Abstract
Objective
To examine the functioning of fronto-striatal brain circuits that support self-regulatory capacities including conflict resolution and sequential processing in unmedicated adults with Obsessive-Compulsive Disorder (OCD).
Methods
We compared fMRI BOLD response in 22 adults with OCD to 22 healthy, age-matched controls during performance of a Simon Spatial Incompatibility task. We used general linear modeling to compare groups in their patterns of brain activation during correct responses to conflict-laden stimuli and explore the effects of trial sequence on group differences.
Results
Behavioral performance on the Simon task did not differ between groups. In response to conflict-laden stimuli, OCD participants activated fronto-striatal regions significantly more than controls, specifically a right hemisphere cluster encompassing the putamen, insula and inferior frontal gyrus. Their activation of this cluster was driven not by conflict on a current trial but by their response to the alternation of stimulus congruence (incongruent or congruent) across trial sequences (i.e., current and preceding trials), and was most accentuated in participants with more severe symptoms in the doubt/checking dimension. Functional connectivity from the putamen to other fronto-striatal regions was also greater in the OCD compared to control participants.
Conclusion
When engaging the self-regulatory control necessary to resolve conflict and process alternating stimuli, OCD participants displayed excessive activation in a fronto-striatal circuit that differs from the OFC-ACC-caudate circuit typically implicated in OCD. Dysfunction in this circuit was associated with processing changes in the stimulus context. We speculate that this dysfunction may be related to the cognitive inflexibility typical of persons with OCD.
Keywords: Obsessive-Compulsive Disorder, Self-Regulation, Cognitive Conflict, fMRI, Simon task, Fronto-striatal Systems
INTRODUCTION
OCD is characterized by intrusive thoughts, images, or impulses (i.e., obsessions) and repetitive acts that are performed to prevent or reduce distress (i.e., compulsions). Obsessions and compulsions are hypothesized to result from a failure to inhibit or control thoughts and behaviors, respectively(1). Indeed, neuroimaging evidence suggests that the fronto-striatal circuits supporting inhibitory control processes are structurally(2–5), metabolically(6–8), and functionally(9) abnormal in OCD. Most findings implicate the orbitofrontal (OFC) and anterior cingulate (ACC) cortices and caudate nucleus in the pathophysiology of OCD. However, inhibitory control processes involve additional fronto-striatal brain areas that may also be dysfunctional in OCD.
Previous fMRI studies have examined the functioning of fronto-striatal circuits in adults with OCD during performance of inhibitory control and conflict tasks requiring the inhibition of irrelevant or conflicting information(10–17). Discrepant findings across studies of increased and decreased activation of OFC and ACC relative to controls are likely due to differences in the tasks used (e.g., Stroop, Go/No-go), task designs (e.g., block, event-related), and performance variables (e.g., errors, correct responses). Moreover, most samples were small (<15), with patients on medication. PET findings suggest that SSRIs attenuate metabolic activity in fronto-striatal regions in OCD participants(18) and fMRI findings suggest that SSRIs attenuate fronto-striatal activations associated with inhibitory control in other disorders(19). Only one prior fMRI study of adult OCD patients assessed brain function during an inhibitory control task before and after symptom improvement with SSRI (n=4), reporting no changes in fronto-striatal activations compared to baseline(20). Thus, the effect of SSRIs on fronto-striatal activations in OCD remains unclear, and the functioning of these circuits in unmedicated adults warrants further investigation.
The Simon Spatial Incompatibility task(21) requires ignoring a task-irrelevant feature of a stimulus (the side of the screen on which an arrow appears) when it conflicts with a more task-relevant one (the direction the arrow points). When responding correctly on incongruent (i.e., conflict-laden) trials, healthy individuals activate fronto-striatal regions including dorsolateral/dorsomedial prefrontal and AC cortices, supplementary motor areas, caudate, and putamen(22–24). Behaviorally, healthy individuals respond more slowly to incongruent stimuli that are preceded by congruent stimuli than to incongruent stimuli preceded by incongruent stimuli because conflict on a preceding incongruent trial enhances inhibitory control and facilitates processing on a current incongruent trial(25, 26). Fronto-striatal activations also depend on trial sequence(27–29). For example, we measured brain activation in healthy individuals during their performance of a Simon task variant that included congruent and incongruent appearing equally often, thereby allowing us to distinguish neural activity associated with the conflict resolution on a current trial from activity associated with effects of trial sequence (i.e., the alternation or repetition of congruence between current and preceding stimuli). Activation of frontal regions increased with increasing levels of conflict, with the greatest magnitude in response to post-congruent stimuli (i.e., incongruent preceded by congruent stimuli)(30). This task variant eliminates potential oddball effects associated with the infrequent presentation of incongruent stimuli, reduces priming effects associated with long repeated trials of congruent stimuli, and is easier than other Simon task versions(22, 23, 31, 32), allowing for group comparisons of brain activity that are not confounded by performance differences(33).
