Abstract
In the generation of a traditional immune response against invading pathogens, innate cells guide T cells by programming their differentiation. However, here we demonstrate that αβ T cells play an essential role in priming innate immunity in the lung after Staphylococcus aureus enterotoxin A (SEA) inhalation. We found that SEA induces waves of cellular activation, cytokine production, and migration into the lung tissue and airways. However, this innate response was completely inhibited in the absence of αβ T cells. Specifically, we found that IL-17A was required for the recruitment of neutrophils and monocytes into the lung. The cellular source of IL-17A was γδ T cells, which increased their IL-17A production following SEA, but only in an αβ T cell-dependent manner. Thus, rapid T cell activation orchestrates innate immunity and may be a new point of therapeutic intervention for acute lung injury.
INTRODUCTION
During infection, pathogens stimulate pattern recognition receptors (PRRs) on innate cells inducing the expression of costimulatory molecules and pro-inflammatory cytokines1,2. The activated innate cells process and present antigen to T cells resulting in protective immunity. However, some pathogens also release superantigenic enterotoxins which have the unique ability to bypass antigen processing and presentation by directly stimulating αβ T cells based on TCR Vβ chain expression3. Staphylococcus aureus enterotoxins exemplify this process, but how they are capable of impacting innate cells in the absence of direct receptor engagement remains unclear. For example, granulocytes such as neutrophils and mast cells are recruited into the lung, and such cellular infiltration has been observed with enterotoxin challenge in mice. This is thought to model the hallmarks of either human asthma (in the case of S. aureus enterotoxin B [SEB])4 or acute lung injury (in the case of S. aureus enterotoxin A [SEA])5. In addition, evidence is emerging that enterotoxins can impact human NALT6 based on the presence of S. aureus-specific IgE antibodies in atopic patients7, the colonization of nasal polyps by S. aureus8, TCR Vβ T cell expansion in BAL from asthmatic patients9,10, and toxic shock observed after endonasal surgery11. Thus, it appears that when T cells are stimulated by S. aureus enterotoxins, they can trigger long lasting and perhaps dangerous immune responses. In fact, recent data suggests a correlation between intestinal colonization with S. aureus strains producing enterotoxins and sudden unexpected death in infancy12,13. Therefore, a perplexing facet of enterotoxin disease pathogenesis is determining how a T cell stimulated by enterotoxins induces multiple aspects of innate immunity with sustained power and across many different innate cell types.
In this report we have begun to elucidate the mechanism through which enterotoxin-stimulated αβ T cells mediate immunity in the lung. Our data demonstrates that the rapid stimulation of αβ T cells, both CD8+ and CD4+, induces a powerful innate response by initiating innate cell recruitment into the lung followed by their activation. This includes an increase in neutrophils and monocytes, as well as NK cells, in both lung tissue and airways. As seen in infectious disease models14,15, IL-17A was needed for neutrophil recruitment after intranasal enterotoxin challenge, and we found that IL-17A was produced largely by Vγ1−Vγ2− γδ T cells. Rather than enterotoxins directly stimulating γδ T cells, we demonstrate that lung γδ T cells completely relied on αβ T cells to produce IL-17A. Thus, S. aureus enterotoxins induce αβ T cell activation which launches a sustained innate immune response that relies on a specific cytokine network and results in pulmonary inflammation.
RESULTS
T cell-mediated NK cell recruitment
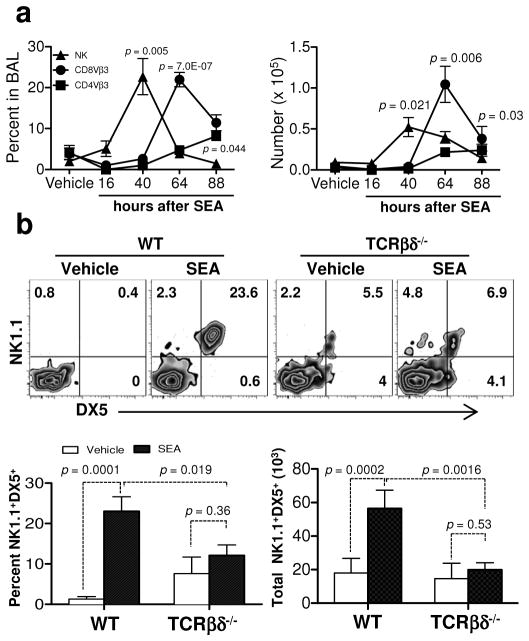
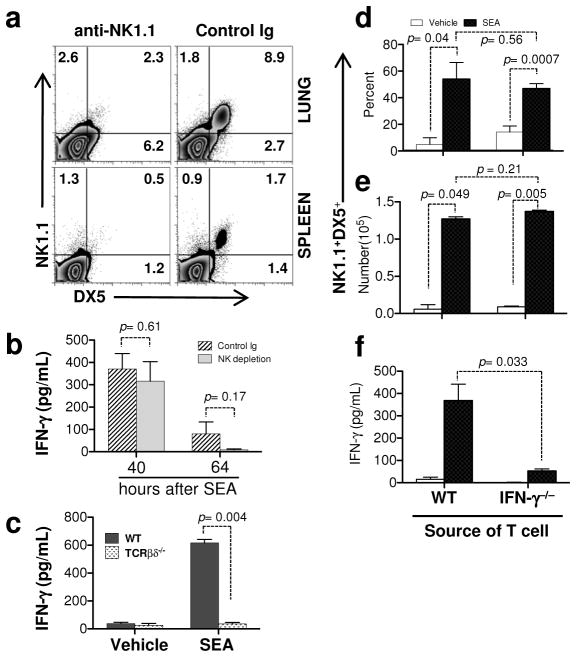
To investigate interactions between enterotoxin-stimulated αβ T cells and cells of the innate immune system, we performed kinetic studies following intranasal (i.n.) SEA challenge of wild type (WT) C57BL/6 mice. Since SEA stimulates TCR Vβ3 T cells through an MHC II-dependent process16, we predicted that clonal expansion of these cells would precede innate cell accumulation in lung airways. However, we found that NK cells appeared first in the BAL after SEA challenge (Figure 1a). These data suggested that perhaps SEA might activate NK cells independently of T cells. To test this notion, TCR βδ−/− mice, which lack both αβ and γδ T cells, were challenged with SEA and our results showed that SEA administration significantly increased NK cell numbers in WT but not TCR βδ−/− mice (Figure 1b). Thus, while NK cell accumulation in the BAL was T cell-dependent, our results did not exclude the possibility that NK cells could influence the overall T cell response. IFN-γ is one of the primary cytokines produced by NK cells17, and in vivo the presence of IFN-γ in BAL fluid is used as a biomarker of lung injury18,19. Therefore, to examine if NK cell-derived IFN-γ positively regulated the αβ T cell response, we used monoclonal antibodies to deplete NK cells 24 h prior to SEA challenge. We found that NK cell depletion (Figure 2a) did not affect IFN-γ levels in BAL fluid at 40 h and was only slightly lower at 64 h (Figure 2b). However, IFN-γ was reduced to background levels when TCR βδ−/− mice were challenged with SEA (Figure 2c). These data suggest that NK cells are neither the primary source of IFN-γ present after SEA nor required to induce IFN-γ production by alternative sources. Since T cells themselves can be a source of IFN-γ, we addressed the reciprocal possibility: is T cell-derived IFN-γ responsible for shaping the NK cell response? We isolated splenic T cells from IFN-γ−/− mice and WT controls and transferred 106 cells into TCR βδ−/− mice 1 day prior to SEA challenge. Our results showed that NK cell accumulation (Figures 2d and e) and IFN-γ levels in the BAL were rescued (Figure 2f) when T cell-deficient mice were reconstituted with WT cells. Importantly, we demonstrated that IFN-γ−/− T cells were sufficient to rescue NK cell accumulation in the BAL despite the significantly reduced total levels of IFN-γ in the BAL fluid (Figure 2d and f). Thus, while NK cell mobilization into lung following SEA preceded that of T cells, their accumulation was dependent on the presence of T cells but independent of the ability of those T cells to produce IFN-γ.
