Abstract
Background
Drug experimentation during adolescence is associated with increased risk of drug addiction relative to any other age group. To further our understanding on the neurobiology underlying such liability, we investigate how early adolescent cocaine experience impacts the overall medial prefrontal cortex (mPFC) network function in adulthood.
Methods
A non-contingent administration paradigm was used to assess the impact of early adolescent cocaine treatment (rats; postnatal days -PD- 35-40) on the overall inhibitory regulation of mPFC activity in adulthood (PD65-75) by means of histochemical and in vivo electrophysiological measures combined with pharmacological manipulations.
Results
Cocaine exposure during early adolescence yields a distinctive hyper-metabolic PFC state that was not observed in adult (PD75-80)-treated rats. Local field potential recordings expand upon these findings by showing that early adolescent cocaine exposure is associated with an attenuation of mPFC GABAergic inhibition evoked by ventral hippocampal stimulation at beta and gamma frequencies that endures throughout adulthood. Such cocaine-induced mPFC disinhibition was not observed in adult-exposed animals. Furthermore, the normal developmental upregulation of parvalbumin immunoreactivity observed in the mPFC from PD35 to PD65 is lacking following early adolescent cocaine treatment.
Conclusion
Our data indicate that repeated cocaine exposure during early adolescence can elicit a state of mPFC disinhibition resulting from a functional impairment of the local prefrontal GABAergic network that endures through adulthood. A lack of acquisition of prefrontal GABAergic function during adolescence could trigger long-term deficits in the mPFC that may increase the susceptibility for the onset of substance abuse and related psychiatric disorders.
Keywords: adolescence, prefrontal cortex, GABA, cocaine, electrophysiology, parvalbumin
Introduction
Adolescence is a vulnerable period not only for the onset of major psychiatric disorders such as schizophrenia and depression, but also for drug use and abuse (1–3). For instance, drug experimentation during this developmental stage is associated with increased risk of drug addiction relative to any other age group (2, 4, 5). Although the neurobiology underlying such vulnerability is not well understood, it has been proposed that drug exposure during adolescence may elicit maladaptive modifications within the mesocorticolimbic-prefrontal cortex (PFC) loop (1–3, 6), which is known to mediate a variety of addiction-related behaviors including enhanced drug-cue associations and drug-seeking (7–10). Importantly, the PFC continues to undergo major structural and functional changes throughout the adolescent transition to adulthood (11–15). Repeated drug exposure during this critical period could therefore interfere with key neurodevelopmental processes required for normal prefrontal maturation, a disruption that could endure through adulthood and trigger long-lasting impairments in the PFC.
At the cellular level, the maturation of PFC activity during adolescence is dependent on the remodeling of local inhibitory circuits by the influence of glutamatergic inputs from the ventral hippocampus and the recruitment of dopamine facilitatory action on GABAergic interneurons (16, 17). Among the different subsets of GABAergic interneurons in the PFC, the parvalbumin (PV)-positive/fast-spiking cells play an important role in determining the timing and spatial selectivity of pyramidal cell firing (18). Thus, the acquisition of an increased prefrontal GABAergic function during the normal adolescent period (19, 20) is thought to be associated with the maturation of cognitive abilities such as working memory, decision making and impulse control (21, 22). Consequently, a developmental impairment of GABAergic transmission in the PFC is expected to trigger prefrontal disinhibition and deficits in PFC-dependent functions as revealed by studies from animal models (17, 23–25). Here, we sought to determine whether a disinhibited PFC would emerge following repeated non-contingent cocaine exposure during adolescence, and if such a disruption results from a developmental alteration of the local GABAergic network. The non-contingent administration paradigm was chosen to determine the pharmacological action of cocaine. We assessed the impact of early adolescent cocaine treatment on the overall inhibitory regulation of medial PFC (mPFC) activity in adulthood by means of histochemical measures and in vivo electrophysiological local field potential recordings combined with pharmacological manipulations. Specifically, changes in medial prefrontal processing of frequency-dependent facilitation and depression of local field potential responses exerted by ventral hippocampal stimulation were compared across age and treatment groups. As shown recently (26), this approach is suitable for assessing changes in mPFC network responses resulting from a developmental disruption of local GABAergic transmission.
Materials and Methods
All experiments were carried out according to the USPHS Guide for Care and Use of Laboratory Animals, and were approved by the Rosalind Franklin University IACUC. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) except for Indiplon, which was obtained from Tocris Bioscience (Ellisville, MO). Different age groups of male Sprague-Dawley rats (Harlan, Indianapolis, IN) were used. They were group housed (2–3 rats/cage) under conditions of constant temperature (21–23°C) and humidity in a 12:12 hour light/dark cycle with food and water available ad libitum. All animals were allowed to acclimate for at least 5 days before receiving any experimental manipulation.
Experimental groups
Early adolescent (postnatal day -PD- 35) rats were randomly assigned to receive daily single non-contingent (home cage) injections of cocaine (20 mg/kg in saline, i.p.) or saline for 5 consecutive days. All histochemical and electrophysiological measures were conducted within PD65-75. To determine if the effects of cocaine are age dependent, prefrontal changes in adult (PD75)-treated rats following an equivalent duration of exposure and washout period (25–35 days, PD105-115) were compared to those from the early adolescent-treated group. To control for the age of testing, electrophysiological recordings from another cohort of early adolescent-treated rats were conducted within the PD105-115 range.
Cytochrome oxidase histochemistry and densitometry
Brain metabolic activity was determined by means of cytochrome oxidase staining using a previously reported protocol (Supplement 1) (27, 28). A single value per structure per animal was obtained by averaging measurements from at least 3 sections per rostrocaudal level.
Medial PFC local field potentials evoked by ventral hippocampal stimulation in vivo
All recordings were conducted following a previously reported protocol (Supplement 1) (26). Briefly, ventral hippocampal train stimulation at 10, 20 and 40 Hz were used to assess whether the altered mPFC network response could result from a developmental disruption of local GABAergic transmission (26). Furthermore, changes in prefrontal local field potential responses following ventral hippocampal high-frequency stimulation (50 pulses at 100Hz/15s x 4) were examined to determine whether a history of early adolescent cocaine exposure is sufficient to alter plasticity within the hippocampal-mPFC pathway. It is well known that changes in synaptic plasticity within the ventral hippocampal-prefrontal pathway can be assessed in vivo by means of local field potentials (29).
Local prefrontal microinfusions of picrotoxin and Indiplon
The experimental procedure for local microinfusions of picrotoxin (GABA-A receptor antagonist) and Indiplon (GABA-A receptor positive allosteric modulator) was conducted using a 28-gauge cannula (Plastics One Inc., Roanoke, VA) secured to the mPFC recording electrode, as previously described (28). The cannula was filled with aCSF-containing vehicle, picrotoxin (503M/0.1% DMSO) or Indiplon (53M/0.02% DMSO). All microinfusions (1μl) were performed at 0.13l/min and changes in mPFC local field potential responses were assessed within the 35 min post-injection period.
