Abstract
Thyrotropin-releasing hormone (TRH), a neuropeptide contained in neural terminals innervating brainstem vagal motor neurons, enhances vagal outflow to modify multisystemic visceral functions and food intake. Type 2 diabetes (T2D) and obesity are accompanied by impaired vagal functioning. We examined the possibility that impaired brainstem TRH action may contribute to the vagal dysregulation of food intake in Goto-Kakizaki (GK) rats, a T2D model with hyperglycemia and impaired central vagal activation by TRH. Food intake induced by intracisternal injection of TRH analog was reduced significantly by 50% in GK rats, compared to Wistar rats. Similarly, natural food intake in the dark phase or food intake after an overnight fast was reduced by 56–81% in GK rats. Fasting (48 h) and refeeding (2 h)-associated changes in serum ghrelin, insulin, peptide YY, pancreatic polypeptide and leptin, and the concomitant changes in orexigenic or anorexigenic peptide expression in the brainstem and hypothalamus, all apparent in Wistar rats, were absent or markedly reduced in GK rats, with hormone release stimulated by vagal activation, such as ghrelin and pancreatic polypeptide, decreased substantially. Fasting-induced Fos expression accompanying endogenous brainstem TRH action decreased by 66% and 91%, respectively, in the nucleus tractus solitarius (NTS) and the dorsal motor nucleus of the vagus (DMV) in GK rats, compared to Wistar rats. Refeeding abolished fasting-induced Fos-expression in the NTS, while that in the DMV remained in Wistar but not GK rats. These findings indicate that dysfunctional brainstem TRH-elicited vagal impairment contributes to the disturbed food intake in T2D GK rats, and may provide a pathophysiological mechanism which prevents further weight gain in T2D and obesity.
Keywords: food intake, type 2 diabetes, the vagus nerve, thyrotropin-releasing hormone, brainstem
INTRODUCTION
Body metabolic homeostasis is tightly regulated by the central nervous system through precise adjustments of sympathetic and parasympathetic outflow to multiple visceral organs. Vagal cholinergic processes, in particular, control hunger, meal initiation, and food digestion, through cholinergic (muscarinic) regulation of gastric secretory and motility functions, especially gastric ghrelin biosynthesis and secretion (Ao et al., 2006; Toshinai et al., 2001). The vagal role can be shown by blockage of food deprivation-induced plasma ghrelin elevation following subdiaphragmatic vagotomy and atropine treatment (Williams et al., 2003). In addition, circulating ghrelin levels in humans are increased or decreased by cholinergic agonists or antagonists, respectively (Broglio et al., 2004).
Attenuated vagal motor activity and exaggerated sympathetic discharge are common in T2D and obese patients (Oida et al., 1999), and develop as part of the mechanisms for re-equilibration of metabolism in these conditions (Chaput et al., 2012). Vagal impairment further enhances the unbalanced sympathetic dominance. Albeit increasing energy expenditure (Straznicky et al., 2012a), sympathetic hyperactivity mediates the development of insulin resistance, hyperglycemia, reduced insulin response to glucose, lipid dysregulation, hypertension, and high cardiovascular mortality (Lindmark et al., 2003; Oida et al., 1999; Perin et al., 2001), thus is critical in the pathogenesis of obesity-associated T2D (Esler et al., 2001; Straznicky et al., 2012b). Impaired vagal function underlies dysregulation of food intake and digestion in obese and T2D subjects, who commonly have gastroparesis-associated early satiety, fullness, bloating, and suppressed plasma ghrelin levels (Boaz et al., 2011; Williams et al., 2006). Understanding the mechanisms of impaired vagal function on food intake regulation is essential to determine processes underlying T2D and obesity.
Thyrotropin-releasing hormone (TRH), a neuropeptide originally found in the hypothalamus, is synthesized also in the brainstem caudal raphe nuclei. The raphe nuclei innervate vagal and sympathetic motor neurons located in the brainstem and the spinal cord. Dense TRH-containing nerve fibers appear in the nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMV), area postrema (AP), and nucleus ambiguus (Amb), all nuclei involved in vagal regulation, as well as in the spinal sympathetic intermediolateral cell column (IML) (Manaker and Rizio, 1989; Rinaman et al., 1989; Taché and Yang, 1994). TRH microinjected into the DMV, or endogenously released into the DMV after chemical stimulation of neurons in the raphe nuclei, induces potent vagal-mediated visceral responses (Ishikawa et al., 1988; Yang et al., 1993; Yang et al., 2002). Substantial evidence shows that TRH is the only brain peptide fulfilling all of the criteria as a neurotransmitter and/or neuromodulator activating vagal motor neurons in the DMV, where it assists vagal regulation of food digestion and nutrition metabolism by increasing gastric/intestinal secretory/motility and stimulating gut/pancreatic hormone release (Ao et al., 2006; Ao et al., 2010; Ao et al., 2005a; Taché and Yang, 1994; Yang et al., 2012). Activation of brainstem TRH receptors increases food intake by stimulating vagal-cholinergic pathway-mediated gastric ghrelin release (Ao et al., 2006). Furthermore, brainstem TRH mRNA levels increase during body energy deficiencies or when energy demand is increased, such as fasting and cold exposure (Ao et al., 2006; Yang et al., 1994). The collective evidence suggests that TRH plays an essential role in mediating vagal control of energy intake and nutrition digestion, and should be a focus of attention in any examination of food regulation, especially in T2D models.
We tested the hypothesis that impaired vagal function in T2D is associated with food intake dysregulation in T2D Goto-Kakizaki (GK) rats. The GK rat is a “non-obese” T2D model generated by selective breeding from non-diabetic Wistar rats with poor glucose tolerance, and shows basal hyperglycemia, hypertension, and insulin resistance (Goto et al., 1988; Yang et al., 2012). We found impaired vagal regulation of visceral organs in GK rats, a consequence of reduced vagal efferent outflow activation by brainstem TRH (Ao et al., 2010; Ao et al., 2005a; Yang et al., 2012). TRH analog intracisternal injection (ic), at doses that did not influence blood glucose levels and heart rate in non-diabetic Wistar rats, induced persistent sympathetic activation-mediated increases in glucose levels, blood pressure and heart rate in GK rats, concomitant with remarkably damaged vagal-counterregulation on insulin stimulation and cardiac inhibition (Ao et al., 2010; Ao et al., 2005a; Yang et al., 2012). The extreme and prolonged hyperglycemia and acute heart failure-induced cardiovascular mortality in GK rats indicate severely damaged TRH action on DMV vagal motor neurons (Ao et al., 2010; Ao et al., 2005a; Yang et al., 2012). In this study, we first established body adipose tissue content in the GK and Wistar rats and then evaluated the inappropriately management of central and peripheral signaling in response to fasting and refeeding in T2D GK rats, with focus on brainstem TRH dysfunction-associated impairment of vagal regulation.