Figure 1.
NK cell recruitment in BAL is T cell dependent in response to SEA. (a) WT (C57BL/6) mice were challenged i.n. with BSS alone (vehicle) or SEA. BAL fluid was collected at 16, 40, 64, and 84 h after SEA challenge, and BAL cells were analyzed by flow cytometery. After gating on live lymphocytes, the percent (left) and number (right) of NK1.1+DX5+, CD8+Vβ3+ and CD8−Vβ3+ cells were quantified at each time point after SEA. Data were combined from 3 independent experiments with n = 3 for vehicle and n = 6 for SEA. (b) Representative plots of BAL cells show NK1.1 and DX5 staining 40 h after SEA treatment. Below bar graphs indicate the percent (left) and number (right) of NK cells from WT and TCRβδ−/− mice. Data were combined from 3 independent experiments with n = 3 for vehicle and n = 9 for SEA. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA and WT versus TCRβδ−/− mice was evaluated by two tailed Student’s t tests with all p values <0.05.
Figure 2.
IFN-γ producing T cells are required for IFN-γ in BAL fluid. (a) WT mice were NK depleted (αNK1.1) or not (control Ig) and 1 day later treated i.n. with SEA. At 40 h, lung (top) and spleen (bottom) cells were stained for DX5 and NK1.1 to confirm depletion. (b) BAL fluid was collected at 40 and 64 h after SEA from NK depleted and control Ig treated mice to determine IFN-γ levels. Bar graph of ELISA data show Mean +/− SEM of IFN-γ (pg/ml). Data were combined from 2 independent experiments with n = 4 for NK depletion and n = 4 for control Ig. (c) BAL fluid was collected at 40 h after SEA from WT and TCRβδ−/− mice and bar graph show Mean +/− SEM of IFN-γ (pg/ml). Data were combined from 3 independent experiments with n = 3 for vehicle and n = 9 for SEA. (d – f) Nylon wool column enriched WT or IFN-γ−/− deficient T cells (~106) were separately transferred into TCRβδ−/− mice, and 1 day after transfer mice were treated i.n. with SEA or vehicle alone. 72 h after SEA, BAL cells were analyzed by NK1.1 and DX5 for NK cell percentages (d) and total numbers (e), and IFN-γ levels in BAL fluid were determined by ELISA (f). Bar graph shows Mean +/− SEM from 2 independent experiments with n = 3 for vehicle and n = 5 for SEA.
Early appearance of monocytes and neutrophils
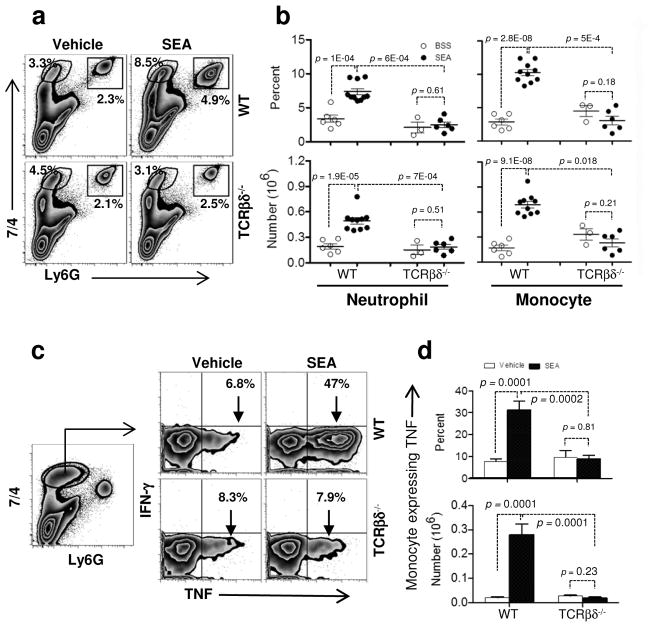
Since we found that NK cell recruitment was T cell-dependent, we hypothesized that other innate cells known to be involved in acute lung injury could be impacted by the presence or absence of T cells. Since monocytes and neutrophils are amongst the earliest cells recruited into the lung after acute injury20,21, we tested if these cells were equally recruited to SEA-induced lung inflammation in WT and TCR βδ−/− mice. Eight hours after SEA challenge, monocytes (Ly6G− 7/4hi) and neutrophils (Ly6Ghi 7/4hi)22,23 were significantly increased in lung tissue (Figures 3a [upper panels] and b). Monocytes upregulated MHC II, which represents a necessary step in their differentiation to macrophages, when exposed to SEA (Supplementary Figure S1). This increase was dependent on the presence of T cells since the percent and absolute numbers of monocytes and neutrophils did not change in TCR βδ−/− mice following SEA administration (Figures 3a [lower panels] and b). Moreover, we found that monocyte function was significantly impaired in the absence of T cells based on the failure of lung-resident monocytes to upregulate TNF production compared to vehicle-treated controls (Figure 3c and d). Thus, pulmonary inflammation can be orchestrated by a T cell-specific response even in the absence of any known PAMPs or factors that directly induce innate cell activation.
Figure 3.
T cells induce innate cell recruitment and cytokine production in the lung. (a) WT and TCRβδ−/− mice were challenged i.n. with vehicle or SEA. Lung cells from WT (upper panel) and TCRβδ−/− mice (lower) were stained for 7/4 and Ly6G to analyze monocytes (7/4+Ly6G−) and neutrophils (7/4+Ly6G+) 8 h after SEA challenge. (b) Monocyte and neutrophil percentages and numbers are shown from WT and TCRβδ−/− mice. Each dot represents an individual mouse treated either with vehicle (open circles: ○) or SEA (closed circles: ●). (c and d) Lung monocytes from 8 h vehicle and SEA treated mice were stimulated ex vivo with media alone or PMA/I with brefeldin A for 4 h and stained for IFN-γ and TNF (c) and were quantified for monocyte expressing TNF percent (d upper) and number (d lower). Data were combined from 4 independent experiments with n = 6 for vehicle, n = 10 for SEA (WT) and from 2 independent experiments with n = 3 for vehicle, n = 6 for SEA (TCRβδ−/−). Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Based on these data, we tested whether αβ or γδ T cells alone were responsible for inducing the innate response. To test this idea, we compared SEA responses in WT and TCR β−/− or TCR δ−/− mice. Our results indicated that the recruitment of Ly6G− 7/4hi monocytes and Ly6Ghi 7/4hi neutrophils was entirely dependent upon the presence of TCR β chain-bearing T cells with no apparent role for γδ T cells (Supplementary Figure S2). Thus, recruitment of monocytes and neutrophils into lung after SEA challenge was dependent upon αβ T cells but did not explain what factors were responsible for recruitment.