Immunohistochemical analyses of parvalbumin immunofluorescence
See Supplement 1 for details. Briefly, coronal sections of 50μm-thick containing the mPFC were mounted on Superfrost Plus slides (VWR, Batavia, IL), dried under the hood for 20 min, and subsequently treated with 1% glycine in 0.1M PBS. Sections were then permeabilized and blocked using 5% normal donkey serum, 1% BSA and 0.2% TX-100 (in 0.1M PBS). After rinsing, the mounted sections were incubated with rabbit anti-parvalbumin antibody (1:2000, Swant, Switzerland) for 18 hours followed by 2 hour incubation in donkey anti-rabbit IgG Alexa488-labeled antibody (1:500; Life Technologies). Digital images corresponding to prelimbic, infralimbic, and forceps minor (white matter) regions were obtained using a Nikon E400 microscope with a 10X objective connected to an ORCA-AG deep cooled digital camera (Hamamatsu, Bridgewater, NJ). The final mean fluorescence intensity of the prelimbic and infralimbic areas was corrected by subtracting the background fluorescence signal from the forceps minor using ImageJ (http://rsb.info.nih.gov/ij/). A single value per structure per animal was obtained by averaging mean fluorescence intensity from 3 mPFC sections within the 2.7mm anterior to bregma.
Statistical analysis
Data are expressed as mean ± SEM. Differences among experimental groups were considered statistically significant at p<0.05. Changes in frontal cortical cytochrome oxidase staining following cocaine exposure were assessed by two-way ANOVA (age x region). A similar two-way ANOVA design was used to determine changes in prefrontal local field potential responses across treatment conditions and age groups (treatment x pulse number or age x pulse number). Within each experimental group (age or treatment), the one-way ANOVA design was used to assess the pattern of the evoked local field potential at a given frequency of train stimulation. Similarly, changes in mPFC parvalbumin immunoreactivity following cocaine exposure were determined by one-way ANOVA.
Results
We first investigated the effects of repeated cocaine treatment on frontal cortical metabolic activity by means of cytochrome oxidase I (CO-I) histochemistry. Relative changes (to the saline group; see Table S1 and Figure S1 in Supplement 1 for details) in CO-I staining were assessed by densitometry from 3 rostrocaudal levels of the frontal cortex (Fig 1A). Regional analyses revealed an age x region effect following repeated cocaine injection (Fig. 1B). In particular, cocaine exposure during early adolescence elicited a distinct elevation of CO-I staining in the rostral level of the adult frontal cortex (bregma +2.7) whereas an overall downregulation of CO-I staining was observed in the adult-treated group across the 3 rostrocaudal levels of the frontal cortex. Further analyses indicate that metabolic activity in all PFC areas (i.e., frontal cortex at bregma +2.7) became elevated in adulthood following adolescent cocaine exposure (Fig 1C). Together, these results indicate that the postnatal age at which the drug exposure occurs determines the distinctive pattern of metabolic activity observed in the PFC.
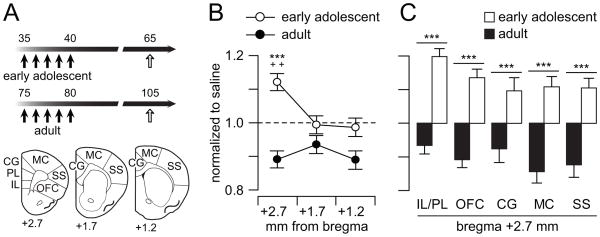
Figure 1.
Cocaine exposure induces age and region specific metabolic changes in the frontal cortex. (A) Diagram illustrating the experimental design. Five non-contingent injections of saline or cocaine were performed in early adolescent (PD35-40) and adult (PD75-80) rats. Metabolic changes in the frontal cortex (3 rostrocaudal levels: +2.7, +1.7, +1.2 mm from bregma) were assessed by means of cytochrome oxidase (CO-I) histochemistry on PD65 in the adolescent-treated group and PD105 in the adult-treated group. (B) Regional analyses revealed that cocaine exposure during early adolescence elicited a distinct metabolic activity elevation in the rostral frontal cortex (i.e., bregma +2.7), whereas an overall downregulation of CO-I staining was observed in adult-treated animals (n=10–12 per group; main age effect F(1,66)=26.29, p<0.00001; age x region interaction F(2,66)= 4.32, p=0.017; ***p<0.0005 vs. adult, ++p<0.005 vs. bregma +1.7 and +1.2, LSD post-hoc test after significant two-way ANOVA). (C) CO-I staining across different areas in the PFC (i.e., rostral frontal cortex, bregma +2.7). Two-way ANOVA analysis revealed that metabolic activity in all PFC areas becomes elevated when cocaine exposure occurred during early adolescence. In contrast, an opposite pattern of CO-I staining in all PFC areas was observed in the adult-treated group (main age effect F(1,110)=137.2, p<0.00001; ***p<0.0005 vs. adult, LSD post-hoc).
We next examined whether the increased CO-I staining observed in the PFC following a history of early adolescent cocaine treatment is associated with a hyperactive PFC state by means of electrophysiological measures in vivo. Medial PFC (mPFC: infralimbic and prelimbic regions) local field potential (LFP) responses to ventral hippocampal stimulation (Fig 2A) were compared between saline- and cocaine-treated animals. All electrophysiological measures were collected within the PD65-75 age period (i.e., 25–35 days from the last saline or cocaine injection; Fig 2B) following the same non-contingent treatment protocol used to assess CO-I levels. We observed that the amplitude of prefrontal LFP responses elicited by increasing current intensities of single hippocampal stimulation becomes enhanced following early adolescent exposure to cocaine (Fig 2C). Such increase LFP response was lack in the caudal level of the frontal cortex where the CO staining remained unchanged (Figure S2 in Supplement 1). Furthermore, no apparent changes were found in the mPFC of adult-treated animals (Fig 2D), indicating that the hyper-prefrontal response observed following early adolescent cocaine is age dependent.
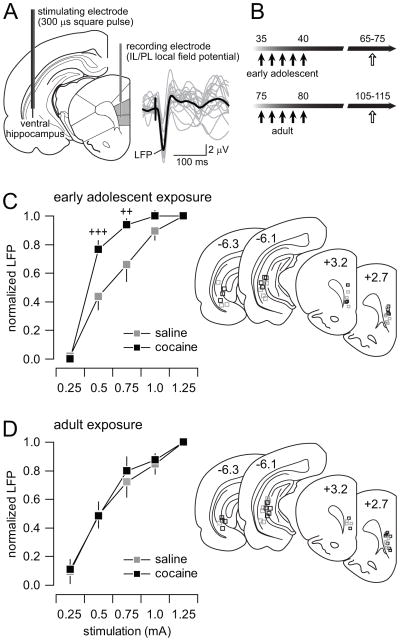
Figure 2.
Adolescent cocaine exposure enhances the mPFC response to ventral hippocampal stimulation. (A) Recording arrangement employed to study the hippocampal-to-prefrontal cortex transmission in vivo. Traces are examples of hippocampal stimulation-induced local field potential (LFP) responses recorded in the mPFC. (B) Diagram summarizing the timeline of the experimental design employed to study the enduring electrophysiological changes (white arrows) following non-contingent cocaine exposure (black arrows). (C) Ventral hippocampal-induced LFP responses in the PFC at different current intensities revealed a significant facilitation of the evoked response only in the adolescent-treated rats (n=9 per group; main treatment effect F(1,60)=15.06, p=0.00026; main intensity effect F(4,60)=99.68, p=0.00001; treatment x intensity interaction F(4,60)=3.86, p=0.00747; ++p<0.005, +++p<0.0005 vs. saline, LSD post-hoc test after significant two-way ANOVA). (D) No apparent changes were observed in the adult-treated groups (n=9 per group).
