Abstract
BACKGROUND & AIMS
Indoleamine 2,3 dioxygenase-1 (IDO1) catabolizes tryptophan along the kynurenine pathway. Though IDO1 is expressed in inflamed and neoplastic epithelial cells of the colon, its role in colon tumorigenesis is not well understood. We used genetic and pharmacologic approaches to manipulate IDO1 activity in mice with colitis-associated cancer and human colon cancer cell lines.
METHODS
C57Bl6 wild type (control), IDO1−/−, Rag1−/−, Rag1/IDO1 double knockout mice were exposed to azoxymethane and dextran sodium sulfate (DSS) to induce colitis and tumorigenesis. Colitis severity was assessed by measurements of disease activity, cytokine levels and histologic analysis. In vitro experiments were conducted using HCT116 and HT29 human colon cancer cells. 1-methyl tryptophan and small interfering RNA were used to inhibit IDO1. Kynurenine pathway metabolites were used to simulate IDO1 activity.
RESULTS
C57Bl6 mice given pharmacologic inhibitors of IDO1 and IDO1−/− mice had lower tumor burdens and reduced proliferation in the neoplastic epithelium following administration of DSS and azoxymethane than control mice. These reductions were also observed in Rag1/IDO1 double knockout mice compared to Rag1−/− mice (which lack mature adaptive immunity). In human colon cancer cells, blockade of IDO1 activity reduced nuclear and activated β-catenin, transcription of its target genes (cyclin D1 and Axin2), and ultimately proliferation. Exogenous administration of IDO1 pathway metabolites kynurenine and quinolinic acid led to activation of β-catenin and proliferation of human colon cancer cells, and increased tumor growth in mice.
CONCLUSIONS
IDO1, which catabolizes tryptophan, promotes colitis-associated tumorigenesis in mice, independent of its ability to limit T-cell mediated immune surveillance. The epithelial cell-autonomous survival advantage provided by IDO1 to colon epithelial cells indicate its potential as a therapeutic target.
Keywords: mouse model, metabolism, ulcerative colitis, IBD
INTRODUCTION
Biologic pathways linking malignancy and the immune system represent novel targets for cancer therapy. Indoleamine 2,3 dioxygenase-1 (IDO1), the initial enzyme in tryptophan catabolism along the kynurenine pathway, is one such target. In tumor draining lymph nodes, IDO1 activity in specialized antigen presenting cells (APC) is postulated to promote immune tolerance and facilitate tumor escape from immunosurveillance.1 Although IDO1 expression is reported in human colon and rectal cancer (CRC),2–4 the function of IDO1 activity in this disease is not well understood.
Much of what is known about IDO1 in the colon is from the study of colitis. In the homeostatic state, IDO1 expression in the colon occurs at low levels and is identified primarily in cells of the lamina propria.5 However, in human inflammatory bowel disease (IBD) and in animal models of colitis, IDO1 is highly upregulated and its expression occurs both in the epithelium and lamina propria APCs.6–8 In these inflammatory states, IDO1, rather than the related IDO2, is the dominant enzyme.9 During active IBD, IDO1 expression and enzymatic activity correlate with disease severity.10, 11 Inhibition of IDO1 accentuates colitis severity in some animal models,12, 13 though IDO1 null mice do not exhibit spontaneous colitis. Conversely, pharmacologic induction of IDO1 reduces colitis severity and supports epithelial restitution.9 Taken together, these findings suggest that IDO1 expression in the gut limits the perpetuation of acute colitis activity by promoting immune tolerance and mucosal healing.5
Patients with chronic colon inflammation, as occurs in human IBD, are at increased risk of developing an aggressive form of CRC termed colitis-associated cancer (CAC).14 Multiple lines of evidence suggest a role for IDO1 in CAC, a disease in which the host’s immune system mediates decisive roles across all phases of tumor development and progression.14, 15 In colitis, IDO1 is upregulated and acts as a natural brake to the inflammatory response. However, in chronic colitis states (including human IBD) IDO1 overexpression persists and may contribute pathologically to tumorigenesis.
To test this hypothesis, we used genetic and pharmacologic approaches to examine IDO1 function in a model of chronic colitis-associated tumorigenesis. IDO1 null mice and those receiving an IDO1 inhibitor exhibited a significantly reduced tumor burden and proliferation in the neoplastic epithelium. Surprisingly, this phenotype held in mice lacking intact adaptive immunity, suggesting that IDO1 functions to support tumor growth independent of its role in modifying tumor immune surveillance. This finding was further investigated in vitro using IDO1 expressing human colon cancer cell lines. This approach revealed a cell-autonomous mechanism by which IDO1 tryptophan catabolites (kynurenine and quinolinic acid) directly promote cancer cell proliferation. These findings demonstrate a previously unrecognized role for IDO1 expression by tumors and support the need for directed investigation of IDO1 inhibition as adjunctive therapy for both colitis-associated and IDO1-expressing sporadic colon cancers.
METHODS
Mice
Wild type (WT), IDO1 knock-out (IDO1−/−), and Rag1−/−, all on the C57BL/6J, were originally purchased from The Jackson Laboratory (Bar Harbor, ME) and then bred in-house to generate comparator groups. IDO1/Rag1 double knock-out (DbKO) mice were generated from these strains. At start of experiments all mice were 6–12 weeks old and weighed 16–22 grams. Age-matched female mice were used preferentially in experiments. Animals were maintained in a specific pathogen-free facility and all comparator groups were sex and age-matched as well as co-caged. Animal procedures and protocols were conducted in accordance with the institutional review board at Washington University School of Medicine.
