Abstract
Mutations in the gene superoxide dismutase 1 (SOD1) are causative for familial forms of the neurodegenerative disease amyotrophic lateral sclerosis. When the first SOD1 mutations were identified they were postulated to give rise to amyotrophic lateral sclerosis through a loss of function mechanism, but experimental data soon showed that the disease arises from a—still unknown—toxic gain of function, and the possibility that loss of function plays a role in amyotrophic lateral sclerosis pathogenesis was abandoned. Although loss of function is not causative for amyotrophic lateral sclerosis, here we re-examine two decades of evidence regarding whether loss of function may play a modifying role in SOD1–amyotrophic lateral sclerosis. From analysing published data from patients with SOD1–amyotrophic lateral sclerosis, we find a marked loss of SOD1 enzyme activity arising from almost all mutations. We continue to examine functional data from all Sod1 knockout mice and we find obvious detrimental effects within the nervous system with, interestingly, some specificity for the motor system. Here, we bring together historical and recent experimental findings to conclude that there is a possibility that SOD1 loss of function may play a modifying role in amyotrophic lateral sclerosis. This likelihood has implications for some current therapies aimed at knocking down the level of mutant protein in patients with SOD1–amyotrophic lateral sclerosis. Finally, the wide-ranging phenotypes that result from loss of function indicate that SOD1 gene sequences should be screened in diseases other than amyotrophic lateral sclerosis.
Keywords: amyotrophic lateral sclerosis, motor neuron disease, superoxide dismutase 1, loss of function
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by the progressive loss of upper and lower motor neurons. Its clinical course is relentlessly progressive and typically causes death within 3 to 5 years of onset, mostly due to respiratory failure (Haverkamp et al., 1995). Similar to other major neurodegenerative disorders, such as Alzheimer’s disease and Parkinson’s disease, ALS is typically sporadic, but 5–10% of cases have an autosomal dominant pattern of transmission and are termed ‘familial’ (Rothstein, 2009). In 1993 the first causative mutations were found within the gene encoding the enzyme, superoxide dismutase 1 (SOD1) (Deng et al., 1993; Rosen et al., 1993). Since then, over 155 SOD1 mutations have been described (although pathogenicity has not been shown for all of these changes) and these mutations account for up to 20% of familial ALS cases (Fig. 1) and 3% of sporadic ALS cases (Pasinelli and Brown, 2006; Acevedo-Arozena et al., 2011; Andersen and Al Chalabi, 2011).
Figure 1.
Diagram of human SOD1 mutations, variants and activity in the current literature. The amino acid sequence of SOD1 is shown, with the location of introns (A). One hundred and fifty-five SOD1 mutations described in patients with ALS are annotated; data are taken from the ALS online database (ALSoD, http://alsod.iop.kcl.ac.uk, January 2013) and additional literature. Note that only variations that are predicted to affect the amino acid sequence of the protein have been included. Pathogenicity has not been shown for all mutations. Mutations listed on ALSoD, InsAexon2 and E133del are the same as mutations V29insA and E133delGAA, respectively, and so have not been annotated separately. Similarly, we believe the mutation D125TT to be L126delTT and mutation E133insTT to be E132inTT. Information about highlighted structural elements was from Wang et al. (2006). Additional references are Pramatarova et al. (1995) and Kobayashi et al. (2012). †Locations where two nucleotide changes results in the same amino acid substitution; ¶Mutations which result in a frameshift and premature stop codon. (B) Diagram of human SOD1 mutations and overall enzyme activity measured in red blood cells, fibroblast and lymphoblast cell lines. Measurements from patients carrying 48 SOD1-familial ALS mutations between 1993 and December 2012; original references are cited in Supplementary Table 1. All measures fall below 100% normal activity. Three mutations found in homozygous individuals are shown on the right hand side of the figure. Red circles show measures of intrinsic activity where these are known. We note that all mutations shown here are familial, not sporadic, and have supporting data indicating they are ALS causative (Supplementary Table 1). Where more than one publication shows overall activity for an individual mutation the value from the report with the highest sample size has been plotted. Refer to supporting references for details. Het = heterozygous; Hom = homozygous.
SOD1 is ubiquitously expressed and highly conserved across species (Fridovich, 1995). The gene is composed of five exons encoding a 153 amino acid metalloenzyme, also referred to as Cu/Zn superoxide dismutase. The protein localizes to the cytoplasm, nucleus, lysosomes and intermembrane space of mitochondria (Chang et al., 1988; Keller et al., 1991; Crapo et al., 1992; Sturtz et al., 2001). It binds copper and zinc ions and forms a homodimer whose main known function is as a dismutase removing dangerous superoxide radicals by metabolizing them to molecular oxygen and hydrogen peroxide, thus providing a defence against oxygen toxicity. Recently, SOD1 has been found to be critical for repressing respiration and directing energy metabolism through integrating responses to O2, glucose and superoxide levels (through casein kinase signalling); this role is independent of its function in oxidative stress (Reddi and Culotta, 2013). Other described functions are nitration of proteins (Beckman et al., 1993), copper buffering (Culotta et al., 1997), phosphate activation (Wang et al., 1996), zinc homeostasis (Wei et al., 2001), thiol oxidation, and immunomodulation—modulation of SOD1 activity may differentially affect the NO-dependent microbicidal activity and release of cytokines by activated macrophages (Marikovsky et al., 2003). Further, SOD1 is produced at high levels that have not yet been explained by known functions, therefore, it may well play other roles both neuron-specific and generally (Reddi and Culotta, 2013).
The finding of loss of dismutase activity in patients with ALS and the distribution of ALS-causative mutations spread throughout the SOD1 gene initially suggested loss of function as a mechanism (Deng et al., 1993; Rosen et al., 1993). However, evidence for a gain of function mechanism was quick to follow from analysis of mutant SOD1 transgenic (tgSOD1) mouse models. The first of these, tgSOD1G93A, carries an ALS-causative point mutation resulting in a glycine to alanine substitution at residue 93 (Gurney et al., 1994). Currently there are more than 12 different published human mutant SOD1 transgenic strains (Joyce et al., 2011) and most of them (including tgSOD1G93A) have increased dismutase activity because they greatly overexpress transgenic mutant human SOD1 in addition to endogenous mouse SOD1 and they develop a progressive adult-onset motor phenotype, accompanied by a striking loss of lower motor neurons.
Thus a loss of function mechanism became less favoured compared with gain of function because: (i) in humans, a lack of correlation was found between SOD1 dismutase activity and aggressiveness of clinical phenotypes (Ratovitski et al., 1999); (ii) in mice, a lack of overt ALS-like phenotype was found in Sod1 null (Sod1−/−) animals, the first of which was published by Reaume et al. (1996); whereas (iii) transgenic mouse models over-expressing mutant human SOD1 have increased SOD1 activity and a loss of motor neurons that models human ALS.
The death knell for loss of function came in a seminal study published by the Cleveland laboratory (Bruijn et al., 1998) who analysed survival time in mice carrying a mutant SOD1 transgene (tgSOD1G85R) on a normal mouse background (i.e. with two copies of the endogenous mouse Sod1 gene) compared with the same transgene on a Sod1−/− background. They found no change in survival of the mice, thus concluding that survival was entirely due to a gain of function mechanism, and independent of mouse SOD1 loss of function. These findings essentially ended the debate for a role of loss of function as a contributor to SOD1-familial ALS (Bruijn et al., 1998).