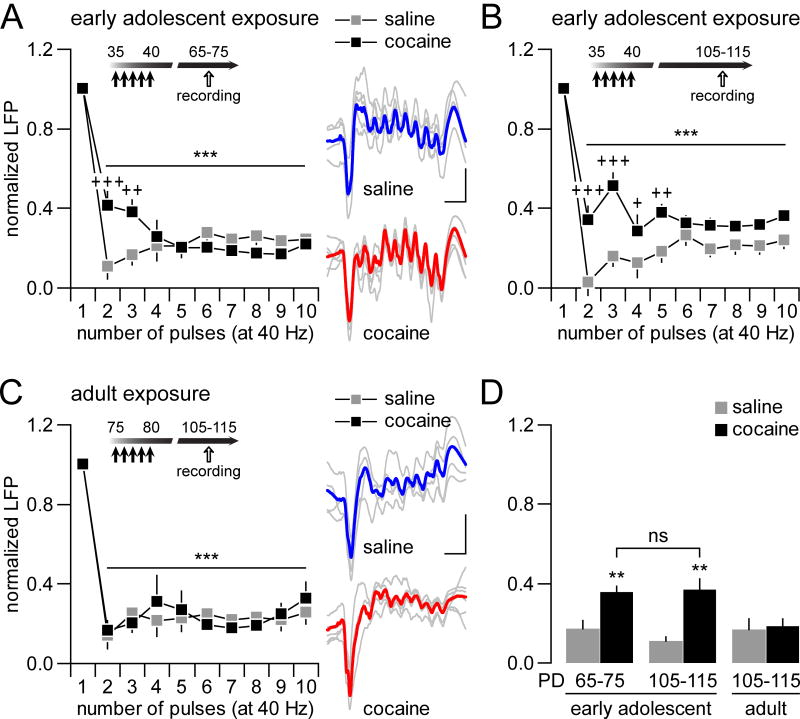
To further assess how adolescent exposure to cocaine impacts mPFC processing of ventral hippocampal inputs, changes in hippocampal train stimulation-induced facilitation and depression of LFP responses were examined across age and treatment groups following the design described in Fig 2B. Typically, a sustained facilitation of mPFC LFP response emerges in response to hippocampal 10Hz train stimulation (Fig 3). We found no apparent disruption of such 10Hz-induced prefrontal facilitation following early adolescent (Fig 3A) or adult (Fig 3B) exposure to cocaine. At 20Hz, a transient attenuation of mPFC LFP response was observed in saline-treated animals when recorded within the PD65-75 and the PD105-115 age ranges (Fig 4A,B). Following early adolescent exposure to cocaine, such LFP inhibition is no longer detectable. Instead, a marked facilitation of LFP response to 20Hz ventral hippocampal stimulation emerged in the adult mPFC at both PD65-75 and PD105-115 (Fig 4A,B). In contrast, this abnormal facilitation was not observed in the mPFC of adult-treated animals since the pattern of the 20Hz-induced prefrontal response in cocaine-treated animals was indistinguishable from the saline controls (Fig 4C,D). Finally, at 40Hz, a distinct pattern of sustained suppression of prefrontal LFP responses was observed in both saline- and cocaine-treated animals (Fig 5). However, the magnitude of this inhibition was significantly reduced in the mPFC of PD65-75 and PD105-115 rats with a history of early adolescent cocaine (Fig 5A,B). Once again, cocaine exposure during adulthood did not disrupt the normal pattern of 40Hz-induced LFP depression in the mPFC (Fig 5C,D). In sum, these results indicate that ventral hippocampal stimulation-induced frequency-dependent inhibition of mPFC LFP responses are selectively diminished by early adolescent cocaine experience, a disruption that endures throughout adulthood from PD65 to PD115.
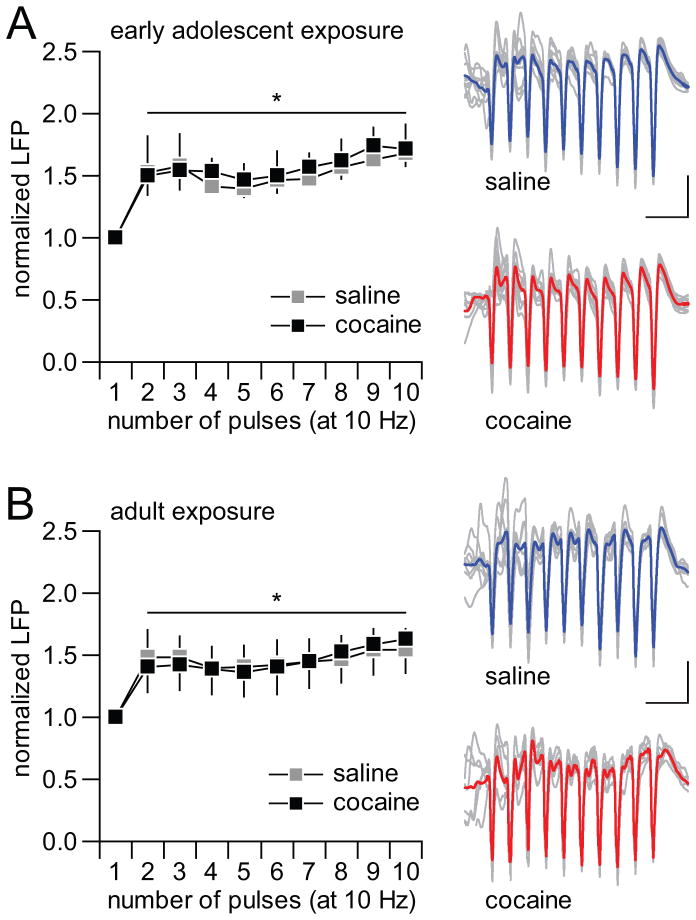
Figure 3.
Hippocampal-induced facilitation of LFP response in the mPFC is not affected by cocaine exposure. (A) All recordings were conducted within the PD65-75 age period, which corresponds to 25–35 days from the last saline or cocaine injection (see Fig 2B). Ventral hippocampal train stimulation at 10Hz typically induces a sustained facilitation of LFP responses in the mPFC. Both saline and cocaine-treated animals exhibited similar pattern of prefrontal responses to the 10Hz-train stimulation (n=9 per group; main pulse number effect F(9,160)=2.27, *p<0.022; no significant treatment effect or treatment x pulse number interaction, two-way ANOVA). (B) Similarly, adult-treated rats exhibited similar degrees of LFP facilitation in response to hippocampal train stimulation at 10Hz (n=9 per group; main pulse number effect F(9,160)=2.29, *p=0.019; no significant treatment or treatment x pulse number effects, two-way ANOVA).
