Abstract
The olfactory bulb (OB) has been recently identified as a circadian oscillator capable of operating independently of the master circadian pacemaker, the suprachiasmatic nuclei of the hypothalamus. OB oscillations manifest as rhythms in clock genes, electrical activity, and odor sensitivity. Dopamine, norepinephrine, and serotonin have been shown to modulate olfactory information processing by the OB and may be part of the mechanism that underlies diurnal changes in olfactory sensitivity. Rhythmic release of these neurotransmitters could generate OB rhythms in electrical activity and olfactory sensitivity. We hypothesized that these monoamines were rhythmically released in the OB. To test our hypotheses, we examined monoamine levels in the OB, over the course of a day, by high-performance liquid chromatography coupled to electrochemical detection. We observed that dopamine and its metabolite, DOPAC, rhythmically fluctuate over the day. In contrast, norepinephrine is arrhythmic. Serotonin and its metabolite HIAA appear to rhythmically fluctuate. Each of these monoamines has been shown to alter OB circuit behavior and influence odor processing. Rhythmic release of serotonin may be a mechanism by which the suprachiasmatic nuclei communicate, indirectly, with the OB.
The olfactory bulb (OB) is one of the few identified circadian oscillators in the brain that is capable of continued rhythmicity without input from the suprachiasmatic nuclei of the hypothalamus (SCN), called the “master circadian pacemaker.” However, the mechanisms that generate the OB’s oscillations are unknown. The purpose of this study was to determine if the content of dopamine (DA), norepinephrine (NE), or serotonin (5-HT) varied across 24 hours and was therefore a possible mechanism underlying OB rhythmicity. Ablation of the SCN affects the period length of OB rhythms (Granados-Fuentes et al., 2004), yet the SCN do not have a known direct connection to the OB. The two structures may interact via OB connection to the lateral hypothalamus (Scott and Pfaffmann, 1967), the SCN connections to the locus coeruleus (LC), the SCN connections to the dorsal hypothalamus that projects to the raphe nuclei (RN), or the SCN connections to the intergeniculate leaflet that projects to the olfactory tubercle and the lateral olfactory tract (for review, see Morin, 2012). Both the OB and the SCN receive input from the RN and the LC. The RN release 5-HT into the OB and SCN, while the LC releases NE into the OB and SCN; the OB cell types and interactions are depicted in Figure 1 (McLean and Shipley, 1987, 1991; Gómez et al., 2005). 5-HT and 5-HT agonists affect SCN rhythms (Prosser et al., 1993) and, in the OB, affect learning and memory (for review, see Fletcher and Chen, 2010) and synaptic transmission (Hardy et al., 2005, Petzold et al., 2009). Rhythmic release of 5-HT into the OB would likely affect how well animals perform on memory tasks as well as affecting OB electrical responses to odorants, depending on the time of day. 5-HT and NE may have additive effects (Yuan et al., 2003), but NE has many varied effects in the OB by itself (for review, see Linster et al., 2011). NE alters odor discrimination and habituation (Guerin et al., 2008), affects learning and memory (Fletcher and Chen, 2010), affects cell death and survival (Veyrac et al., 2005), and affects OB synaptic transmission (Ciombor et al., 1999; Gire and Schoppa, 2008; Jiang et al., 1996; Nai et al., 2010; Trombley and Shepherd, 1992). Rhythmic release of NE by the LC into the OB would alter synaptic transmission and OB circuit dynamics, depending on the time of day that NE was released.
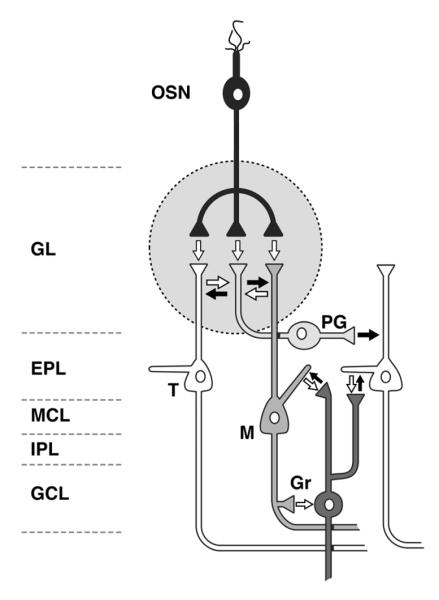
Figure 1.