SOD1 mutations remained the only known cause of ‘classical’ ALS until causative mutations in the gene TARDBP were found (Sreedharan et al., 2008), and therefore have been studied extensively in a variety of animal and cellular models (Ilieva et al., 2009). The causative gain of function is indisputable and several mechanisms by which this occurs have been proposed and comprehensively reviewed (Turner and Talbot, 2008; Ilieva et al., 2009; Rothstein, 2009). However, in recent years a number of laboratories have further investigated Sod1 knockout mice, examining their neuromuscular involvement and non-neurological features. Data from these investigations and recent experiments using transgenic SOD1 overexpressing mice have pointed to the possibility of a modifying role played by loss of dismutase activity on familial ALS disease course.
Here we review what is known about SOD1 loss of function and the evidence to suggest it may play a role in ALS pathogenesis after all, possibly through increased susceptibility to neurodegeneration. It is important and timely to consider these data because they are relevant to current therapeutic strategies to reduce the level of mutant familial ALS-SOD1 in heterozygous individuals. Such strategies could provide badly needed approaches to ameliorating the ALS phenotype, but clearly SOD1 loss has effects on both neuronal and non-nervous system tissues. SOD1 loss of function data also strongly suggest that SOD1 should be screened in disorders other than ALS.
SOD1 dismutase activity is greatly reduced in patients with SOD1-familial amyotrophic lateral sclerosis
Dismutase activity is the best characterized function of SOD1. Two other dismutases, encoded by separate genes, have been identified in mammals: SOD2 (Mn-SOD), which has manganese (Mn) as a cofactor and localizes to the mitochondrial matrix (Fridovich, 1986; Zelko et al., 2002) and SOD3 (Fe-SOD), which exists as a homotetramer and is mainly extracellular (Marklund et al., 1982). The existence of three enzymes with dismutase activity may complicate measures of SOD1 activity alone.
In patients with ALS, SOD1 activity has most commonly been measured using two methods, generally either (i) the ‘activity assay’ which measures total dismutase activity of all three enzymes and then subtracts SOD2 and SOD3 activity, to leave only SOD1 activity; or (ii) the ‘gel assay’ in which the three enzymes are separated by electrophoresis and then dismutase activity is measured within the band of the correct size for the SOD1 dimer (Box 1).
Box 1 Methods of measuring SOD1 dismutase activity.
| Activity assay: | A sample is collected from the tissue of interest, such as red blood cells. Xanthine–xanthine oxidase is added to the sample to generate superoxide anions ( ), and then a chromagen is used as an indicator of ), and then a chromagen is used as an indicator of  production. In the presence of SOD, production. In the presence of SOD,  concentrations are reduced, resulting in decreased colorimetric signal. However, all three SOD isoforms contribute to the activity measured, and so SOD1 activity is obtained indirectly by subtracting SOD2 and SOD3 activity from total SOD activity. This is achieved by running a parallel assay with the addition of potassium cyanide which preferentially inhibits SOD1 (Roe et al., 1988). concentrations are reduced, resulting in decreased colorimetric signal. However, all three SOD isoforms contribute to the activity measured, and so SOD1 activity is obtained indirectly by subtracting SOD2 and SOD3 activity from total SOD activity. This is achieved by running a parallel assay with the addition of potassium cyanide which preferentially inhibits SOD1 (Roe et al., 1988). |
| Gel assay: | Proteins from the tissue of interest are separated by electrophoresis in a native gel which is subsequently stained using a solution of nitro blue tetrazolium and riboflavin. Riboflavin is a source of  when exposed to light. The superoxide anions interact with nitro blue tetrazolium, reducing the yellow tetrazolium within the gel to a blue precipitate. This reduction reaction stains the gel blue; however, SOD inhibits this reaction, resulting in colourless bands where SOD is present. As the intensity of these bands is relative to the amount of SOD present, quantification can be inferred by measuring the intensity of the bands at the correct molecular weight using digital software (Weydert and Cullen, 2010). when exposed to light. The superoxide anions interact with nitro blue tetrazolium, reducing the yellow tetrazolium within the gel to a blue precipitate. This reduction reaction stains the gel blue; however, SOD inhibits this reaction, resulting in colourless bands where SOD is present. As the intensity of these bands is relative to the amount of SOD present, quantification can be inferred by measuring the intensity of the bands at the correct molecular weight using digital software (Weydert and Cullen, 2010). |
Intrinsic and overall SOD1 activity
The dismutase activity of SOD1 can be measured in two ways depending on whether the focus is on (i) the ‘intrinsic’ SOD1 activity, which reflects the enzymatic efficiency of the protein and is obtained by measuring the activity of recombinant SOD1 protein normalized to its quantity; or (ii) the ‘overall’ activity within a tissue sample, which may be affected by various factors in the cellular environment (as described below), and is obtained by normalizing dismutase activity to the quantity of tissue. This last measure has generally been used with patients with familial ALS, and is a reflection of the amount of activity present in a biological context.
Intrinsic activity influences the overall activity, but is only one of the determinants; the others being any factor that affects the quantity, biological availability and functionality of SOD1. Amongst these are SOD1 messenger RNA half-life, SOD1 protein half-life, correct folding of SOD1,  -loading of SOD1 and other post-translational modifications (Wilcox et al., 2009). In general, the ‘overall’ activity is an unbiased measure that takes into account known and unknown influences on SOD1 enzyme activity. Measurements are generally expressed relative to normal control samples.
-loading of SOD1 and other post-translational modifications (Wilcox et al., 2009). In general, the ‘overall’ activity is an unbiased measure that takes into account known and unknown influences on SOD1 enzyme activity. Measurements are generally expressed relative to normal control samples.
Overall SOD1 activity is reduced in human amyotrophic lateral sclerosis samples
Intrinsic SOD1 activity has been measured in at least eight mutant proteins, giving diverse results ranging from 0–150% of human wild-type SOD1 activity. Correlation between these values and clinical aspects of the disease was assessed and did not show significant links (Borchelt et al., 1994; Ratovitski et al., 1999).
However, overall SOD1 activity has most commonly been measured in red blood cells, fibroblasts and lymphoblastoid cell lines derived from patients carrying at least 48 different mutations, and has proved to be more homogeneous than measures of intrinsic activity. We have searched reports of SOD1 activity from 1993 to 2012 in patients with SOD1–familial ALS and found that the majority of 48 tested mutations all have a reduction of overall activity. The average loss of activity is notable and averages 58% (±17, SD) of normal values (Fig. 1). These results are of strikingly consistency given the variability arising from different laboratories performing the measurements and the naturally occurring variation in activity documented in blood samples (de Lustig et al., 1993; Borchelt et al., 1994; Robberecht et al., 1994).
Overall SOD1 activity is normal or only slightly reduced in two mutations: the D90A in both homozygous and heterozygous patients, and the L117V in heterozygotes; although measurement from a homozygous patient showed a reduction of 67% SOD1 activity compared with control subjects (Andersen et al., 1995; Synofzik et al., 2012). Both these mutations are atypical, for example (i) SOD1 misfolding is not detected in cells derived from patients carrying these mutations (see below for association between SOD1 misfolding and SOD1 activity); (ii) the disease allele is homozygous in the majority of patients with D90A ALS and in one of four reported patients with L117V ALS; (iii) penetrance in heterozygotes is low; and (iv) disease progression is unusually slow (Synofzik et al., 2012). Possibly these mutants have a slightly increased propensity to aggregate that is sufficient to start the disease process, although with reduced frequency, in the CNS, but is not enough to determine misfolding and loss of dismutase activity in the periphery.
