Abstract
A growing interest in sensory system plasticity in the natural context of motherhood has created the need to investigate how intrinsic physiological state (e.g., hormonal, motivational, etc.) interacts with sensory experience to drive adaptive cortical plasticity for behaviorally relevant stimuli. Using a maternal mouse model of auditory cortical inhibitory plasticity for ultrasonic pup calls, we examined the role of pup care versus maternal physiological state in the long-term retention of this plasticity. Very recent experience caring for pups by Early Cocarers, which are virgins, produced stronger call-evoked lateral-band inhibition in auditory cortex. However, this plasticity was absent when measured post-weaning in Cocarers, even though it was present at the same time point in Mothers, whose pup experience occurred under a maternal physiological state. A two-alternative choice phonotaxis task revealed that the same animal groups (Early Cocarers and Mothers) demonstrating stronger lateral-band inhibition also preferred pup calls over a neutral sound, a correlation consistent with the hypothesis that this inhibitory mechanism may play a mnemonic role and is engaged to process sounds that are particularly salient. Our electrophysiological data hints at a possible mechanism through which the maternal physiological state may act to preserve the cortical plasticity: selectively suppressing detrimental spontaneous activity in neurons that are responsive to calls, an effect observed only in Mothers. Taken together, the maternal physiological state during the care of pups may help maintain the memory trace of behaviorally salient infant cues within core auditory cortex, potentially ensuring a more rapid induction of future maternal behavior.
Keywords: inhibitory plasticity, maternal behavior, vocalization, auditory cortex, sensory plasticity, spontaneous rate
An individual’s internal state impacts ongoing perception and cortical processing of sensory stimuli (Gandelman, 1983; Parlee, 1983; Critchley and Rolls, 1996; Steinmetz et al., 2000; Supèr et al., 2003; Balcetis and Dunning, 2006; Niell and Stryker, 2010). Since such states are often mediated by neurochemical mechanisms that also contribute to experience-dependent cortical plasticity (Gu, 2002), the neural correlates of sensory memories acquired under distinct states are likely also different. Yet the neurobiological connection between internal states and sensory memories is not fully understood, particularly at the level of sensory cortex, where neural traces of sensory experience can be found (Weinberger, 2004; Ivanova et al., 2011). For example, a putative cortical signature of associative learning is sensory map plasticity, and internal factors like motivation during learning can affect the relative magnitude of this plasticity (Rutkowski and Weinberger, 2005). However, learned behaviors can persist in the absence of sensory map expansion (Reed et al., 2011), and other forms of neural plasticity may also contribute to the longer-term memory trace of a sensory experience (Polley et al., 2004; Schnupp et al., 2006; Razak et al., 2008). Inhibitory plasticity is one such experience-dependent mechanism that has gained recent attention (Pallas et al., 2006; Galindo-Leon et al., 2009; Woodin and Maffei, 2011), though little is known about how internal states affect its long-term expression. Developing an understanding of how intrinsic factors influence forms of cortical plasticity can shed new light on how our sensory systems shape the inputs that lead to each individual’s unique interpretation of the external world from prior experience.
Neuroplasticity during motherhood provides an opportune natural system in which to explore this question. Motherhood is associated with long-term behavioral (Fleming et al., 1994; Kinsley et al., 1999; Lambert et al., 2005; Pawluski et al., 2006) and neurophysiological (Fleming and Korsmit, 1996; Kinsley et al., 2006; Swain et al., 2007; Kim et al., 2010) changes, including plasticity in inhibitory systems (Maguire and Mody, 2008; Maguire et al., 2009). Dramatic hormonal changes accompany pregnancy and parturition, and the degree to which this intrinsic maternal hormonal environment contributes to adaptive plasticity for infant care has been of long-standing interest (Terkel and Rosenblatt, 1972; Bridges, 1984; Fleming and Sarker, 1990; Maguire and Mody, 2009). Such questions now extend to sensory systems, given recent findings of sensory cortical plasticity during motherhood (Xerri et al., 1994; Liu et al., 2006; Rosselet et al., 2006; Liu and Schreiner, 2007; Kim et al., 2010; Cohen et al., 2011). However, much less is known about how physiological state and experience might combine to establish and maintain functionally adaptive cortical plasticity.
We investigate maternal sensory plasticity in a mouse model of acoustic communication between offspring and adult females. Mouse pups emit bouts of ultrasonic whistles that are recognized as behaviorally relevant to mothers but not pup-naïve virgins (Ehret et al., 1987; Ehret and Koch, 1989). Neural correlates for this behavioral difference have been demonstrated in the auditory cortex of both anesthetized (Liu et al., 2006; Liu and Schreiner, 2007; Cohen et al., 2011) and awake, head-restrained animals (Galindo-Leon et al., 2009). In particular, after weaning pups, awake mothers show a stronger single unit (SU) pup call-evoked inhibition compared to virgins, an effect hypothesized to improve the functional detection of pup calls (Galindo-Leon et al., 2009). Rather than a reflection of the maternal physiological state, this long-term inhibitory cortical plasticity could simply be a result of experience with pups. We tested that possibility here using SU recordings in cocaring females, which are virgin mice that raise pups with a littermate mother. Importantly, cocarers do not undergo the same physiological changes as mothers, although they still behaviorally recognize pup ultrasounds after sufficient pup care experience (Ehret et al., 1987).
Experimental Procedures
Experiments were carried out on adult CBA/CaJ female mice, all between 14 and 24 weeks old. Animals were housed under a reversed light cycle (14 hours light/10 hours dark), had access to food and water ad libitum, and were tested during their dark cycle. In the course of our study, distinct Animal Groups were used: Mothers, which were studied after weaning their pups at postnatal day P21; Virgins, which had no adult contact with pups and no breeding experience; Cocarers, which were adult virgins given the same full term (21 postnatal days) of experience caring for pups as a Mother (but without the physiological changes due to pregnancy, parturition and lactation) and studied after pups were weaned; and Early Cocarers, which were adult virgins with experience caring for pups with a Mother at least through postnatal day P6, and studied between P6–11. In the subsequent text, these terms when capitalized refer to our specific study groups; when not capitalized, they refer to generic animals of their respective types.
All Mothers, Cocarers and Early Cocarers used in electrophysiological studies were screened to check that they would successfully retrieve scattered pups in the home cage over a 10 minute period on P5 or P6 (retrieval screening for behavioral studies described below). This ensured that our animals not only had some minimum baseline of experience with ultrasonically vocalizing pups – verified by an ultrasound detector (U30, Ultra Sound Advice, London, United Kingdom) – but also showed comparable levels of maternal responsiveness.
Electrophysiological recordings
A head post surgery under isoflurane anesthesia was performed on animals a few days before chronic, restrained electrophysiological recordings began. During surgery, a stereotaxically targeted grid of small recording holes covering core auditory cortex was marked with india ink on the skull for later opening (~150 um diameter) before each recording session. Animals were given 2–3 days recovery before electrophysiological recording sessions began, at which point their weight and activity levels appeared normal. Before each day’s recording session, animals were very briefly re-anesthetized with isoflurane and placed in the nose clamp of the stereotax so that small holes (about 150 microns diameter) could be drilled with an insect needle held by a pin vise (Fine Science Tools, Foster City, CA) through the holes defined by the grid. Two to three hours after recovering from this brief procedure, the mouse was handled for 10 to 15 minutes to reduce stress and then prepared for electrophysiology recordings by placing them into a two-piece (top and bottom halves), plastic tube (~3 cm diameter) lined with soft foam. The animal’s head post was affixed into a stationary metal bar while its restraint tube was suspended by rubber bands. This helped to ensure that any animal movement would not place torque on the head post, and to minimize any movement-related recording artifacts. If an animal did not acclimate well to the restraint, it was removed; no recordings were collected under sedation. Further details on the methods for head post implantation and lesions for electrophysiological studies may be found in (Galindo-Leon et al., 2009).
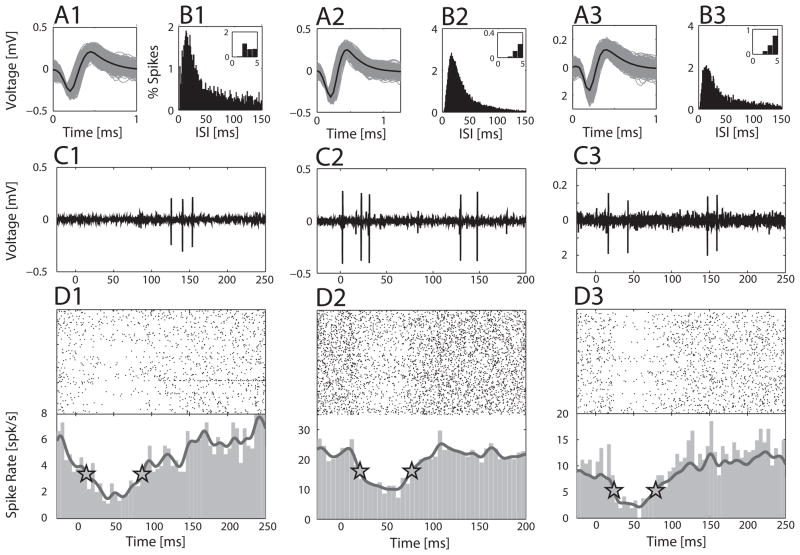
Electrophysiological recordings were performed on a vibration isolation table (TMC 63-533, Peabody, MA) in a 9′-4″ × 10′-6″ double wall anechoic chamber (IAC, Bronx, NY) using 6 MΩ, tungsten electrodes (FHC, Bowdoin, ME), targeting the ultrasound field (UF), primary auditory field (A1), and the anterior auditory field (AAF) in the left auditory cortex (Galindo-Leon et al., 2009). An electrode was advanced using a hydraulic microdrive (FHC), while monitoring the activity on a computer speaker. A reference depth was established at the dural surface by listening for a change in the electrode channel noise level, and all single unit (SU) and local field potential (LFP) recordings were located between 300 and 700 um below this. SUs were isolated by adjusting the electrode depth in 1–5 um steps until a SU was detected and its background noise ratio was optimized. For any detected SU, we played back pure tones and pup calls regardless of whether it was excited, inhibited, or non-responsive. Additional SU isolation methods are described in our previous studies (Galindo-Leon et al., 2009; Lin and Liu, 2010). Figure 1A–C shows example SUs with their respective spike waveforms, interspike intervals (ISI), single trial examples, and their pooled peri-stimulus time histogram (PSTH) responses.