We report an event-related fMRI study in which we used this Simon task to investigate the neural substrates of inhibitory control and conflict resolution in unmedicated adults with OCD. We hypothesized that despite their normal performance on the task, OCD participants would activate fronto-striatal regions to a greater extent than controls when responding correctly to incongruent stimuli, reflecting their greater reliance on this circuit. Our analyses focused on the post-congruent conflict effect (incongruent compared to congruent stimuli preceded by congruent stimuli) because this contrast is associated with the most conflict and greatest magnitude of activation in fronto-striatal regions in healthy individuals. Given their cognitive inflexibility and tendency to `get stuck' in the face of changing environmental contingencies, we suspected that OCD participants would demonstrate greater reliance on, and hence greater activation of, fronto-striatal circuits than controls in response to post-congruent conflict. We also explored general conflict effects, trial sequence effects, group differences in task-related functional connectivity within fronto-striatal circuits, and associations of fronto-striatal activations with OCD symptom dimensions.
METHODS
Participants
Unmedicated adults with OCD and healthy control participants (group-matched by age, sex, and ethno-racial groups) were recruited through flyers, internet advertisements, and word-of-mouth. Participants with a history of neurological illness, past seizures, head trauma with loss of consciousness, mental retardation, pervasive developmental disorder, or current Axis I disorders (other than OCD for the OCD participants) were excluded. Controls had no lifetime Axis I disorders. Formal diagnoses of OCD and the presence of comorbid Axis I diagnoses were established by a psychiatric evaluation and confirmed with the Structured Clinical Interview for DSM-IV(34). On the day of the MRI scan, a trained rater assessed OCD severity using the Yale-Brown Obsessive Compulsive Scale (Y-BOCS)(35, 36) and depressive severity using the Hamilton Depression Scale(37). The Y-BOCS Symptom Checklist was used to ascertain the presence and severity of five different symptom dimensions(38, 39). Full-scale IQs were estimated using the Wechsler Abbreviated Scale of Intelligence(40). Movement within the scanner was assessed for each participant by calculating the average displacement in each translational and rotational axis. The totals of those averages were then compared across groups. The Institutional Review Board of the New York State Psychiatric Institute approved this study. Participants provided written informed consent.
FMRI Paradigm
Stimuli were presented through non-magnetic goggles (Resonance Technologies, Inc) using EPRIME software. A series of white arrows pointing left or right were displayed against a black background to the left or right of a white gaze fixation cross-hair positioned at midline. Stimuli subtended 1 vertical and 3.92 horizontal degrees of the visual field. Stimuli were “congruent” (pointing in the same direction as their position on the screen), “incongruent” (pointing opposite their position on the screen), or `blank' (a cross-hair positioned at midline).
Participants were instructed to respond quickly to the direction of the arrow by pressing a button on a response box, using the index finger of their right hand for a left-pointing arrow and the middle finger of that hand for a right-pointing arrow. The button press recorded responses and reaction times (RTs) for each trial containing congruent or incongruent stimuli. Stimulus duration was 1300msec, with a jittered interstimulus interval (mean=5352msec, SD=842msec, range=4009 to 6857msec). Each run contained 55 stimuli (5min, 7sec), with 22 congruent stimuli (11 left-pointing arrows presented to the left of midline; 11 right-pointing arrows presented to the right of midline), 22 incongruent stimuli (11 left-pointing arrows presented to the right of midline; 11 right-pointing arrows presented to the left of midline) and 11 blank stimuli (longer periods of fixation, Fig. S1 in Supplement). These stimuli were arranged and presented in a pseudorandom order. Each experiment contained 3 runs, totaling 66 congruent and 66 incongruent stimuli. Details of the MRI pulse sequence, image processing, behavioral and exploratory image analyses are described in the online supplement.
Image Analysis
First-level parametric analyses were performed individually for each participant using a modified version of the GLM function in SPM8with a Weighted Least-Squares algorithm (Wellcome Department of Imaging Neuroscience, London, UK; http;//www.fil.ion.ucl.ac.uk/spm/). Preprocessed BOLD time series data at each voxel, concatenated from all 3 runs of the task (420 volumes), were modeled using a GLM with the following predictors corresponding to each trial type: (1) congruent preceded by congruent (cC), (2) congruent preceded by incongruent (iC), (3) incongruent preceded by congruent (cI), and (4) incongruent preceded by incongruent (iI), (5) blank trials, (6) fixation trials, and (7) all incorrect and (8) correct trials (either congruent or incongruent). These events were then convolved with the canonical hemodynamic response function(41). A first-order autoregression with restricted maximum likelihood algorithm was used to estimate parameters for each independent variable and remove serial correlations in the fMRI time series. The parameter estimates for the 3 runs were averaged to produce beta maps for each trial type for each participant.
The resulting beta maps were entered into a second-level mixed model analysis in SPM8: a 2 × 2 × 2 repeated-measures factorial ANOVA with within-subjects factors (1) current congruence (congruent, incongruent) and (2) trial sequence (congruence repeated or alternating between the preceding and current trial). The between-subjects factor was diagnosis (OCD, Control). We assessed the main effects of these factors and their interaction as well as pairwise contrasts. We applied parametric inference and report findings that were identified on group contrast maps using a corrected P-value <0.05. Based on a Monte Carlo simulation with 10,000 iterations implemented in AlphaSim, we selected for our a priori hypothesis test a height threshold of uncorrected P ≤ 0.005 combined with a cluster filter of at least 61 adjacent voxels (3×3×3 mm each). The combined application of a statistical threshold and cluster filter minimizes the false-positive identification of activated regions at any given threshold(42) because clustering can distinguish between true regions of activation that tend to occur over adjacent voxels, and noise that has less tendency to cluster.