Of note is the non-correspondence between the overall patient activity and the intrinsic activity; for example, the SOD1G37R mutant has 150% intrinsic activity but only 40% overall activity compared with normal control subjects. Given that the intrinsic activity is only one of the determinants of the overall activity, other factors, such as the stability of mutant SOD1 protein, have been investigated to try to account for this loss of activity.
Different mutant SOD1 proteins have been shown to have a variable half-life, but this is consistently reduced compared to the wild-type form (Sato et al., 2005). Calculations combining the measurement of intrinsic activity and half-life of six SOD1-familial ALS mutant proteins predicted that the overall activity would have been only 50% of normal levels (Borchelt et al., 1994). Other studies and Fig. 1 are in accord with this finding (Deng et al., 1993; Birve et al., 2010). Further, most measures of SOD1 activity from patients with ALS have been taken in red blood cells, and we note that these cells have no active protein synthesis and are on average 60 days old, making the system particularly responsive to detecting protein half-life changes (Broom et al., 2008). Nevertheless, when tested in post-mortem brain and spinal cord, SOD1 activity was found to be reduced to levels similar to those measured in red blood cells (Bowling et al., 1993; Rosen et al., 1994; Watanabe et al., 1997; Browne et al., 1998; Jonsson et al., 2004), and when activity in red blood cells and CNS was compared in the same subset of patients, results were strikingly concordant (Rosen et al., 1994).
Is SOD1 dismutase activity reduction exacerbated in motor neurons?
SOD1 activity has not been specifically measured in motor neurons and other affected cell types from SOD1–familial ALS post-mortem material, owing to technical difficulties in conducting these assays on limited micro-dissected material. The level of activity reduction in these cell types is therefore unknown; however, there is evidence that the dismutase activity reduction may be enhanced in motor neurons.
RNA
SOD1 messenger RNA was shown to form tissue-specific complexes with ribonucleoproteins from brain and spinal cord and these interactions prolong its half-life in these tissues. However, complex formation appears to be impaired when SOD1 messenger RNA carries ALS-causing mutations, therefore potentially reducing the half-life of mutant SOD1 messenger RNA preferentially in CNS of patients with SOD1-familial ALS (Ge et al., 2006).
Aggregation
Indirect evidence indicates that SOD1 aggregation reduces dismutase activity. For example, data from cell experiments in which SOD1G93A and amyloid-β were co-expressed, suggesting that aggregation results in reduction of SOD1 activity (Yoon et al., 2009). Further, two transgenic mouse lines over-expressing human wild-type-SOD1 at a high level and at 2-fold this level, were analysed for aggregation and SOD1 activity. In liver and muscle samples, aggregation was not found to be present, SOD1 protein levels were increased, as expected, 2-fold and SOD1 activity increased accordingly. However, in spinal cord and brain, where SOD1 aggregation was clearly found, protein levels had a similar increase as in muscle and liver, but importantly SOD1 activity did not show an increase, strongly suggesting a link between protein aggregation and activity (Graffmo et al., 2013). Furthermore, even if SOD1 retains enzymatic activity in aggregates, it may not accomplish the same functions as when correctly targeted to the appropriate cell compartments. SOD1 misfolding and aggregation are a hallmark of SOD1-familial ALS and have been extensively documented in SOD1-familial ALS (Jonsson et al., 2008) and wild-type SOD1 has also been shown to be present in spinal cord aggregates of both patients with familial ALS (Jonsson et al., 2004) and SOD1 mouse models (Deng et al., 2006; Wang et al., 2009a; Prudencio et al., 2010), thus making a dominant negative loss of function plausible. The recent finding of SOD1 aggregation in cases with sporadic ALS (Bosco et al., 2010; Forsberg et al., 2010) makes this mechanism potentially relevant also to sporadic disease.
Oxidative stress
Lastly, oxidative stress induces SOD1 to monomerize as an intermediate step to aggregate formation and it is known that SOD1 does not have dismutase activity in this form (Khare et al., 2004; Rakhit et al., 2004; Ezzi et al., 2007; Wilcox et al., 2009). Motor neurons are known to be particularly susceptible to oxidative stress (Barber and Shaw, 2010) making this process potentially more pronounced in these cell types.
The tissue-specific changes in SOD1 messenger RNA half-life and the effect of SOD1 aggregation and monomerization on dismutase activity, raise the possibility that SOD1 activity in the affected neurons may be lower than that measured from blood. How SOD1 activity in blood relates to that in spinal cord motor neurons is a critical issue that remains to be addressed.
In summary, SOD1 overall activity is consistently reduced in blood samples of patients with SOD1–familial ALS, likely owing to changes in protein activity and alterations in mutant protein half-life. Further, there is an additional possible tissue-specific dismutase activity reduction in neurons.
Sod1−/− mice have neuromuscular, neuronal and extra-neuronal phenotypes
An approach to elucidating the effect of SOD1 loss of function is to assess the phenotype of Sod1 null (Sod1−/−) mice. Homozygous Sod1 null mice have been used to analyse the role of SOD1 in ALS, and for other purposes such as studying oxide radical-mediated toxicity in reproduction and development (Huang et al., 1997; Matzuk et al., 1998). To date five Sod1 knockout mouse lines have been published: Sod1tm1Cpe (Reaume et al., 1996); Sod1tm1Cje (Huang et al., 1997); Sod1tm1Leb (Matzuk et al., 1998); Sod1tm1Ysh (Ho et al., 1998); and Sod1tm1Dkd (Yoshida et al., 2000). All were obtained by targeted deletion of different regions of the Sod1 gene, ranging from a single exon to the entire genomic sequence. For all five lines, no SOD1 protein is detectable in homozygous null mice and SOD1 activity is absent or very low [which may represent the background of the enzyme assay or might be caused by an endogenous superoxide dismutase activity supplied by an alternative scavenging enzyme (Reaume et al., 1996)].
Deletion of different portions of the same gene may result in different phenotypes, but the five Sod1 knockout strains have been compared in a number of studies and are strikingly similar (Huang et al., 1997; Kondo et al., 1997; Kostrominova, 2010). For example, skeletal muscles of three different Sod1 null strains were compared and all developed accelerated age-related muscle denervation (Kostrominova, 2010). Genetic background may also influence phenotypes, however the majority of the studies discussed here, have been carried out on Sod1−/− mice on a congenic C57BL/6 background, making results from different laboratories comparable.
When Sod1−/− mice were first generated, a key issue was whether they developed motor neuron degeneration. The first analysis, from Reaume et al. (1996), found no reduction in motor neuron number at 4 months of age, but an increase in small neurons and astrocytes in spinal cord ventral horns. Subsequent studies confirmed the lack of motor neuron loss at 6, 9 and 17 months (Flood et al., 1999) and lack of vacuolation or chromatolysis, key features of ALS, at 4 and 18 months (Fischer et al., 2012). Microgliosis and ubiquitinated protein accumulation in motor neurons were ruled out, but mild astrocytosis was found at 4 and 18 months (Fischer et al., 2012). Evaluation of lumbar ventral roots confirmed no loss in motor neuron number, but interestingly axonal diameter was reduced at 6 and 19 months and evidence of degenerating and regenerating axons was seen in the ventral L3 root at the latter time point (Flood et al., 1999), although another study did not see these results when analysing the L4 root (Fischer et al., 2012).