Colitis and tumorigenesis protocol
Modeling and analysis of colitis-associated tumorigenesis was performed according to protocol.16. Briefly, mice received intraperitoneal 10 mg/kg azoxymethane (AOM, Sigma, St. Louis, MO) followed by 7-day cycles of sterile filtered dextran sodium sulfate (DSS, TdB Consultancy, Uppsala, Sweden) at 2.25–3% in their drinking water. Rag1−/− mice received two doses of AOM. DSS was alternated with 2-week cycles of autoclaved water as shown in figures. In some experiments, pellets containing 20-day controlled-release L-1-methyl tryptophan (L- 1mT, Innovative Research of America, Sarasota, FL) or placebo were surgically inserted under the dorsal skin prior to the second DSS cycle as previously described.9 Dosage selection was based on prior experience with the goal of setting our focus on IDO1’s role in colon tumorigenesis rather than colitis severity.9, 17 Over the ~2 month protocol, mice were assessed at least 3 times per week for weight loss, rectal bleeding, and diarrhea. A modified disease activity index (DAI) was calculated as the sum of point values for the extent of diarrhea (0–4) or rectal bleeding (0–4) observed, excluding the point value for weight loss as this is reported separately.18
Morphology and histology assessment
At sacrifice, colons were examined for length, diarrhea score, tumor number and size. Digital images were used to confirm tumor dimensions using ImageJ (rsbweb.nih.gov/ij/). Tumors and adjacent non-tumorous tissue was harvested for protein, RNA and immunostaining. TISSUE-TEK O.C.T Compound (Miles, Elkhart, IN) was used for frozen fixation followed by 50:50 acetone:methanol fixation. The entire remaining colon was fixed in 10% buffered formalin, transferred to 70% ethanol, stabilized in 2% agar and embedded in paraffin.9, 16 Colitis severity was determined using a previously described 16-point system where the sum of crypt damage (0–4), crypt damage extent (0–4), inflammatory infiltrate (0–4), and inflammatory infiltrate extent (0–4) create a single score.18, 19 The distal and middle colon segments were examined specifically as these regions demonstrate the greatest damage with DSS. A single histology score was calculated as the average of three 40x fields within each segment. Dysplastic and cancerous lesions were also counted for each 40x field and classified based on previous protocols.20 This number is reported with the understanding that it would be impossible for a single longitudinal section of the colon to encompass all lesions. All histological assessments were performed in random order in a blinded fashion by two of the authors (AIT, MSR).
Protein and RNA expression analysis
Western blotting and immunoflorescence were performed as previously described.9, 21 Antibodies included: polyclonal rabbit anti-mouse and human IDO112, monoclonal rat anti-mouse IDO1 (BioLegend, San Diego, CA), mouse anti-human IDO1 (Millipore, Billerica, MA), anti-β-Catenin (BD Biosciences cat# 610153, San Jose, CA), phospho-β-catenin (ser552 and ser33/37/Thr41, Cell Signaling #9566 and #9561), anti-CyclinD1 and D3 (Cell Signaling #2926 and #2936), and anti-β-actin and fibrillarin (both from Santa Cruz Biotechnology, Santa Cruz, CA). Vectashield with DAPI (Vector Laboratories) was used for nuclear counterstaining. Sections were viewed with a Zeiss Axiovert 200 scope and images captured with an AxioCam MRm camera. Quantitative RT PCR was performed as previously described with the primers listed in supplementary table 1.9 Poly-A purification was performed on total RNA prior to performing qRTPCR to enable amplification.22
Reagents and assays
Epithelial proliferation for in vitro experiments was assayed as previously described21 using bromodeoxyuridine (BrdU) injected 90 minutes prior to euthanasia. Stock solutions of L-1mT, D-1mT, picolinic acid, L-kynurenine and quinolinic acid (Sigma-Aldrich, St Louis, MO) were made at 25mM concentration in warm PBS, sterile filtered and then added to media to reach working concentrations. L-1mT and D-1mT were solubilized with 0.1 molar NaOH and subsequently pH adjusted to 7.5 with HCl according to supplier recommendation. Human IFNγ was purchased from eBioscience (#14-8319, San Diego, CA). HCT116 and HT29 colon cancer cell lines were acquired from American Type Culture Collection (ATCC #s CCL-247 and HTB-38, Manassas, VA) and grown in high glucose DMEM (Life Technologies, Carlsbad, CA). All assays performed according to manufacturer protocol in quadruplicate per treatment group: MTT cell proliferation assay from ATCC (#30-1010K, Manassas, VA, in 48 well plates), Click-iT EdU cell proliferation assay (C-10420 Life Technologies, >10,000 cell counts/experiment). Dharmacon siGENOME SMART pool siRNA for IDO1 (M-010337-01) and scrambled control (D-001206- 13-05) purchased from Thermo Fisher Scientific (Waltham, MA). Nuclear/Cytosolic protein fractionation kit from Cell Biolabs, Inc (San Diego, CA) used according to protocol.
Statistical analysis
All data presented as mean ± SEM unless described otherwise. Unpaired Student’s ttest was used to compare between groups with P<.05 considered significant. All cell-based experiments were performed in quadruplicate and repeated ≥3 times. Animal experiments had 5–10 mice/group repeated ≥2 times/experiment, data is reported for a single representative experiment. Significance of fold increase in mRNA expression over control was assessed using Student’s t-test. *P<0.05, **P<0.01, ***P<0.001 for all figures unless otherwise described.
RESULTS
IDO1 expression in colitis-associated tumors
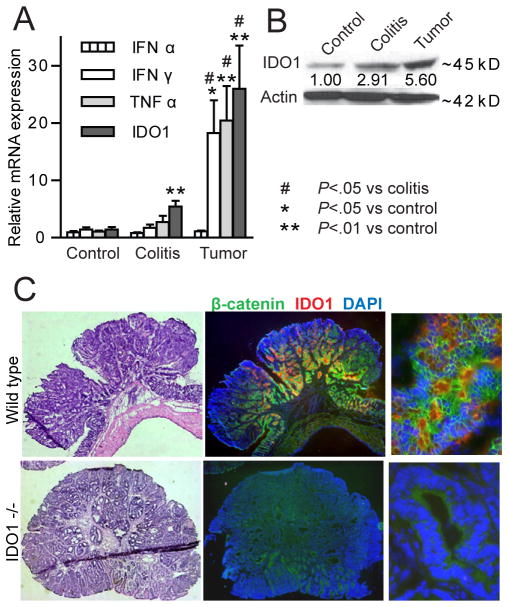
Expression levels of IDO1 and cytokines recognized as IDO1 inducers were measured in tumors and adjacent inflamed tissue and compared to non-colitis control mice. Levels of IFNγ, TNFα and IDO1 mRNA and IDO1 protein were significantly higher in tumor tissue than in inflamed tissue adjacent to the tumors or control (non-colitis) specimens (Figure 1A, B). IFNα, a cytokine known to induce IDO1 in plasmacytoid dendritic cells, was not significantly elevated in either whole chronic colitis tissue or tumors. Tumor immunostaining revealed IDO1 coexpression with CD11c+ cells (dendritic cells, data not shown), but the strongest and most prevalent IDO1 staining was present in the β-catenin positive neoplastic epithelium (Figure 1C) suggesting that epithelial cells are the major source of IDO1 in the colitis-associated tumor microenvironment.