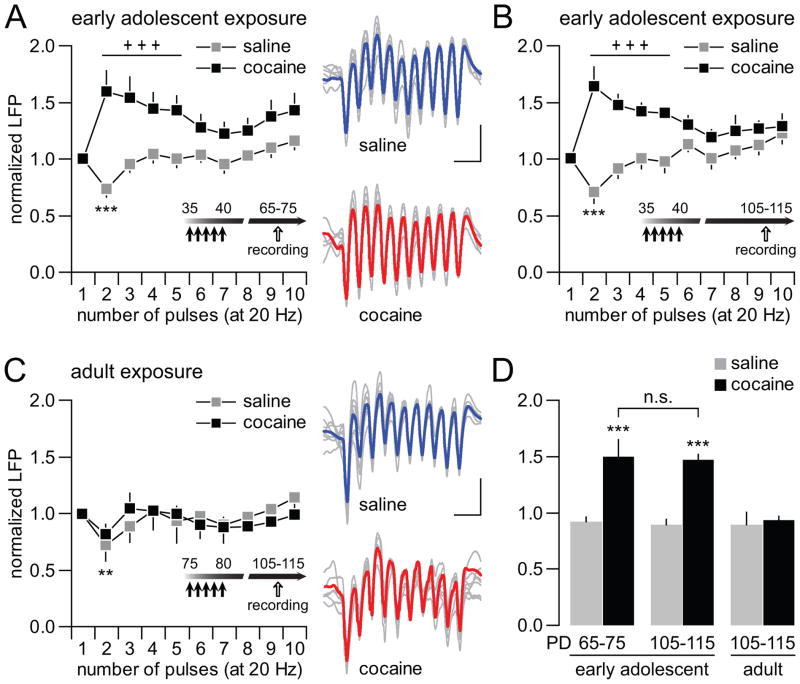
Figure 4.
Disruption of hippocampal-induced transient mPFC LFP inhibition by early adolescent cocaine exposure. (A) Hippocampal stimulation at 20Hz elicited a typical transient inhibition of the evoked LFP in the mPFC of saline-treated rats (n=9; ***p<0.0005 vs. 1st pulse, LSD post-hoc test after significant one-way ANOVA). Such transient LFP depression is lacking in the mPFC of early adolescent cocaine-treated rats (n=9). Instead, a facilitation of the evoked LFP response was observed in the mPFC (main treatment effect F(1,160)=57.95, p<0.00001; treatment x pulse number interaction F(4,160)= 2.56, p=0.0088; +++p<0.0005 vs. saline, LSD post-hoc test after significant two-way ANOVA). (B) Additional recordings revealed that early adolescent cocaine exposure-induced abnormal facilitation of mPFC LFP response at 20Hz is long-lasting and detectable at PD105-115 (n=6 per group; main treatment effect F(1,100)=62.30, p<0.00001; treatment x pulse number interaction F(9,100)=5.31, p<0.00001; +++p<0.0005 vs. saline, LSD post-hoc test after significant two-way ANOVA). In the saline-treated group, a typical transient inhibition of the evoked LFP was observed in the PD105-115 mPFC (***p<0.0005 vs. 1st pulse, LSD post-hoc test after significant one-way ANOVA). (C) In contrast, no apparent changes were observed when cocaine exposure occurred in adulthood (n=9 per group; no significant treatment effect or treatment x pulse number interaction, two-way ANOVA). Both saline- and cocaine-treated groups exhibited the characteristic transient LFP inhibition in the mPFC (**p<0.01 vs. 1st pulse, LSD post-hoc test after significant one-way ANOVA). (D) Bar graph summarizing the mean magnitude of ventral hippocampal-induced LFP responses in the mPFC across treatment and age groups (from pulses 2 to 5 as shown in A,C). All saline-treated animals exhibited similar degree of LFP response irrespectively of the age group. In contrast, a facilitation of the evoked LFP in the mPFC emerges only from animals that received cocaine during early adolescence (main effect of treatment F(1,42)=27.27, p<0.0001; treatment x age interaction F(2,42)=5.58, p=0.007; ***p<0.0005 vs. other age and treatment groups, LSD post-hoc test after significant two-way ANOVA).
Figure 5.
Impact of early adolescent exposure to cocaine on mPFC LFP inhibition following hippocampal train stimulation at 40Hz. (A) Stimulation of the ventral hippocampus at 40Hz elicits a marked suppression of the evoked LFP response in the mPFC of saline and cocaine-treated rats. However, a significant attenuation of the 40Hz-induced LFP suppression in the mPFC was observed in adult rats that were pre-exposed to cocaine during early adolescence (n=9 per group; main pulse number effect F(9,160)=57.52, ***p<0.0005; treatment x pulse number interaction F(9,160)=4.18, p=0.00007; +++p<0.005, ++p<0.005 vs. saline, LSD post-hoc test after significant two-way ANOVA). (B) Additional recordings revealed that early adolescent cocaine-induced attenuation of mPFC LFP inhibition at 40Hz is long-lasting and detectable at PD105-115 (n=6 per group; main pulse number effect F(9,100)=44.49, ***p<0.0005; treatment x pulse number interaction F(9,100)=2.37, p=0.018; +++p<0.005, ++p<0.005, +p<0.05 vs. saline, LSD post-hoc test after significant two-way ANOVA). (C) In contrast, cocaine exposure during adulthood does not disrupt the normal pattern of 40Hz-induced LFP depression (n=9 per group; main pulse number effect F(1,60)=35.24, ***p<0.0005; no significant treatment effect or treatment x pulse number interaction, two-way ANOVA). (D) Summary of ventral hippocampal-induced LFP inhibition in the mPFC across the different treatment and age groups as shown in A–C. A similar degree of mPFC LFP inhibition (mean values calculated from pulses 2 to 5) was observed in response to 40Hz stimulation across all saline groups. The magnitude of such LFP inhibition became markedly attenuated only if cocaine exposure occurred during early adolescence (main effect of treatment F(1,42)=16.25, p<0.0005; treatment x age interaction F(2,42)=4.01, p=0.026; **p<0.005 vs. other age and treatment groups, LSD post-hoc test after significant two-way ANOVA).
We next asked the question whether long-term plasticity within the ventral hippocampal-mPFC pathway is also impaired following a history of cocaine exposure during early adolescence. Thus, a protocol of high-frequency stimulation (HFS; 50 pulses at 100Hz/15s x 4) was applied into the ventral hippocampus to elicit long-term changes in transmission within the hippocampal→mPFC pathway (Fig 6). In saline controls, ventral hippocampal HFS is sufficient to elicit a modest (~20%), but reliable long-term depression (LTD) of the evoked LFP response in the mPFC. Interestingly, a shift from LTD to long-term potentiation (LTP) of the evoked response was observed in animals that received cocaine during early adolescence (Fig 6A). In contrast, neither the magnitude nor the duration of the HFS-induced LTD in the mPFC was affected by cocaine exposure during adulthood (Fig 6B), suggesting that the altered plasticity within the ventral hippocampal-mPFC pathway observed in the early adolescent-treated group is also age dependent (Fig 6C).
Figure 6.
