Abstract
Phosphatidylinositol phosphates are key regulators of vesicle identity, formation and trafficking. In mammalian cells, the evolutionarily conserved class III PtdIns 3-kinase PIK3C3/VPS34 is part of a large multiprotein complex that catalyzes the localized phosphorylation of phosphatidylinositol to phosphatidylinositol-3-phosphate (PtdIns3P). We demonstrate that PIK3C3 has a key function in vesicular trafficking, endocytosis and autophagosome-autolysosome formation in the highly specialized glomerular podocytes.
Keywords: podocyte, Vps34, phosphoinositide 3-kinase, Pik3c3, endocytosis, autophagy, autophago-lysosomal formation, glomerulosclerosis, proteinuria
Postmitotic podocytes are specialized cells of the renal glomerulus that maintain a complex cytoarchitecture to form the glomerular filtration barrier. Previously, we determined that autophagy has a major impact on glomerular disease susceptibility and on maintaining homeostasis in aging podocytes. More recently, it was demonstrated that defects in multivesicular body formation are associated with increased susceptibility to glomerular disease, suggesting the importance of an intact endocytosis machinery for the integrity of the glomerular filter. However, the signaling crosstalk between endocytosis and signal transduction in this specialized cell population remained elusive.
To elucidate the specific role of phosphatidylinositol signaling, endocytosis and vesicle trafficking in podocytes, we generated a podocyte-specific PIK3C3-deficient mouse model (Pik3c3 conditional knockout, cKO). Even though PIK3C3-deficient mice are born without an apparent phenotype, they develop early onset proteinuria and end-stage renal disease, dying within 3 to 9 weeks after birth.
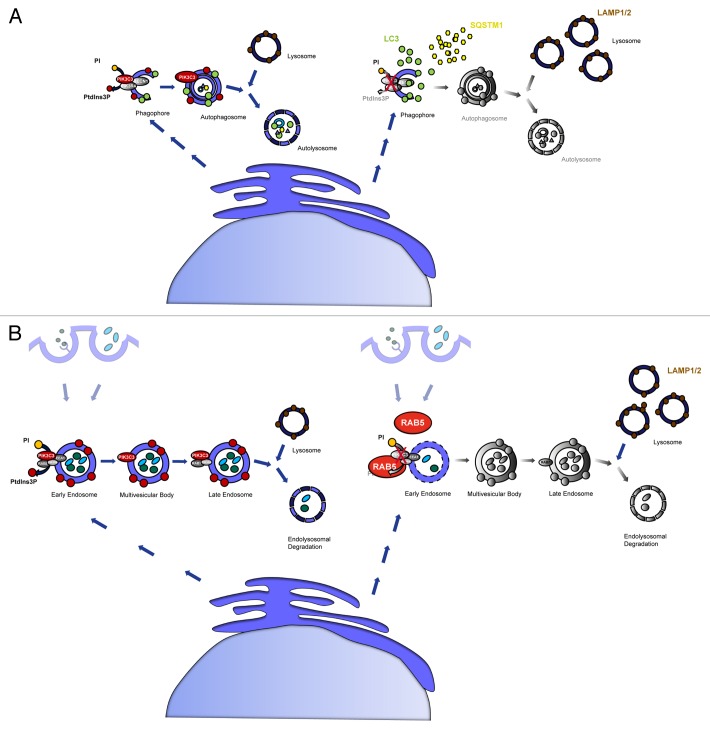
Histologically, PIK3C3-deficient podocytes display substantial cytoplasmic single-membrane vacuoles and show signs of rapid degeneration and glomerulosclerosis. We observed increased levels of LC3-II and the ubiquitin-binding protein SQSTM1/p62 in cKO mice suggesting that autophagosome turnover is impaired, while LC3-I conjugation to phosphatidylethanolamine (PE) can still occur in the absence of PIK3C3 (Fig. 1A). We next performed immuno-gold EM analyses to further define the localization of LC3. Intriguingly, the diffuse cytosolic distribution of anti-LC3 gold particles lack any association with membrane structures indicating that PIK3C3-deficient podocytes had a defect in early autophagosome formation. Consistently, the immensely accumulated vacuoles in PIK3C3-deficient podocytes are not labeled with anti-LC3 gold particles and thus do not represent autophagosomes. In agreement with these observations, confocal imaging demonstrated that LC3 does not colocalize with LAMP1/2 in PIK3C3-deficient podocytes, indicating an absence of autolysosome formation.
Figure 1. Schematic illustration of PIK3C3-deficiency in podocytes. (A) PIK3C3-deficiency leads to incomplete formation of the autophagosomal membrane, resulting in deficient autophagy and autophagosome-lysosome fusion. LC3 can still be converted from LC3-I to LC3-II, but no functional autophagosomes are formed. This leads to an accumulation of non-degraded LC3 and SQSTM1 as well as to an accumulation of vacant lysosomes, and the accumulation of LAMP1/2. The absence of PtdIns3P causes a blockade in autophagosome formation and autophagosome-lysosome fusion. (B) Endosomal trafficking is blocked in PIK3C3-deficient podocytes. Lack of PtdIns3P production inhibits fluid phase uptake, receptor-mediated endocytosis and the maturation of the early endosome to the late endosome resulting in an accumulation of RAB5. RAB7, a marker for the late endosome, is not affected. LAMP1/2, markers for the lysosome, are accumulated indicating unused lysosomes due to insufficient endo-lysosomal fusion.