Rapid T cell activation prior to innate cell recruitment
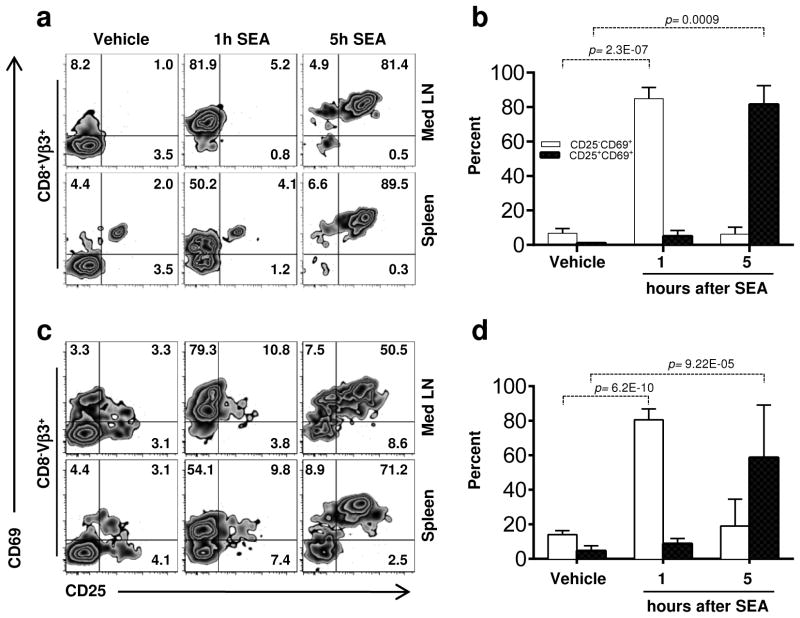
Since innate cell recruitment and activation occurred within a few hours of SEA challenge, we reasoned that if αβ T cells were influencing innate cell recruitment, T cell activation must occur before this time. Therefore, we examined the kinetics of αβ T cell activation following SEA challenge. Within 1 hour, the vast majority of Vβ3 bearing T cells in the lung-draining mediastinal LN upregulated CD69, and by 5 h, they were also CD25+ (Figure 4). This was the case for both the CD8+ and CD8− cells (which we presume to largely be CD4+ T cells). Strikingly, similar results were observed in the spleen supporting the notion that acute lung injury may be initiated locally but rapidly manifest systemically. Since both CD8+ and CD4+ T cells were activated following SEA administration, we used depleting antibodies to test if one subset or the other was primarily involved in mediating innate cell recruitment into the lung. Monoclonal antibodies 2.43 and GK1.5 were administered i.p. 2 days prior to treatment with SEA to deplete CD8+ and CD4+ T cells, respectively. Unlike TCRβ−/− mice, we found no defect in neutrophil or monocyte recruitment in the absence of CD8+ or CD4+ T cells (Supplementary Figure S3a and 3b). Thus either CD8+ or CD4+ T cells are sufficient to drive innate cell recruitment into the lung. However, we were surprised to find a reduction in the percentage of monocytes producing TNF when either CD8+ T cells or CD4+ T cells were depleted (Supplementary Figure S3c). While not reduced to the level of vehicle-treated controls, the reduction in TNF was particularly dramatic in CD4-depleted mice and less so in the CD8-depleted group. Thus, our data implicates a role for CD4+ T cells in maximizing monocyte function in the lung following SEA.
Figure 4.
Monocyte and Neutrophil recruitment into lung depends on TCRβ T cells activation. (a and b) Early activation of CD8+Vβ3+ T cells in mediastinal lymph node (med LN) and spleen was determined by analyzing CD69 and CD25 expression 1 and 5 h after SEA treatment and is quantified in B. Data were combined from 3 independent experiments with n = 5 for vehicle, n = 7 for SEA treated group. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
T cells are known producers of inflammatory cytokines, and once activated, they are a prominent source of IFN-γ and also TNF. Both of these cytokines are present in the inflammatory mileau of the lung following SEA and could function as a type of danger signal. To test if either cytokine was required to mediate innate cell recruitment into the lung, we subjected IFN-γ−/− and TNF−/− mice to i.n. SEA challenge in comparison to WT controls. However, we found that neither IFN-γ−/− nor TNF−/− mice had any defect in monocyte or neutrophil recruitment (Supplementary Figure S4) suggesting that alternative inflammatory molecules mediated the recruitment process.
IL-17A blockade suppresses neutrophil and monocyte recruitment
Cellular recruitment into the lung relies on the action of cytokines and chemokines. Particularly, IL-17A has been shown to play a dominant role in neutrophil recruitment to sites of inflammation24. To answer if IL-17A was involved in innate cell recruitment into the lung following SEA, we antagonized IL-17A or IL-17E (also known as IL-25) with neutralizing monoclonal antibodies25. As expected, treatment with control IgG permitted neutrophil recruitment into the lung after SEA challenge (Figure 5). However, IL-17A blockade prevented an increase in the percent and total numbers of neutrophils at 8 h. Recruitment of monocytes into the lung was also dependent at least partially on IL-17A and IL-17E since neutralizing either cytokine reduced the percentages of monocytes in the lung (Supplementary Figure S5). Thus, IL-17A played a key role in facilitating recruitment of neutrophils and monocytes into lung after SEA challenge.
Figure 5.