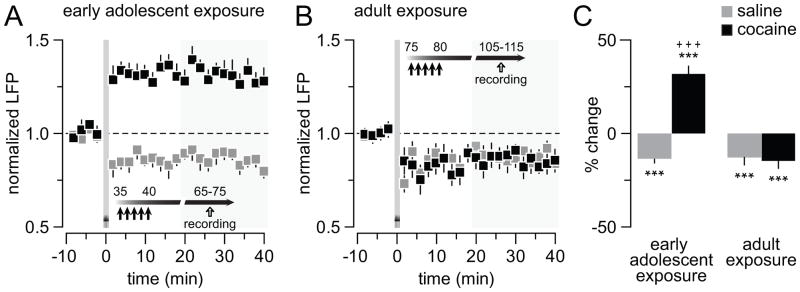
Early adolescent cocaine exposure shifts hippocampal (100Hz)-induced LTD to LTP of the evoked LFP response in the adult mPFC. (A) High-frequency (50 pulses at 100Hz/15 s x 4) stimulation of the ventral hippocampus elicits a characteristic LFP-LTD in the adult mPFC in the saline-treated group (n=8). However, such LTD becomes LTP when cocaine exposure occurred during early adolescence (n=9; +++p<0.0005 vs. saline, unpaired T-test, area marked in gray). (B) In contrast, hippocampal-induced prefrontal LFP-LTD is not affected by cocaine when drug exposure occurred during adulthood. The magnitude and duration of LFP-LTD observed in adult cocaine-treated animals (n=9) resembles that obtained in saline controls. (C) Bar graph summarizing the effects shown in A and B (***p<0.0005 vs. baseline, paired T-test). Two-way ANOVA revealed a treatment x age interaction (F(1,30)=37.87, p<0.0001). Note that mPFC LFP-LTP emerges only following a history of cocaine exposure during early adolescence (+++p<0.0005 vs. other age and treatment groups, LSD post-hoc test).
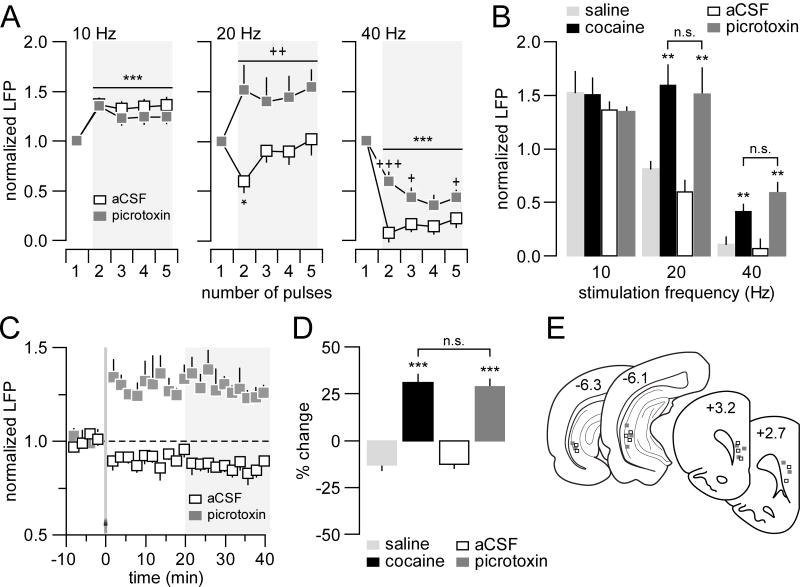
All the above electrophysiological observations point to a common age dependent mechanism underlying the onset of prefrontal disinhibition concurrent to the reduced inhibition of LFP responses within the hippocampal-mPFC pathway, and a switch from LTD to LTP following early adolescent cocaine treatment. Given the critical role of prefrontal GABAergic interneurons in the regulation of local inhibitory responses (19, 20), we assessed whether the disinhibitory mPFC state induced by early adolescent cocaine exposure can be mimicked by an acute prefrontal microinjection of the GABA-A antagonist picrotoxin in adult naïve animals (PD65-75). We found that this was indeed the case. While the pattern of 10Hz-induced prefrontal facilitation remained unaffected, a shift from attenuation to potentiation concurrent with an attenuation of depression of the evoked LFP response was observed following local picrotoxin administration at 20Hz and 40Hz, respectively (Fig 7A,B). Local picrotoxin also favored the abnormal prefrontal LTP over the normal LTD of LFP responses following ventral hippocampal HFS (Fig 7C,D). Thus, an intact prefrontal GABAergic transmission is required for sustaining such distinct frequency-dependent LFP inhibition in the adult mPFC in response to ventral hippocampal train stimulation.
Figure 7.
Medial prefrontal disinhibition induced by local microinjection of the GABA-A antagonist picrotoxin (50μM/0.8μl/8 min) in adult naïve rats. (A) Picrotoxin did not alter the characteristic prefrontal LFP facilitation in response to 10Hz hippocampal train stimulation (n=5 per group; main pulse number effect F(4,40)=9.97, ***p<0.0005; no significant treatment effect or treatment x pulse number interaction, two-way ANOVA). At 20Hz, a shift from the transient attenuation to sustained facilitation of the evoked LFP response was observed in the mPFC after local administration of picrotoxin (n=5 per group; main treatment effect F(1,40)=51.29, p=0.00001; treatment x pulse number interaction F(4,40)=4.17, p=0.0065; ++p<0.005 vs. aCSF, *p<0.05 vs. 1st pulse, LSD post-hoc test after significant two-way ANOVA). Similarly, the 40Hz hippocampal stimulation-induced suppression of LFP responses in the mPFC was markedly decreased after local picrotoxin administration (n=5 per group; main treatment effect F(1,40)=27.10, p<0.00005; main pulse number effect F(4,40)=36.39, ***p<0.0005; treatment x pulse number interaction F(4,40)=3.16, p=0.024; +++p<0.0005, +p<0.05 vs. aCSF, LSD post-hoc test after significant two-way ANOVA). (B) Bar graph summarizing the average magnitude of hippocampal-induced prefrontal LFP responses shown in A. For comparison, data obtained from early adolescent saline- and cocaine-treated groups were also included. Note that the magnitude of LFP facilitation at 20Hz and the level of LFP attenuation at 40 Hz observed after early adolescent cocaine are undistinguishable from those induced by local picrotoxin (**p<0.005 vs. saline or aCSF, Tukey post-hoc test after significant one-way ANOVA). (C) Local prefrontal microinjection of picrotoxin was sufficient to shift hippocampal HFS-induced LFP-LTD to LTP in the adult naïve mPFC. (D) Bar graph summarizing the effects shown in C. Data obtained from early adolescent saline- and cocaine-treated groups were included for comparison (***p<0.0005 vs. saline or aCSF, Tukey post-hoc test after significant one-way ANOVA). The magnitude of prefrontal LTP induced following acute local microinfusion of picrotoxin in control naïve adult rats resembles to that observed in the adult mPFC of early adolescent cocaine-exposed animals. (E) Diagram showing the location of both stimulating and recording sites for this cohort of experiments.