Circuit diagram of the olfactory bulb and distribution of monoamines. This diagram shows the layers, primary neuronal populations, and synaptic connections of the olfactory bulb (OB). The white arrows represent excitatory connections; the black arrows represent inhibitory connections. Noradrenergic fibers from the locus coeruleus terminate in the inner plexiform layer (IPL) and granule cell layer (GCL), with lower levels of innervation to the external plexiform layer (EPL) and mitral cell layer (MCL). There is very little noradrenergic input to the glomerular layer (GL). Serotonergic fibers from the raphe nucleus project to all layers of the OB: the densest innervation is in the GL, and the MCL, IPL, and GCL are also densely innervated. The EPL is only sparsely innervated by raphe axons. There are no outside dopaminergic projections to the OB. Intrinsic dopaminergic neurons in the OB consist of a subpopulation of neurons within the GL. OSN (olfactory sensory neuron); PG (periglomerular neuron); T (tufted cell); M (mitral cell); Gr (granule cell).
Both NE and 5-HT could be released into the OB by SCN activity, and both neurotransmitters have been shown to affect SCN and OB synaptic transmission; we, therefore, hypothesized that these two neurotransmitters affect OB rhythms. However, DA also has multiple effects on neurons within the OB and is released by tyrosine hydroxylase-positive juxtaglomerular cells, which are among the targets of 5-HT and NE in the OB (for review, see Cave and Baker, 2009). DA stimulates subventricular zone neurogenesis (Kim et al., 2010), affects odor discrimination (Escanilla et al., 2009), and affects synaptic transmission in the OB (Berkowicz and Trombley, 2000; Davila et al., 2003; Ennis et al., 2001). Rhythmic changes in DA content may alter OB circuit dynamics across the day. Because DA is co-released with gamma-amino butyric acid (GABA; Maher and Westbrook, 2008), outside serotonergic input may activate dopaminergic (DAergic) cells, stimulate DA and GABA release, and decrease glomerular responses to odorants, in addition to 5-HT’s other effects.
We hypothesized that monoamine content in the OB exhibits daily oscillations, distinct from the bulb’s faster electrical oscillations. To test this hypothesis, we used high-performance liquid chromatography with electrochemical detection (HPLC-EC) to measure the monoamine content in the OB. We found that the DA concentration fluctuates over 24 hours, while NE concentration does not. The concentration of 5-HT follows a 10-12 hour rhythm, with nadir 2 hours prior to lights out. These data suggest that OB circuit dynamics, among other behaviors, are altered across the day and cannot be considered static or immune to circadian influences. Additionally, this work supports the idea that the circadian system affects olfactory function (Granados-Fuentes et al., 2011).
1. Experimental Procedures
1.1. Animals
Male and female Sprague Dawley rat pups (Charles River, Raleigh, NC), aged postnatal days 21-23, were kept in standard rat cages under a 12-hr light, 12-hr dark cycle (lights on at 0700 h EST; ZT0). Water and rat chow were available ad libitum. Tissue from 5-7 individual animals was collected at each time point, for a total of 135 animals used for this study. Animals were anesthetized using isoflurane and killed by decapitation. During the night, tissue collection was performed under dim red light. All procedures were approved by The Florida State University Animal Care and Use Committee.
1.2. Tissue Collection
Olfactory bulbs were quickly dissected and placed singly in 1 mL of cold 0.3N perchloric acid to protect against monoamine degradation. Five to seven pairs of olfactory bulbs were collected for every time point. The bulbs were homogenized using a 60 Sonic Dismembrator (Thermo Fisher, Rockford, IL) to release the chemical contents of the tissue sample. Samples were then centrifuged using a 5810 R (Eppendorf, Hamburg, Germany) for 20 min at 6,000 rpm (3,824*g) at 40 C. The supernatant was transferred to a 0.22 μm filter tube and centrifuged again for 10 min at 10,000 rpm (10,621*g). This filtrate was used for analysis by HPLC-EC. The protein pellet was saved for the protein assay. All samples were stored at −80°C until analysis.
1.3. Chemicals
DA, 3,4-dihydroxyphenylaceticacid (DOPAC), 5-HT, 5-hydroxyindoleacetic acid (HIAA), NE, perchloric acid, and 3-methoxy-4-hydroxyphenylglycol (MHPG) were purchased from Sigma Chemical Company (St. Louis, MO).
1.4. HPLC-EC
The mobile phase consisted of 75 mM monobasic sodium phosphate (EMD USA, Rockland, Massachusetts), 1.7 mM 1-octanesulfonic acid (EMD USA), 5-15% HPLC grade acetonitrile (95% purity, EMD USA), and 25 μM of ethylenedinitrilotetraacetic acid, disodium salt (BDH, Radnor, PA). Phosphoric acid was added to balance pH (EMD USA). Water was purified on a Milli-Q system (Millipore, Bedford, MA).