Figure 1.
IDO1 expression is increased in AOM/DSS model tumors. Tumor and adjacent colitis tissue was compared to colon tissue from untreated (control) mice. A) mRNA levels of IDO1 and IDO1-inducing cytokines are shown (n=6 mice). B) Representative IDO1 protein expression in same comparator groups (numerical fold increase over control shown below IDO1 band). C) H&E (left) and immunofluorescence images of OCT-frozen fixed tissues (25x and 400x original) highlighting epithelial IDO1 expression in β-catenin positive primary tumor cells from a WT mouse (top). IDO1−/− mouse tumor (bottom) shown for comparison.
Chronic colitis-associated cancer modeling in the absence of IDO1 activity
Colitis severity is known to influence tumor development and progression in the AOM/DSS model. To test the effect of IDO1 in this murine model we used both gene deletion (WT vs IDO1−/−) and pharmacologic inhibition (Placebo vs L-1mT) approaches. We previously observed that IDO1 blockade did not result in worse acute DSS colitis severity if low, rather than high, doses of DSS are used.9, 13, 17 To center our investigational focus on IDO1’s role in tumorigenesis, we used DSS at 2–3% and L-1mT at 15mg/day.
Cohabitating, age-matched female mice received azoxymethane prior to cycles of DSS to induce colitis and tumors. Mice were closely monitored for signs of colitis over the course of the protocol and at sacrifice. No significant differences in colitis severity were identified across several parameters. Weight loss and disease activity scores trended similarly with both groups demonstrating transitory weight loss, diarrhea, and hematochezia after each cycle of DSS (Figure 2A, D; S.1A, B). At sacrifice, colon length, stool scores, cytokines and histology were also similar between comparator groups (Figure 2B, E and S.1C, D, F).
Figure 2.
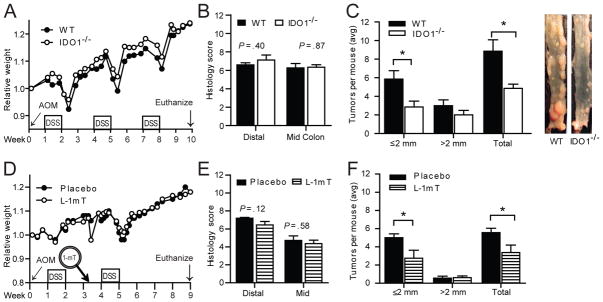
Disrupted IDO1 activity affects tumor burden, but not colitis severity in AOM/DSS. A–C) Age-matched, co-caged, female C57Bl/6 WT and IDO1−/− mice exposed to a single AOM (10 mg/kg) administration followed by 3 cycles of DSS (n=9/group, data from 1 of 3 replicate experiments with similar results). D–F) Female WT mice received either placebo or 20-day control-release 300 mg L-1mT tablet prior to second cycle of DSS (n=8–9/group, data from 1 of 2 replicate experiments with similar results). A, D) Relative change in weight B, E) Colon histology C, F) Average Tumor burden with representative image to right.
Though colitis severity was not worse, tumor development was reduced in mice with genetic disruption or pharmacologic inhibition of IDO1 activity (Figure 2C, F). Average tumor size was similar in WT and IDO1−/− mice (4.01±.10 vs 4.26±.10 mm2 P=.59) and also similar in mice receiving the placebo or L-1mT (2.34±.08 vs 2.18±.07 mm2 P=.74). Microscopically identified non-polypoid dysplasia was also similar between groups (Avg 3.14 vs 2.86 per colon, WT vs IDO1−/−; P=.77)
IDO1 supports epithelial proliferation in vitro and tumor growth independent of effect on adaptive immunity
IDO1-expressing antigen presenting cells in tumor-draining lymph nodes promote immune tolerance. It has been presumed that primary tumor cells of epithelial origin express IDO1 for the same reason. To test whether tumor immune-surveillance may explain the tumor phenotype observed in our model we evaluated tumors for differences in expression of cytokines of known importance in colitis-associated cancer. Though tumors from IDO1−/− mice trended to having higher IFNγ levels and lower TGF- β and IL-10 expression (S.2A), no consistent significant difference in these cytokines was identified. Moreover, tumor-associated expression of CD3e and FoxP3 was not different between WT and KO mice, nor was the CD3e/FoxP3 ratio (S.2B, C).
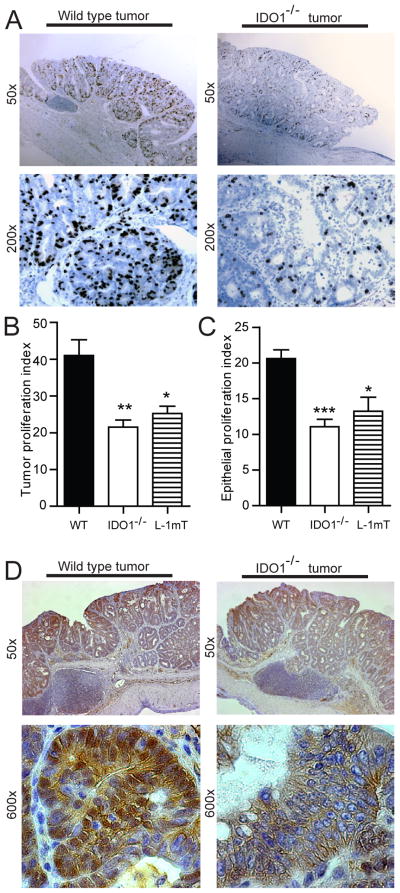
Epithelial proliferation, however, was different based on IDO1 activity. In the setting of disrupted IDO1 activity, tumors and adjacent crypts exhibited a lower epithelial proliferation index (Figure 3). A small difference in colon crypt epithelial proliferation was also noted in non-colitic mice (12.9% vs. 10.6%, WT vs IDO1−/−, P=.03). Furthermore, less intense cytoplasmic and nuclear β-catenin staining was observed in tumors from IDO1−/− and WT mice receiving L-1mT. Together, these findings suggest that there is an epithelial-centric function for IDO1 activity in promoting tumor growth and proliferation.