EXPERIMENTAL PROCEDURES
Animals
Male non-diabetic Wistar rats (280–320 g, 2.5–3 months old) were purchased from Harlan Laboratories (San Diego, CA). Sex- and body weight (BW)-matched T2D GK rats (3 months old) were bred in the animal facilities of Veterans Affairs (VA) Greater Los Angeles Healthcare System (GLAHS) with an Institutional Animal Care and Use Committee (IACUC)-approved breeding protocol. Rats were housed under controlled conditions (21–23°C, lights-on from 06:00 to 18:00) with free access to standard rat chow and tap water. The study protocol was approved by the IACUC of VA GLAHS. Experiments were performed in rats either ad lib fed (normally fed), fasted (over night, 24 h, or 48 h), or refed for 2 h after a 48 h fast. All animal studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemicals
The metabolically stable TRH-analog RX 77368 (p-Glu-His-(3,3′-dimethyl)-Pro-NH2, Reckitt and Colman, Kingston Upon Hull, UK) exerts potent and relatively long-lasting central effects, compared to TRH in rats and mice. RX77368 was freshly diluted with normal saline from an aliquots (30 μg/50 μl in 0.1%BSA/saline) stored at −80°C.
Measurement of body lean and adipose mass quantities, blood glucose, and gut hormone levels
Lean and fat body mass quantities were measured in awake rats by non-invasive magnetic resonance imaging using EchoMRI-4in1 (EchoMedical System, Houston, Texas) and data were automatically calculated by the software installed in the equipment. Blood samples for measuring hormone levels in normally fed, fasted, and refed conditions were collected from rat right heart ventricle immediately before the transcardial perfusion. Sera (20 μl) were kept at −80°C before hormones were measured using a Millipore Luminex with Rat Gut Hormone kit (Merck Millipore, Billerica, MA). Blood glucose levels before and during food intake were measured in other groups of rats by One Touch Ultra Blood Glucose Monitoring System (Lifescan, Milpitas, CA) using ~2 μl of whole blood collected from the tail tip after a 25 G needle puncture of unrestrained rat at each time point. There was no significant influence on food intake by this process compared to rats without receiving the glucose test (Ao et al., 2006).
Measurement of food intake
Rats were housed individually and provided with pre-weighed rat chow and tap water for 2 or 3 h. Food intake was measured each hour by subtracting the weight of the remaining rat chow from the previous weight and expressed as food consumption (g) per 300 g of BW.
Intracisternal injection
Rats were individually positioned on a stereotaxic instrument under short-time (2–3 min) isoflurane-anesthesia. A Hamilton syringe needle was inserted through the skin, posterior atlanto-occipital membrane, dura matter, and the underlying arachnoid membrane into the cistern magna. Successful insertion of the needle into the cisterna magma was verified by aspirating clear cerebrospinal fluid into the syringe before and after injecting the test substance.
Immunohistochemistry of Fos expression in the brainstem
Pentobarbital (70 mg/kg, intraperitoneal injection)-anesthetized rats were transcardially perfused in the morning (10:00–12:00) with fixative [0.1 M sodium phosphate buffer (PB, pH 7.4) containing 4% paraformaldehyde and 14% picric acid]. Blood samples (~2 ml/rat) were collected from the right heart ventricle immediately before perfusion for glucose and gut hormone measures. The brain was collected, post-fixed 4 h in the same fixative, and cryoprotected overnight in 20% sucrose/0.1 M PB. Coronal frozen sections (30 m) at the interaural levels of −4.24 to −5.08 mm were incubated for 24 h at 4°C with a polyclonal rabbit anti-Fos serum (Fos Ab-5, Oncogene Research Products, Cambridge, MA; 1: 10,000) followed by 1 h at room temperature with biotinylated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA; 1:1000) and finally processed using the standard biotin-avidin-horseradish peroxidase methodology (Hsu et al., 1981). The number of Fos-immunoreactive cells was counted, and images were collected using microscopy. In each rat, the mean number of Fos-positive cells per nucleus was calculated from 8–10 brain sections at interaural levels of −4.68 to −5.08 mm according to the atlas of Paxinos and Watson (Paxinos and Watson, 1997). The group mean of Fos-positive cells in each nucleus was calculated from the mean number in individual animals.
Quantitative PCR for gene expression in the hypothalamus and the brainstem
All primers sets were designed by using Primer-BLAST from NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome) to have a unique set among Rattus norvegicus genome with a Tm of 60 ± 0.5°C. The primers are custom-made by Invitrogen (Carlsbad, CA) and listed in Table 1. Total RNAs were extracted using TRIzol Reagent (Invitrogen) from hypothalamic and brainstem tissues collected in rapidly-decapitated rats. The cDNA was synthesized using 1 μg total RNA, 60 μM random primers poly (dN)6, 1 mM dNTPs, 1 U/μl RNase inhibitor and 0.5 U/μl reverse transcriptase (Roche, Indianapolis, IN) in a 30 μl reaction mixture. The mixture was incubated at 50°C for 60 min followed by inactivation at 80°C for 5 min. The cDNA was diluted into 10X and 40X with water. An aliquot of 5 μl from each dilution in duplicates were mixed with equal volumes of 1 μM respective primer sets and 10 μl FastStart SYBR Green Master (Roche). Amplification was carried out at 95°C × 10 min, followed by 40 cycles of 95°C × 15 sec, 60°C × 30 sec and 72°C × 30 sec in a Strategene Mx3000 cycler (Agilent Technologies, Wilmington, DE). Three animal samples were individually evaluated in the same plate. Gene expression levels were calculated by the 2−ΔΔCt method using 18S molecule as a reference (Bustin et al., 2009).
Table 1.
Primer sets used in qPCR
| Genes | ID | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|---|
| AgRP | NM_033650 | GCTGCACGAGTCCTGCTTGGG | AGGGGCGGCTGCAGAGGTTC |
| GHSR | NM_032075.3 | ACCGAGTTCGCTGTGCGCTC | TCCGCGTCTCCGCCATAGCT |
| LepR | NM_012596 | GGTACAGGTTCGCTGCCGGC | GTGGGCAGTACGATGCTTCACCAC |
| MC4R | NM_013099.2 | GACGCCCAGAGCTTCACCGTG | GATCCCGACCCGCCTAACCGT |
| NPY | NM_012614 | GACAATCCGGGCGAGGACGC | AGGGTCTTCAAGCCTTGTTCTGGGG |
| Oxt | NM_012996.3 | ACCGACGGTGGATCTCGGACTG | CAGCAGATGCTCGGCCCGAAG |
| POMC | NM_139326.2 | TCGGGTGGAGCACTTCCGCT | ACTGGCCCTTCTTGTGCGCG |
| TRH | NM_013046 | CCACAAACGACAGCACCCCGG | AGGGGAAGGATCGCCTGCCAG |
| TRHR | NM_013047.3 | AGCAGGCAGCGTGACAGAGTGA | GAGGGCCACGGCTACTTGCG |
| Y1R | NM_001113357 | CTCTGGTTCTCATCGCTGTGGAACG | GATTCGTTTGGTCTCACTGGACCTGTAC |
| Y2R | NM_023968 | AAATGGCCCGGGGAGGAGAAGAG | GTAGTGGTCCATTGCAGCTCCAGG |
Statistical analysis
Data are expressed as mean ± SEM for each group. Statistical comparisons between group-mean values for Wistar and GK rats receiving the same treatment or between RX 77368-treated and vehicle-treated rats of the same strain were performed using unpaired Student’s t-tests. Comparisons between values obtained before and after ic injection in the same group were performed using a paired Student’s t-test. Comparisons among multiple groups were calculated using a one-way or two-way ANOVA. All statistical tests were performed using SigmaStat 2.0 software. P values less than 0.05 were considered statistically significant.
