Abstract
Human Immunodeficiency virus type 1 (HIV-1) disproportionately affects women, accounting for >50% of new HIV infections in adults worldwide. While multiple mechanisms may contribute to a greater degree of HIV infection in women than men, we evaluated the direct effect of 17β-estradiol, the most bioactive form of estrogen in women, on HIV replication in peripheral blood mononuclear cells (PBMCs). We demonstrate that 17β-estradiol, in an ERα dependent manner, inhibits HIV replication by activating β-catenin signaling. Specifically, we show for the first time that 17β-estradiol induces a complex formation between ERα and β-catenin which tether on the HIV LTR at −143 nt site from +1 start site of HIV transcription to repress HIV promoter activity. These studies define a role of 17β-estradiol in inhibiting HIV replication which may impact HIV pathogenesis in women and add to a growing list of viruses that are inhibited by 17β-estradiol through ERα engagment.
Keywords: HIV, women, estradiol, female sex hormones, β-catenin, HIV LTR, transcription, retroviruses, pathogenesis
Background
The current HIV pandemic is heavily skewed against women. HIV infection in women accounts for >50% of new HIV infections in adults worldwide. Once infected, emerging data points to differences in HIV pathogenesis and anti-HIV immune responses in women in comparison to men (Gandhi et al., 2002; Meier et al., 2009; Sterling et al., 2005; Weinberg et al., 2011). Female sex hormones, at least in part, may contribute to gender-based differences in HIV acquisition and pathogenesis. Particularly, female sex hormones regulate immune responses both systemically and in the female genital tract by impacting homing of HIV-susceptible cells to mucosal tissue, regulating cytokine production and antimicrobial peptides induction, and influencing lymphocyte responses (Fahey et al., 1999; Fahey and Wira, 2002; Fung et al., 2013; Kaushic et al., 1998; Lu et al., 2002; Quayle et al., 1998; Rodriguez-Garcia, Patel, and Wira, 2013). This intricate balance between host factors under female sex hormone regulation and HIV is likely to impact HIV transmission and pathogenesis in women.
Some clinical data suggest that lower estrogen level (e.g progesterone dominant stage) is correlated with higher rate of HIV infection and higher plasma viral load. Specifically, during the periovulatroy stage of the menstrual cycle, when estrogen dominates, HIV plasma viral load is decreased (Greenblatt et al., 2000; Hanna, 1999; Money et al., 2003). Further, HIV productive infection of human cervical explant tissue predominately occurs in tissue obtained from women in their secretory phase of the menstrual cycle, a stage where progesterone dominates and counteracts estrogen effects (Saba et al., 2013). Lastly, women on progesterone-based injectable hormonal contraceptives are more likely to become HIV infected and to transmit the virus to their male partners (Heffron et al., 2012). Taken together, these clinical observations suggest that estrogen dominance may be linked to lower susceptibility of cells to HIV infection.
17β-estradiol, the most potent estrogen found in humans, exerts an anti-viral effect for a number of viruses. It inhibits hepatitis C virus (HCV) infection at multiple steps including attachment, entry, replication, and post-replication (Hayashida et al., 2010; Murakami et al., 2013). It also inhibits hepatitis B virus (HBV) transcription (Wang et al., 2012), limits herpes simplex virus (HSV) primary infection and reactivation (Gillgrass et al., 2010; Vicetti Miguel et al., 2010), and inhibits rubella virus replication(Roehrig, Brawner, and Riggs, 1979). 17β-estradiol, on the other hand, promotes the replication of adenovirus type 12 (James, Vanderpool, and Roane, 1992). The signal transduction cascade of estrogen is complex and can be engaged by multiple pathways. Canonical estrogen signaling involves the activation of its nuclear receptors, estrogen receptor α (ERα) or estrogen receptor β (ERβ), which in turn bind to estrogen responsive elements (ERE) located near or within gene promoters to regulate the transcriptional activity of cognate target genes(Marino, Galluzzo, and Ascenzi, 2006). Estrogen also regulates gene expression via noncanonical signaling whereby (Marino, Galluzzo, and Ascenzi, 2006) estrogen receptors bind to other transcriptional factors such as Sp1 or Ap-1, leading to either their stabilization of recruitment of transcriptional co-regulators, which in turn bind to their cognate promoters to regulate gene expression. Also, estrogen can bind to either its nuclear receptors or other receptors to initiate second messenger signaling leading to physiologic effects. These signal transduction pathways include ERK/MAPK, p38/MAPK, or PI3K/AKT, to name a few (Marino, Galluzzo, and Ascenzi, 2006). Interestingly, many of the reported 17β-estradiol reported anti-viral effects are mediated by ERα and not ERβ (Hayashida et al., 2010; Murakami et al., 2013; Wang et al., 2012; Gillgrass et al., 2010; Vicetti Miguel et al., 2010; Roehrig, Brawner, and Riggs, 1979).
β-catenin signaling represses HIV replication in multiple targets, including PBMCs at the level of transcription (Henderson et al., 2012; Kumar et al., 2008; Li et al., 2011). β-catenin is a transcriptional co-regulator and is the central mediator of the canonical Wnt/β-catenin signaling pathway (Al-Harthi, 2012). β-catenin binds to members of the TCF/LEF family of transcription factors (TCF1, TCF3, TCF-4, and LEF) to modulate hundreds of genes, including those that regulate cell proliferation and survival. β-catenin expression is regulated at the protein level by a multi-protein destruction complex (GSK-3β, APC, Axin, and casein kinase 1). This multi-protein complex leads to the phosphorylation, ubiquitination, and proteasomal degradation of β-catenin. When this destruction complex is destabilized, β-catenin is hypophosphorylated and translocates to the nucleus where it binds to TCF/LEF transcription factors, tethering on their cognate DNA binding sites to regulate genes such as CyclinD, C-Myc, Matrix metalloproteinase 7 and 9, and Axin, to name a few (Al-Harthi, 2012).
At least four TCF-4 binding sites have been identified within the HIV promoter at −143 to −136 nt; −336 to −329 nt; +66 to +73 nt; and +186 to +195 nt from the transcription initiation site (Henderson et al., 2012). While all sites have >70% homology to the TCF-4 binding sequence, the −143 site has 100% homology to the TCF-4 core (5’-(A/T)(A/T)CAAAG-3’) and is present in approximately one-third of 500 HIV LTR sequences reported in the Los Alamos gene bank (Henderson et al., 2012). TCF-4 binds at a higher affinity at −143 than at any other site (Henderson et al., 2012). Further, β-catenin is tethered on the HIV LTR at the nt-143 site and knockdown of either TCF-4 or β-catenin enhances HIV transcription (Henderson et al., 2012; Narasipura et al., 2012). Dual knock down of β-catenin and TCF4 does not further enhance HIV LTR activity, indicating that these factors work together to repress HIV transcription.
Given that HIV replication is regulated by a complex interplay between virus and host factors and some epidemiologic data linking an estrogen dominant state to lower plasma viral load and infection rate, we directly assessed the role of estrogen (17β-estradiol) on HIV replication in peripheral blood mononuclear cells (PBMCs). We demonstrate that estrogen through engaging ERα leads to association between ERα and β-catenin that tethers on the HIV LTR at −143 nt from the transcription initiation site to repress HIV transcription. These findings provide the first direct association between ERα and β-catenin on the HIV LTR, impacting HIV transcription. Further, the mechanism revealed here may contribute to some clinical observations linking lower HIV load under an estrogen dominant state (Asin et al., 2008; Greenblatt et al., 2000; Money et al., 2003). Most importantly, these studies add to a growing list of viruses (HCV, HBV,HSV, rubella) that are inhibited by ER through ERα engagment (Gillgrass et al., 2010; Hayashida et al., 2010; Murakami et al., 2013; Roehrig, Brawner, and Riggs, 1979; Vicetti Miguel et al., 2010; Wang et al., 2012).