Figure 1.
Recording examples. (A1–A3) Spike waveform, (B1–B3) inter-spike interval distribution, (C1–C3) single trial, and (D1–D3) pooled response of three SUs to the eighteen different pup vocalizations. The mean spike waveform is shown in black with the gray lines representing all spikes in panel A. Panel D shows the trial-by-trial spike rasters and the 5-ms binned PSTH, for each call-inhibited SU. Trials, grouped by call, are arranged according to the frequency and duration characteristics of the calls. Calls in the lowest frequency range are at the bottom, and highest frequency range at the top, with a further progression from bottom to top of shorter to longer duration calls within each frequency range. The response of call-inhibited SUs was usually not strongly modulated by specific calls, so no attempt is made here to delineate the different calls. The dark gray line is the Gaussian-smoothed PSTH and the stars indicate the start and stop of the duration measure. The stimulus starts at 0 ms and the longest call lasts ~60 ms. (A1–D1) Left column SU 1714 from a Mother. (A2–D2) Middle column SU 1631 from a Cocarer. (A3–D3) Right column SU 1480 from a Virgin.
Acoustic stimulation
For each SU and LFP site, responses were recorded to both pure tones and mouse vocalizations. Sounds were played back to the animal through an Infinity EMIT free-field speaker located 11 cm from the right ear. At this position, the speaker has an uncorrected transfer function that peaks around 15 kHz, and drops smoothly by around 10 dB to 40 kHz, after which it declines more gradually and stays within about +/− 6 dB until beyond 100 kHz. Nevertheless, we equalized the speaker output using the frequency-dependent transfer function to derive an 80-tap Finite Impulse Response (FIR) filter through which all sounds were presented. This completely flattened the speaker output between 5 and 105 kHz, with minimal harmonic distortion.
Tones were played at 60 dBSPL for 60 ms and had 10 ms cos2 onset and offset ramps. To derive frequency response curves, 40 different tones logarithmically spaced ranging from 6.4 to 95 kHz were presented randomly every 600 ms and repeated 15 times through the Brainware software (Tucker Davis Technologies, TDT). We analyzed call responses to eighteen different pup vocalizations that were scaled to a target root mean square (RMS) amplitude of 65 dBSPL. The calls were specifically chosen to vary systematically in average frequency (66 – 81kHz) and duration (12 – 67ms), coming from pups ranging from post-partum day 4 through 14 (Galindo-Leon et al., 2009; Lin and Liu, 2010). Since all calls were selected from a previously recorded database, they were not emitted by pups that were familiar to our study animals. Each call, along with a blank stimulus without a sound, was interleaved in random order for each new recording site, and the cycle of all stimuli was repeated 50 times. All stimuli had the same trial lengths (600 ms), with sounds presented 200 ms into the trial.
For behavioral experiments, two Infinity EMIT free-field speakers were used. They had similar transfer functions with only a shallow, uncorrected roll-off from ~16 kHz to 91 kHz of ~8 dB, measured 60 cm away at the nest. We corrected this with FIR filters and equalized their output. Thus, sound stimulation was matched between the two speakers across the full frequency range. Two different stimuli were used, which consisted of alternating bouts of either a set of pup calls or tones. The pup calls used in each bout were randomly selected from four different vocalizations in our library with an RMS amplitude of 70 dBSPL measured at the nest depression. These four calls varied in both frequency (65, 71, or 74 kHz), and duration (35 or 55ms). Tones were played back from the opposing speaker and designed to match the stimulus variability in the pup call stimuli. Tones had 5 ms cos2 onset and offset functions and were randomly selected from a set that varied in frequency (19, 20, or 21 kHz), and duration (35 or 55ms). The tones were measured at 55 dBSPL at the nest depression to approximately match the sound level differences in the CBA/CaJ hearing thresholds at 20 kHz versus 64 kHz, consistent with earlier behavioral studies (Ehret and Haack, 1982). The test used either a randomized number of calls per bout (2–5 calls), or a fixed number of calls per bout (3 calls) for a given animal, with the start of each bout separated by 1.6 seconds and the inter-stimulus interval within each bout set at an ethologically appropriate 200 ms (Liu et al., 2003).
SU and LFP classification and analysis
Pup call and tone responsive SU PSTH’s were classified using methods previously described (Galindo-Leon et al., 2009) to explicitly detail changes in both the temporal and rate dimensions of the response. Briefly, SU response duration was determined by finding the half-min of the smoothed spike rate (convolution of individual spikes with a Gaussian smoothing function, 5 ms standard deviation), and determining the time over which the smoothed spike rate stayed below the half-min value. To determine if there were differences between animal groups in the pooled spike rate for call-inhibited SUs, each smoothed, time-dependent spike rate function was normalized by the average spontaneous rate during the blank trial and then pooled over SUs. The strength of SU inhibition was quantified by integrating the actual spike count over a period from 5 to 65ms (accounting for the longest duration pup call plus an initial neural delay and any offset responses) and dividing by the spontaneous rate. The normalized spike count and duration of responses were used to create a plot of the bootstrapped mean values for each animal group. Within each animal group, the SU data were randomly sampled with replacement 50000 times to estimate means and 95% confidence ranges. To compute the spontaneous rates for both calls and tones for comparing responsive and non-responsive firing rates, the 200 ms prior to the stimulus onset was used. For calls, this consisted of the average spontaneous firing rate over 900 trials, and for tones it consisted of the rate over 600 trials.
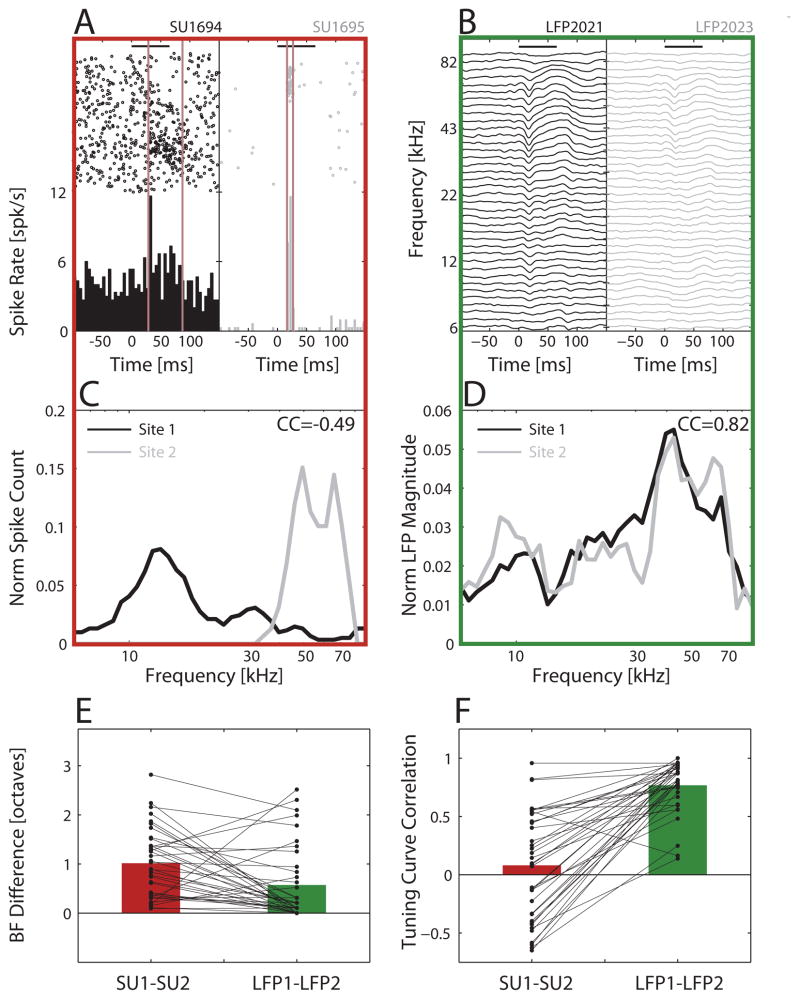
To assign SU recordings to an auditory field, we relied mainly on the best frequency (BF) topography, expecting to observe a reversal of the tonotopic BF gradient at the border between Al and AAF (Stiebler et al., 1997). Since tonotopy in the auditory cortex is most easily seen at a coarser population level, and can be more variable at the SU level (Bandyopadhyay et al., 2010; Rothschild et al., 2010; Hackett et al., 2011), we used the LFP as a more robust way to visualize the BF map. This was validated by comparing the BFs and tuning curves for a subset of data in which we had pairs of SUs and their co-localized LFPs recorded along the same cortical column at different depths (between 300–700 um) in the same electrode penetration (Fig. 2A–D). To compare the variability between SU and LFP BFs within each recording column, the octave difference in BF was measured between the two SUs or LFPs as log2(BF1/BF2), where BF1 (BF2) was the larger (smaller) of the two BFs. SUs were clearly more variable in their BF values within a column compared to LFPs (Fig. 2E). Correlation coefficients between SU or LFP tuning curves further showed that those from LFPs were significantly more similar to each other across sites compared to those from SUs (Fig. 2F). These results therefore justified using the LFP for coarse mapping of recording sites in the awake animal.