RESULTS
Increased adipose content in T2D GK rats
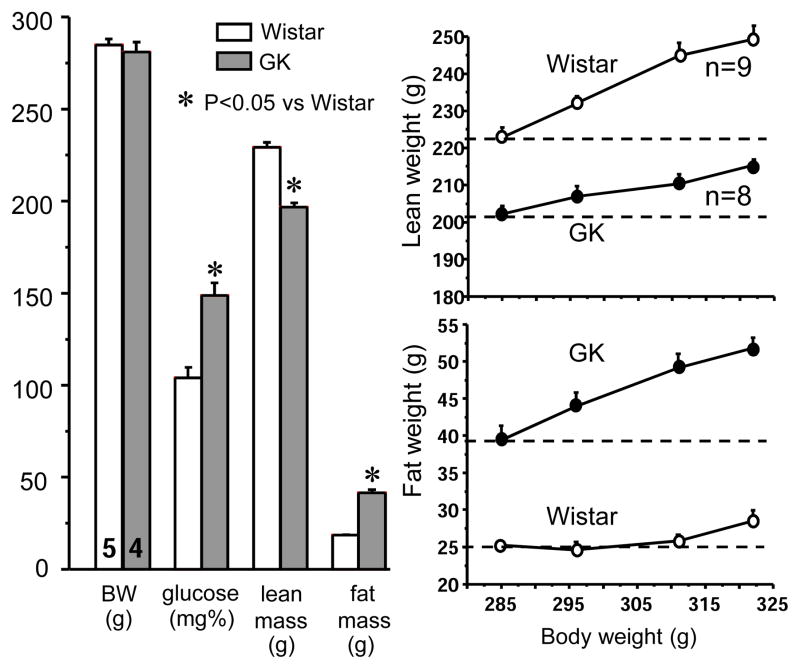
Compared to Wistar rats with similar BW, GK rats had doubled fat mass and significantly less lean mass (Fig. 1). During rat growth from 285 g to 320 g, the increase in Wistar rats was mainly lean weight while that in GK rats was mainly fat weight (Fig. 1).
Figure 1.
Left panel: Body weight (BW), blood glucose, lean mass and fat mass in non-fasted Wistar rats and T2D GK rats. Each column represents mean ± SEM, with the number of rats indicated at the bottom of the first two columns. The lean and fat masses were measured by non-invasive magnetic resonance imaging in awake animals. Right panel: Lean and fat weights in Wistar and GK rats when body weights increased from 280 g to 320 g. Each point represents mean ± SEM, with the number of rats indicated beside the lines.
Reduced food intake in T2D GK rats
Compared to Wistar rats, the body weight matched GK rats showed a remarkable reduction in natural food intake in the dark phase (18:00–21:00) (Fig. 2A1) and in morning food intake (09:00–12:00) after an overnight fast (Fig. 2B1). Despite reduced food consumption, GK rats had more pronounced blood glucose elevation after eating than Wistar rats (Figs. 2A2, 2B2), indicating an impaired insulin response to nutrition digestion and absorption. TRH-analog ic injection-induced food intake (09:00–12:00) was reduced by 50% in normally-fed GK rats (1.68 ± 0.33 vs 3.35 ± 0.28 g, P< 0.001). The stomach weights in the morning, containing food ingested during the dark phase, were 6.83 ± 0.45 g in Wister rats (BW 332 ± 3 g, n = 4) and 3.34 ± 0.26 g in GK rats (BW 333 ± 2 g, n = 5, P<0.001), consistent with the reduced food intake in GK rats.
Figure 2.
Panels A1 and B1: Food intake in the first 3 hours of the dark phase (A1) or in the morning after an overnight fast (B1) in Wistar and GK rats. Each column represents mean ± SEM, with the number of rats indicated at the bottom of the column or above it. Panels A2 and B2: Corresponding blood glucose levels before and after the food consumption in Wistar and GK rats. Each point represents mean ± SEM of the number of rats indicated at the bottom of the column or above it in A1 and B1.
Diminished gut hormonal responses to fasting and refeeding and increased leptin levels in T2D GK rats
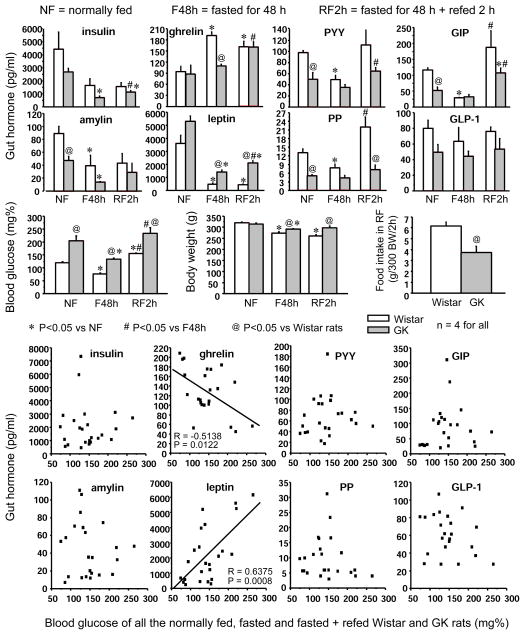
In Wistar rats, a 48-h fast significantly lowered blood glucose levels and increased total serum ghrelin levels by 203% (Fig. 3). GK rats showed significantly higher blood glucose levels and lower serum levels of peptide YY (PYY), glucose-dependent insulinotropic polypeptide (GIP), and pancreatic polypeptide (PP) in normally-fed conditions. Fasting-induced increase in serum ghrelin and decreases in PYY, GIP, and PP levels found in Wistar rats were all absent in GK rats (Fig. 3). Insulin and amylin levels that were lower in GK rats relative to Wistar rats in normally fed conditions were further decreased by fasting in GK rats. In contrast, leptin levels, albeit decreased after a 48 h fast in both strains, were significantly higher (3–4-fold) in GK rats (Fig. 3). Refeeding increased serum PP by 3-fold and GIP by 6.5-fold in Wistar rats while these responses were less pronounced in GK rats (Fig. 3). Serum glucagon-like peptide-1 (GLP-1) levels tended to be lower in GK compared to Wistar rats, but these differences were not significant. When data from Wistar and GK rats with different energy statuses were pooled, blood glucose levels correlated negatively with serum total ghrelin levels, and positively with leptin levels (Fig. 3).
Figure 3.
Top 3 panel rows: Serum levels of insulin, ghrelin, peptide YY (PYY), glucose-dependent insulinotropic polypeptide (GIP), amylin, leptin, pancreatic polypeptide (PP), and glucagon-like peptide-1 (GLP-1) in Wistar and T2D GK rats that were normally fed (NF), fasted for 48 h (F48h), or refed for 2 h after a 48 h fasting (RF2h) and the corresponding blood glucose levels, body weight and the amount of food consumption. Each column represents mean ± SEM of 4 rats. Lowest panel rows: Correlation of blood glucose levels and serum total ghrelin or leptin levels in Wistar and GK rats with different energy status (n = 24).
Abnormal responses of brainstem and hypothalamic neuropeptides to fasting and refeeding in T2D GK rats
In Wistar rats, fasting induced increases (compared to normal feeding) and refeeding induced decreases (compared to fasting) in mRNAs of brainstem TRH and TRH receptor. These changes were absent in GK rats (Fig. 4). Fasting increased hypothalamic gene expression of neuropeptide Y (NPY), agouti-related peptide (AgRP), ghrelin receptor (GHSR), and leptin receptor (LEPR), and decreased that of proopiomelanocortin (POMC) in Wistar rats. These changes also did not appear in GK rats (Fig. 4).