Materials and Methods
Ethics Statement
This study was approved by Rush Institutional Review Board (09040706-IRB01) and participants’ written consent was provided.
Reagents
AZT, 17β-estradiol, PHTPP (4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol), PPT (4,4’,4”-(4-Propyl-[1H]-pyrazole-1,3,5-triyl) trisphenol), anti-total β-catenin antibody, amino acids 768-781 (cat. C2206), and anti-GAPDH antibody (cat. G9545) purchased from Sigma Aldrich (St. Louis, MO, USA). Anti-active β-catenin antibody clone 3C196 purchased from US Biologicals (San Antonio, TX, USA). Anti-ERα antibody clone 62A3 purchased from Cell Signaling (Boston, MA). Anti-ERβ antibody clone 14C8 purchased from Abcam (Cambridge, MA, USA). XAV 939 and IWR-1-endo purchased from Tocris Bioscience (Bristol, United Kingdom).
Isolation and treatment of PBMCs
PBMCs were isolated from venous blood of healthy women of reproductive age and not on hormone contraceptives using lymphocyte separation medium (Lonza Biotech, Allendale, NJ, USA) and density centrifugation. The cells were suspended in phenol red free-RPMI complete medium supplemented with 10% fetal bovine serum (FBS), 1% L-Glutamine, 1% Penicillin, 1% Streptomycin, and 20 U/ml IL-2. PBMCs were left unstimulated or stimulated with 1βg/ml each of anti-CD3/CD28 antibodies (BD Biosciences, Franklin Lake, NJ, USA) for 24h at 37°C under 5% CO2.
Immunofluorescence Staining and Flow Cytometric Analysis
Up to 9-color flow cytometric analyses were performed using a FACSCalibur cytometer with CellQuest (BD) and LSR-II cytometer with Diva software (BD). Aqua Live/Dead staining was used. The following antibodies from BD Biosciences were used for cell surface staining as per instructions: For T cells subsets, CD3- alexafluor 700, CD14-pacific blue, CD8-phycoerythrin-cyanine (PerCP-Cy) 5.5, CD4- allophycocyanin (APC), CD19 APC-H7; for dendritic cell subsets, CD3- PerCP-Cy7, CD123-phycoerythrin (PE), HLA-Dr PerCP, CD14-pacific blue, CD11c-APC, CD56/CD16-alexfluor 700 and CD19/CD20-APC-H7. Proper isotypes were purchased from same vendor for primary stain. Intracellular flow cytometry staining was performed using CytoPerm/fix and CytoWash reagents, according to recommendations of the supplier (BD Biosciences). For ERs staining, 10βl of a FITC-conjugated rabbit monoclonal antibody that recognizes both ERα and ERβ (clone SP1, Abcam, Cambridge, MA, USA) was used. For active β-catenin, 200 μl of 0.05μg dilution in PBS of mouse anti-human active β-catenin un-conjugated antibody (clone 3C196, US Biological, Marbelhead, MA) was used followed by 1μl of a secondary anti-mouse F(ab’)2 FITC-conjugated antibody at 2mg/ml. The cells were subsequently washed and fixed with 2% paraformaldahyde.
Cell viability, HIV Infection, and p24 assay
Cell viability was assessed by standard MTS assay (Promega, Madison, WI). HIV-1Bal or HIVIIIB at 2ng HIV p24/106 cells was added to anti-CD3/CD28 stimulated PBMCs and incubated for 4-6 h at 37°C under 5% CO2. Cells were then washed with PBS three times to remove un-bound virus, suspended in phenol red free-RPMI complete medium with 20 units/ml IL-2, treated as indicated per experimental condition, and plated in 96 well U-bottom plate at 200,000 cells/200μl volume of the media in quadruplicates for 7 days at 37°C, 5% CO2. HIV p24 assay was performed using p24 ELISA kit (SAIC-Frederick, MD) according to the manufacturer's protocol.
Western blot and Immunoprecipitation (IP)
The cells were lysed using RIPA buffer (Sigma) with 10% protease inhibitors and protein content measured by Pierce BCA assay (Thermo Fisher, Barrington, IL). Western blot was performed as described (Narasipura et al., 2012), using anti-active β-catenin antibody at 1:8000, anti-total β-catenin antibody at 1:8000, anti-ERα at 1:1000, anti ERβ at 1:1000, and/or anti-GAPDH at 1:30,000. Secondary antibodies included goat anti-mouse HRP (Pierce) at 1:50,000 and goat anti-rabbit HRP (Cell Signaling) at 1:60,000 for GAPDH. Densitometries were measured by ImageJ software. For IP, cell lysate was pre-cleared with 25μl of protein A/G magnetic beads (Thermo Scientific), cross-linked with specific antibodies using Protein A/G MagSepharose and SpinTrap Buffer Kit (GE Healthcare, Piscataway, NJ) as outlined by manufacturer. Denatured proteins were then resolved on a 12% SDS gel. IgG controls were run in parallel.
RNA Isolation and real time PCR
Total RNA was isolated from PBMCs using Trizol (Life Technologies, Grand Island, NY). Complimentary DNA synthesis was performed using qScript cDNA Supermix (Quanta BioSciences, Gaithersburg, MD). Real-time PCR was performed employing SsoFast EvaGreen Supermix with low ROX (BioRad, Hercules, CA) in a 7500 real time PCR system (Applied Biosystems, Carlsbad, CA) to quantify target genes. Reaction conditions were: 95 °C for 10 min followed by 40 cycles of 95 °C for 15sec, 60 °C for 1min and 95 °C for 30sec. A melting curve stage was included to make sure that single specific products were amplified devoid of non-specific products or primer-dimers. Fold change in product expression was calculated by relative quantification using the comparative CT method with GAPDH as endogenous control. The following primers were used to amplify Wnt/β-catenin pathway target genes: c-Myc, F- 5’-ACGTCTCCACACATCAGCACAACT and R-5’-TGACACTGTCCAACTTGACCCTCT; CyclinD1, F-5’-TCATGGCTGAAGTCACCTGGT and R-5’-TCCACTGGATGGTTTGTCACTGGA; Axin2, F-5’-ACAACAGCATTGTCTCCAAGCAGC and R-5’-GCGCCTGGTCAAACATGATGGAAT and GAPDH, F-5’-TGACTTCAACAGCGACACCCACT and R-5’-ACCACCCTGTTGCTGTAGCCAAAT.
Analysis of HIV reverse transcription, integration and transcription
Stimulated PBMCs were infected with HIVBal and treated or untreated as described above. For HIV reverse transcription analysis, infected cells were harvested at 24h, genomic DNA isolated using DNeasy blood and tissue kit (Qiagen, Valencia, CA), quantitative real time PCR was performed to quantify HIV LTR using F-5’- TCAAGTGAGTGCCCGGTT and R-5’-AGCTCCGGTTTCTCTTTCGCT primers. For HIV integration analysis, infected cells were harvested at 72h AND Alu-PCR was performed as described (Brussel and Sonigo, 2003). A minus Taq and a minus template controls were included throughout. For HIV transcription analysis, at 72h post infection, cDNA was subjected to real time PCR using the following primers to amplify HIV transcripts: Rev, F-5’-TCCTTGGCACTTATCTGGGACGAT and R-5’-TCCCAGAAGTTCCACAATCCTCGT and Env, F-5’-ACGAGGATTGTGGAACTTCTGGGA and R-5’-TGGCATTGAGCAAGCTAACAGCAC. GADPH was used as endogenous control.