Although Sod1 null mice do not develop overt motor neuron degeneration, there is now a considerable literature, summarized below and described in more detail in the Supplementary material, showing that compared with wild-type controls, these mice have a wide range of phenotypes, including several relevant to ALS, such as a slowly progressive motor deficit that manifests from early adulthood and likely involves defects in large motor axons.
Sod1 null mice develop an adult-onset progressive motor axonopathy
Behavioural data
Sod1 null animals appear indistinguishable from littermates at birth through to weaning (Reaume et al., 1996). Weight reduces with age compared with wild-type littermates (Jang et al., 2010; Larkin et al., 2011) and voluntary wheel running diminishes, which is a sensitive test for early locomotor defects (Muller et al., 2006). At 9 months of age Rotarod performance and stride-length worsen (Flood et al., 1999; Muller et al., 2006), although spontaneous locomotion is normal, thus changes in motivation are not likely to contribute to these results (Flood et al., 1999; Muller et al., 2006). At 1 year of age, Sod1−/− mice also have a deficit in grip-strength, and tremors (Fischer and Glass, 2010)—hallmarks of neuromuscular disorders in mice (Muller et al., 2006).
Neurophysiological data
Direct neurophysiological measurement of the response to stimulation of nerve and muscle eliminates behavioural/motivational variability from the outcome. Such studies showed Sod1 null mice have a reduction in muscle strength suggesting a progressive deficit in innervation (Larkin et al., 2011). Motor unit number is reduced at 3 months, with progressive loss with age (Shefner et al., 1999). Complex repetitive discharges found on needle examination indicate a deficit in the terminal part of the motor axon (Shefner et al., 1999). Measures of nerve conduction velocity and latency analysis on sensory and mixed nerves, show a reduction only where a motor component is present, compatible with a deficit in the largest motor axons (Flood et al., 1999). Thus neurophysiological investigations show clear muscle denervation and deficits in motor axons and functional motor units in Sod1 null mice.
Axonal damage and early involvement of neuromuscular junctions
Denervation in Sod1−/− mice has been documented by neuromuscular junction analysis of both fast and slow twitch muscles (Flood et al., 1999; Fischer et al., 2011, 2012; Larkin et al., 2011). Denervation progresses with age, maintaining a more aggressive pattern in fast-twitch rather than slow-twitch muscles (Jang et al., 2010; Fischer et al., 2012). Thus SOD1 is required for maintenance of motor axons and their terminals (Fischer et al. (2011) and without this protein, fast-twitch motor units are lost preferentially, as is also observed in patients with ALS and mouse models of familial ALS (Dengler et al., 1990; Pun et al., 2006). The involvement of neuromuscular junctions as an early pathological target has been extensively documented in mouse models of SOD1–familial ALS (Murray et al., 2010) and was shown to precede motor neuron cell body loss in early disease in a patient with ALS (Fischer et al., 2004).
Muscle pathology is secondary to denervation
Muscle mass is progressively lost (Muller et al., 2006; Jang et al., 2010; Larkin et al., 2011) but has not been reported for organs such as liver, heart or kidney (Muller et al., 2006). Angular muscle fibres, indicating denervation, are present by 2 months of age (Flood et al., 1999) and by later time points a massive reduction in fibre number occurs, preferentially affecting type 2b fibres (fast glycolytic type innervated by large motor neurons), along with an increase in angular fibres (Reaume et al., 1996; Larkin et al., 2011). This muscle profile also occurs in mouse models of familial ALS–SOD1 (Kennel et al., 1996; Frey et al., 2000) and is typical of the neurogenic changes that initially affect larger motor neurons, suggesting that muscle pathology is secondary to axonal events.
Confirmation of muscle pathology being secondary to axonal damage and denervation was obtained by crossing Sod1−/− mice with transgenic mice expressing Sod1 in the CNS but not in muscle. In double mutant progeny, muscle pathology was fully rescued despite the absence of SOD1 in muscle (Flood et al., 1999). Further, in Sod1−/− mice, measurements of steady state redox potential of glutathione (which is routinely used as indicator of the intracellular redox state) in tibial nerve and gastrocnemius muscle showed a selective involvement of the nerve at 4 months, again indicating the primary involvement of the axon (Fischer et al., 2012). Thus muscle changes in Sod1−/− mice are secondary to denervation; they are non-specific and also present in muscle biopsies from patients with ALS (Baloh et al., 2007a).
Sod1−/− motor neurons show increased vulnerability to stress
SOD1 activity is important for motor neuron survival after injury as shown by facial axotomy of Sod1 null mice which resulted in a significant increase in motor neuron loss compared with wild-type controls. This is interesting when considering the potential role for injury and trauma in ALS (Pupillo et al., 2012; Yip and Malaspina, 2012).
Selective susceptibility to damage of the motor system
An important feature of ALS is the selective involvement of motor neurons and their related circuits, and indeed the phenotypes induced by lack of SOD1 in mice preferentially affect motor neurons. Sod1−/− mice have no significant deficits in somatosensory behaviour (Flood et al., 1999). Further, neurophysiology testing showed preferential motor involvement in Sod1−/− mice and, on histopathological examination, L3 dorsal roots at 19 months are normal in contrast to the ventral roots, which have signs of degeneration/regeneration (Flood et al., 1999). Finally, analysis of Sod1 null epidermal nerves, which are the most distal tracts of the sensory axons, showed no abnormality, in contrast to their severely affected motor counterparts, the neuromuscular junctions (Fischer et al., 2012).
Loss of SOD1 affects mitochondrial function
Sod1−/− mice lack superoxide scavenging function in the cytosol and mitochondrial intermembrane space, which contain  generated by complex III (Muller et al., 2004). Sod1 null mitochondria release significantly increased amounts of
generated by complex III (Muller et al., 2004). Sod1 null mitochondria release significantly increased amounts of  and therefore increased oxidative stress in these compartments was hypothesized to account for the neuromuscular phenotype of the mice (Jang et al., 2010). Fischer et al. (2011) demonstrated that mitochondrial density is reduced in Sod1−/− axons and, remarkably, they reversed this loss and the neuromuscular phenotype, by replacing SOD1 selectively in the mitochondrial intermembrane space of these mice.
and therefore increased oxidative stress in these compartments was hypothesized to account for the neuromuscular phenotype of the mice (Jang et al., 2010). Fischer et al. (2011) demonstrated that mitochondrial density is reduced in Sod1−/− axons and, remarkably, they reversed this loss and the neuromuscular phenotype, by replacing SOD1 selectively in the mitochondrial intermembrane space of these mice.
Mitochondrial dysfunction is associated with ALS (Faes and Callewaert, 2011) and mitochondria are important for distal axonal maintenance (Baloh et al., 2007b; Cassereau et al., 2011). Furthermore, mitochondrial transport abnormalities are described in tgSOD1-ALS mouse models (De Vos et al., 2007). Also, abnormal mitochondrial accumulations have been described in lower motor neurons and proximal axons from patients with ALS, post-mortem (Sasaki et al., 2009). Thus it seems likely that damage to mitochondria through raised levels of free radicals may have a significant effect on distal axons of motor neurons.