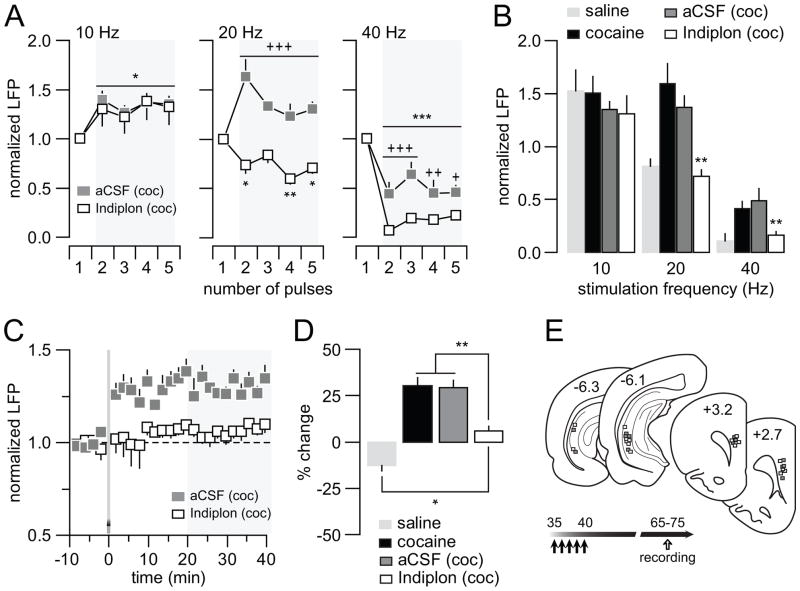
We next asked the question whether a facilitation of GABA-A receptor-mediated transmission in the mPFC is sufficient to normalize the abnormal prefrontal pattern of disinhibited LFP response induced by the repeated early adolescent cocaine treatment. To test this, we determined the effect of acute prefrontal infusion of the GABA-A receptor positive allosteric modulator Indiplon in adult rats (PD65-75 age range) that received repeated cocaine treatment during early adolescence. We found that Indiplon administration into the mPFC failed to alter the characteristic 10Hz-induced prefrontal LFP facilitation whereas the abnormal 20Hz-induced facilitation and the attenuated 40Hz-induced suppression of the evoked LFP responses were restored to saline control levels (Fig 8A,B). Similarly, the characteristic ventral hippocampal HFS-induced prefrontal LTP observed in the adult mPFC of early adolescent cocaine-exposed rats was no longer apparent following local prefrontal microinfusion of Indiplon (Fig 8C,D). These results indicate that a functional upregulation of GABA-A receptor-mediated transmission in the mPFC is sufficient to normalize the abnormal disinhibitory prefrontal state associated with early adolescent cocaine exposure.
Figure 8.
Reversal effects of the GABA-A α1 positive allosteric modulator Indiplon on the abnormal PFC LFP response recorded from early adolescent cocaine-treated rats in adulthood. (A) All recordings were conducted adult rats that received cocaine treatment during early adolescence as described in Fig 2. Relative to the aCSF group (n=6), local prefrontal infusion of Indiplon (10μM/0.8μl/8 min; n=8) did not change the pattern of mPFC LFP response to hippocampal 10Hz stimulation (main pulse number effect F(4,40)=9.97, ***p<0.0005; no significant treatment effect or treatment x pulse number interaction, two-way ANOVA). In contrast, the abnormal 20Hz-induced facilitation of mPFC LFP response observed in early adolescent cocaine-treated rats was not longer apparent following Indiplon infusion (main treatment effect F(1,60)=110.38, p<0.00005; treatment x pulse number interaction F(4,60)= 8.65, p<0.00005; +++p<0.005 vs. aCSF, *p<0.05/**p<0.005 vs. 1st pulse, LSD post-hoc test after significant two-way ANOVA). Similarly, a normalization of the attenuated 40Hz-induced suppression of mPFC LFP was also observed in the early adolescent cocaine-treated group following local prefrontal infusion of Indiplon (main treatment effect F(1,60)=37.49, p=0.00001; main pulse number effect F(4,60)=40.97, ***p<0.0005; treatment x pulse number interaction F(4,60)=3.15, p=0.021; +++p<0.0005, ++p<0.05, +p<0.05 vs. aCSF, LSD post-hoc test after significant two-way ANOVA). (B) Summary of the reversal effects of Indiplon shown in A. Data obtained from early adolescent saline- and cocaine-treated groups were also included for comparison (**p<0.005 vs. cocaine or cocaine + aCSF, Tukey post-hoc test after significant one-way ANOVA). (C) Consistent with the results shown in Fig 6, ventral hippocampal HFS typically elicits a prefrontal LFP-LTP in early adolescent cocaine-treated rats, which remains unaffected by local infusion of aCSF. In the presence of Indiplon, such HFS-induced LFP-LTP in the mPFC was not longer apparent. (D) Bar graph summarizing the effects of local infusion of aCSF and Indiplon shown in C. For comparison, data obtained from early adolescent saline- and cocaine-treated groups were included. Note that Indiplon significantly prevented the expression of prefrontal LFP-LTP, but failed to reverse it to the saline control LTD state (*p<0.05, **p<0.005, Tukey post-hoc test after significant one-way ANOVA). (E) Diagram showing the location of both stimulating and recording sites for this cohort of experiments.
Among the three distinctive subtypes of interneurons expressing Ca++-binding proteins in the PFC, a downregulation of the parvalbumin (PV)-positive fast-spiking interneuron function has been associated with the development of prefrontal disinhibition in animal models (21, 30). PV expression itself is developmentally regulated (31) and activity-dependent (32, 33). In addition, changes in Ca++-binding proteins can define the functional profile of interneurons, which in the case of PV reflects the function of fast-spiking interneurons (34). We therefore conducted PV immunohistochemical measures to determine the impact of early adolescent cocaine exposure on prefrontal PV-positive interneurons in adulthood. Consistent with our recent developmental study (31), we found that PV immunoreactivity in the adult mPFC of saline-treated rats is significantly higher than that from the juvenile mPFC (PD35) (Fig 9A). However, the elevation of PV immunoreactivity is lacking in the mPFC of adult rats (PD65-75) that received cocaine during early adolescence (Fig 9A). Interestingly, the number of PV-positive interneurons per mPFC section remained unchanged (Fig 9B). These results indicate that early adolescent cocaine-induced downregulation of prefrontal PV immunoreactivity cannot be explained by a change in the number of PV-positive interneurons in the mPFC. Instead, the reduced PV immunoreactivity observed in the mPFC suggests that cocaine exposure during early adolescence disrupts the functional maturation of fast-spiking interneurons as reflected by the lack of normative PV upregulation (31).
Figure 9.
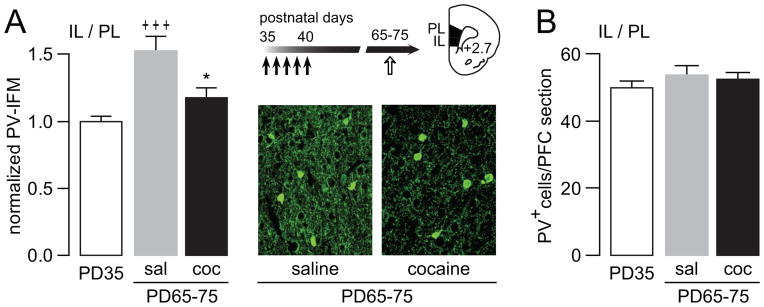
Developmental disruption of PV immunoreactivity in the mPFC by early adolescent exposure to cocaine. (A) Analysis of PV immunoreactivity in the medial PFC by measurement of mean fluorescent intensity (MFI). Relative to the PD35 age group (n=9), a significant increase in PV immunoreactivity was observed in the adult medial PFC (IL: infralimbic; PL: prelimbic) of saline (sal) treated rats (n=6). In contrast, PV immunoreactivity in the early adolescent cocaine-exposed group (coc, n=8) resembles that observed in juvenile animals (*p<0.05 vs. saline; +++p<0.0005 vs. PD 35, Tukey post-hoc test after significant ANOVA, F(2,20)=12.44, p=0.00031). Similar results were obtained when a gray matter control region (i.e., layer I) was used to correct the background signal instead of the white matter (see Figure S3 in Supplement 1). Insets are examples of PV immunofluorescence images (200X magnification) illustrating the reduced PV immunoreactivity observed in the adult mPFC of early adolescent cocaine-treated rats. (B) Bar graph summarizing the cell count of PV-positive (PV+) interneurons data obtained from the same mPFC section samples used in A. No significant changes in the number of PV-positive cells per mPFC section were observed across age and treatment groups.