Figure 4.
Gene expression of TRH and TRH receptor (TRHR) in the brainstem and neuropeptide Y (NPY), agouti-related peptide (AgRP), proopiomelanocortin (POMC), oxytocin, ghrelin receptor (GHSR), leptin receptor (LEPR), neuropeptide receptor 2 (Y2R), and melanocortin receptor 4 (MC4R) in the hypothalamus of normally fed (NF), 48 h fasted (F48h), and 2 h refed after a 48 h fasted (RF2h) Wistar and GK rats. Each column represents mean ± SEM of the number of rats indicated at the bottom of the columns.
Impaired neuronal activation in the dorsal motor nucleus of the vagus by fasting, refeeding, and ic TRH analog in T2D GK rats
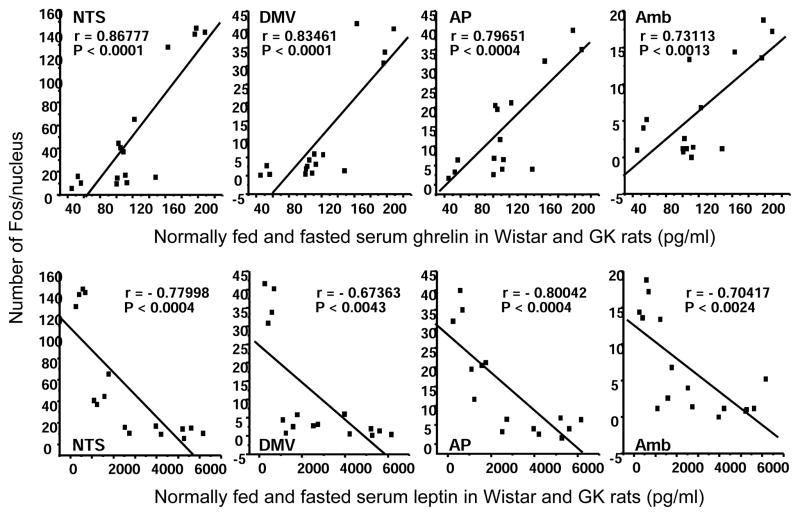
Fos expression in brainstem DMV, NTS, and AP was low in normally fed Wistar rats, but was expressed extensively by a 48-h fast. Such induction was significantly reduced in GK rats by 66% in the NTS, 91% in the DMV, and 49% in the AP (Fig. 5). Refeeding for 2 h in 48 h-fasted rats abolished the fasting-induced Fos-expression in the NTS in both strains, while those in the DMV and AP still appeared in refed Wistar rats, but not in refed GK rats (Fig. 5). The number of Fos-positive cells in brainstem NTS, DMV, AP, and Amb, the nuclei controlling vagal functions, were positively correlated with serum ghrelin levels and negatively with leptin levels (Fig. 6).
Figure 5.
Effects of fasting and refeeding on neuronal activation, shown as Fos expression, in brainstem dorsal vagal complex [DVC, including the dorsal motor nucleus of the vagus (DMV), the nucleus solitary tract (NTS), and the area postrema (AP)] in Wistar and T2D GK rats. Top panel: Diagrams reproduced from the Paxinos and Watson Atlas of The Rat Brain (Paxinos and Watson, 1997) showing the locations of the illustrated fields in the middle panel that were indicated by the rectangles in the diagrams. Middle panel: Light microscopic images showing Fos-immunoreactive cells in brainstem DVC in normally fed (NF), 48 h fasted (F48h) and 2 h refed (RF2h) animals. Lower panel: Quantitative data of Fos expression in brainstem NTS, DMV, and AP in GK and Wistar rats. Each column represents mean ± SEM of 4 rats.
Figure 6.
Correlations of serum total ghrelin or leptin levels and the number of Fos positive cells in brainstem nucleus solitary tract (NTS), dorsal motor nucleus of the vagus (DMV), area postrema (AP), and nucleus ambiguus (Amb) in Wistar and GK rats normally fed, fasted for 48 h or refed 2 h after a 48 h fast (n = 24).
Fos expression in the NTS was higher in GK than in Wistar rats 45 min after saline ic injection. TRH analog RX 77368 ic injection significantly increased Fos expression in the DMV of Wistar rats, but that expression was decreased in T2D GK rats (Fig. 7).
Figure 7.
Effects of intracisternal injection (ic) of saline or TRH analog RX77368 (20 ng) on Fos expression in brainstem nucleus solitary tract (NTS) and dorsal motor nucleus of the vagus (DMV) in Wistar and T2D GK rats. Each column represents mean ± SEM of 4 rats.
DISCUSSION
Food intake disorders are common in obesity and diabetes. A higher prevalence of bulimia nervosa appears in type 1 diabetes (T1D), with increased hypothalamic NPY concentrations that relate to low plasma leptin levels caused by reduced glucose usage in adipose tissue resulting from insulin deficiency (Havel et al., 1998; Mannucci et al., 2005; Williams et al., 1988). Both binge eating and anorexia are reported among the eating disorders in T2D, with anorexia occurs especially in older persons (Aizawa et al., 1997; Herpertz et al., 1998; Morley and Perry, III, 1991). The present study using GK rat model investigated abnormal central and peripheral dysregulation of food intake in T2D and the role of dysfunctional brainstem TRH-induced impairment of vagal efferent function in this dysregulation.
GK rats have more fat mass than Wistar rats for a given BW, reflecting their elevated leptin levels in all energy status. Dyslipidemia appears in GK rats, shown as high plasma levels of very low density lipoprotein-triglyceride and low density lipoprotein-cholesterol (Yamane et al., 1995). Abnormal liver lipid metabolism in GK rats is also present, indicated by different expression of 311 relevant genes between Wistar and GK rats (Almon et al., 2009). Thus, albeit the GK rat is generally recognized as a non-obese T2D model, it possesses severe lipid dysregulation. In humans, despite normal body mass index, a larger amount of visceral adipose tissue, increased intrahepatocellular and intramyocellular lipid content, and higher percentage of body fat associate with low insulin sensitivity and a predisposition for T2D (Gomez-Ambrosi et al., 2011; Lindmark et al., 2005). This relationship is especially apparent in the “thin-on-outside fat-on the inside” Asia subphenotype (Heitmann et al., 2012). Abnormal lipid metabolism results in insulin resistance, which is responsible for derangements of autonomic nervous tone control (Frontoni et al., 2003; Lindmark et al., 2005). On the other hand, sympathovagal imbalance in obesity and T2D further enhances insulin resistance (Frontoni et al., 2005; Lindmark et al., 2005; Lindmark et al., 2003). Given that higher amounts of visceral fat is a sign of a high ratio of sympathetic vs parasympathetic reactivity (Lindmark et al., 2005), the finding of more body fat mass, together with insulin resistance and damaged vagal function in GK rats (Ao et al., 2010; Ao et al., 2005a; Lee et al., 2009; Lee et al., 2012; Li et al., 2012; Liu et al., 2011; Yang et al., 2012), suggests that the GK rat is a realistic model for investigating the impact of sympathovagal imbalance on the pathogenesis of obesity-associated T2D.