Twenty-five μl of thawed olfactory bulb sample was placed into conical autosampler vials. Twenty μl of each sample was injected by an autosampler (Model 542; ESA, Inc., Chelmsford, MA) and delivered to the column by a Prominence degasser piston pump (LC-20AD; Shimadzu, Kyoto, Japan) and a 100 μl injection loop. The flow rate was set at 0.4 ml/min and the pressure was kept at just below 2000 psi. Monoamine separations were performed using a 150mm x 3.2mm C18 reverse phase column, 3 μm particle size (ESA, Part#70-0636), preceded by a guard column with a pore size of 120 angstroms. The sample was oxidized on a conditioning cell (E, +300mV; ESA 5021) and reduced on an analytical cell (E1, +100mV; E2, −225 mV; ESA 5011). Both channels on the analytical cell were set with a gain of 10 nA, 5 s filter, 1 V output and 0% offset. Changes in current were detected by a Colulochem II detector (ESA) and recorded using EZStart 7.3 SP1 software. All monoamines and metabolites were identified based on peak retention times, and the concentration was measured as the area under the curve versus a standard curve. For detection of MHPG, all samples were heated for 5 min at 94° C to break MHPG-sulfate into free MHPG.
1.5. Protein Assay
The total protein content of each individual bulb was measured using the Pierce BCA Protein Assay Kit (Thermo Scientific). Briefly, a standard curve of bovine serum albumin was prepared following kit instructions. Protein pellets were resuspended in 1 ml of PBS. Twenty-five μl of the standard or sample was pipetted, in triplicate, into a 96-well plate. Two hundred μl of working reagent was added to each well. Each plate was left to incubate for 30 min at 37° C. The absorbance of each sample was measured by a V max kinetic microplate spectrophotometer (Molecular Devices, Palo Alto, CA) together with Softmax Version 2.35 software (Molecular Devices) and compared against the bovine serum albumin standard curve. The triplicate measurements were averaged and that average was used to calculate the amount of protein present in the experiment.
1.6. Data Analysis
The data are expressed as a ratio of picomoles (pmol) monoamine per milligram (mg) protein or pmol metabolite/pmol monoamine to reflect turnover and to normalize data. Individual OBs were measured and their contents were averaged between the two bulbs to assess the average per animal. The average amount of monoamine per animal was used for all subsequent data analysis. HPLC-EC data were tested by one-tail ANOVA, using ZT0 as the reference point for the first test (lights on) and ZT12 (lights off) as the reference point for the second test. ANOVA was followed by a Tukey’s Honestly Significant Difference post hoc test, which compared each time point to every other time point. All tests were performed in R (R Core Team, 2012). For all tests, p <0.05 was considered statistically significant and each ANOVA had 23 degrees of freedom.
2. Results
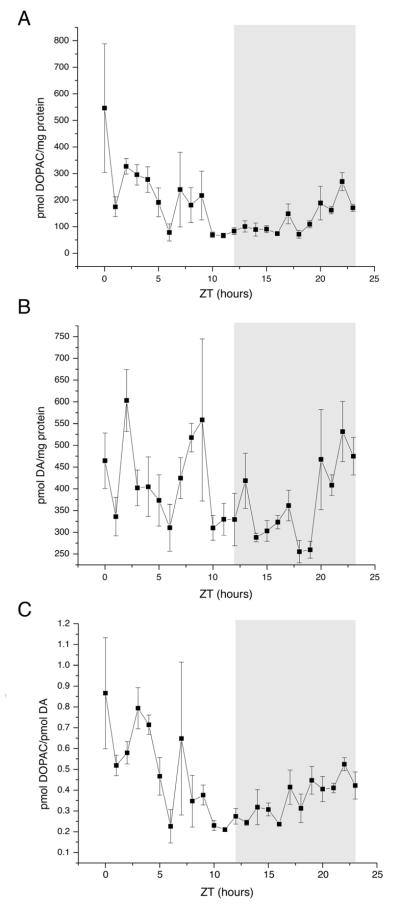
We first examined DA and its metabolite, DOPAC. Because different groups have reported DOPAC/protein (Lookingland et al., 1987) and DOPAC/DA (Hervé et al., 1979) as measures of DAergic activity, we report both here. Figure 2 shows the DOPAC/protein, DA/protein, and DOPAC/DA ratios (A, B, and C, respectively) at hourly time points throughout a day. DOPAC/protein had a single peak at ZT0, when the lights turned on, and DA/protein had multiple peaks throughout the dark and light phases. DOPAC/protein (p = 0.001), DA/protein (p = 0.004), and DOPAC/DA (p = 0.0006) had statistically significant changes as assessed by ANOVA. When we measured NE/protein, two samples stood out. Two of the six samples collected at ZT0 had higher NE/protein ratios than all other samples collected; however, NE/protein did not otherwise fluctuate (Fig. 3). When the two ZT0 outliers were included, NE/protein was statistically significant for ANOVA (p = 0.005). Tukey’s test confirmed that the two animals at ZT0 were the only samples significantly different from the others; when the two outliers were omitted, the ANOVA was not statistically significant, regardless of which reference point was used (p = 0.88). We were unable to acquire consistent readings for MHPG, a metabolite of NE, and so it was not included in our analysis (see Discussion).