Hypothesis Testing
We tested whether participants with or without OCD differed in brain activity in fronto-striatal regions during correct responses on incongruent trials compared with correct responses on congruent trials that were preceded by congruent trials (cI-cC contrast representing post-congruent conflict). Other contrasts were used in exploratory analyses to determine whether observed group differences were associated with the resolution of conflict on a current trial or with effects of trial sequence (Fig. S2 in Supplement). We also explored group differences in task-related functional connectivity within fronto-striatal circuits, and whether the functioning of, and functional connectivity within, fronto-striatal circuits differed across symptom dimensions (see supplement).
RESULTS
Participants
Twenty-two OCD and 22 healthy control participants were scanned. The groups were matched on demographic characteristics (Table I). The majority of the participants in both groups were right-handed. All OCD participants were free of psychotropic medications (14 treatment-naïve and the other 8 had been off an SRI for a mean (SD) of 94 (63) weeks) and free of any current comorbid Axis I disorder; five had a lifetime history of a depressive episode. As in prior studies(43), this was achieved by recruiting OCD subjects from the community (e.g., instead of relying solely upon psychiatrist referral). The target symptoms of the OCD participants were distributed across the five symptom dimensions(39). The two groups did not differ in movement within the scanner, defined by the total (p=0.63) and cumulative (p=0.63) displacement in each translational and rotational axis.
Table I.
Demographic and Clinical Characteristics of Participants
| Participants | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | OCD (n = 22) | Healthy Control (n = 22) | Analysis | ||||
| Mean | SD | Mean | SD | t | df | p | |
| Age, years | 30.00 | 9.09 | 30.14 | 9.35 | −0.05 | 42 | 0.96 |
| WASI IQ Score (Full-4) | 112.73 | 14.06 | 117.05 | 14.22 | −0.99 | 40 | 0.33 |
| Duration of Illness, yrs. | 13.95 | 9.28 | … | … | |||
| Age of OCD Onset, yrs | 16.05 | 7.33 | … | … | |||
| HAM-D scores | 4.81 | 3.65 | |||||
| Y-BOCS Total | 25.91 | 4.20 | |||||
| Obsessions | 12.50 | 2.18 | |||||
| Compulsions | 13.41 | 2.36 | |||||
| N | % | N | % | ||||
| Target Symptoms* | |||||||
| Symmetry/ordering | 5 | 22.72 | |||||
| Doubt/checking | 3 | 13.63 | |||||
| Contamination/cleaning | 6 | 27.27 | |||||
| Taboo thoughts | 7 | 31.81 | |||||
| Hoarding | 1 | 4.54 | |||||
| Sex | |||||||
| Male | 11 | 50.00 | 11 | 50.00 | |||
| Female | 11 | 50.00 | 11 | 50.00 | |||
| Handedness | |||||||
| Right | 18 | 81.82 | 19 | 86.36 | |||
| Left | 4 | 18.18 | 3 | 13.64 | |||
| Ethnicity | |||||||
| Asian | … | … | 1 | 4.54 | |||
| African American | 4 | 18.18 | 4 | 18.18 | |||
| Caucasian | 15 | 68.18 | 14 | 63.64 | |||
| Hispanic | 3 | 13.64 | 3 | 13.64 | |||
Behavioral Performance
No significant main effects of group or interaction of group-by-congruence was detected in either model (p>0.45), indicating that there were no group differences specific to stimulus type. As shown in Table II, RTs and accuracy scores were similar on congruent and incongruent trials, and neither group made many errors. Both groups demonstrated a conflict effect (mean RT incongruent>mean RT congruent) that was greater following congruent than following incongruent trials (congruence-by-sequence interactions, OCD: p=0.02; Control: p=0.05, Fig. S3 in Supplement), and this post-congruent conflict effect did not differ across groups (p=0.37). Both groups also demonstrated a sequence effect (mean RT alternation (trials in which the congruence alternated relative to the preceding trial)>mean RT repetition (trials in which congruence repeated), Table S1 in Supplement). Accuracy correlated inversely with scores on the doubt/checking symptom dimension, indicating that the OCD participants who endorsed the most pathological doubt and checking compulsions performed least accurately (r=−0.48;p=0.02). The conflict and sequence effects correlated positively with doubt/checking symptoms, indicating that the OCD participants who endorsed more of these symptoms took the longest to respond to incongruent versus congruent trials (conflict effect: r=0.59:p<0.003) and to alternating versus repeating trials (sequence effect: r=0.51;p=0.01).
Table II.