Figure 3.
IDO1 expression supports epithelial proliferation in tumors and adjacent crypts. Colon tumors and adjacent upright crypts from WT, IDO−/− and L-1mT mice (from Figure2) were examined for BrdU uptake and β-catenin expression. A) Representative images showing greater tumor epithelial uptake of BrdU in WT than IDO−/− mice. B) Tumor proliferation index (BrdU+/total tumor crypt cells). C) Epithelial proliferation index (BrdU+/total crypt cells) in upright non-dysplastic crypts adjacent to tumors. D) β-catenin staining of WT and IDO1−/− tumors (representative) shown reduced nuclear β-catenin in IDO1−/− tumors. Examination was from 10– 20 crypts/tumor, 5–9 colons/group and 1–3 tumors/mouse for immunostaining and calculation of proliferation index. Tissue sections were formalin-fixed and paraffin embedded.
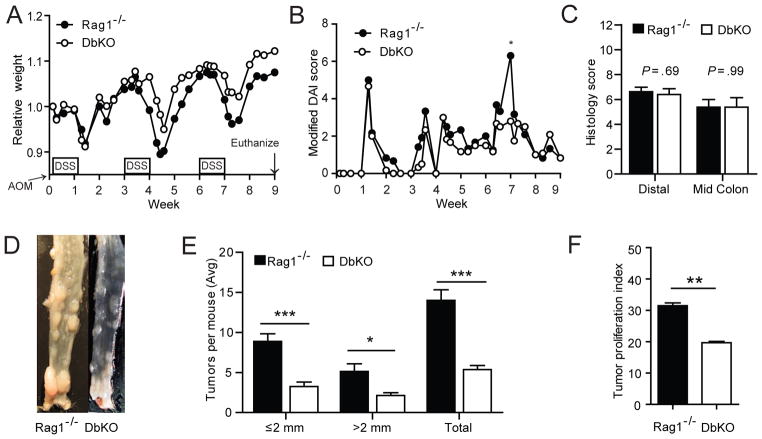
To examine whether IDO1 activity could have an impact on tumor growth independent of its impact on adaptive immune, surveillance we bred IDO1−/− mice to Rag1−/− mice to generate Rag1/IDO1 double gene knockout mice (DbKO). Age-matched, co-caged cohorts of Rag1−/− and DbKO female mice were subjected to the AOM/DSS protocol (Figure 4 and S.1E). Clinical and histologic parameters of colitis severity were similar between these cohorts of mice which lack mature B and T cells. However, mice with disrupted IDO1 activity still exhibited the phenotype of reduced tumor burden. The lower proliferation index (TPI) also persisted in the DbKO vs Rag−/− mice. Compared with WT mice, the TPI of Rag1−/− mice was lower (40% vs 31%), but the TPIs of IDO1−/− and DbKO mice were lower than both (21% and 20% respectively). Thus, both in the presence and absence of adaptive immunity, IDO1 activity supports tumor proliferation and progression in this model of colitis-associated cancer.
Figure 4.
IDO1 activity is associated with increased tumor burden and proliferation independent of influence on adaptive immunity. Rag−/− and Rag−/−/IDO1−/− (DbKO) mice received AOM (twice, one week apart) followed by 3 cycles of DSS. Similar trends were observed in clinical signs of colitis activity including A) Weight loss B) Disease activity index and C) Colon histology scores. However tumorigenesis and tumor proliferation was still lower in the mice lacking intact IDO1 activity. D) Representative colons with polyps E) Tumor quantification and F) Tumor proliferation index. (n=8/group, data shown for 1 of 3 replicate experiments.
IDO1 activity directly promotes colon cancer proliferation in vitro
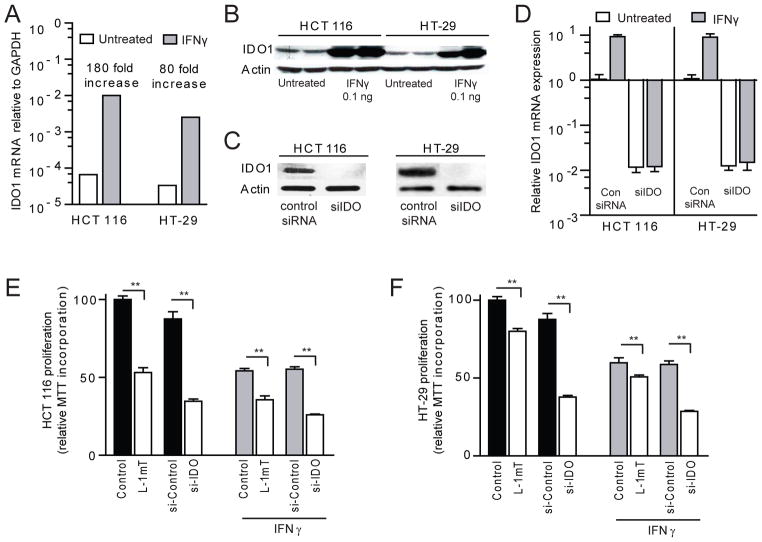
We next used in vitro techniques to further explore the observation that an epithelial cell-autonomous mechanism may explain how IDO1 activity drives tumor proliferation in vivo. HCT 116 and HT-29 human colon cancer cell lines were identified for further testing as they exhibit constitutive and inflammatory cytokine (IFNγ) responsive IDO1 expression (Figure 5A, B). Pharmacologic inhibition and gene silencing with IDO1 specific siRNA (Figure 5C, D) were employed to examine whether disrupted IDO1 activity could directly influence epithelial proliferation in an organ-independent environment.