Figure 2.
Dopamine (DA) and 3, 4-dihydroxyphenylacetic acid (DOPAC) time courses. (A) DOPAC/protein as measured by HPLC. Lights are turned on at ZT0 and turned off at ZT12. Dark phase is indicated by gray bar. Points plotted are mean +/− standard error of the mean (SEM). n = 5-7 animals per time point. (B) As A, but DA/protein as measured by HPLC. (C) As A, but DOPAC/DA as measured by HPLC. All measures are reported as picomoles of monoamine per milligram of protein.
Figure 3.
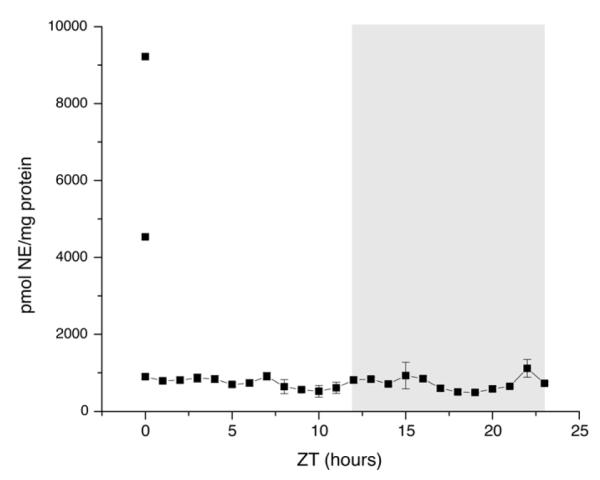
NE time course. Lights are turned on at ZT0 and turned off at ZT12. Dark phase is indicated by gray bar. Points connected by the line are plotted as mean +/− standard error of the mean. The two points not connected by the line are the two outliers at ZT0. n= 5-7 animals per time point. Measured NE is reported as picomoles of NE per milligram of protein.
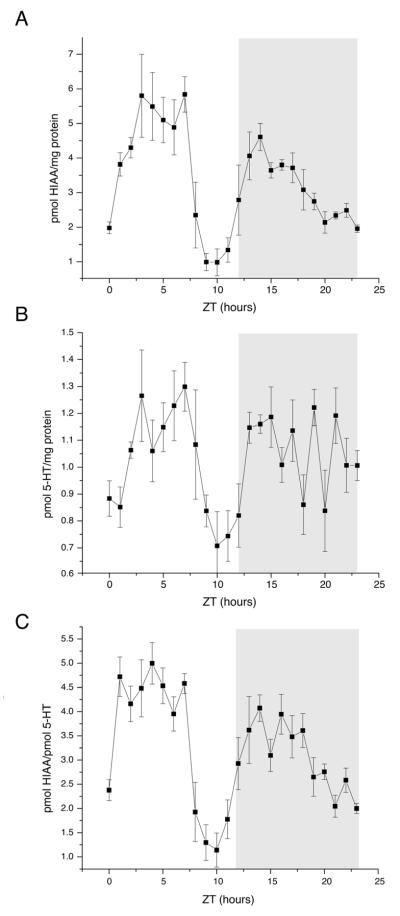
HIAA/protein exhibited a large decline between ZT8 and ZT11, producing a twice-daily profile (Fig. 4, A). 5-HT/protein had a similar profile, but with an amplitude of one-tenth the magnitude (Fig. 4, B). Thus, the amplitude of the time course of 5-HT/protein was almost flat relative to that of HIAA/protein, so the HIAA/5-HT profile (Fig. 4, C) reflects the semi-diurnal HIAA/protein profile. HIAA/protein (p < 0.0001), 5-HT/protein (p = 0.0001), and HIAA/5-HT (p < 0.0001) showed statistically significant variation relative to levels at ZT0 and ZT12 by ANOVA.
Figure 4.
5-HT and hydroxyindole acetic acid (HIAA) time courses. (A) HIAA/protein as measured by HPLC. Lights are turned on at ZT0 and turned off at ZT12. Dark phase is indicated by gray bar. Points plotted are mean +/− standard error of the mean. n = 5-7 animals per time point. (B) As A, but 5-HT/protein as measured by HPLC. (C) As A, but HIAA/5-HT as measured by HPLC. All measures are reported as picomoles of monoamine per milligram of protein.