Group Differences in Bram Activity Associated with Post-Congruent Conflict
| Cluster-level |
Peak-level |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | Side | Ke | Pcorr | T | Puncorr | x | y | z |
| Fronto-striatal cluster | R | 120 | 0.045 | 3.72 | 0.000 | 33 | −4 | 16 |
A Priori Hypothesis Testing
Group Differences in Neural Activity Associated with Post-Congruent Conflict
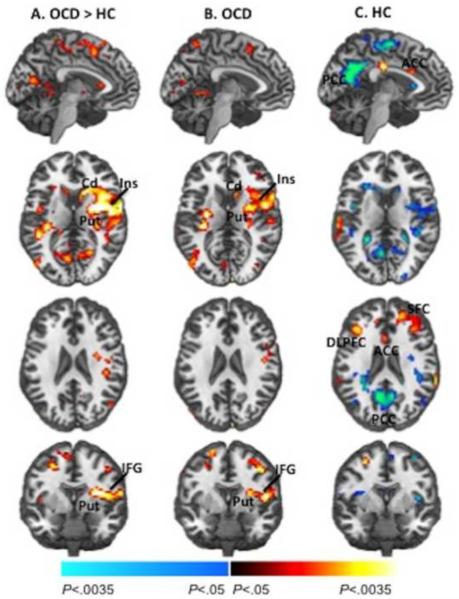
Significant group differences associated with post-congruent conflict were detected in right fronto-striatal regions, including a contiguous cluster encompassing the inferior frontal gyrus (IFG, BA 46), insula (BA 13) and putamen (peak location × y z = 33 −4 16, Fig.1a). These differences derived from greater activation of this cluster in the OCD participants in response to cI versus cC.
Figure 1. Group Average Brain Activations Associated with Post-Congruent Conflict.
(a) Group differences in brain activations associated with the processing and resolution of cognitive conflict preceded by congruent trials (post-congruent conflict, cI-cC) were detected in fronto-striatal (red) and default mode network (blue) regions. Group average brain activations are shown for the OCD (b) and healthy (c) participants. Increases in signal during correct responses to cI relative to cC trials are shown in red, and decreases are shown in blue. For display purposes, these maps (generated with MRIcroN) are thresholded at P = 0.025, uncorrected, with a cluster filter of 25. The within-group effects did not survive our a priori significance threshold (P = 0.005, cluster filter of 61). The between-group effects remained after controlling for age of onset in the OCD group. OCD, Obsessive-Compulsive Disorder; HC, healthy control; IFG, inferior frontal gyrus; Put, putamen; Cd, caudate; PCC, posterior cingulate cortex.
Exploratory Analyses
Conflict Effects
Using a less stringent threshold (p = 0.05, uncorrected), we explored neural activity associated with conflict effects in both groups. Both the OCD and healthy participants activated fronto-striatal regions in response to post-congruent conflict (Fig. 1b&c). In response to all conflict, regardless of the preceding stimulus (congruent or incongruent), both groups activated the pre SMA (BA 6), but healthy participants activated larger expanses of frontal regions including ACC (BA32), bilateral superior frontal gyri (SFG, BA 9), and MFG (BA 8, Fig. S4c in Supplement). These group differences associated with all conflict were significant at the more stringent threshold (p < 0.05, corrected).
Sequence Effects
To determine whether activation of the right fronto-striatal cluster in OCD was associated with the resolution of post-congruent conflict or with trial sequence (i.e., the alternation or repetition of congruence between current and preceding stimuli), we entered beta estimates from the cluster into a repeated-measures factorial ANOVA in SPSS (see supplement). A significant group-by-sequence interaction (p=0.005) derived from the different effects of trial sequence across groups (OCD, p=0.04; Control, p=0.01). Specifically, in OCD participants, activation of the right fronto-striatal cluster was greater during trials in which congruence alternated relative to the previous trial (i.e., cI and iC) than during trials in which congruence repeated (i.e., iI and cC), whereas activation was greater during repeated compared to alternating trials in the control participants (Fig. S5 in Supplement). No significant group-by-current congruence interaction (p=0.70) or main effect of current congruence was detected in either group (p's>0.1), suggesting that greater fronto-striatal activity in the OCD compared to control participants represented sequence rather than conflict-related activity and was associated with their processing of the stimulus context.
Stimulus-Feature Effects
To exclude the possibility that group differences in fronto-striatal activations were driven by differences in the processing of stimulus features within the sequence (e.g., the repetition or alternation of the position and/or direction of arrow stimuli across trials), we ran an extended model with additional regressors (position repetition, position alternation, direction repetition, direction alternation; see supplement). Neither the within-group effects nor the between-group differences in fronto-striatal activations were driven by the sequence of single stimulus features (all p>0.05, uncorrected).
Functional Connectivity
To explore whether greater activation of the right fronto-striatal cluster could reflect greater connectivity within fronto-striatal circuits in OCD participants, we used the putamen (sphere centered at MNI coordinates (x, y, z): 33, −4, 16mm with a 1mm radius) as the seed region in a connectivity analysis. These coordinates corresponded to those of the peak-level of significance of the right hemisphere cluster associated with post-congruent conflict in our a priori hypothesis test (Table 2). This analysis was a variant of a psychophysiological interaction (PPI) analysis(44) in SPM8 that allowed assessment of group differences in condition-dependent and condition-independent functional connectivity during performance of the task (also see supplement). There was significantly greater connectivity in the OCD compared to control participants between the putamen and large expanses of fronto-striatal and parietal areas (Fig. 2; Table S2 in Supplement), including right superior frontal gyrus (BA 10), inferior parietal lobule (BA 7) and caudate, left cingulate gyrus (BA 31) and thalamus, and the bilateral precuneus (BA 7/19). Putamen connectivity did not interact with task conditions (i.e., the PPI analysis) either between or within groups (see supplement).
Figure 2. Functional Connectivity.