Figure 5.
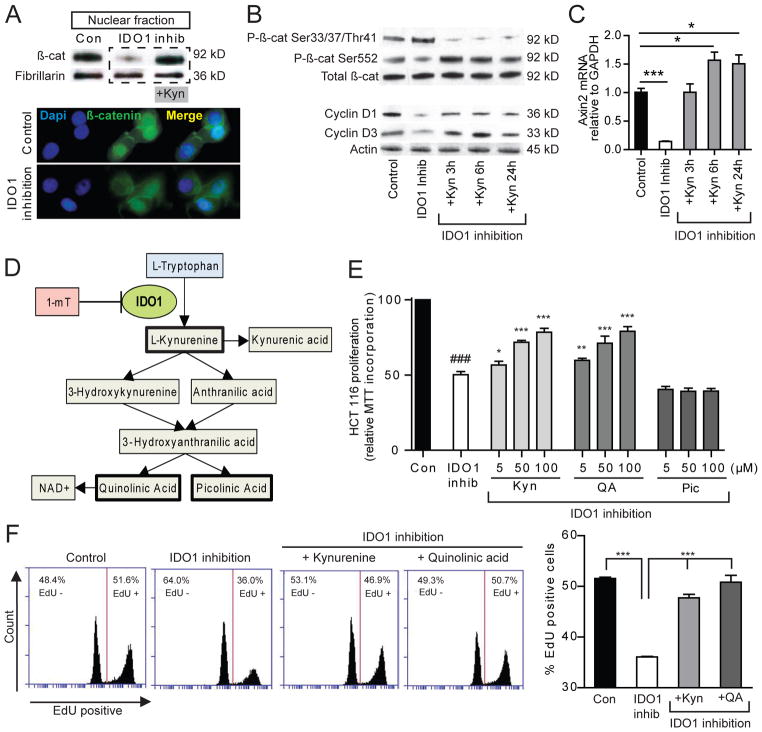
IDO1 supports proliferation in constitutively expressing human colon cancer cells. A) IDO1 mRNA and B) protein is constitutively expressed by HCT 116 and HT-29 cells and is upregulated in response to IFNγ stimulation [0.1ng/ml ×48 hours]. C, D) Gene silencing with IDO1 specific siRNA (siIDO) effectively suppresses expression of IDO1 protein and mRNA in both cell lines [control siRNA or siIDO × 24 hours, followed by ± 24 hours of IFNγ 0.1 ng/ml]. E, F) Inhibition with L-1mT or gene silencing of IDO1 reduces cell proliferation in HCT 116 and HT-29 based on MTT incorporation. Cells grown in 48-well plates were exposed media alone, L-1mT (500mM), control siRNA or siIDO for 72 hours in the absence or presence of IFNγ 0.1ng/ml for 24 hours.
Cell proliferation was examined initially by MTT incorporation assay. Both the D- and L-isomers of the IDO1 inhibitor 1mT led to dose-dependent suppression of cell proliferation which was not observed with tryptophan alone (S.3). The effect was greater for L-1mT than D-1mT. The suppression of proliferation was also additive to that of IFNγ (Figure 5E, F). Silencing-RNA confirmed that IDO1 activity mediated the pro-proliferative effect rather than an off-target influence of 1mT. These findings clarify the role of IDO1 inhibition as a potent cell-centric strategy to reduce colon tumor epithelial cell proliferation.
Kynurenine pathway metabolites activate β-catenin signaling and promote malignant epithelial proliferation
The mechanisms of IDO1’s immunomodulatory effects are attributed to both tryptophan depletion and the generation of kynurenine pathway metabolites. To explore the mechanism by which IDO1 supports epithelial cell-autonomous pro-proliferative effects, we examined β-catenin signaling and proliferation in response to IDO1 blockade and kynurenine pathway metabolites. Changes in β-catenin phosphorylation at Ser33/37/Thr41 (reduced) and Ser552 (increased) correlate with nuclear localization and increased transcriptional activity.23 The reverse is also true. We found that IDO1 inhibition in HCT 116 cells resulted in β-catenin expression pattern consistent with reduced activation and a related reduction in cell cycle regulating genes Cyclin D1 and D3 (figure 6AB). Axin2, a negative feedback regulator of Wnt pathway activation24, was also down regulated with IDO1 inhibition. The addition of kynurenine reversed these effects.
Figure 6.
Metabolites of the IDO1 pathway stimulate β-catenin activation and proliferation in HCT 116 colon cancer cells. HCT 116 cells were incubated for 48 hours in control siRNA or siIDO + 500mM L-1mT ± IDO1 metabolites as indicated. A) Western blot [nuclear fraction] and immunofluorescence demonstrate that IDO1 inhibition reduces nuclear β-catenin, which is restored by addition of kynurenine (100 μM). B) Whole cell fraction Western blot for total and phosphorylated β-catenin [stabilization pathway = Ser 552; degradation pathway = Ser33/37/Thr41] as well as cell-cycle regulating genes Cyclin D1 and D3. C) Relative Axin2 mRNA a transcriptional target of Wnt/β-catenin/Tcf signaling. D) IDO1 pathway of tryptophan catabolism. E, F) Kynurenine and quinolinic acid but not picolinic acid rescue HCT 116 cell proliferation in setting of inhibited IDO1 activity based on MTT incorporation (E) and percent EdU incorporation (F) as marker of S-phase cells. Flow cytometry for EdU included >15,000 events/group, 4 replicates/group, data from 1 of 3 replicate experiments with similar results. P-values *,**,*** vs IDO1 inhibition state; ### (P<0.001) vs scramble siRNA control.
