Abstract
Evasion of apoptosis, which enables cells to survive and proliferate under metabolic stress, is one of the hallmarks of cancer. We have recently reported that SH3GLB1/Bif-1 functions as a haploinsufficient tumor suppressor to prevent the acquisition of apoptosis resistance and malignant transformation during Myc-driven lymphomagenesis. SH3GLB1 is a membrane curvature-inducing protein that interacts with BECN1 though UVRAG and regulates the post-Golgi trafficking of membrane-integrated ATG9A for autophagy. At the premalignant stage, allelic loss of Sh3glb1 enhances Myc-induced chromosomal instability and results in the upregulation of anti-apoptotic proteins, including MCL1 and BCL2L1. Notably, we found that Sh3glb1 haploinsufficiency increases mitochondrial mass in overproliferated prelymphomatous Eμ-Myc cells. Moreover, loss of Sh3glb1 suppresses autophagy-dependent mitochondrial clearance (mitophagy) in PARK2/Parkin-expressing mouse embryonic fibroblasts (MEFs) treated with the mitochondrial uncoupler CCCP. Interestingly, PARK2-expressing Sh3glb1-deficient cells accumulate ER-associated immature autophagosome-like structures after treatment with CCCP. Taken together, we propose a model of mitophagy in which SH3GLB1 together with the class III phosphatidylinositol 3-kinase complex II (PIK3C3CII) (PIK3R4-PIK3C3-BECN1-UVRAG) regulates the trafficking of ATG9A-containing Golgi-derived membranes (A9+GDMs) to damaged mitochondria for autophagosome formation to counteract oncogene-driven tumorigenesis.
Keywords: SH3GLB1/Bif-1, MYC, MCL1, lymphoma, apoptosis, autophagy, mitophagy, malignant transformation, DNA damage, chromosome instability
It is generally acknowledged that macroautophagy, hereafter referred to as autophagy, plays an important role in tumor cell growth by fueling cells and maintaining functional organelles. However, accumulating evidence also suggests that defects in autophagy promote tumorigenesis under certain circumstances (e.g., apoptosis defects). We have previously reported that loss of Sh3glb1/Bif-1, a gene encoding a proapoptotic BAX interacting factor, not only suppresses autophagy but also accelerates spontaneous tumor development in mice. To further understand the mechanism behind the promotion of tumorigenesis by Sh3glb1 deficiency, we utilized the Eμ-Myc mouse lymphoma model, in which expression of the Myc oncogene is deregulated by the IgH enhancer. We found that allelic loss of Sh3glb1 suppresses apoptosis in lymphomatous lymph nodes and accelerates tumor onset and mortality in Eμ-Myc transgenic mice, thus identifying Sh3glb1 as a haploinsufficient tumor suppressor gene in Eμ-Myc-induced lymphomagenesis. Notably, at the prelymphomatous stage, Sh3glb1 hemizygous Eμ-Myc splenocytes show enhanced, rather than suppressed, CASP3 activation, suggesting that loss of the BAX activating function of SH3GLB1 is insufficient to suppress apoptosis under metabolic stress. In contrast, these cells display enhanced expression of the antiapoptotic proteins MCL1 and BCL2L1, an increase in mitochondrial mass and an accumulation of genomic damage. As the autophagic machinery is essential for efficient mitochondrial turnover, defects in this process (i.e., loss of Sh3glb1) can lead to the excessive production of reactive oxygen species and chromosomal instability, thereby facilitating the acquisition of apoptosis resistance and accelerating Myc-driven tumorigenesis. In agreement with this concept, loss of Sh3glb1 suppresses CCCP-induced autophagic clearance of mitochondria as measured by a decrease in mitochondrial matrix proteins and DNAs in PARK2-expressing MEFs.