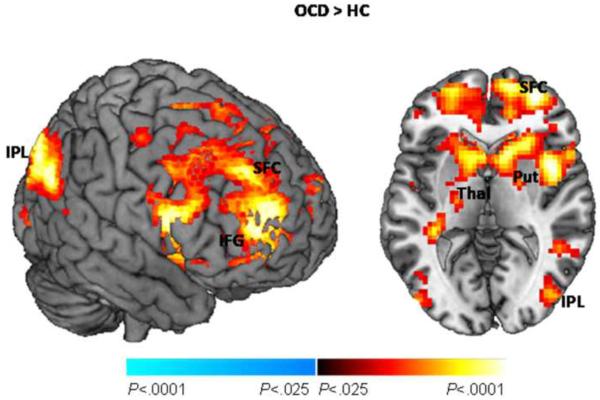
Group differences in seed connectivity from the putamen. This map is thresholded at P = 0.025, uncorrected, with a cluster filter of 25. Also shown are the cluster- and peak-level statistics corresponding to these group differences in connectivity. OCD, Obsessive-Compulsive Disorder; HC, healthy control; Put, putamen; Cd, caudate; Thal, thalamus; SFG, superior frontal gyrus; CG, cingulate gyrus; Pcu, precuneus; IPL, inferior parietal lobule.
Symptom Severity Correlates
In the right fronto-striatal cluster, activity associated with post-congruent conflict and trial sequence correlated positively with doubt/checking symptoms (conflict: r=0.4;p=0.06, sequence: r=0.56;p=0.006), suggesting that the OCD participants who endorsed more of these symptoms also activated fronto-striatal regions more in response to post-congruent conflict and especially in response to the alternation of stimuli (i.e., during trial sequences in which the congruence of stimuli alternated). An outlier test(45) revealed that this positive correlation was not driven by the three OCD participants with the greatest number of doubt/checking symptoms. No significant correlations of brain activation with other symptom dimensions or overall symptom severity (YBOCS scores) were detected (p's>0.2). In addition, no significant correlations of functional connectivity with symptom dimensions were detected (p's>0.05).
DISCUSSION
Unmedicated OCD participants performed similarly to controls on a Simon task with low cognitive demand. Nevertheless, activation of a large right hemisphere cluster of fronto-striatal regions in response to post-congruent conflict was significantly greater in the OCD participants. These regions included the IFG, insula, and putamen. Greater activation of this right fronto-striatal cluster in OCD participants was driven by their response to the alternation of congruence across trials rather than current trial conflict. Functional connectivity between the putamen and large expanses of fronto-striatal regions was also greater in the OCD compared to control participants, suggesting that increased connectivity may contribute to increased activation within fronto-striatal circuits in OCD.
Previous fMRI studies of cognitive control and conflict processing also suggest excessive activation within fronto-striatal circuits in OCD patients compared to controls(8, 13, 14, 16). Those studies report greater activation of the OFC(14, 46) and ACC(8, 13, 16) using different tasks, including Go/No-go(11, 14), continuous performance(13), multisource interference(8) and Stroop-like(10, 16) tasks. Prior studies in healthy individuals indicate that the Simon task does not measure OFC functioning(22, 23, 30) thus it is unsurprising that this region was not implicated in our between- or within-group findings, but that healthy individuals activate the ACC in response to errors(47) and conflict(48) on the Simon task. We did not assess activation associated with errors because our low cognitive demand task did not generate many errors in either group. The healthy, but not our unmedicated OCD, participants activated the ACC in response to post-congruent conflict (Fig. 1c) and all conflict (Fig, S4c in Supplement), consistent with prior findings from healthy individuals(22–24). Importantly, most prior fMRI studies of cognitive control and conflict processing in OCD recruited medicated patients(8, 13, 16), complicating comparison with our data since SSRIs can alter activation of fronto-striatal circuits on various fMRI tasks(49). In addition, prior fMRI studies included OCD patients with comorbid depression and anxiety(11, 14), whereas such participants were excluded from our study.
Our findings implicate instead a right fronto-striatal circuit involving the IFG, insula, and putamen in OCD. Right lateral prefrontal regions, particularly the right inferior frontal cortex, typically activate during successful response inhibition in healthy individuals(50–52). Activation of the right inferior frontal cortex has been associated with correctly rejected high conflict trials on Go/No-go tasks in OCD(11, 14) with one study reporting increased(14) and the other reporting decreased activation compared to controls using a different task design with medicated patients(11). Altered resting-state connectivity from the insula to fronto-parietal and other brain areas(53), reduced serotonin transporter binding in the insula(54), and increased grey matter in the putamen has been reported in OCD compared to control participants(5, 55). However, neither the insula nor the putamen has been implicated in prior fMRI studies of cognitive control or conflict processing in OCD. We speculate that differences between our Simon task and the tasks used previously and our inclusion of only unmedicated OCD participants contributed to our detection of increased activation of the right inferior frontal cortex, insula, and putamen.