Nucleofection and transfection
Stimulated PBMCs were transfected with dominant negative (dn) TCF-4 plasmid (Addgene; Cambridge, MA) and pcDNA backbone (Invitrogen; Carlsbad, CA) using Amaxa T cell Nucleofection kit and Amaxa Program T-23 (Lonza, Walkersville, MA). Jurkat cells were transfected with 0.5μg ERα expression plasmid (pEGFP-C1-ER alpha, Addgene, Cambridge, MA), 0.5μg of either the WT-LTR or Δ-143-LTR previously described (Henderson et al., 2012) using Lipofectamine 2000 (Invitrogen).
Chromatin immunoprecipitation (ChIP) and quantitative real-time PCR
At 48hrs post-transfection, chromatin immunoprecipitation (ChIP) was performed using EMD Millipore EZ Magna ChIPA/G (Temecula, CA) according to manufacturer's protocol with antibodies for total β-catenin (Sigma) and ERα (Thermo). An appropriate IgG (CellSignaling) control antibody was also included. Real time PCR was performed to quantify HIV LTR DNA using above mentioned LTR primers in a 7500 real time PCR system employing SsoFast EvaGreen Supermix with low ROX. Reaction conditions were: 95 °C for 10 min followed by 40 cycles of 95 °C for 15sec, 60 °C for 1min and 95 °C for 30sec. A melting curve stage was included to ensure amplification of a single product. Change in binding was calculated by relative quantification using the comparative threshold cycle (CT) method. Results were reported as fold change relative to IgG control (ΔCT=CT Target – CT IgG control; fold change relative to IgG control = 2−ΔCt).
Statistics
When the data was distributed normally, ANOVA and post-hoc tests were used. When the data was not normally distributed, nonparametric analysis was performed. All tests assumed a two-sided significance level of 0.05. GraphPad Instat 3 software (San Diego, CA) was used for data analysis.
Results
17β-estradiol inhibits HIV replication in PBMCs
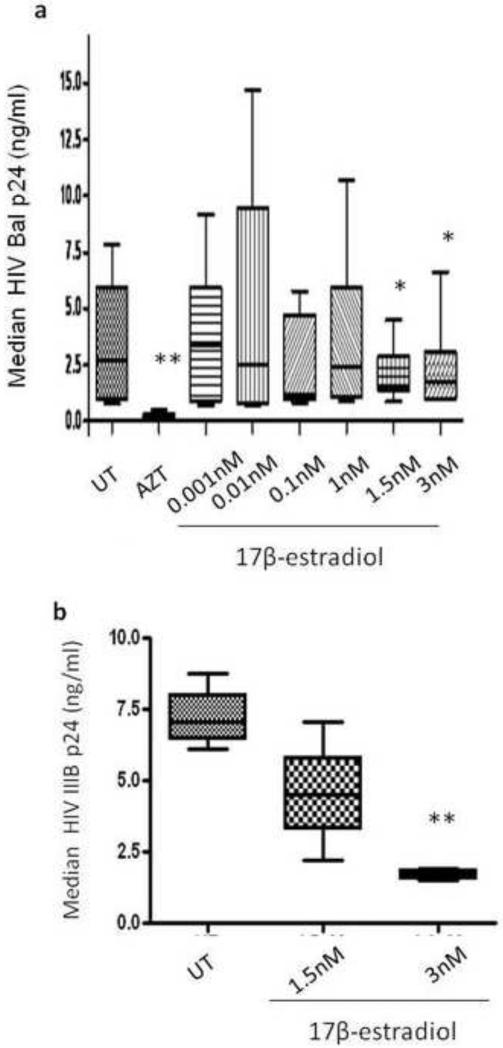
PBMCs were stimulated with anti-CD3/CD28 prior to HIVBal infection then treated with 17β-estradiol (0-3nM) or AZT (1μM). At day seven post infection, HIV replication was monitored by conventional HIV core antigen (p24) ELISA. We demonstrate that 17β-estradiol at 1.5 and 3nM inhibited HIV replication by approximately 40% (Fig.1a). 17β-estradiol at these two doses also inhibited HIV strain (IIIB), a T-tropic/CXCR-4 utilizing strain (Fig.1b). None of these 17β-estradiol doses (0.001-3nM) were cytotoxic to PBMCs, as evaluated by the MTS assay (data not shown).
Fig. 1. 17β-estradiol inhibits HIV replication in PBMCs.
PBMCs were stimulated with anti-CD3/CD28 antibodies for 48h, infected with HIVBal (a) or IIIB (b), re-suspended in phenol red free-RPMI complete medium, and treated with 17β-estradiol (0- 3nM), 1μM AZT, or left untreated. Seven day post-infection, HIV p24 level was measured by conventional ELISA from HIVBal (a) or HIV IIIB (b) infected cells. Data is representative of median value from a minimum of four donors, performed in quadruplicates. The bars represent the data range, where the 25th, 50th and 75% median percentile values are shown and analyzed using Mann-Whitney test. *p < 0.05; **p < 0.01
Robust expression of ERα in PBMCs
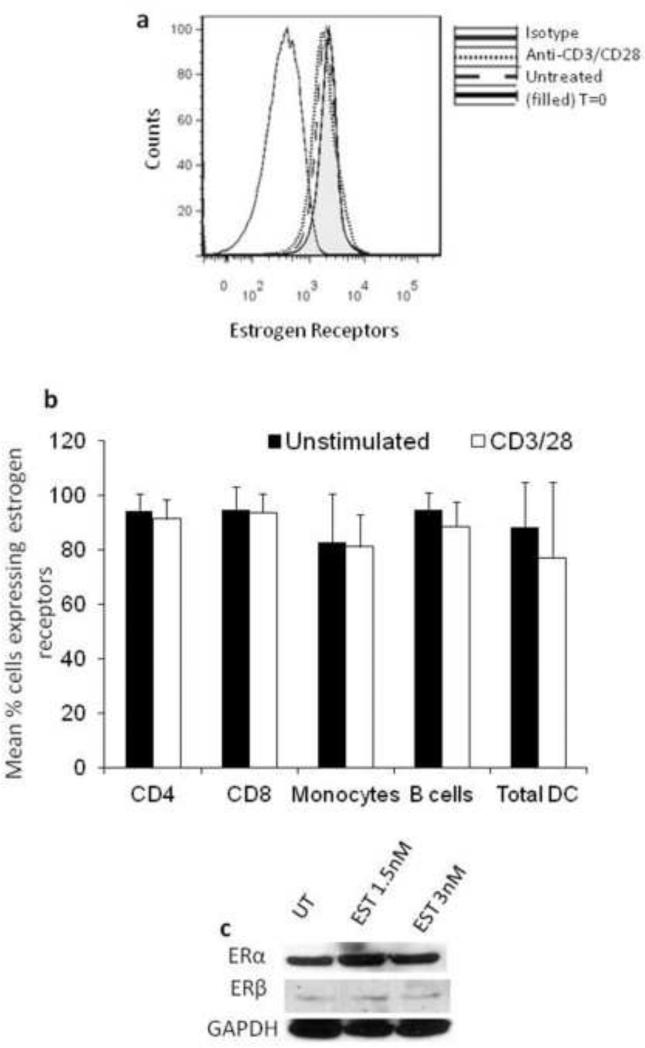
To determine which estrogen receptor (ERα or ERβ) is involved in 17β-estradiol-mediated inhibition of HIV replication, we evaluated ERα and ERβ expression in PBMCs. Using an antibody that recognizes both ERα and ERβ we show that ER expression is >80 % in all lymphocyte subsets and that T cell activation through anti-CD3/CD28 stimulation did not significantly increase ER expression in T cells (Fig. 2a & b). Using an antibody that is specific for either ERα or ERβ, we demonstrate robust expression of ERα in PBMCs and low level of ERβ expression (Fig.2c). 17β-estradiol treatment of PBMCs at doses that inhibited HIV replication (1.5 and 3nM) did not significantly induce ERα or ERβ expression (Fig.2c)
Fig. 2. PBMCs predominately express ERα.