3. Discussion
We observed that DA and 5-HT fluctuate across the day and that NE does not. This implies that many different OB behaviors are, in the intact animal, affected by the time of day and that DA and 5-HT may be part of the mechanism underlying OB rhythms (Granados-Fuentes et al., 2004; 2011). Both DA and 5-HT fluctuations may change how well animals perform in olfactory tasks, either by DA enhancement of olfactory discrimination (Escanilla et al., 2009) or 5-HT-mediated changes in memory (Fletcher and Chen, 2010) via changes in OB synaptic transmission (Davila et al., 2003; Petzold et al., 2009; Ennis et al., 2001; Berkowicz and Trombley, 2000). Release of DA or 5-HT may also synchronize the OB circadian clock as 5-HT does in the SCN or as DA does in the retina (Ruan et al., 2008). However, other parts of the brain may affect neurotransmitter release into the OB, as melatonin decreases NE release into the pineal gland (Chuluyan et al., 1991) and acetylcholine regulates NE release into the OB (El-Etri et al., 1999). We analyzed DA activity using the DA metabolite DOPAC and not the DA metabolite homovanillic acid (HVA). Some groups have reported that DOPAC is more highly concentrated in some brain regions than HVA (Csernansky et al., 1990; Jenkins, 2008; Natividad et al., 2010), and DOPAC has been previously used as a measure of OB DA activity (Dluzen, 1996; Philpot et al., 1998). Thus, we used DOPAC as our measure of DA activity. However, other groups report higher concentrations of HVA than DOPAC in some brain regions (Gomez et al., 2007; Park et al., 2013; Zant et al., 2011), and one report shows higher HVA than DOPAC in specific brain regions but higher DOPAC than HVA overall (Laatikainen et al., 2013). While it is possible that re-analysis of our data using HVA would alter the amplitude of the observed DA/metabolite ratio fluctuations, our interpretations of the results would be unlikely to change.
The peak in NE appears to be only at ZT0 with a high degree of variance, suggesting that NE is not the synchronizing message from the SCN to the OB. The lack of any NE rhythm also indicates that there may not be a ‘best time’ for NE-mediated olfactory learning. We tried to measure MHPG in our system to have an additional assessment of NE activity in the OB. However, MHPG peaks were not consistent in samples or standards, regardless of mobile phase composition, voltage settings, or run times. Because MHPG standard measurements were not consistent in peak size, elution time, or returning to baseline, we did not pursue MHPG analysis further.
5-HT fluctuations in the OB appear to follow a rhythm of less than 24 hours, and 5-HT release depends on stimulation of the dorsal raphe nuclei; therefore, the dorsal raphe nuclei are likely receiving ultradian input that results in the fluctuations of 5-HT that we report. A report from Gracia-Llanes et al. (2010) indicates that roughly half the synaptic contacts of dorsal raphe axons are with type 1 periglomerular cells (GABA-ergic), as defined by Kosaka and Kosaka (2007). In external tufted cells, 5-HT’s effects are mediated partially by 5-HT2A receptors that activate transient receptor potential channels (Liu et al., 2012), as opposed to the 5-HT2C receptors that mediate SCN and some OB responses to 5-HT (Petzold et al., 2009; Varcoe et al., 2003). Tufted cells also express gap junctions composed of connexin 45 and 5-HT has been shown previously to modulate gap junctional electrical communication via 5-HT2 receptors for connexins 43 and 45 in other systems (Bai et al., 2006; Derangeon et al., 2010; Szabo et al., 2010). Electrical coupling via gap junctions significantly influences OB circuits (Christie et al., 2005; Christie and Westbrook, 2006), however, the effects of 5-HT on gap junctional coupling and communication in the OB are unknown. Taken together, the observed fluctuations in 5-HT may activate periglomerular cells, tufted cells, and cells electrically coupled to tufted cells by gap junctions containing connexin45. Such effects could alter OB responses to odorants at multiple levels of the OB circuit.