Using the MTT proliferation assay and EdU incorporation as a marker of S-phase cells, we tested the effects of the first and final IDO1 pathway metabolites (bolded in Figure 6D). Kynurenine and quinolinic acid (QA), but not picolinic acid, rescued cell proliferation in the setting of IDO1 blockade in HCT 116 cells (Figure 6E, F). Similar results were found in HT-29 cells (S.4A). Supporting the physiologic relevance of this finding, the addition of these metabolites to cells with intact IDO activity led to no change in proliferation at low concentrations and toxicity at higher concentrations (S.4B, C).
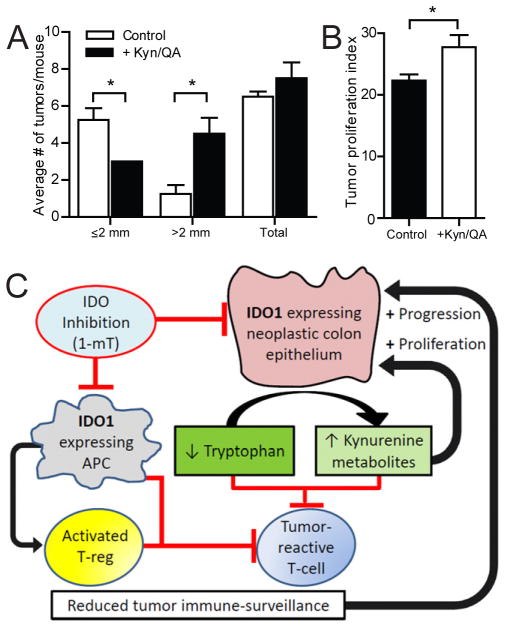
To determine the in vivo significance of this finding we examined the effect of kynurenine and quinolinic acid supplementation on IDO1−/− mice subjected to the AOM/DSS protocol. Larger tumors and more robust tumor proliferation were observed in mice receiving IDO1 pathway metabolites (Figure 7A, B). Clinical signs of disease severity did not differ between treatment and control cohorts (data not shown).
Figure 7.
Kynurenine metabolites promote tumor growth and proliferation of malignant epithelium. Paired littermate female IDO1−/− mice received AOM and two cycles of 3% DSS. Following recovery, mice received kynurenine and QA IP (1mg each) four days/week for 4 weeks or vehicle control (PBS). A) Tumor burden. B) Tumor proliferation index (Brdu+/total crypt cells). C) Schematic depicting dual functions of IDO1 in colon cancer. IDO1 is expressed by the neoplastic epithelium and antigen presenting cells (APC) of the tumor draining lymph nodes or tumor microenvironment. A lowered tryptophan/kynurenine metabolite ratio suppresses tumor reactive effector T-cells and promotes T-reg activation to limit tumor immune-surveillance and thus facilitate tumor progression. Concurrently, elevated local levels of kynurenine metabolites directly stimulate proliferation in the neoplastic epithelium through activated nuclear β-catenin. Inhibition of IDO1 activity (1-mT) reduces tumor progression and proliferation.
In summary, our results identify a novel epithelial-centric, non-immunologic role for IDO1 expression by the neoplastic epithelium where kynurenine metabolites directly activate β-catenin signaling and proliferation. Taken together with current literature, these findings suggest a dual role for IDO1 expression by colonic neoplasms which is to support an immune-tolerant environment and to directly promote tumor growth by stimulating neoplastic epithelial proliferation in a cell-autonomous fashion (Figure 7).
DISCUSSION
Inflammation and immune cell infiltration are present in both sporadic and colitis-associated colon cancers. To support continued growth and progression, it is advantageous for tumors develop adaptive strategies to combat ongoing inflammation and immune assault. Using a murine model of colitis-associated tumorigenesis and human cell lines, we establish a role for indoleamine 2,3 dioxygenase-1 (IDO1) as an adaptive mechanism promoting colon cancer epithelial proliferation.
The most striking finding of this study is that colon epithelial cells themselves exhibit an IDO1 activity-dependent proliferation phenotype. This represents a novel non-immunologic function by which IDO1 expression offers an adaptive advantage to tumor cells. The existing paradigm has been that IDO1 expressing cells (primarily APCs) facilitate tumor progression by promoting tumor-induced tolerance. This is mediated via direct suppression of tumor-reactive T cells and enhancement of local T-reg function.1 Several mechanisms are described for how IDO1 activity promotes T cell tolerance. Local depression in tryptophan contributes via GCN2 kinase activation25, but kynurenine pathway metabolites also mediate immunosuppressive capacities.26
We newly identify tryptophan catabolites to be key mediators of IDO1’s epithelial cell-autonomous pro-proliferative effect in colon cancer. Kynurenine and quinolinic acid enhance proliferation and DNA synthesis in the cancer cell lines after IDO1 inhibition and also increase tumor size and proliferation in the animal model. In vitro kynurenine restores nuclear/activated β-catenin and transcriptional targets of canonical Wnt-β-catenin signaling (Cyclin D1). These data indicated that IDO1 activity and kynurenine metabolites in the tumor microenvironment promote initiation of G1/S phase transition within tumor cells.
It is likely that cell-type specific responses to similar stimuli may explain the different functional outcomes for T and epithelial cells. For example, IDO1 catabolism of tryptophan leads to GCN2 activation. In T-cells, GCN2 activation leads to proliferative arrest and anergy.25 Conversely, in solid human tumors, GCN2 activation (by asparagine or glucose starvation) has been shown to promote cell survival and proliferation.27 Future efforts will be directed at determining if these relationships are relevant in CRC.
Considering the cytokine trends we observed and existing literature examining IDO1 in other cancers, it is likely that IDO1’s capacity to reduce tumor immunosurveillance contributes in part to the phenotype observed in this model of colitis-associated cancer. However, in light of our observations in CRC, it is intriguing to reason that IDO1 expression in many epithelial tumors may have two complementary functions: to promote tumoral immune tolerance and to simultaneously stimulate tumor cell proliferation.
Our phenotypic findings in this model of CAC are similar to those identified in a mouse model of chronic inflammation-associated cutaneous carcinogenesis. Tumor-associated IDO1 expression was increased in WT mice and IDO1 null mice exhibited resistance to tumor formation.28 IDO1 expression in plasmacytoid dendritic cells (pDC) of tumor-draining lymph nodes was the implicated mechanism based on the ability to exert potent T cell suppressor activity. However, the possibility that tumor-based IDO1 expression might drive progression independent of effect on adaptive immunity was not explored.