PBMCs were stimulated with anti-CD3/CD28 antibodies for 48h, left untreated in (a) and (b) or treated with 1.5nM or 3nM 17β-estradiol in (c). Expression of estrogen receptors (ER) was determined by intracellular flow cytometry (a,b) or by Western blot (c). A representative histogram of expression of 17β-estradiol receptors (ERα and ERβ) in PBMCs is shown in (a) and ER expression in leukocyte subsets is shown in (b). Expression of ERα and ERβ detected by Western blot in PBMCs is shown in (c). Data is representative of a minimum of four donors. Error bars represent standard deviation of the mean.
17β-estradiol inhibition of HIV replication is dependent on ERα and is at the level of HIV transciption
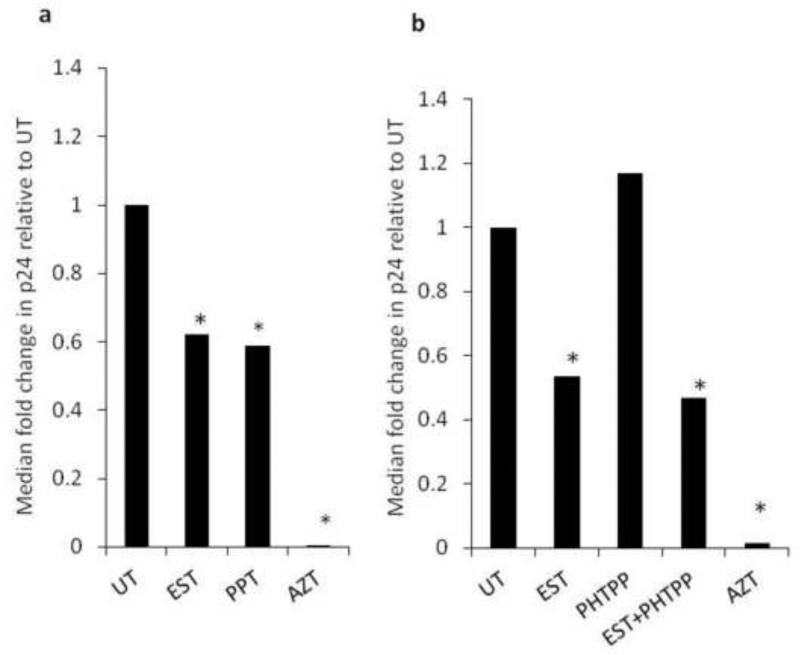
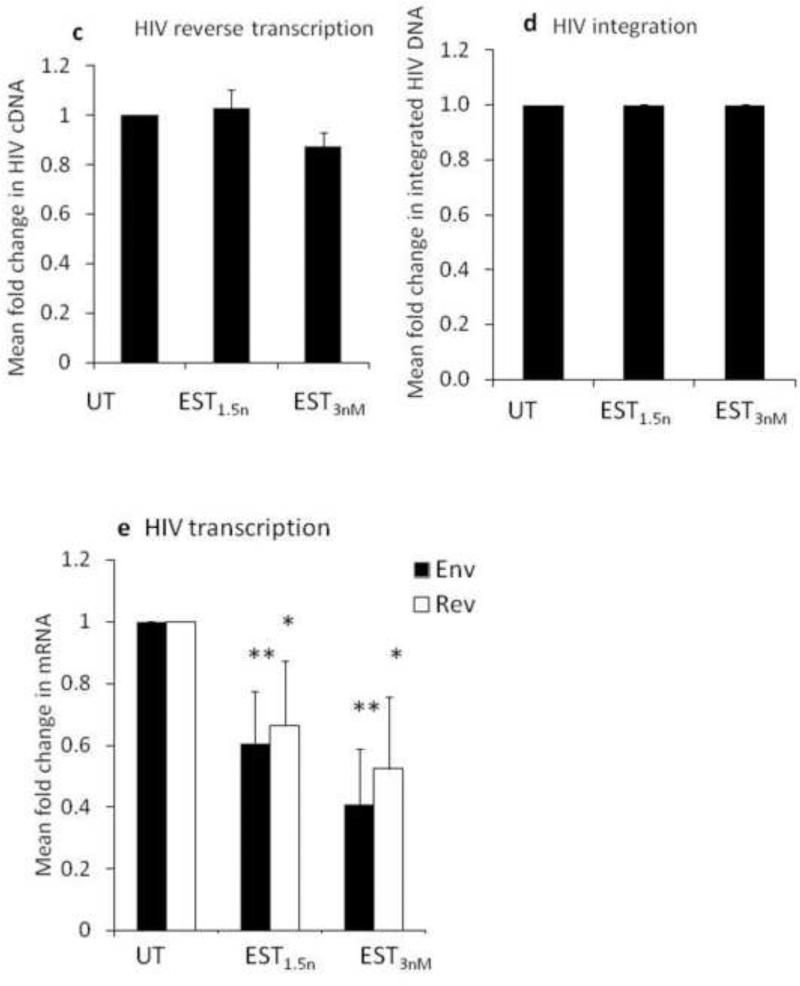
To determine the role of ERα or ERβ in estrogen mediated inhibition of HIV, we used an ERα agonist and an ERβ antagonist. We were unable to use an ERα antagonists in PBMCs because ERα antagonists are highly context dependent and in some cases they function as agonist instead of antagonist. For example, 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (known as MPP) depending on the cell type can be an ERα agonist or antagonist (Shang and Brown, 2002). In PBMCs, ER agonists functioned as antagonist (data not shown). For this reason, we used an ERα agonist (4,4’,4”-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol, PPT) to assess if engaging ERα leads to HIV inhibition. PPT inhibited HIV replication by approximately 40%, a magnitude that was similar to that mediated by 17β-estradiol (Fig.3a). The ERβ antagonist (2-Phenyl-5,7-bis(trifluoromethyl) pyrazolo[1,5-a]pyrimidin-3-yl]phenol, PHTPP) did not abrogate the ability of 17β-estradiol to inhibit HIV replication in PBMCs (Fig.3b). Together, these findings indicate that the mechanism by which 17β-estradiol mediates HIV inhibition is dependent on ERα and is independent of ERβ. To determine the step of the HIV life cycle whereby 17β-estradiol may exert its inhibitory effect, PBMCs were infected with HIVBal and HIV reverse transcription, integration, and transcription were assessed by conventional real time PCR-based assays. 17β-estradiol inhibited HIV transcription by approximately 40% and had no effect on HIV reverse transcription or integration (Fig.4c-e).
Fig. 3. 17β-estradiol engages ERα and not ERβ to repress HIV replication.