In summary Sod1−/− mice appear normal up to the age of weaning, after which they develop a slowly progressive motorneuronopathy that involves primarily the motor neuron axons and neuromuscular junctions and is accompanied by significant secondary denervation pathology in muscles (Supplementary Fig. 1). Sensory involvement is negligible. Although no motor neuron loss has been documented, these are more vulnerable to damage in Sod1−/− mice.
Other neuronal and extra-neuronal Sod1 null phenotypes
In addition to deficits in the motor system, Sod1 null mice develop a range of other disorders including: progressive neuronal hearing loss; progressive retinal degeneration; greatly increased susceptibility to cerebral ischaemia and brain trauma. Most importantly for studies of ALS, these mice have an increased susceptibility to neurodegeneration, for example, when crossed to a mouse model of Alzheimer’s disease (Supplementary Box 1).
Extra neuronal phenotypes are also striking, in particular the well-known susceptibility to hepatocellular carcinoma, a feature that also manifests in the human population; there is a positive correlation between SOD1 activity and postoperative hepatocellular carcinoma survival time, as well as low levels of SOD1 and severity of hepatocellular carcinoma (Elchuri et al., 2005; Takahashi et al., 2002; Casaril et al., 1994; Liaw et al., 1997; Lin et al., 2001). Other non-neuronal features include impaired endothelial-dependent relaxation, thinning of the skin, osteoporosis and female infertility (Supplementary Box 1).
These remarkably wide-ranging phenotypes are perhaps not surprising in an animal that is missing such an important enzyme, but the tissue specificity partly points to effects in tissues with a high production of free radicals, such as the nervous system and liver. Further, the progressive nature of most of these phenotypes is striking and may be relevant to the mechanism of SOD1–familial ALS, both in terms of deficits increasing with age and in terms of targeting of many of the deficits to neuronal tissues.
A SOD1 activity of 50% is not sufficient for normal neuronal function
Sod1+/− mice have abnormal phenotypes including within the motor system
Sod1 null mice are invaluable for investigating in vivo consequences of SOD1 loss of function, and may provide clues for the effects of reduced enzyme activity in ALS. However, the 100% loss of enzyme activity is a different setting from the average 57% reduction in patients with ALS. Thus, although Sod1 null mice clearly indicate a susceptibility of specific tissues to the effects of a loss of SOD1 function, any discussion in the context of ALS must look at phenotypes that arise in Sod1+/− animals (Supplementary Fig. 1) that retain 50% SOD1 activity (Reaume et al., 1996) and so mimic the physiological levels described in patients with SOD1–familial ALS. Although Sod1+/− mice clearly do not develop an ALS-like syndrome, a wide range of studies show that Sod1+/− mice have abnormal phenotypes involving progressive cellular damage and deficits in reaction to injury and toxic stimuli. Here we consider how these may have implications for human ALS.
Sod1+/− motor neurons are more susceptible to cell death after axon injury
Sod1+/− mice suffer significantly more motor neuron loss in response to facial nerve axotomy than wild-type mice. This result is intermediate between Sod1−/− and control mice, suggesting a dose dependence of this effect and demonstrating that 50% SOD1 activity is not sufficient for a normal function of motor neurons in response to injury (Reaume et al., 1996).
Facial nerve axotomy was also performed on copper chaperone for SOD1 null mice (Ccs−/−), that retain only 20% of SOD1 activity, owing to the lack of this crucial protein for delivery of copper to SOD1 (Box 2). Motor neuron survival was significantly reduced in Ccs−/− mice (Subramaniam et al., 2002). This result, and the similar result from Sod1+/− mice, is important in light of the potential role for injury and trauma as a trigger in ALS pathogenesis (Pupillo et al., 2012; Yip and Malaspina, 2012).
BOX 2 Ccs−/− null mice model SOD1 partial loss of activity.
Another mouse model that is relevant to studying the effects of reduced SOD1 activity is the ‘copper chaperone for SOD1’ (Ccs) null mouse. Copper chaperones shuttle copper, which is toxic for cells in its free form, to intracellular target proteins. CCS delivers copper to SOD1 by direct protein–protein interaction and is required for full activation of SOD1 (Culotta et al., 1997, 1999). Mice lacking CCS (Ccs−/−) were generated by gene targeting and retain only 20% of normal SOD1 activity; CCS-independent copper loading into SOD1 probably accounts for the remaining activity. Although the dismutase activity is impaired, there is no difference in the levels of SOD1 protein among wild-type, Ccs+/− and Ccs−/− littermates (Wong et al., 2000).
Spontaneous denervation, motor neuron sensitivity and reduction in mitochondrial numbers are not significant in Sod1+/− mice but all show trends
Although Sod1+/− mice have been less studied than null animals, there have been comprehensive investigations of denervation in these mice (Fischer et al., 2011, 2012). At 18 months, 80% of tibialis anterior muscle (neuromuscular junctions) were innervated in Sod1+/− compared with 92% in controls (Fischer et al., 2011). This result did not reach statistical significance, but this trend was repeated in a following study (Fischer et al., 2012). Currently, there is no evidence for spontaneous denervation in Sod1+/− mice. Experiments were conducted up to the time-point of 18 months, the maximum limit when investigating Sod1−/− mice—which develop and die of liver cancer at this stage—but could be extremely informative at later time-points for the Sod1+/− mice. Whether increased sample size or a later time-point would show a significant result, remains to be determined.
Glutamate toxicity is enhanced in Sod1+/− mice
Glutamate toxicity is implicated in disease in patients with ALS and in animal models (Ilieva et al., 2009). The role of SOD1 in neuronal sensitivity to glutamate toxicity was assessed in vivo by intrastriatal injection of N-methyl-d-aspartic acid and kainite glutamate receptor agonists. Sod1+/− mice were more susceptible to the neurotoxic effects of both stimuli and had reduced glutamic acid decarboxylase and choline acetyltransferase activities compared with controls (Schwartz et al., 1998). Thus SOD1 partial loss of function could play a role in facilitating damage from glutamate toxicity, which may have relevance to ALS.
Increased susceptibility to cerebral ischaemia in Sod1+/− mice
Sod1+/− mice have decreased survival after induced focal cerebral ischaemia, along with increased early blood–brain barrier disruption and increased infarct volume causing brain swelling. Apoptotic neuronal death is also increased demonstrating enhanced ischaemia–reperfusion injury (Kondo et al., 1997). Intriguingly, an important mechanism involved in ischaemia–reperfusion injury is glutamate excitotoxicity, which as discussed above, is postulated to play a role in ALS pathogenesis (Beal, 1992).
We note that blood–brain barrier alterations are found in tgSOD1-ALS mouse models and that indirect evidence of disruption, such as increased cerebrospinal fluid (CSF) albumin/plasma albumin ratios, has been documented in patients with ALS (Leonardi et al., 1984; Apostolski et al., 1991).
Increased memory deficits and plaque formation in an Alzheimer’s disease model on a Sod1+/− background
Overexpression in mice of the APP gene carrying the Swedish mutation causes behavioural deficits and plaque formation and thus models Alzheimer’s disease (Bodendorf et al., 2002). When this mutation is expressed on a Sod1+/− background, it results in increased deficits in behavioural tests used to assess memory and in increased senile plaque formation, thus showing that lack of 50% of SOD1 activity does indeed increase the development of a neurodegenerative phenotype in vivo.