Activation of this right fronto-striatal circuit was driven by OCD participants' neural responses to the alternation of congruence across trials. This activation was not driven by conflict per se, nor by their differential responses to the alternation or repetition of lower-order stimulus features such as the position and direction of the arrow stimuli, but rather to their differential responses to sequential changes in conflict. Perhaps the OCD participants needed to engage these fronto-striatal regions more to compensate for their difficulty processing the alternating stimulus context, consistent with their phenomenological difficulty processing changing environmental contingencies and their overall cognitive inflexibility(1). These ideas are consistent with a neuro-computational model(56) used to explain the neural basis of cognitive inflexibility in OCD(57). The model quickly learns and actively maintains new sequences, flexibly shifting between them but can get `stuck,' becoming unable to shift from certain sequences so that it exhibits repetitive patterns of activity that are similar to the repetitive thoughts and behaviors of OCD. The model highlights the importance of the balance between direct (dis-inhibitory) and indirect (inhibitory) fronto-striatal pathways in OCD(57). Thus, the differential processing of alternating stimulus sequences and excessive activity within fronto-striatal circuits in OCD participants during Simon task performance may also be due to this imbalance between the direct and indirect pathways. These pathways are implicated in sequence learning, the acquisition and maintenance repetitive behaviors, and in OCD pathophysiology(58).
The pattern of functional connectivity from the putamen that we detected in both groups is consistent with prior functional connectivity studies of healthy individuals(59). Our finding of greater connectivity of the putamen with frontal and parietal brain areas in OCD is consistent with findings of greater resting-state connectivity within these circuits in OCD(53). Evidence from fMRI and animal lesion studies suggests that the putamen plays a key role in switching between stimuli on tasks of cognitive flexibility(60, 61). Furthermore, frontal stimulation by transcranial magnetic stimulation disrupts fMRI signal associated with stimulus switching in the putamen and reduces fronto-putamen functional connectivity in healthy individuals(62). These findings suggest that greater functional connectivity of the putamen with frontal and parietal areas in OCD participants may contribute to their greater activation of the putamen in response to the alternation (or switching) of stimuli on the Simon task. By analogy, greater connectivity within these circuits in persons with OCD may also contribute to their overall cognitive inflexibility. Alternatively, the greater connectivity may be a compensatory strategy, allowing them to perform as well as control participants despite their difficulty processing the alternating stimuli on the Simon task and possibly allowing them to function in a world of changing environmental contingencies despite their general cognitive inflexibility.
Prior neuroimaging studies report distinct neural correlates of different symptom dimensions(63–66). We found that OCD participants who endorsed the most doubt/checking symptoms experienced the most cognitive conflict (i.e., greatest conflict effect: mean RT incongruent>mean RT congruent) and performed least accurately on the task. Activation of the right hemisphere fronto-striatal cluster was also greatest in these participants, especially in response to the alternation of stimulus congruence(Fig. 5). Pathological doubt and checking compulsions are associated with a high degree of intolerance for uncertainty(67). Thus, participants who endorse more of these symptoms possibly had to engage fronto-striatal circuits the most to compensate for their difficulty processing the uncertainty of the stimulus context (the alternating stimuli). The right fronto-striatal cluster included the insula, a region that activates in association with the intolerance of uncertainty in healthy individuals(68). Recent findings suggest that morphometric alterations within the right insula (enlarged anterior and reduced posterior insular volumes compared to healthy individuals) are most pronounced in OCD patients with predominant checking symptoms(69). Thus, both functional and structural characteristics of the insula may differentiate doubt/checking from other dimensions.
Although one of the largest studies of inhibitory control and conflict resolution in unmedicated adults with OCD, this study is still limited by its modest sample size, inclusion of OCD subjects (8/22) with past exposure to psychotropic medication, and the absence of general anxiety measures. However, general anxiety was likely to be very low since we excluded OCD participants with current comorbid Axis I disorders, including anxiety and depressive disorders.
Our findings suggest important avenues for future research. For example, the inclusion of additional tasks of response inhibition and conflict resolution in future studies would confirm that our findings generalize to other measures of these processes. Future studies should also investigate other capacities that rely on these circuits, such as stimulus-response (habit) learning that relies on the dorsolateral putamen(70). Perhaps an over-reliance on a right hemisphere fronto-striatal circuit involving the putamen allows the compulsive behaviors of individuals with OCD to crystalize into maladaptive habits, consistent with current theories suggesting that an abnormal reliance on habits may contribute, in part, to compulsions in OCD(71).
In summary, when engaging the control processes necessary to resolve conflict and process the alternating stimulus context, OCD participants displayed excessive activation in the putamen, insula, and IFG. This dysfunction was associated with the processing of changes in contextual information and we speculate may be related to the cognitive inflexibility that is typical of persons with OCD.
Supplementary Material
Figure 3. Main Effects of Symptom Severity in the OCD Participants.
A scatterplot of the association of sequence-related neural activity in the right fronto-striatal cluster with scores on the doubt/checking symptom dimension of the Y-BOCS.
Acknowledgements
This work was supported in part by NIMH grants R21MH093889-09 (RM & HBS), K01-MH077652 (RM), K24 MH091555 (HBS), K02 MH74677 (BSP), and funding from the Alicia Koplowitz fellowship (GH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures Dr. Simpson has received research funds for clinical trials from Janssen Pharmaceuticals (2006–2012), Transcept Pharmaceuticals (2011-present), and Neuropharm, Ltd (2009), served on a Scientific Advisory Board for Pfizer (for Lyrica, 2009–2010) and Jazz Pharmaceuticals (for Luvox CR, 2007–2008), consulted for Quintiles, Inc (on therapeutic needs for OCD, 2012), and receives royalties from Cambridge University Press and UpToDate, Inc. All other authors report no biomedical financial interests.
