey RJ, et al. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–2216. [PubMed] [Google Scholar]
- 62.Manitz MP, et al. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 64.Achouiti A, et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. Plos Pathog. 8:e10029872012. doi: 10.1371/journal.ppat.1002987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hazlett LD, McClellan S, Kwon B, Barrett R. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000;41:805–810. [PubMed] [Google Scholar]
- 66.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.