Ganglion neuron loss is increased with ageing in Sod1+/− mice
A 50% reduction in SOD1 activity results in reduced neuronal survival in vivo with respect to ganglion cell density, although this does not cause an apparent hearing deficit (Keithley et al., 2005).
DNA methylation is reduced in Sod1+/− mice
The effects of reduced mouse SOD1 activity could be relevant to ALS because DNA methyltransferases, the enzymes involved in DNA methylation, and 5-methylcytosine, the end-product of DNA methylation, were found to be upregulated in human ALS, suggesting that aberrant regulation of DNA methylation is part of the pathobiology of ALS (Chestnut et al., 2011). DNA methylation was significantly reduced at 2 months of age in Sod1+/− mice, although this study focused on prostate tissue (Bhusari et al., 2010).
Sod1+/− mice exhibit a contractile vascular phenotype with ageing
High levels of superoxide play a major role in contractile vascular dysfunction and loss of a single copy of Sod1 is enough to increase vascular superoxide levels and produce vascular contractile dysfunction with ageing (Didion et al., 2006).
Loss of mouse SOD1 activity to 50% of normal levels does not cause death of motor neurons but may be relevant to human SOD1–familial amyotrophic lateral sclerosis
Sod1+/− mice show an increased loss of specific neuronal sub-types with ageing and an increased susceptibility to injury and toxic stimuli. These results are relevant to SOD1-familial ALS given the similarity of enzyme activity levels between patients with SOD1-familial ALS and Sod1+/− models. The vulnerability shown in motor neurons after injury, the susceptibility of neurons to glutamate toxicity, and the blood–brain barrier alterations seen in these mice are significant elements since they are mechanisms and alterations postulated to be involved in ALS pathogenesis.
Overall Sod1−/− and Sod1+/− animals do not recapitulate mouse ALS, but they have a wide range of phenotypes that are both related to ALS directly (for example, denervation, increased susceptibility to glutamate toxicity, increased susceptibility to axonal damage) and more generally to neuronal degeneration (for example, loss of ganglion and retinal cells) and therefore this raises the question of a contribution of SOD1 loss of function to disease. A further point to consider is that although several transgenic mice carrying SOD1 mutations have motor neuron degeneration and characteristics of human ALS, other mouse strains with well-characterized pathogenic mutations in different ‘ALS genes’ (for example, TARDBP) have phenotypes less clearly reminiscent of the human disease (Joyce et al., 2011). Thus it remains debatable as to how a mouse–ALS syndrome might manifest, and so considering all phenotypes that develop in the CNS of these models is essential for understanding how ALS mutations cause disease.
SOD1 activity and its influence on SOD1–familial amyotrophic lateral sclerosis mouse models
SOD1 loss of function does not influence survival of transgenic SOD1 disease models
The possibility that SOD1 loss of function contributes to ALS pathogenesis has been investigated by analysing the double mutant progeny of Sod1−/− or of Ccs−/− mice crossed to three tgSOD1-ALS lines overexpressing the human mutations, G93A, G37R and G85R. These crosses produce double mutant mice in which either the transgenic human mutant protein is the only SOD1 present (in the case of Sod1−/− crosses) or both the endogenous and transgenically expressed SOD1 are mostly inactive due to the Ccs−/− background.
As both G93A and G37R mutant SOD1 retain dismutase activity (Fig. 1) they are not informative for the effect of SOD1 dismutase loss of function when on a Sod1 null background. Further, transgenes often form multiple copy concatamers and indeed both the tgSOD1G93A and the tgSOD1G37R lines have an increase of >6-fold of mouse SOD1 activity, compared with non-transgenic control mice (Bruijn et al., 1997; Subramaniam et al., 2002; Deng et al., 2006). As a result, even on a Ccs−/− background, these two transgenic lines still have SOD1 activity levels comparable with those of non-transgenic wild-type mice and so are not useful for examining the effects of SOD1 loss of function on disease course (Subramaniam et al., 2002).
However, two experiments do examine the effect of mouse SOD1 loss of function on the disease developed by tgSOD1-ALS mouse models. These assess progeny from crosses of Sod1−/− and Ccs−/− with tgSOD1G85R, a human ALS mutation that has no detectable intrinsic activity (Borchelt et al., 1994; Bruijn et al., 1997). Activity is predicted to fall to 0% when crossed with Sod1−/− (Bruijn et al., 1998) and shown to be 20% when crossed with Ccs−/−, as expected, given the residual SOD1 activity of the Ccs−/− line (Subramaniam et al., 2002).
The tgSOD1G85R line shows clinical signs of disease between 8–10 months of age, which aggressively progress to paralysis within a few weeks (Bruijn et al., 1997). The disease onset is much later than many other tgSOD1-ALS mouse models, this is appropriate for evaluating any potential modifying effect of SOD1 loss of function. Crosses to both Sod1−/− (n = 5 double mutant progeny) and Ccs−/− (n = 10 double mutant progeny) did not show significant effects on lifespan (Bruijn et al., 1997; Subramaniam et al., 2002).
Both of these studies resulted in seminal papers that have been extremely important to the field and have answered critical questions about SOD1 gain of function in ALS. However, neither paper answered the separate question about whether loss of function modifies ALS, presumably and quite reasonably because that was not the focus of the papers. Both studies were performed using a cohort size too small to detect potential subtle changes [n = 5 in Bruijn et al. (1997) and n = 10 in Subramaniam et al. (2002)] and the sex of the mice analysed was not reported, although it is clear that in both humans and mouse, gender plays a role in ALS natural history (Acevedo-Arozena et al., 2011; Joyce et al., 2011). Furthermore, neither study evaluated the age of disease onset or performed behavioural analysis therefore not addressing the possibility that SOD1 loss of function may have an impact on onset and disease course. Lastly, both studies lack a quantitative pathology analysis: Bruijn et al. (1997) noted n = 2 for axonal count in the L5 ventral roots, and Subramaniam et al. (2002) perform a qualitative analysis only, at end-stage, therefore missing any potential modifier effect occurring during the disease process.
Thus while it remains unclear if SOD1 loss of function modifies important disease characteristics such as age of onset or progression, it appears not to affect lifespan. The lack of effect on survival in the absence of mouse SOD1 activity is an important result because it shows that life expectancy in this line is determined uniquely by mutant SOD1. It has been also speculated that the lack of an effect on lifespan is due to the insufficient levels of endogenous SOD1 to counteract the oxidative stress present in the tgSOD1G85R mice, and because the tgSOD1G85R mice die before developing a significant oxidative stress-mediated motor axonopathy (Wang et al., 2012). With respect to onset and disease course, a larger cohort would be required to note differences, particularly if they are subtle.
SOD1 activity may influence disease course of transgenic SOD1 disease models
While the effect of mouse SOD1 activity on disease onset and progression of tgSOD1-ALS models is unclear from the crosses to Sod1 null mice, there are other experimental data indicating that SOD1 activity may play a role in modifying SOD1-familial ALS.