For further in vitro analyses of dynamic cellular mechanisms of PIK3C3 we generated podocyte-specific, GFP-positive PIK3C3-deficient mice. Primary cell cultures of FACS-sorted PIK3C3-deficient podocytes display substantial perinuclear vacuolization compared with GFP-positive control cells. In line with our observations in kidney sections of cKO and control mice, LC3 and SQSTM1 accumulate in KO podocytes. Upon starvation, chloroquine treatment of wild-type control cells leads to an accumulation of LC3-II, representative of autophagic flux. KO podocytes, in contrast, already have high LC3-II levels under normal conditions, and show only a small incremental increase upon treatment with chloroquine, confirming that autophagic flux is largely disrupted in PIK3C3-deficient podocytes.
Our earlier studies demonstrated that specific disruption of macroautophagy in podocytes by deletion of Atg5 leads to late-onset proteinuria and glomerulosclerosis. Conditionally PIK3C3-deficient mice, however, develop large-sized vacuoles and unprocessed endosomes within the podocyte cytoplasm resulting in rapid cell degeneration and early lethality of the animals. To test whether defective autophagy is the underlying mechanism explaining the severe phenotype in PIK3C3-deficient mice, we generated Atg5 Pik3c3 double-cKO (dcKO) mice and compared them to autophagy-deficient single Atg5 cKO and Pik3c3 cKO mice. Atg5 Pik3c3 dcKO mice exhibit an analogous phenotype to Pik3c3 cKO mice. Impaired autophagy is comparable in Pik3c3 cKO mice and Atg5 Pik3c3 dcKO. The late-onset degeneration of podocytes due to ATG5 deficiency is thus both histologically and phenotypically utterly distinct from that caused by PIK3C3 deficiency, indicating that lack of autophagy is not the main reason underlying the rapid degeneration of PIK3C3-deficient podocytes. Due to the disparities of PIK3C3 and autophagy-deficient mice, we hypothesized that PIK3C3-mediated endocytosis might be of particular importance for podocyte maintenance.
To functionally address the question of impaired endocytosis in PIK3C3-deficient podocytes, we performed FITC-dextran fluid phase uptake and receptor-mediated streptavidin uptake assays to monitor endosome/macropinosome formation. Both dextran uptake as well as receptor-mediated streptavidin uptake into GFP-positive Pik3c3 KO primary podocytes are impaired in the presence of (biotinylated) epidermal growth factor (EGF) indicating disturbed fluid phase uptake and disturbed receptor-mediated endocytosis in PIK3C3-deficient podocytes. We observed that KO podocytes demonstrate substantial accumulation of RAB5, while no significant differences of the late endosomal marker RAB7 are seen, indicating a block in early endosomal maturation (Fig. 1B). Endocytic mechanisms are highly conserved features of filtering cells. To assess whether PIK3C3-mediated vesicle maturation is a general theme of filtering cells, we confirmed the disruption of early endosomal sorting in Garland cell nephrocytes (GCN), the so-called “storage kidneys” in Drosophila melanogaster. In fact, immunofluorescence staining with ZFYVE20/Rabenosyn-5, a marker for the early endosome in D. melanogaster, demonstrate an unequivocal disruption of the early endosomal compartment upon Pik3c3 knockdown.
A large number of distinct endocytic pathways exist to allow the internalization of nutrients, solutes and growth factors. The disruption at the early steps of these endocytosis mechanisms is likely to abrogate podocyte-specific functions. To this end, it is not clear which exact subset of endocytic mechanisms is mostly affected in podocyte physiology. Several studies have previously demonstrated that growth factor pathways are significantly regulated by endocytosis of their respective receptors. Hence, essential growth factor signaling axes, such as vascular epithelial growth factor (VEGF) and epidermal growth factor (EGF) pathways, might be affected by the disruption of PIK3C3-controlled endocytosis in podocytes. However, in our study, we not only observed an almost complete abrogation of receptor-mediated endocytosis in PIK3C3-deficient podocytes but also observed fluid phase uptake to be seriously compromised in PIK3C3-deficient podocytes. Thus, we speculate that our data point to the importance of receptor-mediated endocytosis and fluid phase uptake for the maintenance of the filtration barrier.
In summary, we demonstrated that PIK3C3 controls autophagic flux in the kidney glomerulus and elucidated its major role in regulating endo-lysosomal pathways in podocytes (Fig. 1). Our comparative studies of podocyte-specific PIK3C3-deficient and autophagy-deficient as well as double-deficient mice suggest that defective autophagy is not primarily responsible for the early phenotype caused by the loss of PIK3C3. Most importantly, we highlight the fundamental role of endocytosis and fluid phase uptake for the maintenance of the glomerular filtration barrier.
The specific effect of PIK3C3 depletion upon other signaling pathways such as nutrient-sensing pathways upstream of MTOR activation in podocytes will need to be addressed in future investigations.
Acknowledgments
This work was supported by the Marie Curie Career Integration Grant (W.B.), the Margarete von Wrangell Habilitations-Stipendium (W.B.) and by the Deutsche Forschungsgesellschaft DFG grants KFO 201 (Project P7, T.B.H.), SFB 992 (Project B5, T.B.H.) as well as the Joint transnational Grant—BMBF (T.B.H.). In addition, the study was supported by the Excellence Initiative of the German Federal and State Governments EXC 294 (to T.B.H.) and BMBF GerontoSys2-Project NephAge (to T.B.H.) and GSC-4 (Spemann Graduate School, to C.S.).
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24634