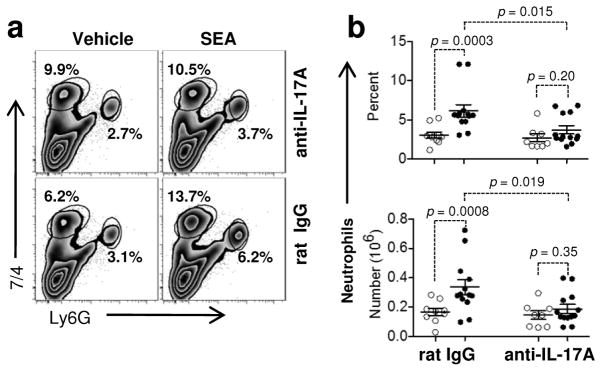
IL-17A neutralization inhibits neutrophil recruitment into the lung after SEA. (a) IL-17A was neutralized in WT mice with anti-IL17A mAb (upper panel) or control Ig (lower) prior to SEA treatment. Lung cells were harvested 8 h after SEA and stained for the presence of monocytes and neutrophils using 7/4 and Ly6G as above. (b) Control Ig and IL-17A neutralized mice were quantified for neutrophil percentages (upper) and total numbers (lower). Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 6 independent experiments with n = 9 for vehicle and n = 13 for SEA treated group. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
αβ and γδ T cells cooperate to facilitate IL-17A secretion
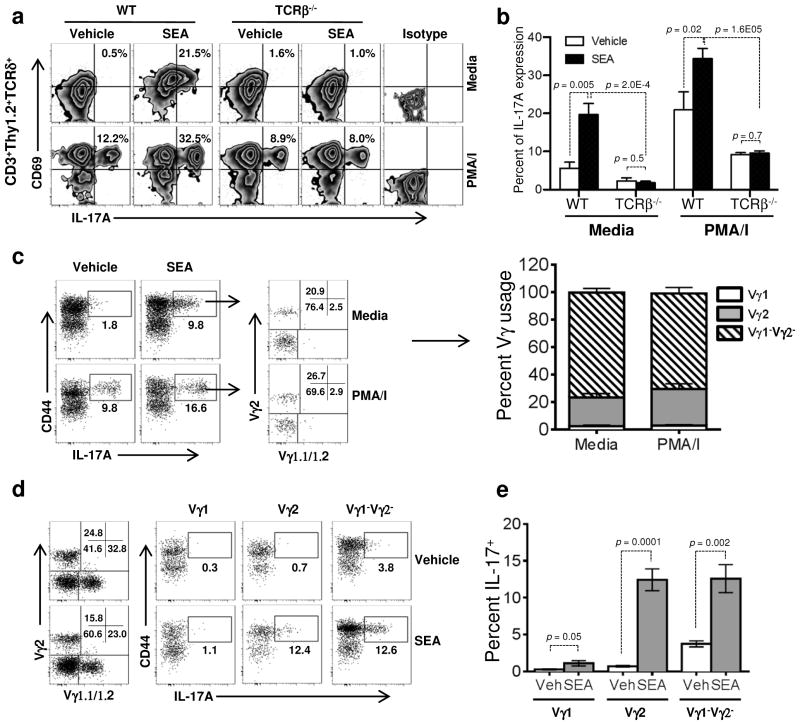
Next we sought to determine the cellular source of IL-17A following SEA challenge. Due to the rapidity of the responses against SEA, we reasoned that it would be unlikely for αβ T cells to have enough time to differentiate into IL-17A-producing cells. Secondly, when clonally expanded lung T cell populations were re-stimulated in vitro, they made high amounts of IFN-γ and TNF, but very little IL-17A (not shown). Thus, while IL-17A played a role, its source was unlikely to be αβ T cells. Therefore, not surprisingly, when lung tissue was isolated 6 h post SEA challenge to examine IL-17A production, neither CD8+ nor CD8− TCRβ+ cells made IL-17A (Supplementary Figure S6a, left). Interestingly, a significant amount of IL-17A was found in the TCRβ− fraction of the total CD3+ cells. Further analysis revealed that the IL-17A producers were CD8− TCRδ+ T cells (Supplementary Figure S6a, right). We then compared the ability of γδ T cells to produce IL-17A following SEA challenge versus vehicle-treated controls and found a significant upregulation in the percentage of IL-17A-producing γδ T cells in SEA challenged mice (Figure 6a). While some γδ T cells have the capacity to produce IL-17A in the absence of TCR engagement (i.e. following in vitro culture with PMA/ionomycin), IL-17A producers were present in the lung even in the absence of mitogen (Supplementary Figure S6a [grey histogram versus dotted line], 6a and 6b). This suggested that γδ T cells were constitutively producing IL-17A early after SEA challenge. Considering the known differences in cytokine production by individual subsets of γδ T cells26, we examined which subset of γδ T cells was being activated using antibodies specific for individual Vγ TCRs. Using antibodies against Vγ1 and Vγ2 allowed us to identify 3 populations of γδ T cells: Vγ1+, Vγ2+, and Vγ1−Vγ2−. When we gated on the population of CD44highIL-17+γδ T cells induced by SEA administration, we found that between multiple experiments about 80% of the total lung γδ T cells lacked expression of Vγ1 and Vγ2 (Figure 6c). Based on the restricted anatomical localization of γδ T cells, it is likely that these cells utilize the Vγ4 TCR27. However, when we analyzed each individual subset for their ability to produce IL-17A after SEA challenge, we found that both Vγ2+ and Vγ1− Vγ2− γδ T cells significantly increased their overall level of IL-17A production compared to vehicle-treated controls (Figure 6d and 6e). Thus, we have identified a previously unappreciated role for γδ T cells as producers of IL-17A in the orchestration of immunity following exposure to SEA.
Figure 6.
Crosstalk between TCRαβ and TCRγδ T cell. (a and b) Lung cells were isolated at 6 h from vehicle or SEA treated WT (left) or TCRβ−/− mice (right) and stimulated with media alone (top) or PMA/I (bottom). Cells were stained for CD3, Thy1.2, TCRδ, CD69 and IL-17A, and the CD3+Thy1.2+TCRδ+ T cells were analyzed for CD69 expression and IL-17A production. Bar graphs indicate the percentage of CD3+Thy1.2+TCRδ+ T cells positive for IL-17A. Data were combined from 3 independent experiments with n = 4 for vehicle n = 8 for the SEA treated group in both WT and TCRβ−/− mice. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05. (c) Following a 4 h stimulation with media or PMA/I, we gated on SEA-induced CD44highIL-17+ cells within the GL3+CD3+TCRβ− population and stained for individual γδ TCR usage. The percentage of cells expressing Vγ1, Vγ2, or lacking expression of both, are shown graphically. (d and e) Isolating and identifying γδ T cells as above, we gated on the total population of Vγ1+, Vγ2+, and Vγ4+ cells present in the lung tissue at 6 h to assess the percent IL-17+ by individual subsets of γδ T cells following vehicle versus SEA challenge. Representative dot plots are shown at left with data quantified in the graph at right. Data are representative of 3 independent experiments with n=12 for both vehicle and SEA. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Based on our findings thus far, we hypothesized that αβ T cells were required to trigger IL-17A production by γδ T cells. To test this idea, we challenged TCR β−/− versus WT mice with SEA and asked if γδ T cells could effectively increase IL-17A production in the absence of αβ T cells. We gated on Thy1.2+CD3+TCRδ+ lung tissue cells and found a significant increase in the percentage of γδ T cells producing IL-17A in mice challenged with SEA, both with and without PMA/ionomycin (Figure 6a and b). On the other hand, γδ T cells from TCR β−/− mice failed to constitutively upregulate IL-17A production (media) in the SEA treated group relative to the vehicle control-treated mice even though they effectively responded to PMA/ionomycin (Figure 6a and b). However, depletion of neither CD8+ nor CD4+ T cells alone significantly altered IL-17 production by γδ T cells (Supplementary Figure S3d). Thus, in a pattern similar to all of the innate cells examined thus far, αβ T cells, either CD8+ or CD4+, were required to initiate the response of γδ T cells, which included the production of IL-17A.
Nevertheless, it was difficult to reconcile how we observed neutrophil recruitment in the absence of γδ T cells (Figures S3a and d). Specifically, if γδ T cells supply IL-17A, how could neutrophil recruitment occur in TCRδ−/− mice? Since we found that IL-17A production was responsible for neutrophil recruitment (Figure 5), we considered the possibility that IL-17A production was compensated for by other cells when γδ T cells were absent. Therefore we further examined IL-17 production in TCRδ−/− mice and found that after SEA challenge, CD3+CD8−TCRδ− cells produced high levels of IL-17A demonstrating a strong compensatory effect within TCRδ−/− mice (Supplementary Figure S6b and c). These cells expressed CD25 and, similar to γδ T cells, were capable of producing IL-17A both directly ex vivo and in response to mitogen. Therefore, the critical role of IL-17A production following SEA challenge was further illustrated by the ability of αβ T cells to produce IL-17A and compensate for the lack of γδ T cells in order to mediate neutrophil recruitment.