SOD1 overexpression and influence on disease
The effect of overexpression of wild-type human SOD1 on disease course has been tested by crossing mutant tgSOD1-ALS animals with transgenic mice overexpressing wild-type human SOD1 (tgSOD1-WT). Double mutant progeny carry both the wild-type and mutant human SOD1 transgenes, with two copies of the endogenous mouse Sod1 in the genetic background (Bruijn et al., 1998; Jaarsma et al., 2000; Fukada et al., 2001; Deng et al., 2006; Wang et al., 2009a, b; Prudencio et al., 2010). Generally, a worsening of both age of onset and survival have been reported, compared with single mutant tgSOD1-ALS littermates. However, we note that tgSOD1-WT mice spontaneously develop motor neuron and axon loss and have misfolded SOD1 accumulations (Jaarsma et al., 2000) and develop an ALS-like disease when expression is further increased (Graffmo et al., 2013), making the results of these crosses hard to interpret.
Of note, transgenic mice over-expressing CCS have also been generated and crossed to different tgSOD1 lines, and results have ranged from no effect to a significant worsening of the phenotype. However, CCS over-expression was shown to have biological effects in the absence of SOD1 enzymatic activation and also to have an influence on the reduced state of SOD1, making these results not helpful for dissecting the role of dismutase activity on disease (Proescher et al., 2008; Son et al., 2009; Graffmo et al., 2013).
Tissue specific expression and inactivation of mutant SOD1 points to a modifying role for dismutase activity
To address questions regarding the cell-autonomy of SOD1-familial ALS, investigators have used Cre-loxP technology to conditionally eliminate mutant SOD1 expression in different cell lineages or used specific promoters to overexpress mutant SOD1 in selected cell types. Analysis of their results is beyond our scope here, but generally demonstrates a central role for neurons in the determination of age of onset and disease progression in SOD1-familial ALS, and has also pointed to a role for other cell types, such as astrocytes and microglia in influencing the course of the disease (Ilieva et al., 2009).
Cre-loxP experiments were conducted using two mutant tgSOD1 lines: conditional tgSOD1G37R, which retains intrinsic dismutase activity, and conditional tgSOD1G85R, which lacks activity. Neuronal excision experiments in both lines showed a beneficial effect on disease onset and survival. Interestingly, for consideration of the effects of dismutase activity, significant differences were found between the conditional tgSOD1G37R and conditional tgSOD1G85R lines after mutant SOD1 excision from microglia and from astrocytes.
A more profound amelioration was observed with the tgSOD1G85R line in both experiments, with an effect on early disease with microglia excision and also on disease onset with excision in astrocytes; neither result occurred in the tgSOD1G37R experiments (Ilieva et al., 2009; Wang et al., 2009b, 2011). A possible explanation for these divergent results has been proposed to lie in differences in dismutase activity (Wang et al., 2012). If in addition to the toxic gain of function effects, tgSOD1G37R has neuroprotective effects in microglia and astrocytes due to its enzymatic activity, then a knockdown of tgSOD1G37R expression would have less ameliorative effects on the disease course than knockdown of the inactive SOD1G85R (Wang et al., 2009b, 2011).
In support of a modifying effect of SOD1 dismutase activity are findings obtained with the excision of tgSOD1G37R from Schwann cells. Excision from the tgSOD1G37R made the disease progression in the mice more severe. Lobsiger et al. (2009) proposed that SOD1G37R activity in Schwann cell had a neuroprotective effect. Conversely, excision of the inactive SOD1G85R caused a delay in disease onset, an increased survival and an amelioration of pathology (Wang et al., 2012). Furthermore, increasing SOD1G93A expression specifically in Schwann cells of the tgSOD1G93A mouse had a beneficial effect on disease (Turner et al., 2010).
In conclusion, the experimental data overall point to a protective role of SOD1 dismutase activity, at least in some cell types, on non-cell autonomous degeneration and disease in SOD1–ALS.
Conclusions
SOD1 loss of function models share many commonalities with amyotrophic lateral sclerosis indicating specific cell-type sensitivities
SOD1 loss of function was initially thought to play a role in ALS due to the discovery of disease-causing mutations in the SOD1 gene and due to the well-established link between oxidative stress and neurodegeneration (Smith et al., 1991; Stadtman 1992; Stadtman and Berlett, 1997). Indeed free radical damage has been shown in CSF, serum and urine from patients with ALS (Smith et al., 1998; Simpson et al., 2004; Mitsumoto et al., 2008) and proteins, lipids and DNA were shown to have elevated oxidative damage in ALS post-mortem material (Shaw et al., 1995; Fitzmaurice et al., 1996; Shibata et al., 2001). As SOD1-familial ALS undoubtedly arises primarily from SOD1 toxic gain of function, the loss of dismutase activity may play a modifying role. The most useful tools to study this possibility in vivo have been the Sod1−/− mice. These mice are a model of chronic oxidative stress, but do not develop a disease that models human ALS.
Long term studies of Sod1−/− mice show striking features related to ALS. Notably, these mice develop a progressive distal motor axonopathy and ALS has indeed been postulated to start by affecting the distal portions of the neurons including neuromuscular junctions and axons (Murray et al., 2010). The most affected motor units in Sod1−/− mice are fast-twitch, which is in accordance with observations in ALS models (Frey et al., 2000). Motor neurons in Sod1 null mice have an increased susceptibility to injury and importantly, stimuli such as trauma, could play a role in initiating ALS (Pupillo et al., 2012) where humans, even if carrying disease-causing mutations, are healthy for decades. Further, in Sod1−/− mice, motor neurons are preferentially affected compared to sensory neurons, recapitulating the selectivity observed clinically and pathologically in ALS.
The neuromuscular phenotype in Sod1 null mice has been demonstrated to be caused by the lack of SOD1 in the mitochondrial intermembrane space and a related decrease in axonal mitochondrial density (Fischer et al., 2011). The involvement of mitochondria in ALS and other forms of motor neuron disease such as spinal muscular atrophy, has been shown in animal and cellular models and indirectly in post-mortem material (Baloh et al., 2007b; De Vos et al., 2007; Acsadi et al., 2009; Sasaki et al., 2009; Wen et al., 2010; Faes and Callewaert, 2011). As described, Sod1−/− mice have other phenotypes that underline the importance of this gene in neuronal ageing and in neurodegeneration. Among these are the spontaneous progressive loss of retinal cells and auditory ganglion neurons, the increased susceptibility to APP induced neurodegeneration and the increased susceptibility to apoptotic cell death following brain trauma and ischaemic injury.
SOD1 activity is greatly reduced in human SOD1-familial amyotrophic lateral sclerosis
SOD1 activity is generally reduced to approximately half of normal in patients with SOD1-familial ALS, as measured in red blood cells, lymphoblastoid cells and fibroblasts (Fig. 1). Indirect evidence raises the possibility that a more severe reduction could occur in susceptible tissues and cell types, owing to reduced mutant SOD1 messenger RNA half-life in the CNS and due to possible effects of SOD1 protein misfolding and aggregation on activity.
Data from other human diseases involving loss of enzyme activity shows many of these are recessive and heterozygotes are generally unaffected (Mitchell et al., 2011). However, this is clearly not the case in the Sod1+/− mouse; these animals have increased neuronal loss and increased susceptibility to injury. Further, the stimuli to which these mice are more susceptible are motor neuron axonal damage and glutamate toxicity, both closely related to ALS pathogenesis. In addition, the blood–brain barrier is more permeable following injury in these mice; indirect evidence of a similar state has also been described in patients with ALS and in mouse models of ALS. Exacerbation of neurodegenerative phenotypes in an Alzheimer’s disease mouse model when on a Sod1+/− background also indicates a potential predisposition to neurodegeneration of mice with a 50% reduction in SOD1 activity. Lastly, as Sod1+/− mice show a spontaneous loss of spiral ganglion cells this confirms that decreased dismutase activity has direct consequences on neuronal survival.