PBMCs were stimulated with anti-CD3/CD28 antibodies, infected with HIVBal, and treated with 17β-estradiol (1.5nM) with or without an ERα agonist (PPT) at 1mM (a) or an ERβ antagonist (PHTPP) at 100 nM (b). HIV p24 ELISA was performed at day seven post infection. Data is representative of a minimum of four donors, performed in quadruplicate. Data was analyzed by Student T-test; *p <0.05.
Fig. 4. 17β-estradiol inhibits HIV transcription.
PBMCs were stimulated with anti-CD3/CD28 antibodies, infected with HIVBal and treated with 17β-estradiol (1.5nM or 3nM) or left untreated. Genomic DNA was isolated at day one post infection to measure HIV reverse transcription by PCR (a). Genomic DNA was isolated at day three post infection to measure HIV integration by Alu PCR (b). Cellular RNA was isolated at day three post infection to measure HIV transcripts by RT-PCR (c). Data in a-c is representative of a minimum of three experiments. Data was analyzed by Student T-test; *p <0.05; **p <0.01.
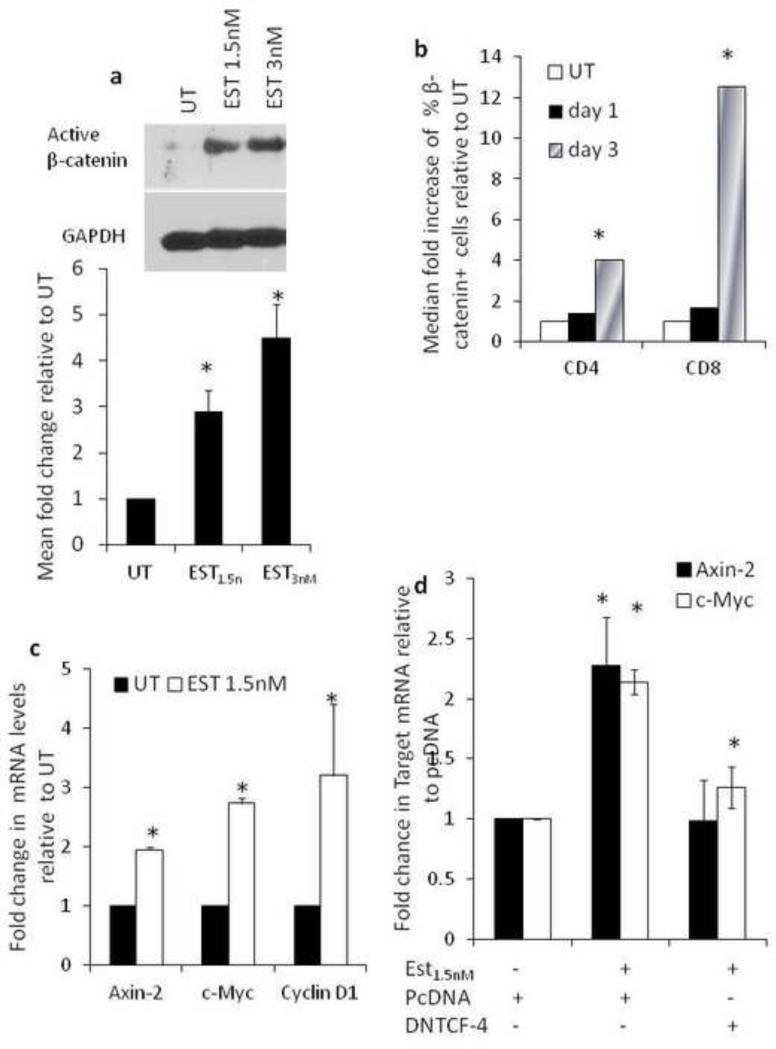
17β-estradiol induces β-catenin signaling
Given that 17β-estradiol inhibited HIV transcription and we defined β-catenin through its interaction with TCF-4 as a potent repressor of HIV transcription (Henderson et al., 2012; Kumar et al., 2008; Narasipura et al., 2012), we evaluated the relationship between 17β-estradiol and β-catenin signaling. 17β-estradiol induced active β-catenin expression in PBMCs as evaluated by western blot (Fig. 5a) and intracellular staining for β-catenin (Fig.5b). 17β-estradiol also induced classical target genes of β-catenin-mediated transcription such as axin-2, c-Myc, and cyclinD1 (Fig. 5c). Inhibition of TCF-4 through transfection with a dominant negative TCF-4 construct abrogated the ability of 17β-estradiol to induce axin-2 mRNA, indicating that 17β-estradiol-mediated induction of axin-2 is β-catenin specific (Fig. 5d). Collectively, these data demonstrate that 17β-estradiol activates β-catenin signaling. This is the first study, to our knowledge, to demonstrate 17β-estradiol activation of β-catenin signaling in PBMCs.
Fig. 5. 17β-estradiol induces β-catenin signaling.
PBMCs were stimulated and treated with 17β-estradiol (1.5nM) for three days. Western blot and densitometry quantification for active -catenin measured by Western blot is shown in (a). Flow cytometric analysis of active β-catenin expression in CD4+ and CD8+ T cells is shown in (b). Level of downstream targets of Wnt/β-catenin pathway (axin-2, c-Myc, cyclin D-1) post 17β estradiol treatment performed by real time RT-PCR is shown in (c). GAPDH was used as endogenous control and data shown as fold change relative to untreated cells. In (d) PBMCs were stimulated with antiCD3/CD28 for 48h and nucleofected with a dominant negative plasmid for TCF-4 (dnTCF4) or backbone vector (pcDNA). Post nucleofection the cells were treated with 17β-estradiol (1.5nM) for three days and axin-2 and c-myc mRNA level was evaluated by real-time RT-PCR. GAPDH was used as endogenous control. Data is representative of a minimum of three experiments and analyzed by Student T-test; *p<0.05.
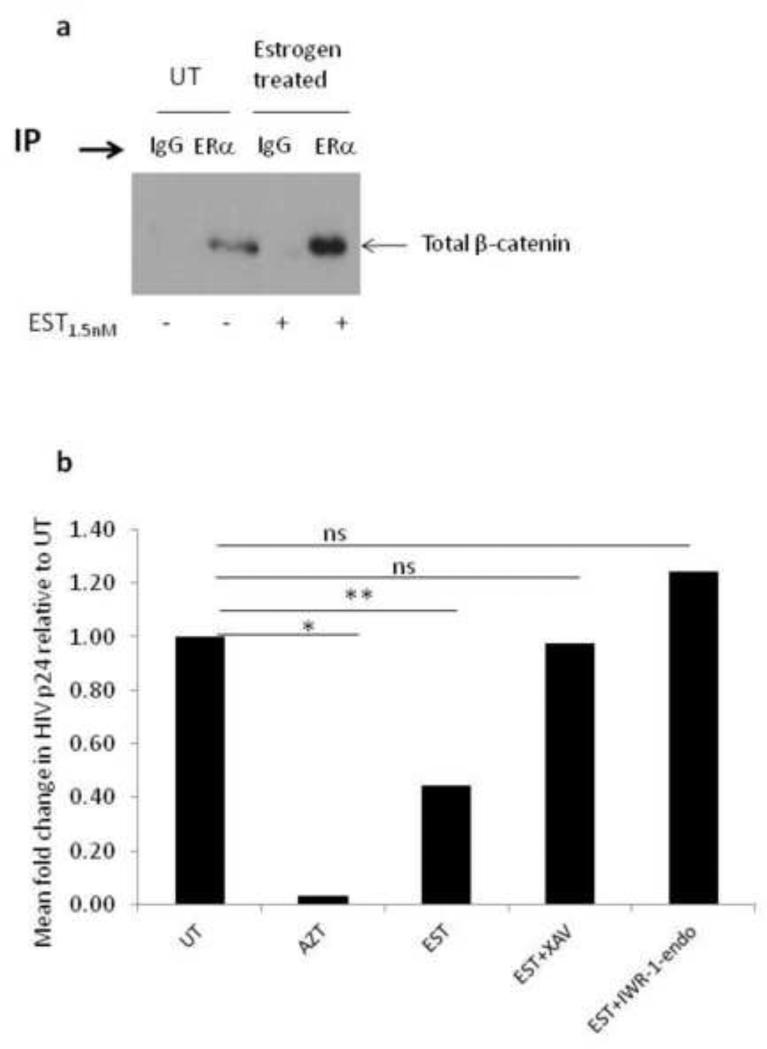
17β-estradiol induces the stable association of β-catenin and ERα
We assessed the interaction between ERα and β-catenin in PBMCs by immunoprecipitation (IP) and western blot (WB). While β-catenin was pulled down by ERα IP, the level of β-catenin pulled down by ERα IP was significantly higher when the cells were treated with 17β-estradiol (Fig.6a). These data indicate that 17β-estradiol potentiates the interaction between ERα and β-catenin, which maybe driven by higher level of β-catenin in response to 17β-estradiol or stabilization of β-catenin in presence of 17β-estradiol to promote its interaction with ERα.