Discussion
The aim of the present study was to determine how early adolescent cocaine experience impacts the overall mPFC network function in adulthood. We found that repeated non-contingent cocaine exposure during early adolescence yields a distinctive hyper-metabolic prefrontal state and a frequency-dependent attenuation of ventral hippocampal-induced inhibition of mPFC LFP responses that endures throughout adulthood. Such a liability to cocaine-induced prefrontal disinhibition was not observed in adult-exposed animals. In vivo LFP recordings combined with local pharmacological manipulations show that the disinhibited mPFC state induced by early adolescent cocaine exposure is associated with an attenuation of local prefrontal GABA-A receptor-mediated transmission. Immunohistochemical analyses further demonstrate that a developmental disruption of PV expression in the mPFC accompanies the prefrontal disinhibition induced by early adolescent cocaine treatment. Together, these results indicate that repeated cocaine exposure during early adolescence is sufficient to elicit a state of disinhibited mPFC resulting from a functional impairment of the local prefrontal GABAergic network that endures through adulthood.
Measures of basal cortical metabolic function by means of CO-I histochemistry revealed that repeated non-contingent cocaine exposure triggers opposing frontal cortical neuroadaptations in early adolescent vs. adult rats. At 25 days from the last cocaine injection, a hyper-metabolic state was observed in the early adolescent-treated group whereas an overall hypo-metabolic state was found in the PFC when cocaine was given during adulthood. This latter observation from the adult-treated group is consistent with the bulk of well-documented human studies showing a similar frontal cortical hypoactivity during protracted withdrawal from chronic cocaine abuse (35–39). Traditionally, these neuroimaging findings have often been interpreted as a state of hypofrontality associated with PFC-dependent cognitive deficits (40–44). Similar impairments in prefrontal-dependent executive functions have been observed after long-term exposure to psychostimulants during adolescence (2, 3, 45). At first, our findings of increased PFC metabolism following early adolescent cocaine do not appear to be consistent with the traditional concept of hypofrontality. However, hypofrontality is currently perceived as a state of functional impairment characterized by a failure of the PFC to support behavior (46, 47). In fact, both cortical metabolic hyperactivity and hypoactivity have been shown to contribute to hypofrontality (47–50) due to the non-linear relationship between PFC activation and task demand (46, 47). Thus, in light of this conceptual framework, the hyper-metabolic PFC observed in the early adolescent cocaine-treated group could be a reflection of reduced prefrontal functional capacity as two non-mutually exclusive pathophysiological conditions.
Cocaine exposure during early adolescence elicited a distinct elevation of CO-I staining only in the rostral level of the adult frontal cortex (Fig 1). Interestingly, such an effect was observed in functionally diverse cortical regions within the same rostrocaudal level (i.e., limbic/associative vs. sensorimotor areas). Since this effect was not observed in adult-treated animals, it is likely that the restricted pattern of alterations resulting from early adolescent exposure to cocaine is due to the shared developmental trajectory of inhibitory elements in the rostral regions of the frontal cortex (31, 73). Together with the distinct rostro-caudal gradient of cortical dopamine innervation (74, 75), we hypothesize that the rostral level of the frontal cortex is highly susceptible to the effects of cocaine or other psychostimulants when administered during adolescence. Future studies are warranted to determine the effects of these interactions.
Electrophysiological measures of prefrontal LFP responses to ventral hippocampal stimulation also uncovered an age-dependent effect of cocaine. Consistent with our recent study (26), a proper functioning of local GABA-A receptor-mediated transmission is required in the normal adult mPFC for sustaining the characteristic frequency-dependent inhibition of prefrontal LFP responses to ventral hippocampal stimulation at 20Hz and 40Hz. Recordings conducted from animals exposed to cocaine during early adolescence revealed a selective attenuation of such high frequency-induced mPFC inhibition, a disruption that was not found in the adult-treated group. It is therefore very likely that a disinhibitory mechanism resulting from a developmental impairment of local prefrontal GABAergic network contributes to maintaining the hyper-metabolic mPFC state observed following early adolescent cocaine treatment. Similarly, a switch from hippocampal HFS-induced LTD to LTP was observed in the mPFC of early adolescent cocaine-exposed rats as well as following acute local GABA-A receptor blockade in adult naïve rats, suggesting a common GABAergic mechanism underlying the development of prefrontal disinhibition and the abnormal hippocampal-mPFC plasticity. These findings are consistent with a recent study conducted in PFC brain slices showing that drug withdrawal from cocaine-sensitized juvenile rats favors the expression of activity-induced glutamatergic LTP onto pyramidal neurons, an effect thought to be triggered by a downregulation of local GABA-A receptor-mediated inhibition (51). Accordingly, we found that single in vivo prefrontal microinfusion of the GABA-A α1 receptor positive allosteric modulator Indiplon was sufficient to acutely restore the impaired high frequency-dependent inhibition of LFP in the mPFC and prevent the abnormal expression of ventral hippocampal-induced prefrontal LTP induced by early adolescent cocaine treatment. While ventral hippocampal inputs to the mPFC play a critical role in the control of working memory, decision making and impulsive behavior (52, 53), afferent information from the basolateral amygdala is required for the integration of emotionally salient information (54, 55). Thus, a developmental impairment of local prefrontal GABAergic transmission could compromise mPFC processing of inputs originated from these regions and trigger the onset of a variety of prefrontal-dependent cognitive dysfunctions and reduced impulse control. Future studies will assess the behavioral consequences of the early adolescent cocaine exposure and determine the input specificity of the altered prefrontal state induced by adolescent cocaine.
Developmental alterations of GABAergic interneurons in dopamine-rich cortical regions (e.g., mPFC) have been repeatedly observed following prenatal cocaine exposure (56, 57). Among cortical interneurons, deficits in PV cell function have been mechanistically linked to the onset of prefrontal disinhibition in developmental animal models of psychiatric disorders (21, 30). By means of immunohistochemical analyses, we found that the developmental upregulation of mPFC PV immunoreactivity observed during the normal periadolescent transition (31) is lacking in adolescent cocaine-treated rats. Since the functional profile of PV-positive interneurons and the PV expression level are tightly correlated (34, 58), the reduced PV immunoreactivity observed in the mPFC after early adolescent cocaine could be therefore attributable to a developmental disruption of PV interneuron maturation and function that endures through adulthood. PV-containing cells, often referred as fast-spiking GABAergic interneurons, are functionally positioned to exert fast, stable and timed feedforward inhibition onto pyramidal output cells in respond to high-frequency excitatory inputs (see review by (59)). Thus, an age-dependent developmental dysregulation of PV-positive interneuronal function is likely to contribute to the frequency-dependent GABAergic disinhibition found in the mPFC when cocaine was given during early adolescence. It remains to be determined whether such impairment is associated with altered dopamine modulation of interneuronal function in the mPFC, as recently shown in cocaine-sensitized rats 24 hours post-receiving a challenge dose of cocaine (60).