This data also sheds new light on observational studies linking IDO1 expression in human tumors with worse clinical prognosis. Ferdinande found high IDO1 expression at the tumor invasion front of localized CRC to associate with reduced overall and metastases-free survival.4 Though IDO1’s ability to promote tumor immune tolerance was postulated as an explanation for this observation, no significant correlation between IDO1 expression and the presence of CD3 or CD8 positive T-cells was identified. It has separately been reported that increased nuclear β-catenin expression at the invasion front of CRCs correlates with poor prognosis.29 Our data show that IDO1 pathway metabolites promote nuclear β-catenin and neoplastic epithelial proliferation providing a mechanistic link for these observations. They also support the observation that IDO1 expression may be an early transforming step in colon carcinogenesis.30
It is possible that IDO1 activity and kynurenine metabolites may have multiple functions within the colon epithelium. We show in cell lines and the CAC model that there is activation of the β-catenin complex and proliferation in colonic neoplasia. Transcriptional upregulation of Cyclin D1 and Axin2 suggests that kynurenine activates canonical Wnt signaling. However, the demonstration of β-catenin phosphylation at Serine552 also suggests the possibility of AKT-mediated transcriptional activation31, a pathway implicated in CAC.32 We also link Cyclin D3 expression to IDO1 activity and kynurenine. Cyclin D3 facilitates G1/S transition, promotes intestinal epithelial cell proliferation33, is over-expressed in colon cancers and is associated with liver metastasis;34, 35 however, it is not a classic canonical Wnt target gene and understanding of its regulation in colonic epithelial cells is incomplete. Additional mechanisms by which kynurenine could regulate cell proliferation include stimulation of the human AHR36, 37 or to serve as a de novo source for NAD+ synthesis.38 Support for the latter mechanistic possibility comes from the observation that picolinic acid, a kynurenine metabolite outside of the NAD+ synthesis pathway, does not rescue proliferation in the colon cancer cells.
The inflammatory milieu and colitis severity contribute to tumor development and propagation. The function of IDO1 has been studied in several models of colitis. In T-cell dependent models of colitis, IDO1 acts as a natural brake to developing Th1 and Th17 inflammatory responses.9, 12, 13 In the DSS model, where T-cells affect colitis severity but are not required,39 the role of IDO1 is less well elucidated. We previously reported that 1-mT alone did not worsen acute colitis severity at 7 days, though prophylactic induction of IDO1 limited colitis and was associated with greater epithelial proliferation.9 Matteoli and colleagues found that a high 1mT dose (30 mg/day) worsened colitis severity during the recovery phase of epithelial reconstitution after severe injury with 5% DSS.13 Together with the current study, these data suggest that IDO1 activity is important in epithelial reconstitution after severe injury and it is likely that effect is mediated through kynurenine metabolites.
IDO1’s role in chronic DSS colitis is not previously described. To focus our investigation on IDO1’s role in chronic colitis-associated tumorigenesis, we used lower doses of DSS (2–3%) and 1-mT (15mg/day). Across several experiments, IDO1 null mice did not develop identifiably worse colitis than WT mice using this protocol. A controlled release 1mT product was administered prior to the second DSS cycle, allowing us to examine its effect on tumor growth and colitis severity. IDO1 inhibition with 1mT did not worsen colitis severity, but did reduce tumor burden. A recent report assessing potential cardiac and gastrointestinal liabilities of IDO1 deficiency showed in a single experiment that exposure to the genotoxin 1,2-dimethylhydrazine followed by one 7-day course of DSS led to more tumors in IDO1 null mice.40 Whether greater colitis severity, as proposed, or differences in environmental exposure influenced this inconsistent result is not clear. Regardless, careful monitoring for toxicity will be important in clinical trials testing IDO inhibitors as well as agents that induce IDO1 expression.
Our results demonstrate that IDO1 expression plays a key role in colon cancer progression. These findings provide mechanistic evidence to support the retrospective observational studies associating high IDO1 expression to poor prognosis in human colorectal cancer. The current paradigm suggests that tumor based IDO1 activity drives tryptophan depletion and kynurenine metabolite accumulation; this in turn promotes tumoral evasion from host immunity by limiting antigen-dependent T-cell activation.41 Our results provide new insight into the adaptive survival advantage of IDO1-expressing colon tumors by showing that IDO1 activity also directly promotes tumor growth and proliferation of the neoplastic epithelium in a cell-autonomous fashion via the generation of kynurenine metabolites and activation of β-catenin signaling. These findings have important implications for IDO1 inhibitors as therapy for IDO1- expressing colitis-associated and sporadic colonic neoplasms. Moreover, manipulation of the kynurenine pathway within tumors may represent a new therapeutic target for colon cancer and potentially other malignancies.
Supplementary Material
Acknowledgments
Funding: This research was funded in part by NIH grants DK089016 (MAC), DK064798 (RDN), DK075713 (WFS) and P30-DK52574 (Washington University DDRCC). AIT was a medical student research fellow of the Howard Hughes Medical Institute. MAC received a Central Society for Clinical Research Early Career Development Award.
MAC extends great appreciation to George Prendergast PhD, Nicholas Davidson MD and Thaddeus Stappenbeck MD, PhD who serve as advisors on his career development award (DK089016).
Abbreviations
- APC
Antigen Presenting Cell
- AOM
azoxymethane
- DSS
dextran sodium sulfate
- DbKO
double gene knockout mouse for IDO1 and Rag 1
- IDO
Indoleamine 2,3 dioxygenase
- IBD
inflammatory bowel disease
- 1-mT
1-methyl tryptophan
- QA
Quinolinic acid
- TDO
Tryptophan-2,3-dioxygenase
- Kyn
Kynurenine
Footnotes
Conflicts of Interest statement: The authors have no potential conflicts to declare.