DISCUSSION
The concept of pattern recognition formulated by the late Charles Janeway Jr. has proven to be prophetic28. Specifically, ligation of pattern recognition receptors (PRR) on innate cells induces cellular activation, thus allowing innate immune cells to direct adaptive immunity1,29. However, in some cases, this canonical process is subverted, and T cell activation occurs in the absence of PRR engagement. Here we show that when such T cell responses, as initiated by exposure to S. aureus enterotoxin A, precede innate immunity, they can control the initiation and magnitude of the ensuing innate immune response. We provide evidence that this process begins with rapid αβ T cell stimulation (Figure 4). The αβ T cells then initiate innate cell recruitment into the lung tissue and cytokine synthesis, which ultimately results in waves of immune cells entering the airways (Figures 1 – 3). In this model, neutrophil and monocyte recruitment into the lung is dependent on IL-17A (Figure 5), and innate-like γδ T cells are able to synthesize IL-17A only when αβ T cells are present (Figure 6). Thus, αβ T cells can spring effective innate immunity.
An important event observed in our studies was the speed with which the SEA-specific T cells became activated (Figures 4). While pulmonary infectious disease models demonstrate T cell activation over a period of days, our results showed systemic T cell activation within 1 hour. This is likely an outcome of superantigen binding to MHC II followed by bridging the TCR with no need for Ag processing30. Interestingly, the rapid increase of CD69 and CD25 did not translate into clonal expansion until days after S. aureus enterotoxin challenge (Figure 1a). In fact, we were surprised to identify so many immunological parameters occurring prior to αβ T cell expansion, which were nonetheless dependent upon the presence of T cells themselves. For example, the expansion of NK cells in the lung was T cell-dependent. On the other hand, while NK cell function can foster T cell differentiation in certain infection models31, NK cells seemed to have little impact on T cell function following SEA. Thus, the role of NK cells in SEA-induced lung injury is still unknown. Neutrophils and monocytes also required T cell activation in order to accumulate in the lung. Even TNF synthesis by recruited monocytes was dependent upon T cells, especially CD4+ T cells (Supplementary Figure S3c), and the magnitude of TNF production was particularly striking. However, it is possible, and perhaps likely, that multiple T-dependent mechanisms are responsible for the waves of innate cell recruitment that occur in the given time frame which could explain the approximate 30 h delay in NK cell recruitment into the lung following neutrophils and monocytes. Taken together, our data demonstrates that T cell activation can guide early innate cell recruitment and activation, and the importance of these findings may help understand the mechanism of toxic shock syndrome and severe food poisoning in patients with S. aureus enterotoxin exposure. While it is clear that T cells produce cytokines in response to S. aureus enterotoxins, perhaps more attention should be paid to the role innate cells play during assembly of the cytokine storm32, which could ultimately enhance the development of therapeutics against toxic shock.
An observed outcome of the cytokine storm was rapid cell recruitment into the lungs, and we focused our studies on a likely list of potential candidates. TNF was a clear possibility since TNF release occurs rapidly after T cell activation33. However, neither TNF nor IFN-γ were responsible, leaving cytokines that were most likely not synthesized by recently activated naive T cells as the best contenders. As such, IL-17A can mediate the recruitment of neutrophils into the lung34. In recent years, numerous mouse models have been used to demonstrate the requirement for IL-17A to drive neutrophil recruitment into sites of inflammation, including the lung15,26,35. Our results showed that IL-17A was detected directly ex vivo in non-αβ TCR T cells (Supplementary Figure S6a), and similar to Mycobacterium tuberculosis (TB) and Listeria monocytogenes infection, we found that γδ T cells were specifically responsible for IL-17A production14,36. Interestingly, using Bacillus subtilus to model another form of lung inflammation termed hypersensitivity pneumonitis, Simonian et al. found that CD4+ T cells could compensate for the loss of IL-17A-producing γδ T cells in TCRδ−/− mice by increasing their production of IL-17A37. A comparison between SEA and TB, a pathogen of the lung, raises several interesting points. For one, TB infection occurs over weeks in a chronic fashion which is dramatically different from the acute response observed after enterotoxin exposure. Yet in both inflammatory settings, IL-17A mediates neutrophil recruitment into the lung, thus leading to pathology. A second interesting similarity between the two systems is the ability of IFN-γ to counteract the action of IL-17A both after infection38 and after enterotoxin5. Thus, while the chronic and acute inflammatory systems are very different there is a heavy reliance on lung γδ T cells to trigger inflammation. Future studies are required to determine if the response of the γδ T cells following SEA depends on a similar cytokine milieu, particularly IL-2 responsiveness, as is true after TB infection39. Since IL-2 is released early after TCR ligation, we postulate that T cell derived IL-2 may be an unappreciated bridge to stimulating the innate immune system. While others have shown that IL-2 has an inhibitory effect on CD4+ Th17 cells, IL-2 can have a positive role on the survival and proliferation of γδ T cells40,41. Ultimately, understanding how IL-17A is induced in γδ T cells following SEA is of particular interest since the upregulation of IL-1β and, to a lesser extent, IL-18 and IL-23p19, was also detected by real-time PCR following SEA (Supplementary Figure S7). IL-1β is likely involved since it remained upregulated in TCR δ−/− mice but not TCR β−/− mice. In total, manipulating both arms of the immune system with combinatorial therapy may impede acute inflammatory reactions that we contend rely on T cell activation as well as innate cell function. For example, inhibition of IL-2 and IL-17A would block early neutrophil and monocyte recruitment while IL-2 neutralization could impede T cell expansion and activation. In contrast to other translational approaches, which focus on a major cytokine from either the innate or adaptive arm of the immune response, such combinatorial therapy could further hinder compensatory efforts of the immune system since a key cytokine from both arms would be inhibited.
A perplexing question raised by our findings is how does a T cell response induce cell recruitment into the lung via IL-17A in just hours, specifically without the presence of a known PAMP. While one can never definitively state that the administered SEA is free of any contaminating products, the lack of a response in TCR βδ−/− mice, strongly suggests a lack of any PAMPs known to activate innate cells independently of T cells. In several models, TLR stimulation is known to trigger IL-17A production, typically through the action of IL-1, IL-23 and other factors42. One example is a human cell culture model of mycobacterium infection where IL-17A synthesis is dependent on TLR4 and dectin-1 expression43. In vivo responses to N. gonorrhoeae infection depend on TLR4 for IL-17A production44, and our previous data support this idea since responses to LPS helped expand and maintain Th17 cells45. This concept is also not limited to cases of infection since TLR6 stimulation mediates protective IL-17A responses counteracting lung pathology in an asthma mouse model46. Thus, evidence that IL-17A production is initiated by PRR stimulation is abundant. Our data, however, showed that direct αβ T cell stimulation also triggers early IL-17A synthesis, but perhaps surprisingly by γδ T cells. Interestingly, we found no evidence that i.n. SEA provoked Th17 differentiation since the expanded BAL Vβ3+ T cells produced IFN-γ and TNF, but not IL-17A (S. Kumar and A. Vella unpublished data). In this way, one could liken SEA stimulation of T cells to PRR stimulation of innate cells. Importantly, both situations result in analogous outcomes such as cellular activation, the release of cytokines and chemokines, and recruitment of cells into solid organs.
In sum, our data support the notion that enterotoxins can trigger T cell-dependent innate immunity and suggest that blocking cytokines with a penchant for adaptive and innate cells might foster stronger inflammation abatement in vivo.