DA content appears to fluctuate over time within the OB, with peaks in the light and dark phases. However, DA fluctuations did not synchronize to 5-HT release, indicating that DA rhythms were not the direct result of the reported 5-HT rhythm and presumed 5-HT2A receptor activation. Activation of DAergic neurons and subsequent DA release may be a result of mitral cell rhythmicity, first identified by Granados-Fuentes et al. (2004). If DAergic neurons receive rhythmic inputs, rhythmic activation could synchronize glomeruli that are connected by these cells (Kosaka and Kosaka 2008). DA has been shown to affect the phosphorylation state of gap junctions in the retina, which modulates their ability to provide direct electrical coupling with adjacent neurons (Kothmann et al, 2009). Direct electrical coupling of OB neurons, via gap junctions, contributes to the synchronization of OB neurons projecting to the same glomerulus and is thought to be critical to odor processing by the OB. Whether DA has similar effects in the OB is currently being examined. The difference in light phase/dark phase olfactory sensitivity reported by Granados-Fuentes et al. (2011) may be the result of DA synchronization of the olfactory circuit or 5-HT’s effects on multiple cell types in the olfactory circuit.
4. Conclusions
DA and 5-HT levels in the rat OB fluctuate throughout the day, while NE levels are mostly constant. The 5-HT levels have a semi-diurnal pattern, while the DA fluctuations are less structured. Rhythmic changes in the content and/or release of these neurotransmitters could influence the cellular changes in excitability that underlie circadian rhythms in the OB via modulation of synaptic transmission and electrical coupling.
Highlights.
Dopamine content fluctuates in the rat olfactory bulb
Serotonin content fluctuates in the rat olfactory bulb
Norepinephrine content does not fluctuate in the rat olfactory bulb
Acknowledgements
The authors thank C. Badland for helping us generate the figure artwork, D. Fiore and D. McKee for technical assistance, and J. Olcese for ideas and helpful discussions.
Abbreviations
- DA
dopamine
- DOPAC
3, 4-dihydroxyphenylacetic acid
- HPLC-EC
high-performance liquid chromatography using electrochemical detection
- MHPG
3-methoxy-4-hydroxyphenylglycol
- NE
norepinephrine
- 5-HT
serotonin
- HIAA
hydroxyindoleacetic acid
- SCN
suprachiasmatic nuclei of the hypothalamus
- OB
olfactory bulb
- LC
locus coeruleus
- RN
raphe nuclei
- pg
picograms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: JT Corthell and PQ Trombley designed experiments; JT Corthell, AM Stathopoulos, and CC Watson collected samples and performed experiments; JT Corthell analyzed the data and performed statistical analysis; JT Corthell, AM Stathopoulos, R Bertram, and PQ Trombley wrote the manuscript.
Monoamine changes in rat olfactory bulb
References
- Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) J Pharmacol Exp Therapeutics. 2006;319(3):1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- Berkowicz DA, Trombley PQ. Dopaminergic modulation at the olfactory nerve synapse. Brain Res. 2000;855:90–99. doi: 10.1016/s0006-8993(99)02342-2. [DOI] [PubMed] [Google Scholar]
- Cassone VM, Chesworth M, Armstrong SM. Entrainment of rat circadian rhythms by daily injections of melatonin: synchronization in constant light and dependence upon the suprachiasmatic nuclei. Soc Neurosci Abstr. 1985;11:1140. [Google Scholar]
- Cave JW, Baker H. Dopamine systems in the forebrain. Adv Exp Med Biol. 2009;651:15–35. doi: 10.1007/978-1-4419-0322-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JM, Bark C, Hormuzdi SG, Helbig I, Monyer H, Westbrook GL. Connexin36 mediates spike synchrony in olfactory bulb glomeruli. Neuron. 2005;46(5):761–772. doi: 10.1016/j.neuron.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Christie JM, Westbrook GL. Lateral excitation within the olfactory bulb. J Neurosci. 2006;26(8):2269–2277. doi: 10.1523/JNEUROSCI.4791-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuluyan HE, Rosenstein RE, Chang SM, Gálvez MM, Cardinali DP. Presynaptic effects of melatonin on norepinephrine release and uptake in rat pineal gland. J Pineal Res. 1991;10:165–173. doi: 10.1111/j.1600-079x.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neurosci. 1999;90(2):595–606. doi: 10.1016/s0306-4522(98)00437-0. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Bellows EP, Barnes DE, Lombrozo L. Sensitization versus tolerance to the dopamine turnover-elevating effects of haloperidol: the effect of regular/intermittent dosing. Psychopharmacol. 1990;101:519–524. doi: 10.1007/BF02244231. [DOI] [PubMed] [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. Dopamin e modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol. 2003;90:395–404. doi: 10.1152/jn.01058.2002. [DOI] [PubMed] [Google Scholar]