Author Contributions:
Study concept and design: MAC, TAK, RDN, WFS
Acquisition of data: AIT, MSR, KSB, LF, JMM, MAC
Analysis and interpretation of data: MAC, AIT, KSB
Drafting of the manuscript: MAC, AIT
Critical revision of the manuscript for important intellectual content: RDN, WFS
Obtained funding: MAC, WFS
Approved final manuscript: All authors
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–54. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–51. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 3.Gao YF, Peng RQ, Li J, et al. The paradoxical patterns of expression of indoleamine 2,3- dioxygenase in colon cancer. J Transl Med. 2009;7:71. doi: 10.1186/1479-5876-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferdinande L, Decaestecker C, Verset L, et al. Clinicopathological significance of indoleamine 2,3- dioxygenase 1 expression in colorectal cancer. Br J Cancer. 2012;106:141–7. doi: 10.1038/bjc.2011.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciorba MA. Indoleamine 2,3 dioxygenase in intestinal disease. Curr Opin Gastroenterol. 2013;29:146–52. doi: 10.1097/MOG.0b013e32835c9cb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferdinande L, Demetter P, Perez-Novo C, et al. Inflamed intestinal mucosa features a specific epithelial expression pattern of indoleamine 2,3-dioxygenase. Int J Immunopathol Pharmacol. 2008;21:289–95. doi: 10.1177/039463200802100205. [DOI] [PubMed] [Google Scholar]
- 7.Dieckgraefe BK, Stenson WF, Korzenik JR, et al. Analysis of mucosal gene expression in inflammatory bowel disease by parallel oligonucleotide arrays. Physiol Genomics. 2000;4:1–11. doi: 10.1152/physiolgenomics.2000.4.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Hansen JJ, Holt L, Sartor RB. Gene expression patterns in experimental colitis in IL-10-deficient mice. Inflamm Bowel Dis. 2009;15:890–9. doi: 10.1002/ibd.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciorba MA, Bettonville EE, McDonald KG, et al. Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol. 2010;184:3907–16. doi: 10.4049/jimmunol.0900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf AM, Wolf D, Rumpold H, et al. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Gupta NK, Thaker AI, Kanuri N, et al. Serum analysis of tryptophan catabolism pathway: Correlation with Crohn’s disease activity. Inflamm Bowel Dis. 2012;18:1214–20. doi: 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurtner GJ, Newberry RD, Schloemann SR, et al. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–73. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Matteoli G, Mazzini E, Iliev ID, et al. Gut CD103+ dendritic cells express indoleamine 2,3- dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 14.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 15.Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 16.Thaker AI, Shaker A, Rao MS, et al. Modeling Colitis-Associated Cancer with Azoxymethane (AOM) and Dextran Sulfate Sodium (DSS) J Vis Exp. 2012;67:4100. doi: 10.3791/4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaker AI, Bettonville EE, Stenson WF, et al. Indoleamine-2,3-Dioxygenase-1 Exerts Distinct Non- T-Cell Mediated Protective Functions in Acute Colitis. Gastroenterology. 2010;138:S261, S1726. [Google Scholar]
- 18.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 19.Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper HS, Murthy S, Kido K, et al. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–68. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 21.Ciorba MA, Riehl TE, Rao MS, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut. 2012;61:829–38. doi: 10.1136/gutjnl-2011-300367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerr TA, Ciorba MA, Matsumoto H, et al. Dextran sodium sulfate inhibition of real-time polymerase chain reaction amplification: a poly-A purification solution. Inflamm Bowel Dis. 2012;18:344–8. doi: 10.1002/ibd.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voronkov A, Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr Pharm Des. 2013;19:634–64. doi: 10.2174/138161213804581837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jho EH, Zhang T, Domon C, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munn DH, Sharma MD, Baban B, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–42. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Fallarino F, Grohmann U, Vacca C, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol. 2003;527:183–90. doi: 10.1007/978-1-4615-0135-0_21. [DOI] [PubMed] [Google Scholar]
- 27.Ye J, Kumanova M, Hart LS, et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–96. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A. 2008;105:17073–8. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldus SE, Monig SP, Huxel S, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–6. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa K, Hara T, Shimizu M, et al. Suppression of azoxymethane-induced colonic preneoplastic lesions in rats by 1-methyltryptophan, an inhibitor of indoleamine 2,3-dioxygenase. Cancer Sci. 2012;103:951–8. doi: 10.1111/j.1349-7006.2012.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He XC, Yin T, Grindley JC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–98. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee G, Goretsky T, Managlia E, et al. Phosphoinositide 3-kinase signaling mediates beta-catenin activation in intestinal epithelial stem and progenitor cells in colitis. Gastroenterology. 2010;139:869–81. 881, e1–9. doi: 10.1053/j.gastro.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko TC, Pan F, Sheng H, et al. Cyclin D3 is essential for intestinal epithelial cell proliferation. World J Surg. 2002;26:812–8. doi: 10.1007/s00268-002-4057-1. [DOI] [PubMed] [Google Scholar]
- 34.Mermelshtein A, Gerson A, Walfisch S, et al. Expression of D-type cyclins in colon cancer and in cell lines from colon carcinomas. Br J Cancer. 2005;93:338–45. doi: 10.1038/sj.bjc.6602709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanami H, Tsuda H, Okabe S, et al. Involvement of cyclin D3 in liver metastasis of colorectal cancer, revealed by genome-wide copy-number analysis. Lab Invest. 2005;85:1118–29. doi: 10.1038/labinvest.3700312. [DOI] [PubMed] [Google Scholar]
- 36.Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 37.Kawajiri K, Kobayashi Y, Ohtake F, et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A. 2009;106:13481– 6. doi: 10.1073/pnas.0902132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garten A, Petzold S, Korner A, et al. Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol Metab. 2009;20:130–8. doi: 10.1016/j.tem.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen GY, Nunez G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology. 2011;141:1986–99. doi: 10.1053/j.gastro.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang MY, Smith C, Duhadaway JB, et al. Cardiac and gastrointestinal liabilities caused by deficiency in the immune modulatory enzyme indoleamine 2,3-dioxygenase. Cancer Biol Ther. 2011;12:1050–8. doi: 10.4161/cbt.12.12.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–21. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.