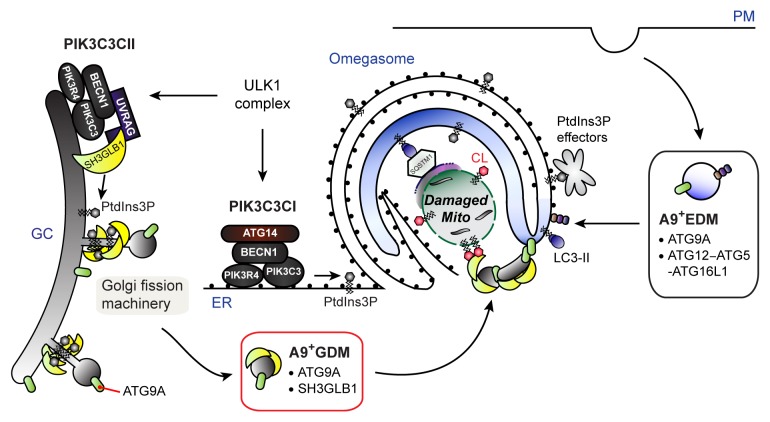
Although Sh3glb1 appears to be indispensable for autophagy-dependent mitochondrial turnover, we observe that LC3-positive autophagosome-like structures accumulate and surround CCCP-treated damaged mitochondria regardless of SH3GLB1 expression. Interestingly, loss of Atg9A or inhibition of ULK1, a pro-autophagic kinase required for the post-Golgi trafficking of ATG9A, have also been reported to have minimal effects on CCCP-induced autophagic processing of LC3. How then does SH3GLB1 regulate mitophagy? Some phagophores, the precursor structures of autophagosomes, grow within Ω-shaped ER cisternae (omegasomes) and are released from the cradles upon membrane closure. Our data shows that loss of Sh3glb1 results in the accumulation of ER-associated immature autophagosomes and suppresses the maturation of autophagosomes. Interestingly, our previous data demonstrate that SH3GLB1 and the PIK3C3CII mediate Golgi fission to regulate the trafficking of ATG9A-containing Golgi-derived membranes (A9+GDMs) for autophagosome formation. Therefore, A9+GDMs may play a key role in the completion of autophagosome formation and/or the maturation of nascent autophagosomes rather than in the formation of phagophores (Fig. 1). Notably, while the activation of PIK3C3 is required for phagophore formation, only the PIK3C3CI (PIK3R4-PIK3C3-BECN1-ATG14), but not the PIK3C3CII, can localize at or near the omegasomes to regulate this process. Each PIK3C3 complex may thus have a distinct role in the regulation of autophagosome formation during mitophagy. Interestingly, a fraction of ATG9A-positive structures localizes at autophagosome formation sites independent of ULK1 and PIK3C3. Furthermore, the plasma membrane contributes to phagophore formation, and ATG9A shuttles between the Golgi complex and endosomes. Therefore, we propose that ATG9A-containing endosome-derived membranes (A9+EDMs) may deliver ATG9A and the ATG12–ATG5-ATG16L1 complex to the omegasome for the generation and expansion of phagophores during mitophagy (Fig. 1). In this scenario, A9+EDMs and A9+GDMs can be envisioned to contain distinct factors that are required for phagophore formation and autophagosomal membrane closure, respectively. Importantly, A9+GDMs contain SH3GLB1, which is capable of interacting with the inner mitochondrial membrane lipid, cardiolipin. Since SH3GLB1 accumulates on outer mitochondrial membrane-ruptured mitochondria during CCCP treatment, SH3GLB1 may also play an important role in the delivery of A9+GDMs to cardiolipin-exposed damaged mitochondria. In addition, the self-association of Atg9 is required for the expansion of autophagosome precursors in yeast, suggesting that ATG9A on the phagophore may mediate the fusion with the A9+GDMs in a similar manner to SNARE-mediated membrane fusion. These hypotheses are currently under investigation in order to understand the precise mechanism of SH3GLB1-mediated mitophagy for the future development of cancer therapeutics.
Figure 1. A hypothetical model of SH3GLB1-mediated mitophagy. In this model, we propose that the PIK3C3CI and CII differentially regulate autophagosome formation to sequester damaged mitochondria. In response to mitochondrial damage, the PIK3C3CI generates PtdIns3P to recruit PtdIns3P effectors, and promotes omegasome formation to grow the phagophore in a ubiquitin-like conjugation systems-dependent manner to sequester damaged mitochondria. Simultaneously, SH3GLB1 together with the PIK3C3CII generates A9+GDMs by inducing the tubulation and fission of the Golgi membrane. The resultant A9+GDMs containing SH3GLB1 fuse with the phagophore and are critical for the completion of autophagosomes. The ULK1 complex may be required for specifying the activation sites of each PIK3C3 complex. Although the PtdIns3P effectors for the PIK3C3CII remain unidentified, our preliminary in vitro data indicate that SH3GLB1 can directly associate with PtdIns3P. In addition, SH3GLB1 may direct the A9+GDM to damaged mitochondria by associating with cardiolipin. Moreover, the recognition of damaged mitochondria by the phagophore may be mediated by the interaction of LC3 with LC3-interacting adaptor proteins, including SQSTM1/p62. ER, endoplasmic reticulum; GC, Golgi complex; PM, plasma membrane.
Acknowledgments
We thank Dr. Tatsuya Saitoh and Dr. Shizuo Akira (Osaka University, Japan) for the Atg9A−/− MEFs and Dr. Arthur Günzl (University of Connecticut Health Center) for the cDNA encoding PTP. This work was supported by National Institutes of Health Grant CA129682.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/24817