Profound impairments in executive functions associated with frontal cortex dysregulation such as impulsivity, disinhibition, and working memory deficits are often observed in chronic cocaine users (40–44), many of which can be mimicked by acute blockade of GABA-A receptor-mediated transmission in the PFC in animal models (61–63). As cortical interneurons are critical for determining the timing and spatial selectivity of pyramidal cell firing (18, 64–66), the increased sensitivity to cocaine-induced disruption of prefrontal GABAergic transmission during early adolescence is likely to impair the fine-tuning of prefrontal output activity and PFC-dependent functions. For example, a recent study by the Marinelli group (67) using a self-administration paradigm demonstrated that adolescent onset of cocaine use produces greater stress-induced relapse after prolonged withdrawal, compared with adult-onset of cocaine use. Interestingly, stress-induced relapse has been shown to be, at least in part, dependent upon the PFC (10, 68, 69). We therefore speculate that a disruption of prefrontal GABAergic function during adolescence triggers long-term functional impairments in the mPFC that could increase the susceptibility for the onset of substance abuse and related psychiatric disorders. In conclusion, our present findings have uncovered for the first time that local GABAergic circuits in the mPFC is highly susceptible to cocaine during early adolescence, a distinct neurobiological mechanism from previously established molecular events associated with the periadolescent vulnerability to psychostimulants (3, 70–72).
Supplementary Material
Acknowledgments
This research was supported by Rosalind Franklin University and National Institutes of Health Grants R01 DA004093 and R01-MH086507 to KYT. We thank Dr. Anthony West and Dr. Michela Marinelli for helpful comments and suggestions.
Footnotes
Conflict of Interest
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzia JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- 4.Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- 5.SAMHSA. National Survey on Drug Use and Health: Summary of National Findings. 2010 http://oas.samhsa.gov/NSDUH/2k10NSDUH/12k10Results.htm.
- 6.Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86:189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rebec GV, Sun W. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Anal Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 12.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 14.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 15.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 16.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng KY, O’Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007;61:843–850. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37:484–492. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O’Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomases DR, Cass DK, Tseng KY. Periadolescent Exposure to the NMDA Receptor Antagonist MK-801 Impairs the Functional Maturation of Local GABAergic Circuits in the Adult Prefrontal Cortex. J Neurosci. 2013;33:26–34. doi: 10.1523/JNEUROSCI.4147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng KY, Amin F, Lewis BL, O’Donnell P. Altered prefrontal cortical metabolic response to mesocortical activation in adult animals with a neonatal ventral hippocampal lesion. Biol Psychiatry. 2006;60:585–590. doi: 10.1016/j.biopsych.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng KY, Caballero A, Dec A, Cass DK, Simak N, Sunu E, et al. Inhibition of striatal soluble guanylyl cyclase-cGMP signaling reverses basal ganglia dysfunction and akinesia in experimental parkinsonism. PLoS One. 2011;6:e27187. doi: 10.1371/journal.pone.0027187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurden H, Takita M, Jay TM. Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Structure and Function. 2013 doi: 10.1007/s00429-013-0508-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B, Sun QQ. A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A. 108:12131–12136. doi: 10.1073/pnas.1105296108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philpot BD, Lim JH, Brunjes PC. Activity-dependent regulation of calcium-binding proteins in the developing rat olfactory bulb. J Comp Neurol. 1997;387:12–26. [PubMed] [Google Scholar]
- 34.Eggermann E, Jonas P. How the ‘slow’ Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci. 2011;15:20–22. doi: 10.1038/nn.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 39.Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 40.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- 42.Cunha PJ, Bechara A, de Andrade AG, Nicastri S. Decision-making deficits linked to real-life social dysfunction in crack cocaine-dependent individuals. Am J Addict. 2011;20:78–86. doi: 10.1111/j.1521-0391.2010.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, et al. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users--an ERP study. Eur J Neurosci. 2011;33:1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, et al. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia. 2011;49:1660–1669. doi: 10.1016/j.neuropsychologia.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 47.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 48.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 49.Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg TE, Berman KF, Fleming K, Ostrem J, Van Horn JD, Esposito G, et al. Uncoupling cognitive workload and prefrontal cortical physiology: a PET rCBF study. Neuroimage. 1998;7:296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 51.Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. J Neurosci. 2012;32:10915–10924. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 56.Morrow BA, Elsworth JD, Roth RH. Prenatal exposure to cocaine selectively disrupts the development of parvalbumin containing local circuit neurons in the medial prefrontal cortex of the rat. Synapse. 2005;56:1–11. doi: 10.1002/syn.20121. [DOI] [PubMed] [Google Scholar]
- 57.Stanwood GD, Washington RA, Shumsky JS, Levitt P. Prenatal cocaine exposure produces consistent developmental alterations in dopamine-rich regions of the cerebral cortex. Neuroscience. 2001;106:5–14. doi: 10.1016/s0306-4522(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 58.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 59.Bartos M, Elgueta C. Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol. 2012;590:669–681. doi: 10.1113/jphysiol.2011.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroener S, Lavin A. Altered dopamine modulation of inhibition in the prefrontal cortex of cocaine-sensitized rats. Neuropsychopharmacology. 2010;35:2292–2304. doi: 10.1038/npp.2010.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 62.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficit in monkeys by locally disturbed prefrontal neuronal activity by bicuculline. Behav Brain Res. 1988;31:193–198. doi: 10.1016/0166-4328(88)90023-x. [DOI] [PubMed] [Google Scholar]
- 63.Sawaguchi T, Matsumura M, Kubota K. Delayed response deficits produced by local injection of bicuculline into the dorsolateral prefrontal cortex in Japanese macaque monkeys. Exp Brain Res. 1989;75:457–469. doi: 10.1007/BF00249897. [DOI] [PubMed] [Google Scholar]
- 64.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 65.Szabadics J, Lorincz A, Tamas G. Beta and gamma frequency synchronization by dendritic gabaergic synapses and gap junctions in a network of cortical interneurons. J Neurosci. 2001;21:5824–5831. doi: 10.1523/JNEUROSCI.21-15-05824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 67.Wong WC, Ford KA, Pagels NE, McCutcheon JE, Marinelli M. Adolescents are more vulnerable to cocaine addiction: behavioral and electrophysiological evidence. The Journal of Neuroscience (in revision) 2012 doi: 10.1523/JNEUROSCI.1371-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 69.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 71.Carlezon WA, Jr, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47(Suppl 1):47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stanwood GD, Washington RA, Levitt P. Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex. 2001;11:430–440. doi: 10.1093/cercor/11.5.430. [DOI] [PubMed] [Google Scholar]
- 73.Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65:278–286. doi: 10.1002/syn.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- 75.Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T. Dopamine. Handbook of Chemical Neuroanatomy. 2005;21:1–588. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.