All authors report no potential conflicts of interest.
REFERENCES
- 1.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci Biobehav Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg DR, Keshavan MS. Toward a neurodevelopmental model of of obsessive--compulsive disorder. Biol Psychiatry. 1998;43:623–640. doi: 10.1016/s0006-3223(97)00443-5. [DOI] [PubMed] [Google Scholar]
- 4.Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, et al. Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry. 2009;65:75–83. doi: 10.1016/j.biopsych.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Sumitani S, Harada M, Kubo H, Ohmori T. Proton magnetic resonance spectroscopy reveals an abnormality in the anterior cingulate of a subgroup of obsessive-compulsive disorder patients. Psychiatry Res. 2007;154:85–92. doi: 10.1016/j.pscychresns.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Bartha R, Stein MB, Williamson PC, Drost DJ, Neufeld RW, Carr TJ, et al. A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry. 1998;155:1584–1591. doi: 10.1176/ajp.155.11.1584. [DOI] [PubMed] [Google Scholar]
- 8.Yucel M, Harrison BJ, Wood SJ, Fornito A, Wellard RM, Pujol J, et al. Functional and biochemical alterations of the medial frontal cortex in obsessive-compulsive disorder. Arch Gen Psychiatry. 2007;64:946–955. doi: 10.1001/archpsyc.64.8.946. [DOI] [PubMed] [Google Scholar]
- 9.Maia TV, Cooney RE, Peterson BS. The Neural Bases of Obsessive-Compulsive Disorder in Children and Adults. Development and Psychopathology. 2008;20:1251–1283. doi: 10.1017/S0954579408000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. A functional MRI comparison of patients with obsessive-compulsive disorder and normal controls during a Chinese character Stroop task. Psychiatry Res. 2005;139:101–114. doi: 10.1016/j.pscychresns.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Roth RM, Saykin AJ, Flashman LA, Pixley HS, West JD, Mamourian AC. Event-related functional magnetic resonance imaging of response inhibition in obsessive-compulsive disorder. Biol Psychiatry. 2007;62:901–909. doi: 10.1016/j.biopsych.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- 13.Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–353. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- 14.Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Page LA, Rubia K, Deeley Q, Daly E, Toal F, Mataix-Cols D, et al. A functional magnetic resonance imaging study of inhibitory control in obsessive-compulsive disorder. Psychiatry Res. 2009;174:202–209. doi: 10.1016/j.pscychresns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- 17.van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 18.Saxena S, Brody AL, Ho ML, Zohrabi N, Maidment KM, Baxter LR., Jr. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–532. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 19.Wagner G, Koch K, Schachtzabel C, Sobanski T, Reichenbach JR, Sauer H, et al. Differential effects of serotonergic and noradrenergic antidepressants on brain activity during a cognitive control task and neurofunctional prediction of treatment outcome in patients with depression. J Psychiatry Neurosci. 2010;35:247–257. doi: 10.1503/jpn.090081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao T, Nakagawa A, Yoshiura T, Nakatani E, Nabeyama M, Yoshizato C, et al. Brain activation of patients with obsessive-compulsive disorder during neuropsychological and symptom provocation tasks before and after symptom improvement: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:901–910. doi: 10.1016/j.biopsych.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81:174–176. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- 22.Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13:427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22:1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Rubia K, Smith AB, Woolley J, Nosarti C, Heyman I, Taylor E, et al. Progressive increase of frontostriatal brain activation from childhood to adulthood during event-related tasks of cognitive control. Hum Brain Mapp. 2006;27:973–993. doi: 10.1002/hbm.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wuhr P, Ansorge U. Exploring trial-by-trial modulations of the Simon effect. Q J Exp Psychol A. 2005;58:705–731. doi: 10.1080/02724980443000269. [DOI] [PubMed] [Google Scholar]
- 26.Sturmer B, Leuthold H, Soetens E, Schroter H, Sommer W. Control over location-based response activation in the Simon task: behavioral and electrophysiological evidence. J Exp Psychol Hum Percept Perform. 2002;28:1345–1363. doi: 10.1037//0096-1523.28.6.1345. [DOI] [PubMed] [Google Scholar]
- 27.Mansouri FA, Buckley MJ, Tanaka K. Mnemonic function of the dorsolateral prefrontal cortex in conflict-induced behavioral adjustment. Science. 2007;318:987–990. doi: 10.1126/science.1146384. [DOI] [PubMed] [Google Scholar]
- 28.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- 29.Ullsperger M, Bylsma LM, Botvinick MM. The conflict adaptation effect: it's not just priming. Cogn Affect Behav Neurosci. 2005;5:467–472. doi: 10.3758/cabn.5.4.467. [DOI] [PubMed] [Google Scholar]