Figure 2.
Validating LFP over SU to characterize frequency tuning in a cortical penetration. SU frequency responses from the same recording penetration were more variable compared to co-localized LFPs. (A) Raster responses (tone trials ordered low to high frequency from bottom to top) and PSTHs for SUs at two sites in one example penetration, recorded at depths of 483 um (SU 1694) and 584 um (SU 1695). (B) Average pure tone LFP responses (smoothed with nearest neighbor frequencies) co-recorded with the SUs: LFP 2021 with SU 1694, and LFP 2023 with SU 1695. Horizontal black bars indicate start and duration of the tones in (A) and (B). (C) Tuning curves for SU 1694 (black) and SU 1695 (gray), based on counting spikes between the vertical brown lines in (A), when responses deviated from spontaneous activity. Tuning curves were normalized to an area of one. (D) Corresponding tuning curves for LFP 2021 (black) and LFP 2023 (gray), based on the peak negative deflection within 70 ms of tone onset, and normalized to a tuning curve area of one. Correlation coefficients (CC) for the tuning curves of the example SU and LFP pairs are shown in the upper right corners of (C) and (D). (E) BF difference in octaves, log2(BF1/BF2), computed between all pairs of SU or co-recorded LFP recordings (37 pairs, average depth difference between pairs of 121.1 um ± 94.2 um standard deviation). (F) CC, computed between all pairs of SU or co-recorded LFP recordings. In both (E) and (F), bars indicate means for SUs (red) and LFPs (green), and connected dots link values for co-recorded pairs of SUs and LFPs. LFPs from the same penetration generally had much closer BFs (E) and more similar tuning curves (F) compared to SUs in the same penetration.
A recording site’s LFP BF, the bandwidth of the tuning curve, and the BF relative to surrounding recording sites were then used to assign the recording hole and its associated SUs to an auditory field. A hole was generally assigned to UF if it had an LFP BF greater than ~45 kHz and a more localized excitatory response to the ultrasonic frequencies. AAF was typically defined as the holes ventral to UF holes, which showed LFP BFs below ~45 kHz with excitatory responses across a broad frequency range. The localization of UF and AAF helped define the caudally located A1 by tracking the rostral to caudal reversal of the tonotopic progression. Recording sites that were located in the middle of this reversal were labeled as joint AAF/A1. Any recording holes that demonstrated no reliable LFP responses to the pure tones but showed auditory responsiveness to pup calls were considered as non-core auditory fields and were not included in the data analysis. Our analysis of lateral-band fields consisted of recordings from holes assigned to AAF, A1 or AAF/A1, which was carried out blindly to the knowledge of the SU responses within those holes. In addition, our experimental strategy accumulates recordings for the same stimulus set over time in a large database. The current work includes a small subset of all SUs (86 out of 367), including call-inhibited SUs (28 out of 103), that were part of a previous publication (Galindo-Leon et al., 2009), though the vast majority of units included here are new (77% for all SUs, 73% for call-inhibited).
Two-alternative choice behavior
All behavioral studies were conducted inside an 8′-2″ × 10′-6″ double wall anechoic chamber (IAC, Bronx, NY) under dim red light. Animals were tested on an elevated W-maze with the following dimensions (refer to later Fig. 6 for a schematic of the maze): 35.5 cm from ground, 80 cm length, 3.5 cm high walls, 33 cm between the long edge at the base of the W to nest in the y-direction (e.g. perpendicular to the base of the W), and 30 cm from long edge to the end of the open arms. Speakers were located 60 cm away from the nest depression. Two servo-controlled doors (Hitec HS-7950TH, Poway, CA) 24.5 cm long and 13.5 cm high were hinged on stands located 18 cm from the maze in the x-direction (e.g. along the base of the W), and 5 cm from the maze in the y-direction. The doors were controlled remotely using variable duration digital pulses from a System 3 RX5-2 (Tucker Davis Technologies) module, allowing the user to control the position of the doors in real time.
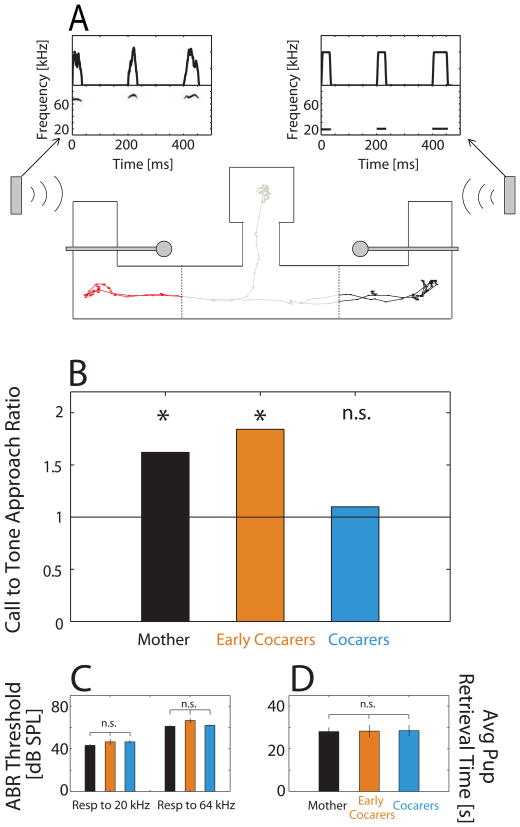
Figure 6.
Maternal physiological state during pup experience contributes to long-term retention of pup call salience. (A) Schematic top view of the W-maze used in two-alternative tests of pup call recognition. Vertical gray boxes represent speakers playing back either behaviorally irrelevant ~20 kHz pure tones (right inset) or~64 kHz pup calls (left inset), depicted as spectrograms (bottom row) and amplitude waveforms (top row). Thin horizontal boxes represent servo-controlled doors positioned in the open configuration. Thin line shows an animal’s actual center-of-mass movement in the center region (gray), past the left arm threshold (red) and past the right arm threshold (black). This animal’s first trajectory is towards the pup call speaker before turning around and proceeding towards the tone speaker. Dots along the trace are spaced 0.33 s apart, and the entire trace represents the first 150 seconds of an experiment. Dashed vertical lines indicate the decision thresholds. A video of another trial from a Mother engaged in the choice behavior (pup calls emitted from the left speaker) can be found at ≪LINK to mom_pupcallatleftspkr.mpg≫. (B) Mothers approached the pup call speaker significantly more often than the tone speaker (47 call:29 tone approaches), as did Early Cocarers (35 call:19 tone). Cocarers did not show a preferential approach to either side (33 call: 30 tone). Black line represents chance. Differences between animal groups were not attributable to (C) ABR hearing thresholds, since these did not differ significantly across groups at either 20 or 64 kHz; or (D) ability to retrieve pups, since average latencies to retrieve also did not differ significantly.
Mothers, Cocarers, and Early Cocarers were tested over three consecutive days with four pups aged P6–P8 days postpartum. For Mothers and Cocarers, the four pups came from a donor litter since the Mother’s pups had been weaned, while for Early Cocarers, the four pups came from the litter in which they were coca ring. Prior to each test day, 70% ethanol was used to wipe down the elevated W-maze, and clean Alpha-Dri bedding was added. The mouse was then placed onto the W-maze with four pups in the nest area for a one hour habituation. During this time, the experimenter remained outside the anechoic chamber while the mouse was habituated to the environment and sound of the servo-controlled doors. Periodically, the experimenter would enter the chamber and displace pups from the nest to motivate retrieval behavior. The number of times this occurred per animal varied, but each animal would perform at least two retrievals prior to the test, with the goal of getting the mouse to retrieve pups in the maze consistently.