- Derangeon M, Bozon V, Defamie N, Peineau N, Bourmeyster N, Sarrouilhe D, Argibay JA, Hervé JC. HT4 and 5-HT2 receptors antagonistically influence gap junctional coupling between rat auricular myocytes. J Mol Cell Cardiol. 2010;48(1):220–229. doi: 10.1016/j.yjmcc.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. Age-related changes in monoamines within the olfactory bulbs of the Fisher 344 male rat. Mech of Ageing and Devel. 1996;91:37–45. doi: 10.1016/0047-6374(96)01774-5. [DOI] [PubMed] [Google Scholar]
- El-Etri MM, Ennis M, Griff ER, Shipley MT. Evidence for cholinergic regulation of basal norepinephrine release in the rat olfactory bulb. Neurosci. 1999;93(2):611–617. doi: 10.1016/s0306-4522(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001;86(6):2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- Escanilla O, Yuhas C, Marzan D, Linster C. Dopaminergic modulation of olfactory bulb processing affects odor discrimination learning in rats. Behav Neurosci. 2009;123(4):828–833. doi: 10.1037/a0015855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher ML, Chen WR. Neural correlates of olfactory learning: Critical role of centrifugal neuromodulation. Learn Mem. 2010;17:561–570. doi: 10.1101/lm.941510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Long-term enhancement of synchronized oscillations by adrenergic receptor activation in the olfactory bulb. J Neurophysiol. 2008;99:2021–2025. doi: 10.1152/jn.01324.2007. [DOI] [PubMed] [Google Scholar]
- Gómez C, Briñón JG, Barbado MV, Weruaga E, Valero J, Alonso JR. Heterogeneous targeting of centrifugal inputs to the glomerular layer of the main olfactory bulb. J Chem Neuroanat. 2005;29:238–254. doi: 10.1016/j.jchemneu.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Gómez C, Briñón JG, Valero J, Recio JS, Murias AR, Curto GG, Orio L, Colado MI, Alonso JR. Sex differences in catechol contents in the olfactory bulb of control and unilaterally deprived rats. Eur J of Neurosci. 2007;25:1517–1528. doi: 10.1111/j.1460-9568.2007.05407.x. [DOI] [PubMed] [Google Scholar]
- Gracia-Llanes FJ, Balsco-Ibáñez JM, Nácher J, Varea E, Liberia T, Martínez P, Martínez-Guijarro FJ, Crespo C. Synaptic connectivity of serotonergic axons in the olfactory glomeruli of the rat olfactory bulb. Neurosci. 2010;169:770–780. doi: 10.1016/j.neuroscience.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Ben-Josef G, Perry G, Wilson DA, Sullivan-Wilson A, Herzog ED. Daily rhythms in olfactory discrimination depend on clock genes but not the suprachiasmatic nucleus. J Biol Rhythms. 2011;26(6):552–560. doi: 10.1177/0748730411420247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin D, Peace ST, Didier A, Linster C, Cleland TA. Noradrenergic neuromodulation in the olfactory bulb modulates odor habituation and spontaneous discrimination. Behav Neurosci. 2008;122(4):816–826. doi: 10.1037/a0012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy A, Palouzier-Paulignan B, Duchamp A, Royet J-P, Duchamp-Viret P. 5-hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neurosci. 2005;131:717–731. doi: 10.1016/j.neuroscience.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Hervé D, Tassin JP, Barthelemy C, Blanc G, Lavielle S, Glowinski J. Difference in the reactivity of the mesocortical dopaminergic neurons to stress in the balb/c and C67 BL/6 mice. Life Sci. 1979;25(19):1659–1664. doi: 10.1016/0024-3205(79)90407-7. [DOI] [PubMed] [Google Scholar]
- Jenkins TA. Effect of angiotensin-related antihypertensives on brain neurotransmitter levels in rats. Neurosci Lett. 2008;444(2):186–189. doi: 10.1016/j.neulet.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16(19):6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wang W-Z, Comte I, Pastrana E, Tran PB, Brown J, Miller RJ, Doetsch F, Molnár Z, Szele FG. Dopamine stimulation of postnatal murine subventricular zone neurogenesis via the D3 receptor. J Neurochem. 2010;114(3):750–760. doi: 10.1111/j.1471-4159.2010.06799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K, Kosaka T. Chemical properties of type 1 and type 2 periglomerular cells in the mouse olfactory bulb are different from those in the rat olfactory bulb. Brain Res. 2007;1167:42–55. doi: 10.1016/j.brainres.2007.04.087. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K. Tyrosine hydroxylase-positive GABAergic juxtaglomerular neurons are the main source of the interglomerular connections in the mouse main olfactory bulb. Neurosci Res. 2008;60:349–354. doi: 10.1016/j.neures.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29(47):14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen LM, Sharp T, Harrison PJ, Tunbridge EM. Sexually dimorphic effects of catechol-O-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. PLoS One. 2013;8(4):e61839. doi: 10.1371/journal.pone.0061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Nai Q, Ennis M. Nonlinear effects of noradrenergic modulation of olfactory bulb function in adult rodents. J Neurophysiol. 