- 30.Horga G, Maia TV, Wang P, Wang Z, Marsh R, Peterson BS. Adaptation to conflict via context-driven anticipatory signals in the dorsomedial prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16208–16216. doi: 10.1523/JNEUROSCI.2783-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerns JG. Anterior cingulate and prefrontal cortex activity in an FMRI study of trial-to-trial adjustments on the Simon task. Neuroimage. 2006;33:399–405. doi: 10.1016/j.neuroimage.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Marsh R, Steinglass JE, Gerber AJ, O'Leary KG, Walsh BT, Peterson BS. Deficient Activity in the Neural Systems that Mediate Self-Regulatory Control in Bulimia Nervosa. Arch Gen Psychiatry. 2009;66:1–13. doi: 10.1001/archgenpsychiatry.2008.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson D, Halligan P. The relevance of behavioural measures for functional-imaging studies of cognition. Nat Rev Neurosci. 2004;5:67–73. doi: 10.1038/nrn1302. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- 35.Goodman WK, Price LH, Rasmusson SA, Mazure C, Delgado P, Henninger GR, et al. The Yale-Brown Obsessive Compulsive Scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 36.Goodman WK, Price LH, Rasmusson SA, Mazure C, Delgado P, Henninger GR, et al. Yale-Brown Obsessive Compulsive Scale: II. Validity. Arch Gen Psychiatry. 1989;46:1012–1018. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton MAX. Development of a Rating Scale For Primary Depressive Illness. British Journal of Social & Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 38.Pinto A, Eisen JL, Mancebo MC, Greenberg BD, Stout RL, Rasmussen SA. Taboo thoughts and doubt/checking: a refinement of the factor structure for obsessive-compulsive disorder symptoms. Psychiatry Res. 2007;151:255–258. doi: 10.1016/j.psychres.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto A, Greenberg BD, Grados MA, Bienvenu OJ, 3rd, Samuels JF, Murphy DL, et al. Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res. 2008;160:83–93. doi: 10.1016/j.psychres.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wechsler D. WAIS-R Manual. Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation. Harcourt Brace Jovanovich, Inc.; San Antonio, TX: 1981. [Google Scholar]
- 41.Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- 42.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 43.Simpson HB, Shungu DC, Bender J, Jr., Mao X, Xu X, Slifstein M, et al. Investigation of Cortical Glutamate-Glutamine and gamma-Aminobutyric Acid in Obsessive-Compulsive Disorder by Proton Magnetic Resonance Spectroscopy. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 45.Grubbs FE. Procedures for Detecting Outlying Observations in Samples. Technometrics. 1969;11:1–21. [Google Scholar]
- 46.Ursu S, Carter CS. An initial investigation of the orbitofrontal cortex hyperactivity in obsessive-compulsive disorder: exaggerated representations of anticipated aversive events? Neuropsychologia. 2009;47:2145–2148. doi: 10.1016/j.neuropsychologia.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 49.Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 50.Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 51.Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, et al. A developmental fMRI study of self-regulatory control. Hum Brain Mapp. 2006;27:848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 53.Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting-State Functional Connectivity between Fronto-Parietal and Default Mode Networks in Obsessive-Compulsive Disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsumoto R, Ichise M, Ito H, Ando T, Takahashi H, Ikoma Y, et al. Reduced serotonin transporter binding in the insular cortex in patients with obsessive-compulsive disorder: a [11C]DASB PET study. Neuroimage. 2010;49:121–126. doi: 10.1016/j.neuroimage.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 55.Valente AA, Jr., Miguel EC, Castro CC, Amaro E, Jr., Duran FL, Buchpiguel CA, et al. Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry. 2005;58:479–487. doi: 10.1016/j.biopsych.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 56.Verduzco-Flores S, Ermentrout B, Bodner M. Modeling neuropathologies as disruption of normal sequence generation in working memory networks. Neural Netw. 2012;27:21–31. doi: 10.1016/j.neunet.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Maia TV, McClelland JL. A neurocomputational approach to obsessive-compulsive disorder. Trends Cogn Sci. 2012;16:14–15. doi: 10.1016/j.tics.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 59.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 60.Cools R, Ivry RB, D'Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- 61.Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Schouwenburg MR, O'Shea J, Mars RB, Rushworth MF, Cools R. Controlling Human Striatal Cognitive Function via the Frontal Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:5631–5637. doi: 10.1523/JNEUROSCI.6428-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rauch SL, Wedig MM, Wright CI, Martis B, McMullin KG, Shin LM, et al. Functional magnetic resonance imaging study of regional brain activation during implicit sequence learning in obsessive-compulsive disorder. Biol Psychiatry. 2007;61:330–336. doi: 10.1016/j.biopsych.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 64.van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, et al. The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain. 2009;132:853–868. doi: 10.1093/brain/awn267. [DOI] [PubMed] [Google Scholar]
- 65.Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, et al. Functional connectivity in fronto-subcortical circuitry during the resting state in obsessive-compulsive disorder. Neurosci Lett. 2010;474:158–162. doi: 10.1016/j.neulet.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 66.Gilbert AR, Akkal D, Almeida JR, Mataix-Cols D, Kalas C, Devlin B, et al. Neural correlates of symptom dimensions in pediatric obsessive-compulsive disorder: a functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2009;48:936–944. doi: 10.1097/CHI.0b013e3181b2163c. [DOI] [PubMed] [Google Scholar]
- 67.Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. J Anxiety Disord. 2003;17:233–242. doi: 10.1016/s0887-6185(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 68.Simmons A, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song A, Jung WH, Jang JH, Kim E, Shim G, Park HY, et al. Disproportionate alterations in the anterior and posterior insular cortices in obsessive-compulsive disorder. PLoS One. 2011;6:e22361. doi: 10.1371/journal.pone.0022361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 71.Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW, et al. Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry. 2011;168:718–726. doi: 10.1176/appi.ajp.2011.10071062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.