METHODS
Mice
C57BL/6 mice, IFN-γ−/−, TNF−/−, TCRβ−/−, TCRδ−/− and TCRβδ−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME) and used between 7–14 weeks of age. All mice were maintained in the Central Animal Facility at the University of Connecticut Health Center in accordance with federal guidelines.
Immunization
Staphylococcus enterotoxin A (SEA) was purchased from Toxin Technology Inc. (Sarasota, FL). According to the certificate of analysis provided by the manufacturer, the level of endotoxin was less than an endotoxin unit/mg by LAL assay. Mice were anesthetized with isoflurane (Vedco Inc., Saint Joesph, MO) in a vaporizing chamber (Vaporizer Sales and Service Inc., Rockmart, GA). After anesthetization, 1 μg of SEA diluted in 50 μl of BSS, or BSS alone (vehicle) was pipetted on the nostrils (i.n. route) of mice, and this volume was inhaled instantly.
Antibodies and Cytokine assays
Anti-mouse IL-17A and anti-mouse IL-17E monoclonal antibodies were a kind gift from Amgen (Thousands Oaks, CA). Antibodies for flow cytometer were purchased from BD Bioscience (San Jose, CA): CD3e (500A2), CD4 (L3T4, GK1.5), CD8 (53-6.7), TCRVβ3 (KJ25), TCRδ (GL3), TCRβ (H57-597), TCR Vγ2 (UC3-10A6), CD90.2 (Thy1.2, 53-2.1), CD69 (H1.2F3), NK1.1 (PK136), NK-CD49b (DX5), CD11b (M1/70), CD44 (IM7), Ly-6G (1A8), IL-17A (TC11-18H10), IFN-γ (XMG1.2), TNF (MP6-XT22); eBioscience (San Diego, CA): CD25 (PC61.5), MHCII (M5/114.15.2), F4/80 (BM8), Ly-6G (RB6-8C5); AbD Serotech (Raleigh, NC): 7/4 (Ly-6B.2); BioLegend (San Diego, CA): TCR Vγ1.1 and Vγ1.2 (4B2.9). ELISA kits for IL-12p70, IFN-γ, TNF, IL-2, were purchased from BD Biosciences IL-17A was purchased from R&D Systems (Minneapolis, MN, USA) and IL-23p19 was purchased from eBioscience.
BAL fluid processing
Lungs were lavaged in situ with 5 ml of physiological buffer saline (PBS), and the BAL fluid was centrifuged at a low speed of 1500 rpm at 4°C for 5 min. The cellular pellet was washed, and cells were counted using a Z1 particle counter (Beckman Coulter, Brea, CA). BAL fluids were further centrifuged at a higher speed 20,000 g at 4°C for 10 min and stored at −20°C. Levels of IFN-γ protein in the BAL fluid were measured by ELISA according to manufacturer’s instruction.
Adoptive T cell transfer
For T cell transfer experiments, splenocytes were enriched using Nylon wool column as described earlier47. Enriched T cells (~106) were adoptively transferred intravenously by retro orbital injection into recipient (TCRβδ−/−) mice.
Immune cell depletion
The following monoclonal antibodies were purchased from BioXCell (West Lebanon, NH): anti-NK1.1 clone PK136, anti-CD8 clone 2.43, and anti-CD4 clone GK1.5. Mice were treated with 50ug ant-NK1.1, 500ug anti-CD8, 200ug anti-CD4, or control IgG. All antibodies were administered i.p., and depletion was confirmed by flow cytometry.
Cytokine neutralization
IL-17A and IL-17E neutralization25 was carried out in WT mice by i.p. injection of 200 μg of either anti-mouse IL-17A or anti-mouse IL-17E mAbs. Mouse or rat IgG was used as control in separate group prior to SEA challenge.
Tissue processing and cell isolation
Lung tissue was perfused with PBS-heparin (75U/ml), cut into small pieces and incubated in the presence of 1.3 mM EDTA at 37°C for 30 min, washed, followed by digestion with collagenase from clostridium histolyticum IV (150U/mL) (Sigma-Aldrich, St. Louis, MO) for 1 hour at 37°C. Subsequently, digested lung tissues were crushed through nylon mesh cell strainers (BD Biosciences) and partitioned between 44% and 67% Percoll gradient to obtain lymphocytes at the interface. Spleens, inguinal, axillary, branchial and mediastinal lymph nodes were homogenized into single-cell suspension by crushing them through nylon mesh cell strainers. Spleen cells were treated with ammonium chloride to lyse RBC. Single-cell suspensions from different tissues were washed with BSS and counted using a Z1 particle counter (Beckman Coulter).
In vitro cell culture
For in vitro stimulation, cells (106) were cultured for 5 h or the indicated time at 37°C and 5% CO2 in 200 μl complete tumor medium, consisting of MEM with 5% fetal bovine serum (FBS), amino acids, salts and antibiotics. Cells were stimulated with media alone or phorbol myristate acetate (PMA) (50 ng/ml; Calbiochem, Darmstadt, Germany) plus ionomycin (1 μg/ml) (Invitrogen, San Diego, CA) and brefeldin A (BFA; 5 μg/ml; Calbiochem), and stained for intracellular cytokines.
Flow cytometry
Surface and intracellular staining was performed as described previously48. Briefly, cells were stained with primary Abs on ice for 30 min, in the presence of a blocking solution containing 5% normal mouse serum (Sigma-Aldrich), 10 μg/ml human γ-globulin (Sigma-Aldrich), and 0.1% sodium azide in culture supernatant from the 2.4.G.2 hybridoma (anti-FcR Abs)49. If secondary incubation was necessary, cells were washed and resuspended in wash buffer, and incubated on ice with streptavidin conjugated flurochrome for 30 min. For intracellular cytokine staining, the cells were stained with primary antibodies, washed and fixed with 2% paraformaldehyde in BSS. The cells were placed in permeabilization buffer (0.25% saponin in wash buffer) for 10 min and stained for intracellular staining with IL-17A, IFN-γ and TNF or an isotype control. After staining, cells were washed in wash buffer containing 3% FBS and 0.1% sodium azide in BSS. Flow cytometery was performed on a FACS-LSRII (BD Biosciences) and data were analyzed using FlowJo software (Tree Star Inc. Ashland, OR).
Quantitative real-time RT PCR
Total RNA was isolated from lung cells (percoll purified) from 2 h vehicle and SEA immunized mice by RNeasy mini kit (Quiagen, Valencia, CA). RNA samples (100 ng) were reverse transcribed into cDNA using a High-Capacity cDNA Archive Kit (Applied Biosystems). mRNA expression level of target genes and the endogenous control gene HPRT1 were assessed by real-time PCR on 7300 Real Time PCR System (Applied Biosystems). The expression levels of target genes: IL-1β (Mm00434228_m1), IL-18 (Mm00434225_m1), IL-23p19 (Mm01160011_g1) were calculated by normalized to HPRT1(Mm01545399_m1) (Applied Biosystems).
Statistical analysis
A two-tailed Student’s unpaired t test was used for data analyses, with values of p < 0.05 used as the significance threshold. All statistical analyses were performed using Microsoft Excel and Prism-GraphPad, La Jolla, CA).
Supplementary Material
Monocyte maturation and cytokine expression. (a) Lung cells were isolated 24 h after SEA treatment from WT mice and stained for monocytes and neutrophils. Pure (7/4highLy6Gnegative) (left panel) and mixed monocytes (7/4lowLy6Gnegative) (right) were stained for MHCIIa-e expression (dashed line) compared to isotype control (gray histogram). (b) The MFI of MHCII expression was quantified for each population in A. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 8 for vehicle, n = 10 for SEA.