2011;105:1432–1443. doi: 10.1152/jn.00960.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Aungst JL, Puche AC, Shipley MT. Serotonin modulates the population activity profile of olfactory bulb external tufted cells. J Neurophysiol. 2012;107:473–483. doi: 10.1152/jn.00741.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lookingland KJ, Jarry HD, Moore KE. The metabolism of dopamine in the median eminence reflects the activity of tuberoinfundibular neurons. Brain Res. 1987;419(1-2):303–310. doi: 10.1016/0006-8993(87)90597-x. [DOI] [PubMed] [Google Scholar]
- Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008;99:1559–1564. doi: 10.1152/jn.00636.2007. [DOI] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Serotonergic afferents to the rat olfactory bulb: II. Changes in fiber distribution during development. J Neurosci. 1987;7(10):3029–3039. doi: 10.1523/JNEUROSCI.07-10-03029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT. Postnatal development of the noradrenergic projection from locus coeruleus to the olfactory bulb in the rat. J Comp Neurol. 1991;304(3):467–477. doi: 10.1002/cne.903040310. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.06.026. doi:10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nai Q, Dong HW, Linster C, Ennis M. Activation of α1 and α2 noradrenergic receptors exert opposing effects on excitability of main olfactory bulb granule cells. Neurosci. 2010;169:882–892. doi: 10.1016/j.neuroscience.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natividad LA, Tejada HA, Torres OV, O’Dell LE. Nicotine withdrawal produces a decrease in extracellular levels of dopamine in the nucleus accumbens that is lower in adolescent versus adult male rats. Synapse. 2010;64(2):136–145. doi: 10.1002/syn.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-Y, Myung S-W, Kim I-S, Choi D-K, Kwon J-S, Yoon S-H. Simultaneous measurement of serotonin, dopamine and their metabolites in mouse brain extracts by high-performance liquid chromatography with mass spectrometry following derivatization with ethyl chloroformate. Biol Pharm Bull. 2013;36(2):252–258. doi: 10.1248/bpb.b12-00689. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Hagiwara A, Murthy VN. Serotonergic modulation of odor input to the mammalian olfactory bulb. Nat Neurosci. 2009;12(6):784–791. doi: 10.1038/nn.2335. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Men D, McCarty R, Brunjes PC. Activity-dependent regulation of dopamine content in the olfactory bulbs of naris-occluded rats. Neurosci. 1998;85(3):969–977. doi: 10.1016/s0306-4522(97)00667-2. [DOI] [PubMed] [Google Scholar]
- Prosser RA, Dean RR, Edgar DM, Heller HC, Miller JD. Serotonin and the mammalian circadian system. I. In vitro phase shifts by serotonergic agonists and antagonists. J Biol Rhythms. 1993;8:1–16. doi: 10.1177/074873049300800101. [DOI] [PubMed] [Google Scholar]
- R Core Team R: a language and environment for statistical computing. 2012 http://www.R-project.org.
- Ruan G-X, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6(10):2248–2265. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, Pfaffmann C. Olfactory input to the hypothalamus: electrophysiological evidence. Science. 1967;158(3808):1592–1594. doi: 10.1126/science.158.3808.1592. [DOI] [PubMed] [Google Scholar]
- Szabo TM, Caplan JS, Zoran MJ. Serotonin regulates electrical coupling via modulation of extrajunctional conductance: H-current. Brain Res. 2010;1349:21–31. doi: 10.1016/j.brainres.2010.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci. 1992;10:3985–3991. doi: 10.1523/JNEUROSCI.12-10-03985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcoe TJ, Kennaway DJ, Voultsios A. Activation of 5-HT 2C receptors acutely induces Per gene expression in the rat suprachiasmatic nucleus at night. Molec Brain Res. 2003;119:192–200. doi: 10.1016/j.molbrainres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Didier A, Colpaert F, Jourdan F, Marien M. Activation of noradrenergic transmission by α2-adrenoceptor antagonists counteracts deafferentation-induced neuronal death and cell proliferation in the adult mouse olfactory bulb. Exper Neurol. 2005;194:444–456. doi: 10.1016/j.expneurol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH. Mitral cell β1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem. 2003;10:5–15. doi: 10.1101/lm.54803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zant JC, Leenaars CHC, Kostin A, Van Someren EJW, Porkka-Heiskanen T. Increases in extracellular serotonin and dopamine metabolite levels in the basal forebrain during sleep deprivation. Brain Res. 2011;1399:40–48. doi: 10.1016/j.brainres.2011.05.008. [DOI] [PubMed] [Google Scholar]