Natural food intake in the dark phase and the morning food intake after an overnight fast were remarkably reduced in T2D GK rats, compared to Wistar rats. Reduced food intake explains the slow growth and the “non-obese” appearance of GK rats (Almon et al., 2009; Xue et al., 2011; Yasuda et al., 2002). However, these findings somewhat disagree with those of Maekawa et al (Maekawa et al., 2006), who reported that GK rats at the age of 6–12 weeks have hyperphagia, hyperglycemia, hyperleptinemia and increased visceral fat accumulation (Maekawa et al., 2006). These phenomena were also found in our study, except the hyperphagia. The different outcomes in food intake of GK rats between the two studies may result from differences between the two GK groups used. Our GK rats are interbred, and may express different characteristics from other GK rats. There are variations in the reports of GK rat growth rate among different research groups. GK rats body weights did not differ from Wistar rats at the same age in the Maekawa study (Maekawa et al., 2006), but were significantly lower than Wistar rats in other studies (Almon et al., 2009; Xue et al., 2011; Yasuda et al., 2002). Our GK rats also have lower body weights when compared to same aged Wistar rats. Reduced food intake in GK rats is coincident with profound alterations in gut/adipose hormones and hypothalamic neuropeptides in response to fasting and refeeding, compared to Wistar rats. Low fasting ghrelin and elevated leptin levels in GK rats result in an absence of fasting-induced increases in hypothalamic gene expression of NPY/AgRP and decreases in that of POMC/ghrelin receptor, the well-established food intake regulatory pathways that appeared in Wistar rats. The changes in ghrelin receptor mRNA in the hypothalamus are consistent with previous findings that those values are decreased by leptin and increased by ghrelin in the arcuate nucleus (Nogueiras et al., 2004). These abnormalities comprise the mechanisms responsible for the reduced food intake in T2D GK rats.
Impaired vagal efferent activation by brainstem TRH may play an important role in the dysregulation of food intake in GK rats. TRH actions at brainstem/spinal nuclei to integrate sympathovagal activity are critical for controlling ghrelin and other gut hormone release, gastric acid secretion, and gut motility before and after a meal (Ao et al., 2006; Taché and Yang, 1994). Previous studies have found that GK rats have accentuated sympathetic and diminished vagal activation by brainstem TRH administration, compared to Wistar rats, with consequences of potentiated increases in blood glucose, blood pressure and heart rats, and reduced insulin secretion (Ao et al., 2010; Ao et al., 2005a; Yang et al., 2012). This study shows that impaired TRH function in the DMV may also be involved in the dysregulation of food intake in GK rats, who showed a decrease in Fos expression in the DMV and a 50% reduction in food-intake response to ic TRH analog, compared to Wistar rats. The absence in the DMV and the remarkable reduction in the NTS and AP, the natural TRH targeting nuclei, of Fos expression after fasting and refeeding suggest impaired vagal motor neuronal responses to endogenous TRH in GK rats. These findings coincided with the impaired vagal regulation of gastric secretory and motility functions, such as the diminished ghrelin elevation in fasted T2D GK rats observed in the present study and the gastroparesis during food digestion found in T2D patients. In addition, gut hormone release in response to vagal activation, such as that of insulin, amylin, ghrelin, and PP, was significantly decreased in either fasting or food-digesting states. Damaged vagal regulation of food intake occurs in GK rats receiving ventromedial hypothalamic (VMH) lesions (Yoshida et al., 1996). Such lesions induce vagal hyperactivity while reduce sympathetic discharge, resulting in increases of food intake, serum insulin and BW in non-diabetic rats (Bray, 1984; Kiba et al., 1996). By contrast, in GK rats, VMH lesions enhance hyperglycemia, reduce pancreatic insulin content and BW, and increase triglyceride levels and mesenteric fat weight, which are signs of damaged vagal function in mediating the metabolic effects of VMH lesions (Yoshida et al., 1996).
Central TRH neurons are metabolic sensors, and TRH gene expression is regulated by nutrition states (Hollenberg, 2008; Lechan and Fekete, 2006). Brainstem TRH mRNA levels increase after fasting and when the body needs more energy, such as during cold exposure (Ao et al., 2006; Yang et al., 1994). Reduction in TRH synthesis in brainstem raphe nuclei and an absence of its elevation after fasting likely contribute to the impaired DMV neuronal activation in GK rats. How altered nutritional/hormonal signals in the T2D condition impact TRH gene expression in brainstem raphe nuclei remains to be investigated. Besides reducing TRH gene expression, the abnormal neural and hormonal signals in T2D conditions may diminish the excitatory action of TRH on vagal motor neurons in the DMV. The AP and portions of the NTS possess a leaky blood-brain barrier, and are central targets of peripheral hormonal/nutritional signals, including ghrelin, cholecystokinin, and leptin, all hormones regulating food intake (Date et al., 2002; Grill et al., 2002; Gross et al., 1990; Hussain and Bloom, 2012; Kanoski et al., 2012; Zhang et al., 2004). These hormones, and also inflammatory factors, such as interleukin-1β, modulate the vagal-stimulatory action of TRH on DMV neurons (Saperas et al., 1990; Taché and Yang, 1994). These interactions may contribute to the mechanisms operating in T2D GK rats (Xue et al., 2011). For example, tumor necrosis factor (TNF)-α, which is elevated in T2D patients (Lindmark et al., 2006), is an important mediator of cancer anorexia and acts in NTS/DMV neurons to suppress the vagal efferent activatory action of TRH (Bernstein, 1996; Emch et al., 2002; Hermann et al., 1999). In addition, insulin resistance in the DMV neurons may impair vagal motor neuronal function, albeit this needs to be studied.
Hyperglycemia in GK rats is a causative factor for the reduced food intake. Despite the complex interactions among neurotransmitters/neuropeptides and hormonal/nutritional signals occurred in the dorsal vagal complex (DVC), blood glucose levels play an important role in regulating vagal efferent activity. Hypoglycemia-induced vagal-dependent stimulation of gastric motility, a component of the feeling of hunger, is prevented by microinjection of glucose into the DVC, indicating a direct influence of glucose on DVC neurons (Sakaguchi et al., 1994). Indeed, the DVC contains glucose sensors and DVC neuronal activation is induced by hypoglycemia and inversely correlated with glucose levels (Ao et al., 2005b; Ritter et al., 1981; Yuan and Yang, 2002). Vagal activation stimulates gastric ghrelin release (Ao et al., 2006; Broglio et al., 2004; Toshinai et al., 2001). Oral or intravenous glucose administration significantly decreases plasma ghrelin concentrations in normal subjects (Shiiya et al., 2002). These findings indicate that sensing glucose levels in brainstem vagal-regulatory circuits and increasing vagal outflow when glucose levels is lower than the normal range may play an initial role triggering food intake behaviour. In this process, brainstem TRH contained in nerve fibers innervating the DMV neurons may contribute to activate vagal motor neurons. The vagal-efferent activation by ic TRH analog, which induces food intake, is dose-dependently prevented by intravenous glucose infusion (Ao et al., 2006; Doong and Yang, 2003). In the present study, blood glucose levels before and after the food intake were significantly higher in GK than in Wister rats, consonant with reduced food intake in GK rats. A possible mechanism in GK rats that hyperglycemia suppressed DVC neuronal response to TRH and subsequently lowered ghrelin response to fasting is suggested by several of our findings. Glucose levels were negatively correlated with serum ghrelin levels, which were positively correlated with neural activation in brainstem vagal-regulatory circuits, where TRH is an excitatory neurotransmitter. Moreover, the reduced Fos expression in the DMV of GK rats after ic TRH analog, compared to Wister rats, can be attributed to the extreme hyperglycemia induced by this treatment in GK rats, that resulted from sympathetic overactivation (Ao et al., 2010; Ao et al., 2005a; Yang et al., 2012). The present finding of a brainstem mechanism of food intake dysregulation in T2D GK rats provide supporting evidence for the glucostatic theory of appetite control (Chaput and Tremblay, 2009; MAYER, 1953).