Fig. 6. 17β-estradiol-mediated inhibition of HIV replication is β-catenin-dependent.
PBMCs were stimulated with anti-CD3/CD28 antibodies for 48h then left untreated or treated with 17β-estradiol (1.5nM). At day three, the cells were harvested, lysed, and the protein immunoprecipitated (IP) using ERα antibodies or IgG control then probed with β-catenin (a). In (b) the ability of small molecule inhibitors of β-catenin to abrogate 17β-estradiol-mediated inhibition of HIV replication is shown. Specifically, PBMCs were stimulated with anti-CD3/CD28 , infected with HIVBal , left untreated or treated with 17β-estradiol at 1.5nM with or without small molecule inhibitors of β-catenin (XAV at 1mM and IWR-1-endo at 2.5mM ) or treated with 1μM AZT. HIV p24 was measured by ELISA on day seven post-infection. HIV p24 levels form XAV and IWR-1-endo treatments alone, without 17β-estradiol, was not statistically different than untreated cultures. Data is representative of a minimum of three experiments and analyzed by Student T-test; *p<0.05, NS indicate not significant (p>0.05).
Inhibiting β-catenin abrogates the ability of 17β-estradiol to inhibit HIV replication
XAV and IWR-1 Endo are two small molecules described to inhibit β-catenin signaling by stabilizing axin-2, a scaffolding protein that is part of the β-catenin destruction complex (Huang et al., 2009). By stabilizing axin-2, β-catenin is phosphorylated, ubiquitinated, and tagged for proteasomal degradation. Treating HIV infected PBMCs in the presence of 17β-estradiol and XAV or IWR-1endo abrogated 17β-estradiol inhibition of HIV replication (Fig. 6b). Collectively, these data indicate that the mechanism by which 17β-estradiol mediates HIV inhibition is both ERα dependent and β-catenin dependent.
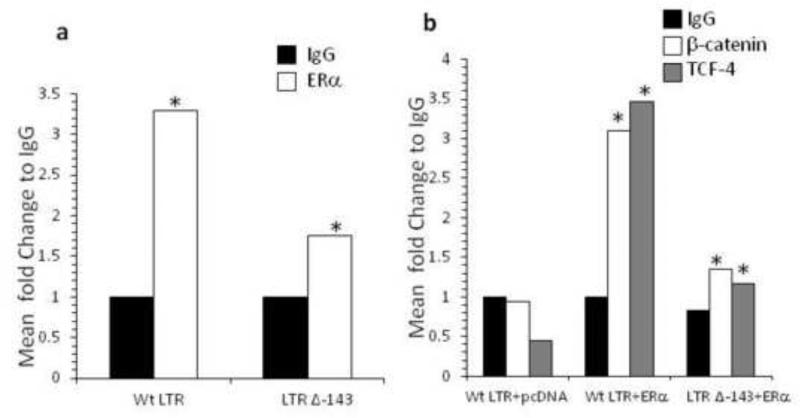
ERα, β-catenin, and TCF-4 associate with the HIV LTR at −143 nt site from transcription initiation site
We evaluated whether ERα, β-catenin, and TCF-4 are associated with the HIV LTR to repress transcription. β-catenin does not bind DNA directly rather acts as a transcription co-factor by binding to TCF/LEF transcription factors. Recently several TCF-4 binding sites were identified on the HIV LTR (Henderson et al., 2012). One in particular, at −143 from +1 transcription start site (−143 to −136 nt) is where TCF-4 and β-catenin associate with the nuclear matrix protein SMAR1 to pull the HIV DNA towards the nuclear matrix and away from RNA pol II transcriptional machinery (Henderson et al., 2012). We assessed whether ERα tethers at −143 by chromatin immunoprecipitation. Jurkat cells were transfected with ERα plasmid and WT-LTR or an LTR deleted in −143 from the +1 start site (LTR Δ −143). The chromatin was pulled down using IgG or ERα antibody and the DNA amplified for HIV LTR. We show that deletion of −143 dramatically diminished the association of ERα with the HIV LTR at the −143 site (Fig. 7a). Further, β-catenin and TCF-4 were confirmed to associate with the HIV LTR at −143 (Fig. 7b). Collectively, these data demonstrate that ERα, β-catenin, and TCF-4 all associate at −143 nt site of the HIV LTR.
Fig. 7. Tethering of ERα, β-catenin, and TCF-4 at −143 site on the HIV LTR.
Jurkat cells were transfected with wild-type (WT) LTR or LTR deleted in −143 (LTR Δ −143, deletion from −143 to −136 nt from +1 site) with or without ERα plasmid. ChIP was performed with either pull down with ERα antibody (a) or β-catenin or TCF-4 antibodies (b) and DNA amplified for HIV LTR. Data represent fold change in comparison to IgG of at least two independent experiments. Asterisks denote p<0.05 in comparison to IgG determined by Student T-test.
Discussion
17β-estradiol levels greatly fluctuate during the menstrual cycle, reaching peak levels in the preovulatory phase (>690 pmol/l to <2,120pmol/l) and declining in the luteal phase (>300 to <710 pmol/l) (Harlow and Ephross, 1995). The level of 17β-estradiol is also highly variable among women and within the same woman from one menstrual cycle to the next. For this reason, we evaluated a wide dose of 17β-estradiol effects on HIV replication in PBMCs. We show here that 17β-estradiol at a physiologic dose reported during the preovulatory phase of women of reproductive age (1.5nM equivalent to 1500pmol/l) (Harlow and Ephross, 1995) inhibits HIV replication in PBMCs at the level of transcription by engaging the association between β-catenin, TCF-4 and ERα at −143 nt site of the HIV LTR. The −143 nt site of the HIV LTR is of a particular importance because it was recently demonstrated to be associated with β-catenin/TCF-4/and SMAR protein to inhibit POLII docking and repress HIV transcription (Henderson et al., 2012). Based on our data we propose that under low level of 17β-estradiol minimal interaction between ERα and β-catenin occurs, which does not protect β-catenin from degradation. However, under a 17β-estradiol dominant state, the interaction between ERα and β-catenin is induced leading to stabilization of β-catenin, its subsequent translocation into the nucleus, and its association with TCF-4 to repress HIV LTR activity. Although the LTR has estrogen responsive elements (EREs) at 343 nt and −231 nt from transcription initiation site, those sites are not relevant to the association between β-catenin and ERα on the HIV LTR, as deletion of −143nt site alone abrogated the tethering of β-catenin, TCF-4, and ERα on the HIV LTR.