Following habituation on each day, two sound preference tests were performed, spaced approximately 1 hour apart. Prior to each sound test, we motivated the mouse by again scattering pups and eliciting a retrieval response, following the successful protocol in earlier behavioral studies of mouse phonotaxis (Ehret and Haack, 1982). Two pups were placed on the left and right sides of the nest depression at equivalent distances to avoid a left-right bias, and the mouse was monitored using the TopScan video tracking software (Cleversys, Reston, VA). If during this test, a female mouse did not retrieve within 10 minutes, the mouse was excluded from further testing of sound preference. For mice that did retrieve, we allowed them to explore the W-maze post-retrieval, and waited until she had returned to the nest for at least 30 seconds before closing the servo-controlled doors. Once the doors closed, two pups were removed from the nest, and then the two-alternative choice test began. We played back bouts of pup calls from a speaker (randomly chosen) positioned on one side of the maze, alternating with a speaker placed on the opposite side playing back behaviorally irrelevant pure tones centered around 20 kHz (see Acoustic Stimulation, above). The animal was kept in the nest by the doors to ensure that it listened to the laterally presented sounds before making a choice. We remotely opened the doors after approximately 30 seconds of sound playback, and scored whether the mouse moved towards the pup call or tone speaker by crossing a threshold of 18 cm from the center of the maze. Once that threshold was crossed, both speakers were switched off, and full access to the ends of the W arms was prevented by the repositioned doors. These steps reduced the likelihood that animals would learn that no real pup was the source of the sounds. Using a W-maze instead of a straight running board (Ehret and Haack, 1982) helped minimize the chance that animals would simply move reflexively towards the speaker they happened to be pointing at when the doors opened. In total, we collected data from at most six pup retrieval and sound preference tests for each animal.
Hearing tests in behavioral animals
Auditory Brainstem Responses (ABRs) were measured only on those animals used in the behavioral tests to confirm each animal’s threshold of hearing for different tone frequencies. Animals were anesthetized using a mixture of Ketamine and Xylazine (initial dose of 4 parts 100 mg/kg Ketamine : 1 part 5 mg/kg Xylazine, maintained subsequently by 6 parts Ketamine : 1 part Xylazine). Once the animal showed no reflexive response to a light toe pinch, it was placed onto a Styrofoam bed with its right ear 11 cm from a speaker on the vibration-isolation table in the electrophysiology rig. Subdermal needles connected to a RA4LI headstage (Tucker Davis Technologies) were used to record the ABR with the ground placed ventral lateral to the left external pinna, the reference electrode ventral lateral to the right external pinna, and the recording electrode at the vertex of the skull. The brainstem signals were recorded at 25 kS/s and band pass filtered from 100 Hz to 3 kHz. Stimuli were played 500 times presented at a rate of 19 Hz and in 5 dB steps to obtain an averaged ABR for a specific tone frequency at different amplitude levels. The threshold was then defined as the lowest amplitude level that could evoke an auditory response.
Statistical analysis
To compare SU responses, parametric and non-parametric statistical tests were used. Parametric tests (ANOVA) were used if all groups were normally distributed and did not statistically differ in their variances. If any of the groups violated normality, the Ansari-Bradley was used to test for equality of variances. For the non-parametric analysis of variance test we used the Kruskal-Wallis, for paired recordings the Wilcoxon Sign Rank test, and for unpaired recordings the Mann-Whitney U test. If the ANOVA or Kruskal-Wallis test returned p<0.05, post-hoc group comparisons were tested using Fisher’s least significant difference criterion set at the 0.05 level.
For the two-alternative choice behavior, we used a binomial test to investigate whether the proportion of pup call responses differs from chance level (50%). For testing spontaneous rates, we performed a square root transformation of the data, since this has often been used to convert neural activity with a Poisson distribution to a normal distribution (Baker et al., 2002; Prince et al., 2002; Ogawa and Komatsu, 2004; Hayden and Gallant, 2005). This transformation is often applied when the variances of the data are proportional to the means, and is often applied to data when the samples are taken from a Poisson distribution (Zar, 1999).
Results
Long-term inhibitory plasticity is not simply a result of experience
Our starting point was our previous finding that core auditory cortical SUs from awake Mothers and Virgin mice do not show systematic differences in simple measures of pup call-evoked excitation, but do differ significantly in call-evoked lateral-band inhibition (Galindo-Leon et al., 2009). The question addressed here is whether the differences in evoked inhibition are related to extrinsic experience raising pups or intrinsic (e.g. physiological state) changes associated with motherhood. We recorded SUs from the core auditory fields of 13 Mothers, 14 Cocarers, and 14 Virgins. In all, we obtained 322 SUs from core auditory fields, including both pup call-responsive and nonresponsive SUs. Call-inhibited responses (Fig. 1), accounted for 36/114 (32%) SUs in Mothers, 22/100 (22%) in Cocarers, and 27/108 (25%) in Virgins, which we found were not statistically different in their proportions (Pearson χ2 (2, N=322) = 2.7, p > 0.05).
Based on our earlier study (Galindo-Leon et al., 2009), we targeted call-inhibited SUs in lateral-band fields, A1 and AAF. To assign recording sites to auditory fields, we relied on the LFP BF, since call-inhibited SUs did not always have excitatory tonal tuning curves. At a coarse spatial scale across the second row of our stereotaxically laid recording grid (see (Galindo-Leon et al., 2009)), we typically found the expected tonotopic reversal between A1 and AAF (Stiebler et al., 1997) in the second or third column (Fig. 3), corresponding to 60–70% of the distance from Bregma to Lambda. In the top recording row, holes caudal to this reversal point were often associated with either A1 or the ambiguous border between AAF/A1. The most rostral two holes usually contained sites with high BF’s above 40 kHz, corresponding to ultrasound field UF. Occasionally, a more caudally located hole in either row would show a break from the tonotopic gradient with a much higher frequency BF, and/or a much weaker relative LFP response, suggestive of non-core fields. SUs from non-core areas were excluded from all data analyses.
Figure 3.
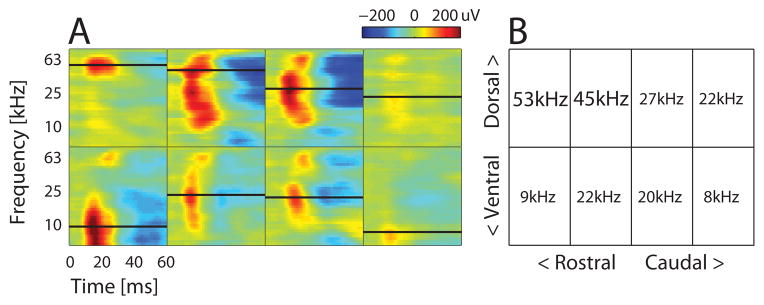
Coarse BF map across stereotaxically targeted grid of holes covering auditory cortex. (A) Time course of LFP amplitudes across all frequencies at each hole (box). Negative LFP deflections are shown as red; positive deflections as blue. Color limits fixed to the maximum and minimum values across the entire map. Black lines in each box indicate the LFP BF for that recording hole, also shown numerically in the grid in (B). High ultrasound BFs in the dorsal-rostral quadrant are from likely UF sites. Note the tonotopic reversal in the second row between AAF to A1.
The distribution of LFP BFs around call-inhibited SUs in A1 and AAF was similar across animal groups (ANOVA, F (2,53) = 1.05, p = 0.36, Fig. 4A), allowing us to compare call-inhibited responses without this potential selection bias. We pooled each SU’s responses to 18 different pup ultrasounds, since unlike call-excited SUs, call-inhibited SUs tended to be inhibited in a similar fashion across calls (Fig. ID). Each SU’s overall PSTH duration and depth of inhibition were then computed. Confirming our earlier result (Galindo-Leon et al., 2009) with a much larger superset of lateral-band SUs, Mothers had significantly greater duration and strength (normalized spike count closer to 0) of lateral-band inhibition when compared to Virgins (Fig. 4B–D). Cocarers were intermediate for duration – not significantly different from either Mothers or Virgins (Fig. 4D) – and much more like Virgins for inhibition strength, where they were significantly different from Mothers (Fig. 4C). These results are evident in the two-dimensional summary plot of the normalized spike count and duration data for each SU, which shows the Cocarer’s 95% confidence region overlapping mainly with the Virgin rather than Mother group (Fig. 4E). Although Fig. 4A shows a slightly greater proportion lower LFP BFs in the Virgin, our inhibitory results did not stem from these differences as we found no significant correlations between the call-inhibited measures and the BF (CCstrength = −0.09, p = 0.47, CCduration = 0.09, p = 0.49). In UF, there was neither an LFP BF (Kruskal-Wallis, χ2 (2,26) = 1.29, p = 0.53), nor an inhibition duration (Kruskal-Wallis, χ2(2,26) = 3.9, p = 0.14) nor inhibition strength (ANOVAN, F(2,26) = 1.6, p = 0.22) difference across animal groups. Hence, consistent with our earlier finding, the most robust changes in call-evoked inhibition arose in AAF and A1, auditory fields with BFs lateral to the frequency of ultrasonic pup calls. More importantly, the Cocarer data indicate that pup experience alone does not explain why post-weaning Mothers differed from Virgins.
Figure 4.
Cocarers were more similar to Virgins than Mothers in their call-inhibited SU responses in lateral-band fields A1 and AAF. (A) Mothers (black), Cocarers (blue), and Virgins (gray) did not differ in their cumulative distribution of LFP BFs for co-recorded call-inhibited SU in A1 and AAF. (B) Population-averaged normalized (by the spontaneous rate) spike rate for call-inhibited SUs. (C) Mothers had a significantly lower normalized evoked spike count (Kruskal-Wallis, χ2 (2,53) = 10.24, p = 0.006, Fisher’s LSD post hoc: M-V, *; M-C, *; C-V, n.s.). (D) Mothers showed significantly longer durations of call-evoked inhibition compared to Virgins, with Cocarers intermediate (Kruskal-Wallis, χ2 (2,53) = 8.06, p = 0.018, Fisher’s LSD post hoc: M-V, *; M-C, n.s.; C-V, n.s.). (E) Combined normalized spike count and duration plot illustrating variability in the characteristics of individual call-inhibited SUs (dots), yet clear separability in the responses of Mothers. Contours represent the 95% confidence regions around the mean (plus) normalized spike count and duration, estimated by bootstrap. NMothers = 21 SUs, NCocarers = 18 SUs, NVirgins = 17 SUs. For this figure and all subsequent figures, an asterisk indicates significance at the 0.05 level for the Fisher’s LSD post hoc test.