CONCLUSIONS
Abnormal metabolic signals, such as hyperglycemia and hyperleptinemia, may influence the synthesis and the vagal-stimulating function of brainstem TRH, leading to sympathovagal imbalance that is responsible for the dysfunction of multisystemic visceral organs, including food intake dysregulation. Increased energy expenditure and reduced food intake resulting from sympathovagal imbalance may form part of the feedback regulatory mechanisms against obesity. However, long term sympathetic overactivation and vagal impairment, due to its profound influence on pancreatic islets, cardiovascular system, liver, and insulin sensitivity (Lindmark et al., 2003; Oida et al., 1999; Perin et al., 2001; Yang et al., 2012)., contribute to create a vicious cycle that accelerates the development of diabetes. Proper control of food intake and taking balanced nutrition to reduce body weight and correct hyperglycemia and hyperleptinemia have beneficial effects to enhance vagal efferent function and to restore sympathovagal balance, and thus important for preventing the development of obesity-related type 2 diabetes. Additional studies are clearly required to demonstrate the association of brainstem TRH dysfunction and the obesity-related T2D development, such as selective ablation of TRH or TRH receptor in the brainstem to determine its effect on autonomic function, leptin, and refeeding-related peptides in the periphery and hypothalamus. Further understanding of the brainstem mechanisms causing vagal impairment in obesity and T2D is warranted to identify new potential targets for development of therapeutic interventions to restore sympathovagal balance and limit T2D complications.
Highlights.
Food intake is reduced in type 2 diabetic Goto-Kakizaki (GK) rats.
Hormonal and neuronal responses to fasting and refeeding are disturbed in GK rats.
Brainstem TRH dysfunction results in vagal dysregulation of food intake.
Acknowledgments
This work was supported by a Department of Veterans Affairs Merit Award (H. Yang), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-41301 (Center for Ulcer Research and Education Center animal Core). The authors thank Dr. Sylvie Bradesi for use of the EchoMRI and Ms. Ai Chen for the technical assistance.
Abbreviations
- AgRP
agouti-related peptide
- Amb
nucleus ambiguus
- AP
area postrema
- BW
body weight
- DMV
dorsal motor nucleus of the vagus
- F48h
48 h fasted
- GHSR
ghrelin receptor
- GIP
glucose-dependent insulinotropic polypeptide
- GK rats
Goto-Kakizaki rats
- GLAHS
Greater Los Angeles Healthcare System
- GLP-1
glucagon-like peptide-1
- IACUC
Institutional Animal Care and Use Committee
- ic
intracisternal or intracisternal injection
- IML
spinal intermediolateral cell column
- LEPR
leptin receptor
- NF
normally fed
- NPY
neuropeptide Y
- NTS
nucleus tractus solitarius
- PB
phosphate buffer
- POMC
proopiomelanocortin
- PP
pancreatic polypeptide
- PYY
peptide YY
- RF2h
2 h refed after a 48 h fasted
- RVLM
rostral ventrolateral medulla
- T2D
type 2 diabetes
- TRH
thyrotropin-releasing hormone
- TRHR
TRH receptor
- VA
Veterans Affairs
Footnotes
CONFLICTS OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizawa M, Sakaguchi H, Fukunaga Y, Ashida C, Koshiyama H. A case of non-insulin-dependent diabetes mellitus associated with anorexia nervosa. Intern Med. 1997;36:380. doi: 10.2169/internalmedicine.36.380. [DOI] [PubMed] [Google Scholar]
- Almon RR, DuBois DC, Lai W, Xue B, Nie J, Jusko WJ. Gene expression analysis of hepatic roles in cause and development of diabetes in Goto-Kakizaki rats. J Endocrinol. 2009;200:331–346. doi: 10.1677/JOE-08-0404. [DOI] [PubMed] [Google Scholar]
- Ao Y, Go VL, Toy N, Li T, Wang Y, Song MK, Reeve JRJ, Liu Y, Yang H. Brainstem Thyrotropin-Releasing Hormone Regulates Food Intake through Vagal-Dependent Cholinergic Stimulation of Ghrelin Secretion. Endocrinology. 2006;147:6004–6010. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- Ao Y, Ko M, Chen A, Marvizon JC, Adelson D, Song MK, Go VL, Liu YY, Yang H. Potent hyperglycemic and hyperinsulinemic effects of thyrotropin-releasing hormone microinjected into the rostroventrolateral medulla and abnormal responses in type 2 diabetic rats. Neuroscience. 2010;169:706–719. doi: 10.1016/j.neuroscience.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao Y, Toy N, Song MK, Go VL, Yang H. Altered glucose and insulin responses to brain medullary thyrotropin-releasing hormone (TRH)-induced autonomic activation in type 2 diabetic Goto-Kakizaki rats. Endocrinology. 2005a;146:5425–5432. doi: 10.1210/en.2005-0553. [DOI] [PubMed] [Google Scholar]
- Ao Y, Wu S, Go VL, Toy N, Yang H. Maintaining euglycemia prevents insulin-induced Fos expression in brain autonomic regulatory circuits. Pancreas. 2005b;31:142–147. doi: 10.1097/01.mpa.0000172562.96168.59. [DOI] [PubMed] [Google Scholar]
- Bernstein IL. Neutral mediation of food aversions and anorexia induced by tumor necrosis factor and tumors. Neurosci Biobehav Rev. 1996;20:177–181. doi: 10.1016/0149-7634(95)00046-h. [DOI] [PubMed] [Google Scholar]
- Boaz M, Kislov J, Dickman R, Wainstein J. Obesity and symptoms suggestive of gastroparesis in patients with type 2 diabetes and neuropathy. J Diabetes Complications. 2011;25:325–328. doi: 10.1016/j.jdiacomp.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Bray GA. Hypothalamic and genetic obesity: an appraisal of the autonomic hypothesis and the endocrine hypothesis. Int J Obes. 1984;8(Suppl 1):119–137. [PubMed] [Google Scholar]
- Broglio F, Gottero C, Van KP, Prodam F, Destefanis S, Benso A, Gauna C, Hofland L, Arvat E, Van Der Lely AJ, Ghigo E. Acetylcholine regulates ghrelin secretion in humans. J Clin Endocrinol Metab. 2004;89:2429–2433. doi: 10.1210/jc.2003-031517. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Doucet E, Tremblay A. Obesity: a disease or a biological adaptation? An update. Obes Rev. 2012;13:681–691. doi: 10.1111/j.1467-789X.2012.00992.x. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Tremblay A. The glucostatic theory of appetite control and the risk of obesity and diabetes. Int J Obes (Lond) 2009;33:46–53. doi: 10.1038/ijo.2008.221. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Doong ML, Yang H. Intravenous glucose infusion decreases intracisternal thyrotropin-releasing hormone induced vagal stimulation of gastric acid secretion in anesthetized rats. Neurosci Lett. 2003;340:49–52. doi: 10.1016/s0304-3940(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. Tumor necrosis factor-alpha inhibits physiologically identified dorsal motor nucleus neurons in vivo. Brain Res. 2002;951:311–315. doi: 10.1016/s0006-8993(02)03178-5. [DOI] [PubMed] [Google Scholar]