Our findings suggest that fluctuations in 17β-estradiol levels during the menstrual cycle and across the reproductive stage of women may impact HIV load and in turn HIV pathogenesis and transmission. Indeed, some studies reported that HIV genital shedding is highest during menses (low estrogen) and declines during the follicular stage (high estrogen). Other studies found that the menstrual cycle leads to a decline in plasma HIV load in the follicular to mid-luteal stage (high estrogen) without impacting HIV vaginal shedding (Greenblatt et al., 2000; Hanna, 1999; Reichelderfer et al., 2000; Reichelderfer et al., 2002; Villanueva et al., 2002). Mid-secretory levels of estrogen and progesterone, together, were also found to inhibit HIV replication in vitro in PBMCs (Asin et al., 2008). However, depending on the estrogen dose, there was either no effect or negligible effect on HIV replication in this study (Asin et al., 2008). This may be reflection of several experimental factors, the most important of which is the use of charcoal stripping in these studies. In our study, the ability of 17β-estradiol to inhibit HIV replication in PBMCs was conducted without charcoal stripping. Historically, charcoal stripping of FBS was performed to remove endogenous steroid hormones, especially in breast and cervical cancer cell lines that are highly enriched in steroid production. However, emerging evidence indicates that charcoal stripping eliminates other metabolites essential for cellular health (Cao et al., 2009). In our experience, charcoal stripping of FBS compromises the survival of PBMCs (data not shown). For this reason and because untreated cultures serve as background for endogenous 17β-estradiol/steroids levels, we have elected not to use charcoal stripping in our cultures and showed that 17β-estradiol at preovulatroy doses inhibits HIV replication and revealed the mechanism by which it does so .
Cross-talk between 17β-estradiol and Wnt/β-catenin has been described in mouse and human epithelial cells (Hou et al., 2004) (Kouzmenko et al., 2004). We provide the first evidence in human PBMCs that 17β-estradiol induce ERα and β-catenin interaction to potentiate HIV repression. In human and mouse uterine epithelial cells, 17β-estradiol induces β-catenin gene expression and activates a number of Wnt proteins, including Wnt4, Wnt5a, and frizzled receptor 2 (Hou et al., 2004). Further, ERα and β-catenin in human colon and breast cancer cells precipitate within the same immune complexes (Kouzmenko et al., 2004), demonstrating that ERα and β-catenin are physically associated with each other. Exogenous 17β-estradiol further enhances this physical interaction of ERα and β-catenin. The result of ERα and β-catenin physical association is a reciprocal regulation of cognate target genes (Kouzmenko et al., 2004).
ERα represses the replication of a number of viruses, including HCV, HBV, HSV, and rubella (Gillgrass et al., 2010; Hayashida et al., 2010; Murakami et al., 2013; Roehrig, Brawner, and Riggs, 1979; Vicetti Miguel et al., 2010; Wang et al., 2012). We show here that ERα also inhibits HIV replication. These findings indicate that ERα engages direct anti-viral pathways to limit replication of some viruses, which may mediate gender-specific differences in susceptibility to virus infection and/or spread. In the case of HCV, women are more likely to clear HCV in acute stage than men and female sex hormones have been suggested as a potential factor for better clearance (Bakr et al., 2006). Some studies have also alluded to women having a 2-6 fold lower plasma viral load than men during acute and chronic HIV infection (Delmas et al., 1997; Gandhi et al., 2002; Lemly et al., 2009; Meditz et al., 2011; Sterling et al., 1999; Sterling et al., 2001), although additional studies are needed to assess the direct contribution of female sex hormones on HIV replication and progression in the clinical setting suggest.
Research Highlights.
17β-estradiol, the most bioactive form of estrogen, inhibits HIV replication in PBMCs
17β-estradiol, in an ERα dependent manner, inhibits HIV through engaging β-catenin
ERα and β-catenin tether on the HIV LTR to repress HIV promoter activity
Our findings add HIV to a growing list of viruses which are inhibit by 17β-estradiol
Acknowledgments
We thank the participants in this study for donating blood. The following reagents were obtained from NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, Frederick, MD: Human recombinant interleukin (rIL-2) provided by Dr. Maurice Gately, Hoffman-LaRoche Inc, HIV-1Ba-L provided by Dr. Suzanne Gartner, Dr. Mikulas Popovic and Dr. Robert Gallo, and HIVIIIB provided from Dr. Robert Gallo to the repository.
Funding: This work was supported by funding from the National Institute of Allergy and Infectious Diseases [PO A108297] and the National Institute of Neurological Disorders and Stroke [R011NS060632].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Harthi L. Interplay Between Wnt/beta-Catenin Signaling and HIV: Virologic and Biologic Consequences in the CNS. J Neuroimmune Pharmacol. 2012;7(4):731–9. doi: 10.1007/s11481-012-9411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asin SN, Heimberg AM, Eszterhas SK, Rollenhagen C, Howell AL. Estradiol and progesterone regulate HIV type 1 replication in peripheral blood cells. AIDS Res Hum Retroviruses. 2008;24(5):701–16. doi: 10.1089/aid.2007.0108. [DOI] [PubMed] [Google Scholar]
- Bakr I, Rekacewicz C, Hosseiny ME, Ismail S, Daly ME, El-Kafrawy S, Esmat G, Hamid MA, Mohamed MK, Fontanet A. Higher clearance of hepatitis C virus infection in females compared with males. Gut. 2006;55(8):1183–7. doi: 10.1136/gut.2005.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussel A, Sonigo P. Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol. 2003;77(18):10119–24. doi: 10.1128/JVI.77.18.10119-10124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, West C, Norton-Wenzel CS, Rej R, Davis FB, Davis PJ. Effects of resin or charcoal treatment on fetal bovine serum and bovine calf serum. Endocr Res. 2009;34(4):101–8. doi: 10.3109/07435800903204082. [DOI] [PubMed] [Google Scholar]
- Delmas MC, Jadand C, De Vincenzi I, Deveau C, Persoz A, Sobel A, Kazatchkine M, Brunet JB, Meyer L. Gender difference in CD4+ cell counts persist after HIV-1 infection. SEROCO Study Group. Aids. 1997;11(8):1071–3. [PubMed] [Google Scholar]
- Fahey JV, Prabhala RH, Guyre PM, Wira CR. Antigen-presenting cells in the human female reproductive tract: analysis of antigen presentation in pre and post-menopausal women. Am J Reprod Immunol. 1999;42(1):49–57. doi: 10.1111/j.1600-0897.1999.tb00465.x. [DOI] [PubMed] [Google Scholar]
- Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185(11):1606–13. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- Fung KY, Mangan NE, Cumming H, Horvat JC, Mayall JR, Stifter SA, De Weerd N, Roisman LC, Rossjohn J, Robertson SA, Schjenken JE, Parker B, Gargett CE, Nguyen HP, Carr DJ, Hansbro PM, Hertzog PJ. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science. 2013;339(6123):1088–92. doi: 10.1126/science.1233321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35(3):313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- Gillgrass A, Chege D, Bhavanam S, Kaushic C. Estradiol limits viral replication following intravaginal immunization leading to diminished mucosal IgG response and non-sterile protection against genital herpes challenge. Am J Reprod Immunol. 2010;63(4):299–309. doi: 10.1111/j.1600-0897.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Greenblatt RM, Ameli N, Grant RM, Bacchetti P, Taylor RN. Impact of the ovulatory cycle on virologic and immunologic markers in HIV-infected women. J Infect Dis. 2000;181(1):82–90. doi: 10.1086/315207. [DOI] [PubMed] [Google Scholar]