Maternal physiological state improves retention of cortical inhibitory plasticity
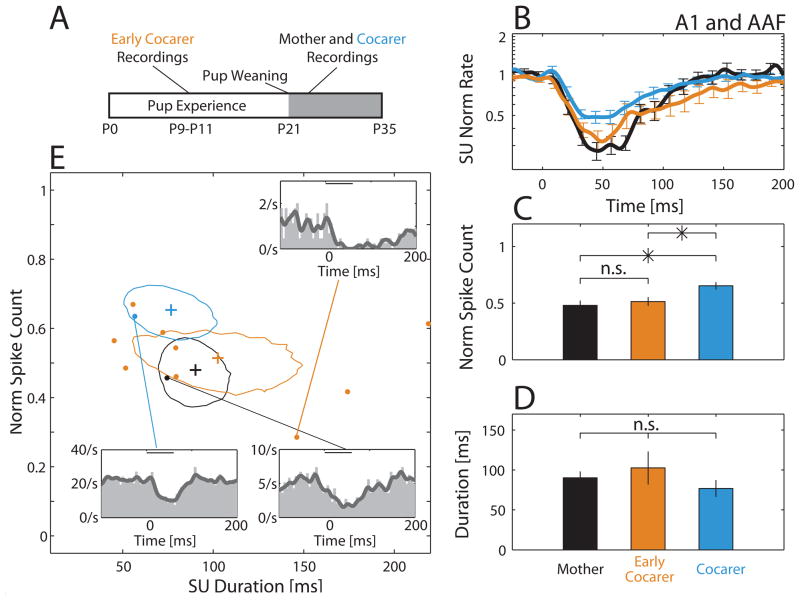
Given the principle of experience-dependent sensory plasticity for behaviorally relevant stimuli, the differences found between Cocarers and Mothers was unexpected. Previous studies have shown that virgin females given 5 days of pup experience by coca ring with a mother behaviorally prefer pup vocalizations over a neutral sound (Ehret et al., 1987), indicating that such cocarers indeed find calls behaviorally relevant. Interestingly though, call recognition behavior reportedly deteriorates in ovariectomized cocarers (but not mothers) when tested one month after pup weaning (Ehret and Koch, 1989). Given this, we wondered whether the similarity between Cocarers and Virgins was because experience never induced cortical plasticity, or if neural changes occurred early during pup experience and later decayed. This motivated follow-up experiments on coca ring virgins during an early stage of their experience with pups to see if they would be more like Mothers. Below, we refer to such animals as Early Cocarers, and reserve as the term, Cocarers, for virgins that had a full term of pup experience (21 days) before post-weaning recordings (see Experimental Procedures).
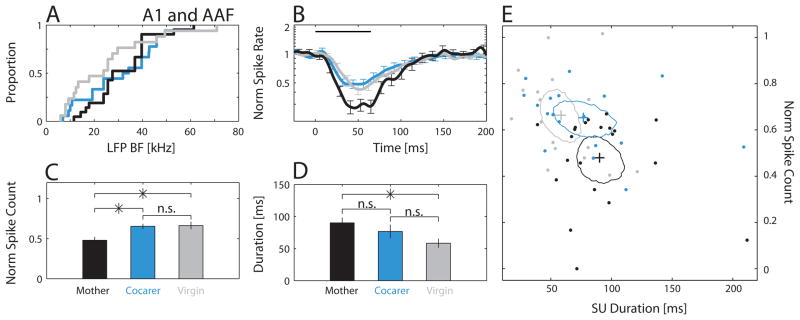
Early Cocarers acquired pup experience through post-parturition day P6, around the peak in pup vocal activity (Haack et al., 1983), before being isolated for surgery and electrophysiology. Chronic recording sessions began on the third day post-surgery and continued for 3 days (Fig. 5A) in six animals, yielding 45 SUs, 14/45 of which were call-inhibited (31%) in core auditory fields, 9/14 of which were designated as A1 and/or AAF sites. Using the same measures as in Fig. 4, we found that Early Cocarers were similar to Mothers but significantly different from Cocarers in their average call-inhibited SU PSTH (Fig. 5B) and strength of inhibition (Fig. 5C). The average duration of inhibition was comparable across the three groups (Fig. 5D). Interestingly though, there was noticeably more variability in the inhibition duration in the Early Cocarer group (note its larger standard error in Fig. 5D), mainly because some SUs had incredibly long durations (Fig. 5E). An example of an Early Cocarer SU with unusually long inhibitory duration is shown in the top right inset of Fig. 5E, along with examples from a Mother and a Cocarer. The three extremely long duration Early Cocarer SUs were found in two different individuals, suggesting that this was not just a case of individual variability. More generally, our call-inhibited SUs were well distributed across subjects, even for groups with small sample sizes. For each group, we found at least one call-inhibited A1 and/or AAF SU in 57% or more of that group’s animals (Early Cocarers: 4/6 animals; Cocarers: 8/14 animals; Virgins: 8/14 animals; Mothers: 11/13 animals). The average number of call-inhibited A1 and/or AAF SUs per animal with such SUs was also not significantly different across groups (Early Cocarers: 1.5±1.4; Cocarers: 1.3±1.5; Virgins: 1.2±1.4; Mothers: 1.8±1.4; Kruskal-Wallis, χ2 (3,43) = 1.72, p = 0.63).
Figure 5.
Early Cocarers were more similar to post-weaning Mothers than Cocarers in their call-inhibited SU responses in lateral-band fields A1 and AAF. (A) Time line showing when Early Cocarers (orange) were recorded compared to post-weaning Mothers (black) and Cocarers (blue). (B) Both Mothers and Early Cocarers showed stronger averaged call-inhibited SU responses when compared to Cocarers. (C) Mothers and Early Cocarers had significantly lower normalized spike counts when compared to Cocarers (Kruskal-Wallis, χ2 (2,45) = 10.34, p = 0.006, Fisher’s LSD post hoc: M-C, *; M-EC, n.s.; C-EC, *). (D) Although both Mothers and Early Cocarers had mean durations higher than Cocarers, the groups were not significantly different (Kruskal-Wallis, χ2 (2,45) = 2.6, p = 0.27). (E) Combined normalized spike count and duration plot illustrating variability in the characteristics of individual call-inhibited SUs (dots). Contours represent the 95% confidence regions around the mean (plus) normalized spike count and duration, estimated by bootstrap. The confidence region for Early Cocarers substantially overlapped with that for Mothers. Insets depict PSTH responses to all 18 calls for three different example SUs taken from each of the three animal groups. NMothers = 21 SUs, NCocarers = 18 SUs, NEarly Cocarers = 9 SUs.
Hence, our data suggest that very recent pup care experience in Early Cocarers can produce measures of call-evoked inhibition similar to Mothers – and in individual cases even more exaggerated than Mothers. However, unlike Mothers that still show significant differences from Virgins after weaning pups, the plasticity in Early Cocarers apparently decays to the Virgin level as pup experience fades into the past after weaning. Finally, given this decay in the cocaring virgin groups, we also assessed whether Mothers might show a decay in inhibition characteristics depending on the time since weaning pups, but did not find correlations that reached significance (CCduration of −0.27, p = 0.11; CCstrength of 0.20, p = 0.24).
Maternal physiological state improves long-term salience of pup calls
The results described above would predict that if the observed lateral-band auditory cortical inhibitory plasticity were to contribute to behavioral recognition of pup calls, then Mothers and Early Cocarers, but not Cocarers after pup weaning, should at least show a preference for calls. To test the call-evoked behavioral response in these groups, we implemented a semi-automated two-alternative choice phonotaxis task in a W-maze (Fig. 6A; see Experimental Procedures for details). We recorded a total of 81 experiments from 14 Mothers, 74 experiments from 13 Cocarers, and 60 experiments from 10 Early Cocarers.
Similar to previous studies (Ehret and Haack, 1982; Uematsu et al., 2007), we evaluated the number of approaches towards the pup call speaker in each animal group. We found that Mothers (Binomial Test z = 2.06, p = 0.05, 2-tailed) and Early Cocarers (Binomial Test, z = 2.2, p = 0.04, 2-tailed) approached the pup call speaker 1.62x and 1.84x more frequently, respectively, than the tone speaker. In contrast, the ratio for Cocarers was 1.10x, which was no different than chance (Binomial Test, z = 0.38, p = 0.80, 2-tailed, Fig. 6B). Additionally, at the individual animal level, 8/14 (57%) Mothers and 6/10 (60%) Early Cocarers performed the call recognition task above chance, while only 4/13 (31%) Cocarers performed better than chance. In fact, there were more experiments when Cocarers did not even respond to sound playback (15%), compared to Mothers (6.2%) and Early Cocarers (10%). Hence, the behavioral difference in pup call approach by Mothers and Early Cocarers versus Cocarers appears to mirror the difference in lateral-band SU inhibitory plasticity found between these animal groups. This supports the possibility that the cortical plasticity contributes to the perception of the calls as behaviorally relevant.