- Esler M, Rumantir M, Wiesner G, Kaye D, Hastings J, Lambert G. Sympathetic nervous system and insulin resistance: from obesity to diabetes. Am J Hypertens. 2001;14:304S–309S. doi: 10.1016/s0895-7061(01)02236-1. [DOI] [PubMed] [Google Scholar]
- Frontoni S, Bracaglia D, Baroni A, Pellegrini F, Perna M, Cicconetti E, Ciampittiello G, Menzinger G, Gambardella S. Early autonomic dysfunction in glucose-tolerant but insulin-resistant offspring of type 2 diabetic patients. Hypertension. 2003;41:1223–1227. doi: 10.1161/01.HYP.0000073062.29546.01. [DOI] [PubMed] [Google Scholar]
- Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15:441–449. doi: 10.1016/j.numecd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Gil MJ, Valenti V, Rotellar F, Ramirez B, Salvador J, Fruhbeck G. Body Adiposity and Type 2 Diabetes: Increased Risk With a High Body Fat Percentage Even Having a Normal BMI. Obesity (Silver Spring) 2011;19:1439–1444. doi: 10.1038/oby.2011.36. [DOI] [PubMed] [Google Scholar]
- Goto Y, Suzuki K, Sasaki M, Ono T, Abe S. GK rat as a model of nonobese, noninsulin-dependent diabetes. Selective breeding over 35 generations. In: Renold AE, Renold, editors. Frontiers in Diabetes Research. Lessons from Animal Diabetes II. London, UK: John Libbey; 1988. pp. 301–303. [Google Scholar]
- Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Pang JJ, Shaver SW, Wainman DS. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am J Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- Havel PJ, Uriu-Hare JY, Liu T, Stanhope KL, Stern JS, Keen CL, Ahren B. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am J Physiol. 1998;274:R1482–R1491. doi: 10.1152/ajpregu.1998.274.5.R1482. [DOI] [PubMed] [Google Scholar]
- Heitmann BL, Westerterp KR, Loos RJ, Sorensen TI, O’Dea K, Mc LP, Jensen TK, Eisenmann J, Speakman JR, Simpson SJ, Reed DR, Westerterp-Plantenga MS. Obesity: lessons from evolution and the environment. Obes Rev. 2012;13:910–922. doi: 10.1111/j.1467-789X.2012.01007.x. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Tovar CA, Rogers RC. Induction of endogenous tumor necrosis factor-alpha: suppression of centrally stimulated gastric motility. Am J Physiol. 1999;276:R59–R68. doi: 10.1152/ajpregu.1999.276.1.R59. [DOI] [PubMed] [Google Scholar]
- Herpertz S, Albus C, Wagener R, Kocnar M, Wagner R, Henning A, Best F, Foerster H, Schulze SB, Thomas W, Kohle K, Mann K, Senf W. Comorbidity of diabetes and eating disorders. Does diabetes control reflect disturbed eating behavior? Diabetes Care. 1998;21:1110–1116. doi: 10.2337/diacare.21.7.1110. [DOI] [PubMed] [Google Scholar]
- Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor. Thyroid. 2008;18:131–139. doi: 10.1089/thy.2007.0251. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Hussain SS, Bloom SR. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond) 2012 doi: 10.1038/ijo.2012.93. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Yang H, Taché Y. Medullary sites of action of the TRH analogue, RX 77368, for stimulation of gastric acid secretion in the rat. Gastroenterology. 1988;95:1470–1476. doi: 10.1016/s0016-5085(88)80065-9. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhao S, Guarnieri DJ, Dileone RJ, Yan J, De Jonghe BC, Bence KK, Hayes MR, Grill HJ. Endogenous leptin receptor signaling in the medial nucleus tractus solitarius affects meal size and potentiates intestinal satiation signals. Am J Physiol Endocrinol Metab. 2012;303:E496–E503. doi: 10.1152/ajpendo.00205.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Tanaka K, Numata K, Hoshino M, Misugi K, Inoue S. Ventromedial hypothalamic lesion-induced vagal hyperactivity stimulates rat pancreatic cell proliferation. Gastroenterology. 1996;110:885–893. doi: 10.1053/gast.1996.v110.pm8608899. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Lee JH, Palaia T, Ragolia L. Impaired insulin-mediated vasorelaxation in diabetic Goto-Kakizaki rats is caused by impaired Akt phosphorylation. Am J Physiol Cell Physiol. 2009;296:C327–C338. doi: 10.1152/ajpcell.00254.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Palaia T, Ragolia L. Impaired insulin-stimulated myosin phosphatase Rho-interacting protein signaling in diabetic Goto-Kakizaki vascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;302:C1371–C1381. doi: 10.1152/ajpcell.00254.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang Y, Zhou Y, Liu J. Gastric bypass surgery alters the mechanisms of insulin resistance in the adipose tissue of GK rats. Mol Med Rep. 2012;6:1111–1116. doi: 10.3892/mmr.2012.1053. [DOI] [PubMed] [Google Scholar]
- Lindmark S, Buren J, Eriksson JW. Insulin resistance, endocrine function and adipokines in type 2 diabetes patients at different glycaemic levels: potential impact for glucotoxicity in vivo. Clin Endocrinol (Oxf) 2006;65:301–309. doi: 10.1111/j.1365-2265.2006.02593.x. [DOI] [PubMed] [Google Scholar]
- Lindmark S, Lonn L, Wiklund U, Tufvesson M, Olsson T, Eriksson JW. Dysregulation of the autonomic nervous system can be a link between visceral adiposity and insulin resistance. Obes Res. 2005;13:717–728. doi: 10.1038/oby.2005.81. [DOI] [PubMed] [Google Scholar]
- Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of Type 2 diabetes patients and control subjects. Diabet Med. 2003;20:399–405. doi: 10.1046/j.1464-5491.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou Y, Wang Y, Geng D, Liu J. Roux-en-Y gastric bypass-induced improvement of glucose tolerance and insulin resistance in type 2 diabetic rats are mediated by glucagon-like peptide-1. Obes Surg. 2011;21:1424–1431. doi: 10.1007/s11695-011-0388-z. [DOI] [PubMed] [Google Scholar]
- Maekawa F, Fujiwara K, Kohno D, Kuramochi M, Kurita H, Yada T. Young adult-specific hyperphagia in diabetic Goto-kakizaki rats is associated with leptin resistance and elevation of neuropeptide Y mRNA in the arcuate nucleus. J Neuroendocrinol. 2006;18:748–756. doi: 10.1111/j.1365-2826.2006.01470.x. [DOI] [PubMed] [Google Scholar]
- Manaker S, Rizio G. Autoradiographic localization of thyrotropin-releasing hormone and substance P receptors in the rat dorsal vagal complex. J Comp Neurol. 1989;290:516–526. doi: 10.1002/cne.902900406. [DOI] [PubMed] [Google Scholar]