Monocyte and Neutrophil recruitment in TCRβ−/− versus TCRδ−/− mice. (a – d) WT and TCRβ−/− mice (a and b) or WT and TCRδ−/− mice (c and d) were challenged i.n. with vehicle alone or SEA for 6 h. Lung cells from WT (upper panel) and T cell-deficient mice (lower) were stained for 7/4 and Ly6G expression. Monocyte and neutrophil percentages (upper) and total numbers (lower) are shown. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 4 for vehicle, n = 6 for SEA for WT versus TCRβ−/− experiments. Data were combined from 3 independent experiments with n = 4 for vehicle, n = 6 for SEA for WT and TCRδ−/− experiments. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Role of CD4+ versus CD8+ T cells in innate cell recruitment. (a–d) WT mice were treated with depleting antibodies specific for CD4+ T cells (anti-CD4) or CD8+ T cells (anti-CD8) or control antibody (control IgG) 2 days prior to i.n. SEA challenge. At 6 h, lung cells were harvested, and analyzed for monocytes and neutrophils as above (a and b), TNF production from monocytes (c), and IL-17 production from CD3+GL3+TCRβ− (d). Data were combined from 3 independent experiments with n = 4 for vehicle (open circles; ○) and n = 9 for SEA (closed circles; ●).
Monocyte and Neutrophil recruitment in TNF−/− and IFN-γ−/− mice. (a – d) WT and TNF−/− mice (a and b) or WT and IFN-γ−/− mice (c and d) were treated with vehicle alone or SEA for 8 h. Lung cells from WT (upper panel) and cytokine-deficientmice (lower) were stained for 7/4 and Ly6G expression. Monocyte and neutrophil percentages (upper) and total numbers (lower) are shown from individual mice. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 3 for vehicle, n = 6 for SEA for each WT and TNF−/− group. Data were combined from 2 independent experiments with n = 4 for vehicle, n = 6 for SEA for each group WT and IFN-γ−/−. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Monocyte recruitment into lung after IL-17A and or IL-17E neutralization. WT mice were treated with control Ig, anti-mouse IL-17A mAb, or anti-mouse IL-17E mAb, prior to SEA treatment. Lung cells were harvested 8 h after SEA and stained for the presence of monocytes. Control Ig, anti-mouse IL-17A or anti-mouse IL-17E treated mice were quantified for percent (upper) and number (lower) of monocytes. Data were combined from 6 independent experiments with n = 8 for vehicle and n = 13 for SEA treated group. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
γδ T cells are the primary source of IL-17A early following SEA. (a) Lung cells from 6 h SEA treated WT mice were stimulated ex vivo with media alone (gray histogram) or PMA/I (dashed line) in the presence of brefeldin A for 4 h. CD3+ T cells were gated for TCRβ and CD8 (left panel) or TCRδ and CD8 (right) and were analyzed for IL-17A production. (b and c) Lung cells from vehicle or 6 h SEA treated WT and TCRδ−/− mice were stimulated ex vivo with media alone or PMA/I in the presence of brefeldin A for 4 h. Lymphocytes were stained with CD3, CD8 and TCRδ, and the CD3+CD8−TCRδ− cells were analyzed for CD25 and IL-17A.
Induction of IL-17-polarizing cytokines after SEA. We subjected WT, TCRδ KO, TCRβ KO, and TCRβδ KO mice to i.n. SEA challenge and harvested the lung after 2 hrs. RNA was isolated from total cells following percoll purification, and mRNA expression levels of IL-1β, IL-18, and IL-23p19 was determined by real-time PCR as described in the Materials & Methods. Data were combined from 3 independent experiments with n = 3 for vehicle, n = 5 for SEA.
Acknowledgments
This work was supported by NIH grant 1PO1-AI05172 (L.L.) Project 3 (A.T.V.).
Footnotes
Supplementary information includes seven figures.
DISCLOSURE
A.L. Budelsky is employed by and owns stock in Amgen, Inc. The remaining authors declared no financial conflict of interest.
References
- 1.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 3.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 4.Herz U, et al. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness--a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Muralimohan G, Rossi RJ, Guernsey LA, Thrall RS, Vella AT. Inhalation of Staphylococcus aureus enterotoxin A induces IFN-gamma and CD8 T cell-dependent airway and interstitial lung pathology in mice. J Immunol. 2008;181:3698–3705. doi: 10.4049/jimmunol.181.5.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez S, Cisney ED, Hall SI, Ulrich RG. Nasal immunity to staphylococcal toxic shock is controlled by the nasopharynx-associated lymphoid tissue. Clin Vaccine Immunol. 2011;18:667–675. doi: 10.1128/CVI.00477-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachert C, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J Allergy Clin Immunol. 2010;126:962–968. 968 e961–966. doi: 10.1016/j.jaci.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Corriveau MN, Zhang N, Holtappels G, Van Roy N, Bachert C. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23:461–465. doi: 10.2500/ajra.2009.23.3367. [DOI] [PubMed] [Google Scholar]
- 9.Hauk PJ, Wenzel SE, Trumble AE, Szefler SJ, Leung DY. Increased T-cell receptor vbeta8+ T cells in bronchoalveolar lavage fluid of subjects with poorly controlled asthma: a potential role for microbial superantigens. J Allergy Clin Immunol. 1999;104:37–45. doi: 10.1016/s0091-6749(99)70111-9. [DOI] [PubMed] [Google Scholar]
- 10.Hodges E, et al. T cell receptor (TCR) Vbeta gene usage in bronchoalveolar lavage and peripheral blood T cells from asthmatic and normal subjects. Clin Exp Immunol. 1998;112:363–374. doi: 10.1046/j.1365-2249.1998.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber R, Keerl R, Hochapfel F, Draf W, Toffel PH. Packing in endonasal surgery. Am J Otolaryngol. 2001;22:306–320. doi: 10.1053/ajot.2001.26499. [DOI] [PubMed] [Google Scholar]
- 12.Weber MA, et al. Infection and sudden unexpected death in infancy: a systematic retrospective case review. Lancet. 2008;371:1848–1853. doi: 10.1016/S0140-6736(08)60798-9. [DOI] [PubMed] [Google Scholar]
- 13.Highet AR, Goldwater PN. Staphylococcal enterotoxin genes are common in Staphylococcus aureus intestinal flora in Sudden Infant Death Syndrome (SIDS) and live comparison infants. FEMS Immunol Med Microbiol. 2009;57:151–155. doi: 10.1111/j.1574-695X.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 14.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 15.Ye P, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan JE, Herman A, Kappler JW, Marrack P. Stimulation of B10. BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990;144:2473–2479. [PubMed] [Google Scholar]
- 17.Catalona WJ, Ratliff TL, McCool RE. gamma-Interferon induced by S. aureus protein A augments natural killing and ADCC. Nature. 1981;291:77–79. doi: 10.1038/291077a0. [DOI] [PubMed] [Google Scholar]
- 18.Bhandari V, Elias JA. Cytokines in tolerance to hyperoxia-induced injury in the developing and adult lung. Free Radic Biol Med. 2006;41:4–18. doi: 10.1016/j.freeradbiomed.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller EJ, Cohen AB, Peterson BT. Peptide inhibitor of interleukin-8 (IL-8) reduces staphylococcal enterotoxin-A (SEA) induced neutrophil trafficking to the lung. Inflamm Res. 1996;45:393–397. doi: 10.1007/BF02252934. [DOI] [PubMed] [Google Scholar]