In reaching this conclusion, additional controls ruled out two potential intrinsic factors that could have acted during the test itself to produce behavioral differences. First, an animal’s threshold for hearing the 60–80 kHz pup calls versus the 20 kHz tones could affect both its proportion of approaches and exploration times. However, we found no interaction effect between animal group and auditory brainstem response thresholds for 64 kHz and 20 kHz tones (repeated measures ANOVA, F(2,31) = 0.82, p > 0.05; NMothers=14; NCocarers=13; NEarly cocarers=10, Fig. 6C). Second, call recognition behavior might be correlated with the animal’s motivation to retrieve pups in our test apparatus, or with its familiarity with the pups (i.e. familiar pups for Early Cocarers vs. foster pups for Mothers and Cocarers). However, we found that the average amount of time an animal took to bring each pup back to the nest during the retrieval test was no different across groups (Kruskal-Wallis, χ2 (2,211) = 0.18, p = 0.91; NMothers=81; NCocarers=74; NEarly cocarers=59, Fig. 6D), suggesting similar levels of maternal responsiveness to live pups. This is consistent with previous reports of pup retrieval latencies by mothers and pup-sensitized virgins (Stolzenberg and Rissman, 2011).
Maternal physiological state correlates with reduced spontaneous activity selectively for call-responsive SUs
What aspects of one’s intrinsic physiological state might contribute mechanistically to maintaining adaptive neural plasticity in mothers? The maternal hormonal milieu during pup experience (Fleming and Sarker, 1990) is an obvious candidate. While a systematic dissection of how individual hormones affect neural coding is beyond the scope of the current work, the above results motivated us to explore potential functional targets on which hormones might act to preserve plasticity in mothers. In particular, spontaneous neural firing is detrimental to the preservation of synaptic plasticity (Zhou et al., 2003; Li et al., 2009), and reproductive hormones have been found to modulate spontaneous neural firing in brain areas such as the amygdala (Schiess et al., 1988), cerebellum (Smith et al., 1988), and barrel cortex (Kisley and Gerstein, 2001). This led us to test whether there were any differences across our animal groups in auditory cortical spontaneous firing rates. Across all core SUs recorded (Fig. 7A), we found no mean differences between groups (ANOVA, F (3,348) = 1.7, p = 0.17). Intriguingly though, by separating SUs according to whether they were call-responsive or not, we found an overall main effect of call-responsiveness (ANOVA, F(1,344) = 35.7, p = 5.7e-9, Fig. 7B1) and a significant interaction between call-responsiveness and animal group (ANOVA, F(3,344) = 4.7, p = 0.003). The main effect indicates that on average, SUs that were call-responsive were more likely to have higher spontaneous firing rates, perhaps because these SUs are closer to threshold and more likely to fire spontaneously and respond to stimuli. However, the interaction indicates that this effect was not the same across all animal groups. In Mothers, post-hoc tests showed that spontaneous rates for call-responsive SUs were similar to those of call-nonresponsive SUs and significantly lower than those of all three virgin groups (Fig. 7B2, left bars). On the other hand, spontaneous rates of call-responsive SUs in Virgins, Cocarers and Early Cocarers were not different from each other. Moreover, spontaneous rates for call-nonresponsive SUs were not significantly different across all groups (Fig. 7B2, right bars).
Figure 7.
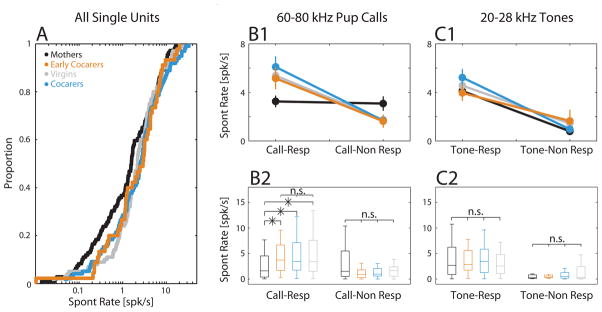
Suppressed spontaneous firing specifically in call-responsive SUs in Mothers. (A) Cumulative distribution of the spontaneous firing rate across all core auditory cortical SUs. The average spontaneous rate did not differ across animal groups. SUs that were call-responsive generally had higher spontaneous activity (B1) than call-nonresponsive SUs. However, (B1) there was a significant interaction between animal group and call-responsiveness, which post-hoc tests showed (B2) was due to a significantly suppressed spontaneous rate in call-responsive SUs only in Mothers compared to Early Cocarers, Cocarers and Virgins, which were not different from each other. Box plots indicate median, 25th and 75th percentiles, with whiskers extending to the most extreme data points not considered outliers. Call-responsive: NMothers = 72 SUs, NCocarers = 68 SUs, NEarly Cocarers = 30 SUs, NVirgins = 59 SUs (Fisher’s LSD post hoc: M-V/M-C/M-EC, *; V-C/V-EC/C-EC, n.s.). Call non-responsive: NMothers = 37 SUs, NVirgins = 41 SUs, NCocarers = 30 SUs, NEarly Cocarers = 15 SUs (Fisher’s LSD post hoc: M-V/M-C/M-EC/V-C/V-EC/C-EC, n.s.). (C1, C2) Classifying SUs instead by responsiveness to 20–28kHz tones (4 tones total), all groups showed a higher spontaneous firing for tone-responsive versus tone-nonresponsive SUs. Tone-responsive: NMothers = 69 SUs, NVirgins = 77 SUs, NCocarers = 67 SUs, NEarly Cocarers = 27 SUs. For tone-nonresponsive: NMothers = 29 SUs, NVirgins = 19 SUs, NCocarers = 27 SUs, NEarly Cocarers = 16 SUs.
Did the decrease in spontaneous activity among call-responsive SUs in Mothers generalize to responsiveness for behaviorally irrelevant sounds? We reclassified SUs depending on whether they were responsive or not to pure tones covering an equivalent logarithmic frequency range as our pup calls, namely 20–28 kHz. Figures 7C1–2 show that while there was again a significant main effect of stimulus-responsiveness (ANOVA, F(1,323) = 62.5, p = 4.3e-14), there was now no significant interaction between group and tone-responsiveness (ANOVA, F(3,323) = 0.29, p = 0.83). Importantly, unlike for call-responsiveness, the relationship between tone-responsiveness and spontaneous rate in Mothers was the same as in other animal groups (Fig. 7C2). Hence, the spontaneous firing rates of SUs involved in call coding were selectively suppressed in Mothers.
Discussion
Understanding how extrinsic experience may interact with intrinsic physiological states to generate sensory plasticity at the level of the cortex is essential to elucidating the nature of our individualized memories. Using behavioral experiments and electrophysiology in awake head-restrained mice to investigate a cortical model of adaptive maternal plasticity for infant cues, we found that a mother’s physiological state and not just her experience rearing pups helps to preserve both the long-term behavioral salience of and auditory cortical inhibitory plasticity elicited by infant vocalizations. This plasticity manifested in the depth and duration of call-evoked SU inhibition. While consistent with our earlier study in Mothers (Galindo-Leon et al., 2009), the result here was derived directly from single units (rather than using local field potentials) in the A1 and AAF auditory cortical fields that represent frequencies “lateral” to those of the ultrasound calls. Our results further demonstrate that pup experience alone in Early Cocarers could produce immediate behavioral and cortical changes. However, these changes were not retained in Cocarers in the weeks following weaning, unlike in Mothers recorded after weaning. Interestingly, Mothers were the only pup-experienced animals to show a selective suppression (relative to pup-naïve Virgins) in spontaneous firing, specifically in neurons involved in coding ultrasound calls. This hints at the possibility that intrinsic cellular excitability might be regulated by hormonal history, and that this could help preserve a long-term memory trace of behaviorally salient communication vocalizations in the core auditory cortex.
The form of experience-dependent cortical plasticity examined here involved stimulus-evoked inhibition, rather than topographic map expansion or excitatory receptive field plasticity, both of which were previously demonstrated for somatosensory stimulation in a maternal context (Xerri et al., 1994; Rosselet et al., 2006). “Inhibition” here referred to the pure reduction in stimulus-locked action potential firing relative to spontaneous firing, so that SUs labeled “call-inhibited” contained no obvious excitatory component in their spiking. We used this term instead of “call-suppressed,” since “suppression” has already been applied in the auditory field to refer to the reduction in sound-driven excitatory responses (Atiani et al., 2009; Otazu et al., 2009; Pienkowski and Eggermont, 2010). Whether plasticity in the strength of inhibitory synaptic potentials (Dorm et al., 2010; Maffei, 2011) provides a mechanism for this response type would need to be tested in the future with intracellular recordings. The alternative possibility that vocalizations produced a sudden decrease in excitatory input cannot yet be ruled out. However, it is notable that a recent study investigating multisensory modulation by pup odors of sound-evoked auditory cortical firing in anesthetized mice found that inhibitory interneurons indeed fired more robustly in Mothers and Early Cocarers (Cohen et al., 2011).