- Mannucci E, Rotella F, Ricca V, Moretti S, Placidi GF, Rotella CM. Eating disorders in patients with type 1 diabetes: a meta-analysis. J Endocrinol Invest. 2005;28:417–419. doi: 10.1007/BF03347221. [DOI] [PubMed] [Google Scholar]
- MAYER J. Glucostatic mechanism of regulation of food intake. N Engl J Med. 1953;249:13–16. doi: 10.1056/NEJM195307022490104. [DOI] [PubMed] [Google Scholar]
- Morley JE, Perry HM., III The management of diabetes mellitus in older individuals. Drugs. 1991;41:548–565. doi: 10.2165/00003495-199141040-00004. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tovar S, Mitchell SE, Rayner DV, Archer ZA, Dieguez C, Williams LM. Regulation of growth hormone secretagogue receptor gene expression in the arcuate nuclei of the rat by leptin and ghrelin. Diabetes. 2004;53:2552–2558. doi: 10.2337/diabetes.53.10.2552. [DOI] [PubMed] [Google Scholar]
- Oida E, Kannagi T, Moritani T, Yamori Y. Diabetic alteration of cardiac vago-sympathetic modulation assessed with tone-entropy analysis. Acta Physiol Scand. 1999;165:129–134. doi: 10.1046/j.1365-201x.1999.00494.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Perin PC, Maule S, Quadri R. Sympathetic nervous system, diabetes, and hypertension. Clin Exp Hypertens. 2001;23:45–55. doi: 10.1081/ceh-100001196. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR, Kreider MS. Ultrastructural localization of thyrotropin-releasing hormone immunoreactivity in the dorsal vagal complex in rat. Neurosci Lett. 1989;104:7–12. doi: 10.1016/0304-3940(89)90320-0. [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Aono T, Ohtake M, Sandoh N. Interaction of glucose signals between the nucleus of the vagus nerve and the portal vein area in the regulation of gastric motility in rats. Brain Res Bull. 1994;33:469–471. doi: 10.1016/0361-9230(94)90292-5. [DOI] [PubMed] [Google Scholar]
- Saperas ES, Yang H, Rivier C. Central action of recombinant interleukin-1 to inhibit acid secretion in rats. Gastroenterology. 1990;99:1599–1606. doi: 10.1016/0016-5085(90)90463-b. [DOI] [PubMed] [Google Scholar]
- Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Eikelis N, Nestel PJ, Dixon JB, Dawood T, Grima MT, Sari CI, Schlaich MP, Esler MD, Tilbrook AJ, Lambert GW, Lambert EA. Baseline sympathetic nervous system activity predicts dietary weight loss in obese metabolic syndrome subjects. J Clin Endocrinol Metab. 2012a;97:605–613. doi: 10.1210/jc.2011-2320. [DOI] [PubMed] [Google Scholar]
- Straznicky NE, Grima MT, Sari CI, Eikelis N, Lambert EA, Nestel PJ, Esler MD, Dixon JB, Chopra R, Tilbrook AJ, Schlaich MP, Lambert GW. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes. 2012b;61:2506–2516. doi: 10.2337/db12-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taché Y, Yang H. Role of medullary TRH in the vagal regulation of gastric function. In: Wingate DL, Butkd TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton: CRC; 1994. pp. 67–80. [Google Scholar]
- Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–5187. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- Williams DL, Grill HJ, Cummings DE, Kaplan JM. Overfeeding-induced weight gain suppresses plasma ghrelin levels in rats. J Endocrinol Invest. 2006;29:863–868. doi: 10.1007/BF03349188. [DOI] [PubMed] [Google Scholar]
- Williams G, Steel JH, Cardoso H, Ghatei MA, Lee YC, Gill JS, Burrin JM, Polak JM, Bloom SR. Increased hypothalamic neuropeptide Y concentrations in diabetic rat. Diabetes. 1988;37:763–772. doi: 10.2337/diab.37.6.763. [DOI] [PubMed] [Google Scholar]
- Xue B, Sukumaran S, Nie J, Jusko WJ, DuBois DC, Almon RR. Adipose tissue deficiency and chronic inflammation in diabetic goto-kakizaki rats. PLoS One. 2011;6:e17386. doi: 10.1371/journal.pone.0017386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane M, Jiao S, Kihara S, Shimomura I, Yanagi K, Tokunaga K, Kawata S, Odaka H, Ikeda H, Yamashita S. Increased proportion of plasma apoB-48 to apoB-100 in non-insulin-dependent diabetic rats: contribution of enhanced apoB mRNA editing in the liver. J Lipid Res. 1995;36:1676–1685. [PubMed] [Google Scholar]
- Yang H, Nyby MD, Ao Y, Chen A, Adelson DW, Smutko V, Wijesuriya J, Go VL, Tuck ML. Role of brainstem thyrotropin-releasing hormone-triggered sympathetic overactivation in cardiovascular mortality in type 2 diabetic Goto-Kakizaki rats. Hypertens Res. 2012;35:157–165. doi: 10.1038/hr.2011.154. [DOI] [PubMed] [Google Scholar]
- Yang H, Ohning GV, Taché Y. TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurons in rats. Am J Physiol. 1993;265:G880–G886. doi: 10.1152/ajpgi.1993.265.5.G880. [DOI] [PubMed] [Google Scholar]
- Yang H, Tache Y, Ohning G, Go VL. Activation of raphe pallidus neurons increases insulin through medullary thyrotropin-releasing hormone (TRH)-vagal pathways. Pancreas. 2002;25:301–307. doi: 10.1097/00006676-200210000-00014. [DOI] [PubMed] [Google Scholar]
- Yang H, Wu SV, Ishikawa T, Taché Y. Cold exposure elevates thyrotropin-releasing hormone gene expression in medullary raphe nuclei: relationship with vagally mediated gastric erosions. Neuroscience. 1994;61:655–663. doi: 10.1016/0306-4522(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nishikawa W, Iwanaka N, Nakamura E, Seino Y, Tsuda K, Ishihara A. Abnormality in fibre type distribution of soleus and plantaris muscles in non-obese diabetic Goto-Kakizaki rats. Clin Exp Pharmacol Physiol. 2002;29:1001–1008. doi: 10.1046/j.1440-1681.2002.03757.x. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yamashita S, Tokunaga K, Yamane M, Shinohara E, Keno Y, Nishida M, Kotani K, Shimomura I, Kobayashi H, Nakamura T, Miyagawa J, Kameda-Takemura K, Odaka H, Ikeda H, Matsuzawa Y. Visceral fat accumulation and vascular complications associated with VMH lesioning of spontaneously non-insulin-dependent diabetic GK rat. Int J Obes Relat Metab Disord. 1996;20:909–916. [PubMed] [Google Scholar]
- Yuan PQ, Yang H. Neuronal activation of brain vagal-regulatory pathways and upper gut enteric plexuses by insulin hypoglycemia. Am J Physiol Endocrinol Metab. 2002;283:E436–E448. doi: 10.1152/ajpendo.00538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Lin TR, Hu Y, Fan Y, Zhao L, Stuenkel EL, Mulholland MW. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol. 2004;559:729–737. doi: 10.1113/jphysiol.2004.064121. [DOI] [PMC free article] [PubMed] [Google Scholar]