- Hanna L. The menstrual cycle and viral load. Beta. 1999;12(2):18. [PubMed] [Google Scholar]
- Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women's health. Epidemiol Rev. 1995;17(2):265–86. doi: 10.1093/oxfordjournals.epirev.a036193. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Shoji I, Deng L, Jiang DP, Ide YH, Hotta H. 17beta-estradiol inhibits the production of infectious particles of hepatitis C virus. Microbiol Immunol. 2010;54(11):684–90. doi: 10.1111/j.1348-0421.2010.00268.x. [DOI] [PubMed] [Google Scholar]
- Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Jolobo E, Ngure K, Kiarie J, Coombs RW, Baeten JM. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LJ, Narasipura SD, Adarichev V, Kashanchi F, Al Harthi L. Identification of Novel T Cell Factor 4 (TCF 4) Binding Sites on the HIV Long Terminal Repeat Which Associate with TCF-4, beta-Catenin, and SMAR1 To Repress HIV Transcription. J Virol. 2012;86(17):9495–503. doi: 10.1128/JVI.00486-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen mediated uterine growth. Mol Endocrinol. 2004;18(12):3035–49. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461(7264):614–20. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- James CB, Vanderpool EA, Roane P. Acceleration of adenovirus replication and increased virion production by treatment with the steroid hormone 17 beta-estradiol. Microbiol Immunol. 1992;36(1):99–103. doi: 10.1111/j.1348-0421.1992.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39(3):209–16. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Kouzmenko AP, Takeyama K. i., Ito S, Furutani T, Sawatsubashi S, Maki A, Suzuki E, Kawasaki Y, Akiyama T, Tabata T, Kato S. Wnt/{beta}-Catenin and Estrogen Signaling Converge in Vivo 10.1074/jbc.C400331200. J. Biol. Chem. 2004;279(39):40255–40258. doi: 10.1074/jbc.C400331200. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zloza A, Moon RT, Watts J, Tenorio AR, Al-Harthi L. Active {beta} catenin signaling is an inhibitory pathway of HIV replication in peripheral blood mononuclear cells. J Virol. 2008;82(6):2813–20. doi: 10.1128/JVI.02498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemly DC, Shepherd BE, Hulgan T, Rebeiro P, Stinnette S, Blackwell RB, Bebawy S, Kheshti A, Sterling TR, Raffanti SP. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;199(7):991–8. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Henderson LJ, Major EO, Al Harthi L. IFN-{gamma} Mediates Enhancement of HIV Replication in Astrocytes by Inducing an Antagonist of the {beta}-Catenin Pathway (DKK1) in a STAT 3 Dependent Manner. J Immunol. 2011;186(12):6771–8. doi: 10.4049/jimmunol.1100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu FX, Abel K, Ma Z, Rourke T, Lu D, Torten J, McChesney M, Miller CJ. The strength of B cell immunity in female rhesus macaques is controlled by CD8+ T cells under the influence of ovarian steroid hormones. Clin Exp Immunol. 2002;128(1):10–20. doi: 10.1046/j.1365-2249.2002.01780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics. 2006;7(8):497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meditz AL, MaWhinney S, Allshouse A, Feser W, Markowitz M, Little S, Hecht R, Daar ES, Collier AC, Margolick J, Kilby JM, Routy JP, Conway B, Kaldor J, Levy J, Schooley R, Cooper DA, Altfeld M, Richman D, Connick E. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203(4):442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15(8):955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money DM, Arikan YY, Remple V, Sherlock C, Craib K, Birch P, Burdge DR. Genital tract and plasma human immunodeficiency virus viral load throughout the menstrual cycle in women who are infected with ovulatory human immunodeficiency virus. Am J Obstet Gynecol. 2003;188(1):122–8. doi: 10.1067/mob.2003.65. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Fukasawa M, Kaneko Y, Suzuki T, Wakita T, Fukazawa H. Selective estrogen receptor modulators inhibit hepatitis C virus infection at multiple steps of the virus life cycle. Microbes Infect. 2013;15(1):45–55. doi: 10.1016/j.micinf.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Narasipura SD, Henderson LJ, Fu SW, Chen L, Kashanchi F, Al Harthi L. Role of beta-catenin and TCF/LEF family members in transcriptional activity of HIV in astrocytes. J Virol. 2012;86(4):1911–21. doi: 10.1128/JVI.06266-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152(5):1247–58. [PMC free article] [PubMed] [Google Scholar]
- Reichelderfer PS, Coombs RW, Wright DJ, Cohn J, Burns DN, Cu-Uvin S, Baron PA, Coheng MH, Landay AL, Beckner SK, Lewis SR, Kovacs AA. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. WHS 001 Study Team. Aids. 2000;14(14):2101–7. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- Reichelderfer PS, Kovacs A, Wright DJ, Landay A, Cu-Uvin S, Burns DN, Cohn J, Coombs RW. The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis. 2002;186(5):726–8. doi: 10.1086/342051. author reply 728-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97(1):74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrig JT, Brawner TA, Riggs HG., Jr. Effects of 17beta estradiol on the replication of rubella virus in an estrogen-responsivef, continuousff cell line. J Virol. 1979;29(1):417–20. doi: 10.1128/jvi.29.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba E, Origoni M, Taccagni G, Ferrari D, Doglioni C, Nava A, Lisco A, Grivel JC, Margolis L, Poli G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295(5564):2465–8. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180(3):666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- Sterling TR, Pisell-Noland T, Perez JL, Astemborski J, McGriff JR, Nutting L, Hoover DR, Vlahov D, Bollinger RC. Sex-based differences in T lymphocyte responses in HIV-1-seropositive individuals. J Infect Dis. 2005;191(6):881–5. doi: 10.1086/427827. [DOI] [PubMed] [Google Scholar]
- Sterling TR, Vlahov D, Astemborski J, Hoover DR, Margolick JB, Quinn TC. Initial plasma HIV-1 RNA levels and progression to AIDS in women and men. N Engl J Med. 2001;344(10):720–5. doi: 10.1056/NEJM200103083441003. [DOI] [PubMed] [Google Scholar]
- Vicetti Miguel RD, Sheridan BS, Harvey SA, Schreiner RS, Hendricks RL, Cherpes TL. 17-beta estradiol promotion of herpes simplex virus type 1 reactivation is estrogen receptor dependent. J Virol. 2010;84(1):565–72. doi: 10.1128/JVI.01374-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva JM, Ellerbrock TV, Lennox JL, Bush TJ, Wright TC, Pratt-Palmore M, Evans-Strickfaden T, Conley LJ, Schnell C, Hart CE. The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis. 2002;185(2):170–7. doi: 10.1086/338447. [DOI] [PubMed] [Google Scholar]
- Wang SH, Yeh SH, Lin WH, Yeh KH, Yuan Q, Xia NS, Chen DS, Chen PJ. Estrogen receptor alpha represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4alpha. Gastroenterology. 2012;142(4):989–998. e4. doi: 10.1053/j.gastro.2011.12.045. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Enomoto L, Marcus R, Canniff J. Effect of menstrual cycle variation in female sex hormones on cellular immunity and regulation. J Reprod Immunol. 2011;89(1):70–7. doi: 10.1016/j.jri.2010.11.009. [DOI] [PubMed] [Google Scholar]