Why would experience produce a greater reduction in the firing of some cortical neurons to a behaviorally relevant stimulus, and how would this serve the perceptual processing of the sounds? In interpreting this, we note that the strongest inhibitory changes were found in the so-called “lateral-band” core auditory fields where sites are tuned to lower frequency sounds than the 60–80 kHz ultrasonic pup calls. We have hypothesized that this reduces activity in neurons whose firing would represent interfering background sounds, thus improving the population level neural activity contrast evoked by ultrasonic calls (Galindo-Leon et al., 2009). This fits with electrophysiological results from awake ferrets trained to detect a target tone in noise in a conditioned avoidance paradigm (Atiani et al., 2009): if the target frequency was far away from the BF of a recorded neuron, then its activity was on average suppressed compared to those with BFs near the target. Interestingly, the harder the detection task as background noise increased, the greater the observed suppression. The magnitude of spiking suppression reported was not large (average -17% for hardest task), yet comparable to the relative decrease we observed in normalized spike count between Virgins and Mothers (average -23%). Those authors attributed their results to selective attention, although general engagement in an auditory task engagement has also been reported to produce a similar magnitude of suppressed auditory cortical firing (Otazu et al., 2009).
It may be then that the salient vocalizations in our paradigm engage attentional or arousal mechanisms in animals that recognize calls, which would explain why the same animal groups showing the strongest lateral band inhibitory plasticity (Mothers and Early Cocarers) also exhibit the strongest behavioral preference for pup calls. Moreover, our behavioral results in Early Cocarers confirm that pup experience by itself can imbue pup vocalizations with behavioral relevance. Incidentally, even though the pups used in testing Early Cocarers were familiar rather than novel (as for Mothers and Cocarers, see Experimental Procedures), there was no difference between groups for retrieving actual pups, suggesting the level of maternal responsiveness for all groups was similar. Nevertheless, the contrast between Mothers and Cocarers after pups are weaned suggests that a Mother’s distinct physiological profile associated with pregnancy, parturition and/or lactation during pup experience may be needed for the continued salience of pup calls after pup care ends.
Our electrophysiological finding that the inhibitory changes in Early Cocarers fade away after pups were weaned (measured in Cocarers) was unexpected for two reasons. The first comes from a cortical plasticity perspective. It was recently discovered that even “passive exposure” of adult animals to sounds continuously can induce a persistent suppression of auditory cortical activity evoked by those sounds, possibly as homeostatic compensation for increased afferent activity (Norena et al., 2006; Pienkowski et al., 2011). In our paradigm, cocaring mice hear ultrasonic calls over the ~2 week period of heightened vocalizing during rearing (Haack et al., 1983), so some long-term change seemed likely. More importantly, cocaring virgins actively respond to calling pups and provide care, so the calls’ behavioral relevance in addition to their prevalence was expected to foster lasting auditory cortical plasticity, as reported in cases of auditory classical conditioning (Weinberger et al., 1993). Nevertheless, these factors did not translate after pups were weaned into the same sustained inhibitory plasticity in Cocarers as seen in Mothers, as would have been expected if sound experience and behavioral relevance per se were sufficient for long-term plasticity in adults. It is possible that something about the quality of the experience with ultrasound vocalizing pups might be different between mothers and cocarers, even though the duration of pup experience and hearing calls was the same. This is difficult to assess, but we did check that our Mothers, Cocarers and Early Cocarers were equivalently effective at retrieving ultrasonically vocalizing, scattered pups during an explicit pup retrieval experience on P5–P6 (see Experimental Procedures). Whether the quality of maternal responsiveness subsequently changes and how that might correlate with auditory cortical activity on an individual animal basis during behavioral call recognition would best be studied by implanting animals with chronic electrodes for recording during behavior – a future direction. This could be done in both cocaring females and mothers, and tracked well after weaning to determine whether any decay in call-evoked inhibition might occur later on for mothers.
The second reason for surprise comes from the maternal behavior perspective. On the one hand, maternal hormonal levels during initial pup experience accelerate subsequent maternal responsiveness, indicating a long-term maternal memory (Fleming and Sarker, 1990; Scanlan et al., 2006) whose basis has been attributed to subcortical mechanisms (Li and Fleming, 2003; Scanlan et al., 2006). On the other hand, recent evidence in mice suggests that sufficient initial experience with pups by itself can also establish a long-term memory of prior maternal behavior gained in the absence of reproductive hormones. Ovariectomized virgin mice with just 2 hours/day of pup experience over 4 days display shorter latencies than pup-naïve virgins for live pup retrieval 1 month later (Stolzenberg and Rissman, 2011). Why then did our phonotaxis tests of approach towards played back calls not reveal retention of maternal memory in pup-experienced, post-weaning Cocarers? We speculate that traditional tests of maternal responsiveness wherein animals fully interact with pups using all their senses engage maternal memories differently than our modality-specific test focused just on the auditory recognition of pup calls. The latter simply asks whether the acoustic cues alone are sufficient to elicit recognition and a preferred approach and thus narrowly focuses on the question of the calls’ acoustic “attractiveness,” without the reinforcing pup cues from other modalities (Cohen et al., 2011) that would be present during physical pup retrieval. The lack of behavioral preference for calls in Cocarers 1–2 weeks post-weaning in our study, and in a previous study performed 1 month post-weaning (Ehret and Koch, 1989), would therefore indicate that behavioral salience of calls alone decays over time if pup experience is gained outside the maternal physiological state. The correlated decay in call-evoked cortical inhibition observed between Early Cocarers and Cocarers then implicates core sensory cortex, often overlooked in maternal behavior studies, in the maternal memory for the salience of a unimodal pup cue. This auditory cortical activity – which our awake, head-restrained studies assess – would then feed into the maternal motivation circuit along with signals from other sensory modalities processing pup cues (e.g. olfaction) to drive behavioral responses.
How might the maternal physiological state promote long-term preservation of pup call salience and cortical plasticity? During pregnancy, parturition, and lactation, mothers undergo wide changes in hormones and neuromodulators such as estrogen, progesterone, prolactin, oxytocin, and dopamine, all of which may contribute to experience-dependent sensory plasticity for infant cues (Miranda and Liu, 2009). Indeed, a recent fMRI study in human mothers supports the idea that auditory cortical processing of baby cries depends on the history of hormones during infant experience (Swain et al., 2008). Specifically, in contrasting 2–4 week post-partum responses to the cries of a mother’s own versus a stranger’s baby, delivery by cesarean section (which avoids vagino-cervical stimulation and associated neurohormonal release known to improve the acquisition of maternal behavior) produced less significant activation in the superior temporal gyrus near Wernicke’s Area compared to mothers that deliver vaginally. In fact, hormones like estrogens can directly modulate neural activity within the auditory system itself, at least in songbirds (Tremere et al., 2009; Remage-Healey et al., 2010; Tremere and Pinaud, 2011) and possibly mice (Charitidi and Canlon, 2010). For this reason, we chose not to record from lactating mothers here so that the hormonal status would be more similar across animal groups. Lactating mothers could have even greater call-evoked differences relative to Early Cocarers or Mothers recorded after weaning (Cohen et al., 2011), potentially reflecting hormone-dependent processes operating during pup rearing that would help cement improved neural processing of salient stimuli (Maney et al., 2006; Tremere et al., 2012).
Regardless of the precise neurochemical origins, our discovery that spontaneous activity specifically in call-responsive cortical neurons was suppressed in Mothers compared to the various virgin groups suggests a possible target through which such mechanisms might act to retain the salience of calls. Suppression of spontaneous rate has previously been implicated in plasticity maintenance and long-term memory, although not at the cortical level. In the developing optic tectum of Xenopus, spontaneous activity rapidly reversed induced long-term potentiation (Zhou et al., 2003). This attests to the detrimental effects of spontaneous firing for maintaining synaptic plasticity. Presumably, higher spontaneous rates would produce faster decays, an idea that has been validated through modeling. A simulation of conditioned fear memory in the lateral amygdala found that increased spontaneous firing resulted in a faster decay of plasticity; those authors hypothesized that low spontaneous firing acts to preserve fear memory through decreased Hebbian weakening (Li et al., 2009). Our data validated this in vivo through electrophysiological recordings of cortical neurons in awake animals. Whether and how maternal modulation of specific neurochemicals might act in conjunction with infant experience to produce selective changes in the spontaneous activity of neurons encoding infant calls would need to be explored further. Furthermore, how these auditory cortical changes interact with subcortical circuits driving maternal responsiveness (Numan, 2006) also requires future investigation. Nevertheless, the current results provide a clear demonstration that intrinsic physiological state during pup care, and not just the extrinsic experience alone, is required for long-term core auditory cortical inhibitory plasticity for salient pup calls in the maternal context.
Supplementary Material
Research highlights.
Recent infant care experience can alter sensory cortical response to infant calls
Infant care experience alone is insufficient for long-term salience of calls
Mom’s physiological state during infant care maintains call salience and plasticity
Spontaneous firing of call-encoding neurons is selectively suppressed in moms
Acknowledgments
The authors thank NIH NIDCD (008343) and NSF CBN (IBN-9876754) for funding, and acknowledge the contributions of Tatsuya Oishi and Maria Luisa Scattoni in the design and testing of an earlier version of our two-choice behavioral apparatus. We also thank K.N. Shepard and S. Banerjee for comments on the manuscript.