- 21.Parsons PE, Gillespie MM, Moore EE, Moore FA, Worthen GS. Neutrophil response to endotoxin in the adult respiratory distress syndrome: role of CD14. Am J Respir Cell Mol Biol. 1995;13:152–160. doi: 10.1165/ajrcmb.13.2.7542895. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 23.Tsou CL, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Rickel EA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 26.Ribot JC, et al. CD27 is a thymic determinant of the balance between interferon-gamma-and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 30.Seth A, et al. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994;369:324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- 31.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 33.Peavy DL, Adler WH, Smith RT. The mitogenic effects of endotoxin and staphylococcal enterotoxin B on mouse spleen cells and human peripheral lymphocytes. J Immunol. 1970;105:1453–1458. [PubMed] [Google Scholar]
- 34.Laan M, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 35.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 36.Hamada S, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonian PL, et al. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–6549. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz A, et al. Cutting edge: IFN-gamma regulates the induction and expansion of IL-17-producing CD4 T cells during mycobacterial infection. J Immunol. 2006;177:1416–1420. doi: 10.4049/jimmunol.177.3.1416. [DOI] [PubMed] [Google Scholar]
- 39.Janis EM, Kaufmann SH, Schwartz RH, Pardoll DM. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989;244:713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- 40.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Ribot JC, Debarros A, Mancio-Silva L, Pamplona A, Silva-Santos B. B7-CD28 costimulatory signals control the survival and proliferation of murine and human gammadelta T cells via IL-2 production. J Immunol. 2012;189:1202–1208. doi: 10.4049/jimmunol.1200268. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 43.van de Veerdonk FL, et al. Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol. 2010;88:227–232. doi: 10.1189/jlb.0809550. [DOI] [PubMed] [Google Scholar]
- 44.Packiam M, et al. Protective role of Toll-like receptor 4 in experimental gonococcal infection of female mice. Mucosal Immunol. 2011;5:19–29. doi: 10.1038/mi.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAleer JP, et al. Potent intestinal Th17 priming through peripheral lipopolysaccharide-based immunization. J Leukoc Biol. 2010;88:21–31. doi: 10.1189/jlb.0909631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreira AP, et al. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J Clin Invest. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trizio D, Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974;113:1093–1097. [PubMed] [Google Scholar]
- 48.McAleer JP, Zammit DJ, Lefrancois L, Rossi RJ, Vella AT. The lipopolysaccharide adjuvant effect on T cells relies on nonoverlapping contributions from the MyD88 pathway and CD11c+ cells. J Immunol. 2007;179:6524–6535. doi: 10.4049/jimmunol.179.10.6524. [DOI] [PubMed] [Google Scholar]
- 49.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monocyte maturation and cytokine expression. (a) Lung cells were isolated 24 h after SEA treatment from WT mice and stained for monocytes and neutrophils. Pure (7/4highLy6Gnegative) (left panel) and mixed monocytes (7/4lowLy6Gnegative) (right) were stained for MHCIIa-e expression (dashed line) compared to isotype control (gray histogram). (b) The MFI of MHCII expression was quantified for each population in A. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 8 for vehicle, n = 10 for SEA.
Monocyte and Neutrophil recruitment in TCRβ−/− versus TCRδ−/− mice. (a – d) WT and TCRβ−/− mice (a and b) or WT and TCRδ−/− mice (c and d) were challenged i.n. with vehicle alone or SEA for 6 h. Lung cells from WT (upper panel) and T cell-deficient mice (lower) were stained for 7/4 and Ly6G expression. Monocyte and neutrophil percentages (upper) and total numbers (lower) are shown. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 4 for vehicle, n = 6 for SEA for WT versus TCRβ−/− experiments. Data were combined from 3 independent experiments with n = 4 for vehicle, n = 6 for SEA for WT and TCRδ−/− experiments. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Role of CD4+ versus CD8+ T cells in innate cell recruitment. (a–d) WT mice were treated with depleting antibodies specific for CD4+ T cells (anti-CD4) or CD8+ T cells (anti-CD8) or control antibody (control IgG) 2 days prior to i.n. SEA challenge. At 6 h, lung cells were harvested, and analyzed for monocytes and neutrophils as above (a and b), TNF production from monocytes (c), and IL-17 production from CD3+GL3+TCRβ− (d). Data were combined from 3 independent experiments with n = 4 for vehicle (open circles; ○) and n = 9 for SEA (closed circles; ●).
Monocyte and Neutrophil recruitment in TNF−/− and IFN-γ−/− mice. (a – d) WT and TNF−/− mice (a and b) or WT and IFN-γ−/− mice (c and d) were treated with vehicle alone or SEA for 8 h. Lung cells from WT (upper panel) and cytokine-deficientmice (lower) were stained for 7/4 and Ly6G expression. Monocyte and neutrophil percentages (upper) and total numbers (lower) are shown from individual mice. Each dot represents an individual mouse treated either with vehicle (open circles; ○) or SEA (closed circles; ●). Data were combined from 3 independent experiments with n = 3 for vehicle, n = 6 for SEA for each WT and TNF−/− group. Data were combined from 2 independent experiments with n = 4 for vehicle, n = 6 for SEA for each group WT and IFN-γ−/−. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
Monocyte recruitment into lung after IL-17A and or IL-17E neutralization. WT mice were treated with control Ig, anti-mouse IL-17A mAb, or anti-mouse IL-17E mAb, prior to SEA treatment. Lung cells were harvested 8 h after SEA and stained for the presence of monocytes. Control Ig, anti-mouse IL-17A or anti-mouse IL-17E treated mice were quantified for percent (upper) and number (lower) of monocytes. Data were combined from 6 independent experiments with n = 8 for vehicle and n = 13 for SEA treated group. Data shown are Mean +/− SEM. Statistical significance between vehicle and SEA was evaluated by two tailed Student’s t tests with all p values <0.05.
γδ T cells are the primary source of IL-17A early following SEA. (a) Lung cells from 6 h SEA treated WT mice were stimulated ex vivo with media alone (gray histogram) or PMA/I (dashed line) in the presence of brefeldin A for 4 h. CD3+ T cells were gated for TCRβ and CD8 (left panel) or TCRδ and CD8 (right) and were analyzed for IL-17A production. (b and c) Lung cells from vehicle or 6 h SEA treated WT and TCRδ−/− mice were stimulated ex vivo with media alone or PMA/I in the presence of brefeldin A for 4 h. Lymphocytes were stained with CD3, CD8 and TCRδ, and the CD3+CD8−TCRδ− cells were analyzed for CD25 and IL-17A.
Induction of IL-17-polarizing cytokines after SEA. We subjected WT, TCRδ KO, TCRβ KO, and TCRβδ KO mice to i.n. SEA challenge and harvested the lung after 2 hrs. RNA was isolated from total cells following percoll purification, and mRNA expression levels of IL-1β, IL-18, and IL-23p19 was determined by real-time PCR as described in the Materials & Methods. Data were combined from 3 independent experiments with n = 3 for vehicle, n = 5 for SEA.