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CI, Behrmann M, Olson CR. Impact of learning on representation of parts and wholes in monkey inferotemporal cortex. Nat Neurosci. 2002;5:1210–1216. doi: 10.1038/nn960. [DOI] [PubMed] [Google Scholar]
- Balcetis E, Dunning D. See what you want to see: Motivational influences on visual perception. Journal of Personality and Social Psychology. 2006;91:612–625. doi: 10.1037/0022-3514.91.4.612. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nat Neurosci. 2010;13:361–368. doi: 10.1038/nn.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS. A Quantitative Analysis of the Roles of Dosage, Sequence, and Duration of Estradiol and Progesterone Exposure in the Regulation of Maternal Behavior in the Rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Canlon B. Estrogen receptors in the central auditory system of male and female mice. Neuroscience. 2010;165:923–933. doi: 10.1016/j.neuroscience.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Cohen L, Rothschild G, Mizrahi A. Multisensory integration of natural odors and sounds in the auditory cortex. Neuron. 2011;72:357–369. doi: 10.1016/j.neuron.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. J Neurophysiol. 1996;75:1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Haack B. Ultrasound recognition in house mice: key-stimulus configuration and recognition mechanism. J Comp Physiol [A] 1982;148:245–251. [Google Scholar]
- Ehret G, Koch M. Ultrasound-Induced Parental Behavior in House Mice Is Controlled by Female Sex-Hormones and Parental Experience. Ethology. 1989;80:81–93. [Google Scholar]
- Ehret G, Koch M, Haack B, Markl H. Sex and parental experience determine the onset of an instinctive behavior in mice. Naturwissenschaften. 1987;74:47. doi: 10.1007/BF00367047. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Sarker J. Experience-hormone interactions and maternal behavior in rats. Physiol Behav. 1990;47:1165–1173. doi: 10.1016/0031-9384(90)90368-e. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M. Plasticity in the maternal circuit: Effects of maternal experience on Fos-lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience. 1996;110:567–582. doi: 10.1037//0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M. Rat Pups Are Potent Reinforcers to the Maternal Animal - Effects of Experience, Parity, Hormones, and Dopamine Function. Psychobiology. 1994;22:44–53. [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory Plasticity in a Lateral Band Improves Cortical Detection of Natural Vocalizations. Neuron. 2009;62:705–716. doi: 10.1016/j.neuron.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman R. Gonadal hormones and sensory function. Neuroscience & Biobehavioral Reviews. 1983;7:1–17. doi: 10.1016/0149-7634(83)90003-9. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Haack B, Markl H, Ehret G. Sound communication between parents and offspring. In: Willott JF, editor. The auditory psychobiology of the mouse. Springfield, IL: Charles C. Thomas; 1983. pp. 57–97. [Google Scholar]
- Hackett TA, Barkat TR, O’Brien BM, Hensch TK, Polley DB. Linking topography to tonotopy in the mouse auditory thalamocortical circuit. J Neurosci. 2011;31:2983–2995. doi: 10.1523/JNEUROSCI.5333-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Gallant JL. Time course of attention reveals different mechanisms for spatial and feature-based attention in area V4. Neuron. 2005;47:637–643. doi: 10.1016/j.neuron.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Matthews A, Gross C, Mappus RC, Gollnick C, Swanson A, Bassell GJ, Liu RC. Arc/Arg3.1 mRNA expression reveals a sub-cellular trace of prior sound exposure in adult primary auditory cortex. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The Plasticity of Human Maternal Brain: Longitudinal Changes in Brain Anatomy During the Early Postpartum Period. Behavioral Neuroscience. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, McLearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kisley MA, Gerstein GL. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 2001;13:1993–2003. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Lambert KG, Berry AE, Griffins G, Amory-Meyers E, Madonia-Lomas L, Love G, Kinsley CH. Pup exposure differentially enhances foraging ability in primiparous and nulliparous rats. Physiol Behav. 2005;84:799–806. doi: 10.1016/j.physbeh.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003;117:426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- Lin FG, Liu RC. Subset of thin spike cortical neurons preserve the peripheral encoding of stimulus onsets. J Neurophysiol. 2010;104:3588–3599. doi: 10.1152/jn.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory Cortical Detection and Discrimination Correlates with Communicative Significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Liu RC, Miller KD, Merzenich MM, Schreiner CE. Acoustic variability and distinguishability among mouse ultrasound vocalizations. J Acoust Soc Am. 2003;114:3412–3422. doi: 10.1121/1.1623787. [DOI] [PubMed] [Google Scholar]
- Maffei A. The many forms and functions of long term plasticity at GABAergic synapses. Neural Plast. 2011;2011:254724. doi: 10.1155/2011/254724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J, Ferando I, Simonsen C, Mody I. Excitability Changes Related to GABA(A) Receptor Plasticity during Pregnancy. Journal of Neuroscience. 2009;29:9592–9601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Cho E, Goode CT. Estrogen-dependent selectivity of genomic responses to birdsong. Eur J Neurosci. 2006;23:1523–1529. doi: 10.1111/j.1460-9568.2006.04673.x. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: hormones and experience in a maternal context. Hear Res. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norena AJ, Gourevitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Komatsu H. Target selection in area V4 during a multidimensional visual search task. J Neurosci. 2004;24:6371–6382. doi: 10.1523/JNEUROSCI.0569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci. 2009;12:646–654. doi: 10.1038/nn.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas SL, Wenner P, Gonzalez-Islas C, Fagiolini M, Razak KA, Kim G, Sanes D, Roerig B. Developmental plasticity of inhibitory circuitry. J Neurosci. 2006;26:10358–10361. doi: 10.1523/JNEUROSCI.3516-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlee MB. Menstrual rhythm in sensory processes: A review of fluctuations in vision, olfaction, audition, taste, and touch. Psychological Bulletin. 1983;93:539–548. [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Eggermont JJ. Passive exposure of adult cats to moderate-level tone pip ensembles differentially decreases AI and All responsiveness in the exposure frequency range. Hear Res. 2010;268:151–162. doi: 10.1016/j.heares.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Munguia R, Eggermont JJ. Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hear Res. 2011;277:117–126. doi: 10.1016/j.heares.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci U S A. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SJ, Pointon AD, Cumming BG, Parker AJ. Quantitative analysis of the responses of V1 neurons to horizontal disparity in dynamic random-dot stereograms. J Neurophysiol. 2002;87:191–208. doi: 10.1152/jn.00465.2000. [DOI] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosselet C, Zennou-Azogui Y, Xerri C. Nursing-induced somatosensory cortex plasticity: temporally decoupled changes in neuronal receptive field properties are accompanied by modifications in activity-dependent protein expression. J Neurosci. 2006;26:10667–10676. doi: 10.1523/JNEUROSCI.3253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat Neurosci. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlan VF, Byrnes EM, Bridges RS. Reproductive experience and activation of maternal memory. Behav Neurosci. 2006;120:676–686. doi: 10.1037/0735-7044.120.3.676. [DOI] [PubMed] [Google Scholar]
- Schiess MC, Joels M, Shinnick-Gallagher P. Estrogen priming affects active membrane properties of medial amygdala neurons. Brain Res. 1988;440:380–385. doi: 10.1016/0006-8993(88)91012-8. [DOI] [PubMed] [Google Scholar]
- Schnupp JW, Hall TM, Kokelaar RF, Ahmed B. Plasticity of temporal pattern codes for vocalization stimuli in primary auditory cortex. J Neurosci. 2006;26:4785–4795. doi: 10.1523/JNEUROSCI.4330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–282. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy A, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature. 2000;404:187–190. doi: 10.1038/35004588. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol [A] 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–354. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supèr H, van der Togt C, Spekreijse H, Lamme VAF. Internal State of Monkey Primary Visual Cortex (V1) Predicts Figure–Ground Perception. The Journal of Neuroscience. 2003;23:3407–3414. doi: 10.1523/JNEUROSCI.23-08-03407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkel J, Rosenblatt JS. Humoral factors underlying maternal behavior at parturition: Cross transfusion between freely moving rats. Journal of Comparative and Physiological Psychology. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- Tremere LA, Pinaud R. Brain-generated estradiol drives long-term optimization of auditory coding to enhance the discrimination of communication signals. J Neurosci. 2011;31:3271–3289. doi: 10.1523/JNEUROSCI.4355-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Jeong JK, Pinaud R. Estradiol Shapes Auditory Processing in the Adult Brain by Regulating Inhibitory Transmission and Plasticity-Associated Gene Expression. J Neurosci. 2009;29:5949–5963. doi: 10.1523/JNEUROSCI.0774-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremere LA, Kovaleski RF, Burrows K, Jeong JK, Pinaud R. Mechanistic Basis and Functional Roles of Long-Term Plasticity in Auditory Neurons Induced by a Brain-Generated Estrogen. Journal of Neuroscience. 2012 doi: 10.1523/JNEUROSCI.3233-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Uematsu A, Kikusui T, Kihara T, Harada T, Kato M, Nakano K, Murakami O, Koshida N, Takeuchi Y, Mori Y. Maternal approaches to pup ultrasonic vocalizations produced by a nanocrystalline silicon thermo-acoustic emitter. Brain Research. 2007;1163:91–99. doi: 10.1016/j.brainres.2007.05.056. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci U S A. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin MA, Maffei A, editors. Inhibitory Synaptic Plasticity. New York: Springer; 2011. [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 4. New Jersey: Prentice-Hall; 1999. [Google Scholar]
- Zhou Q, Tao HW, Poo MM. Reversal and stabilization of synaptic modifications in a developing visual system. Science. 2003;300:1953–1957. doi: 10.1126/science.1082212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.