A human SOD1 loss of function phenotype?
Although both Sod1−/− and Sod1+/− mice develop characteristics that have obvious relevance to ALS, we have found no data from human genetic analyses to suggest that SOD1 loss of function alone causes the human disease. >155 mutations in SOD1 have been described (Fig. 1), but there have been no truncation mutations occurring in the N-terminal part of SOD1 that would generate an effectively null allele (i.e. due to reduced expression from nonsense mediated decay of the messenger RNA or from inactivity of just a short stretch of N-terminal amino acids). We note a frameshift in exon 2 generating a predicted 35 amino acid protein that might be a null, has been reported in a family with ALS, but data regarding segregation are unclear making conclusions uncertain (Hu et al., 2012).
Further, we could find no description of patients with full loss of SOD1 activity, even when SOD1 mutations are found in homozygosity. Of the six homozygous SOD1 mutations (L84F, N86S, D90A, L117V, L126S and G27delGGACCA) described, activities for four (D90A, L117V, L126S and G27delGGACCA) have been measured and vary between 25% and 93% of normal levels (Andersen et al., 1995; Boukaftane et al., 1998; Hayward et al., 1998; Kato et al., 2001; Zinman et al., 2009; Synofzik et al., 2012). Of note, a patient with a CCS homozygous mutation has been described with SOD1 activity of ∼25% of normal. This patient showed a complex neurodevelopmental phenotype; however, this is attributed to a mutation in SLC33A1 and not a loss of SOD1 function (Huppke et al., 2012).
SOD1 gain and loss of function could complement each other in amyotrophic lateral sclerosis pathogenesis
A reduction in SOD1 activity is not causative for ALS (which is certainly what the mouse data show), however, it may modify disease, as suggested by results from the mouse cross experiments described above. It seems likely such modifying effects would come through an increased susceptibility to neurodegeneration either directly through, for example, the increased susceptibility to axonal damage seen in Sod1+/− mice, or indirectly through, for example, effects on respiration in high energy consumers such as motor neurons—note the recent finding that SOD1 is a critical focus for integrating O2, glucose and superoxide levels, through casein kinase signalling, to repress respiration and directing energy metabolism, and that this role is independent of its function in oxidative stress (Reddi and Culotta, 2013). Thus SOD1 loss of function will likely have effects on cellular metabolism and, further, as it is still unclear why this enzyme is produced at such high levels, SOD1 may well have other as yet unknown roles in neuronal function.
Loss and gain of function mechanisms coexist in other neurodegenerative diseases as shown in models of Huntington’s disease (Zuccato et al., 2010), Parkinson’s disease (Winklhofer et al., 2008) and spinocerebellar ataxia 1 (Lim et al., 2008; Crespo-Barreto et al., 2010). Indeed both loss and gain of function have been hypothesized to contribute to pathogenesis in ALS caused by TARDBP and FUS mutations (Lagier-Tourenne and Cleveland, 2009; Guo et al., 2011).
SOD1 has a crucial role in superoxide clearance and its loss of function generates an increased state of oxidative stress. In a tgSOD1-ALS mouse model, SOD1 is itself a major target of oxidization (Andrus et al., 1998) and SOD1 oxidation and glutathionylation, which occurs in response to oxidative stress, both increase the propensity of the dimer to dissociate and become misfolded (Khare et al., 2004; Rakhit et al., 2004; Ezzi et al., 2007; Wilcox et al., 2009).
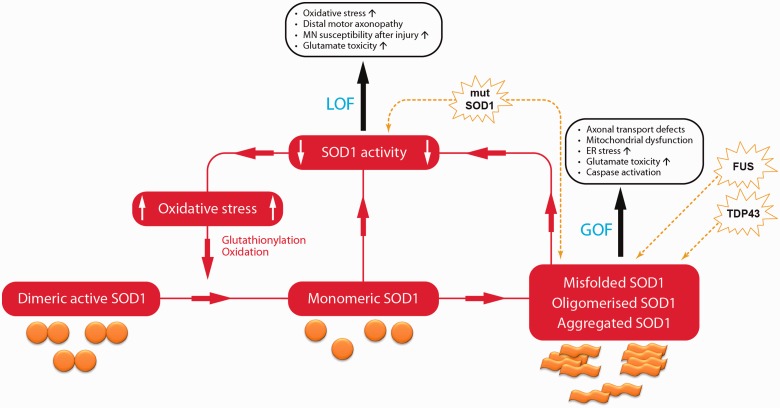
These findings set the scene for a potential co-operation of SOD1 loss and gain of function in ALS pathogenesis. Indeed, a vicious circle can be hypothesized in which oxidized SOD1 has an increased propensity to misfold, causing seeding and aggregation of SOD1 and resulting in a reduction of dismutase activity, which therefore feeds more potential oxidative stress to the start of the loop (Fig. 2). We note that the strong link between SOD1 misfolding and its loss of function (see above) make the two effects very difficult to assess independently.
Figure 2.
The cycle of SOD1 loss of function, schematic representation of a potential co-operation between SOD1 loss and gain of function in SOD1–familial ALS pathogenesis. SOD1 loss of function (LOF) increases levels of oxidative stress, which through glutathionylation and oxidation, can facilitate the monomerisation of dimeric SOD1. Once monomerized, SOD1 is more prone to become misfolded, oligomerized and aggregated. The monomerization of previously active dimeric SOD1 and the recruitment of SOD1 into aggregates further enhance the loss of function, feeding back to the beginning of the loop. In this way the gain of function (GOF) effects of misfolded, oligomerized and aggregated SOD1, which are known to cause motor neuron degeneration, are amplified by the loss of function circle. Mutant SOD1 (mutSOD1) has both a direct effect on reduction of SOD1 activity and induces SOD1 misfolding and aggregation. Mislocalisation of both TDP43 and FUS result in misfolding of SOD1. ER = endoplasmic reticulum; MN = motor neuron.
A number of recent findings have underlined how such a mechanism could be relevant not only to SOD1-familial ALS, but also to sporadic cases. Hyperoxidized and misfolded SOD1 have been demonstrated in sporadic ALS cases (Bosco et al., 2010; Forsberg et al., 2010; Guareschi et al., 2012) and misfolding of SOD1 was shown to be induced by both TDP43 and FUS mislocalization (Pokrishevsky et al., 2012), events that occur in the majority of sporadic patients with ALS (Maekawa et al., 2009; Deng et al., 2010; Matsuoka et al., 2011). Recent studies demonstrating that SOD1 aggregation can be seeded in vitro from mouse tgSOD1G93A spinal cord material (Chia et al., 2010), and ‘transmitted’ between cells (Munch et al., 2011) extend the potential role for these pathogenic mechanisms to the clinical and pathological ‘spread’ of ALS (Ravits et al., 2007; Pokrishevsky et al., 2012). Of note, SOD1 was also found to be oxidized in Alzheimer’s disease and Parkinson’s disease (Choi et al., 2005).
In fact, it is possible to speculate that the absence of SOD1 in the loss of function mouse model omits one of the most important targets of oxidative stress in ALS—that is, SOD1 itself—leaving the pathogenic cascade incomplete.
Finally, we note the lack of studies addressing the expression of the SOD1 trans allele in SOD1–familial ALS and the possibility that this plays a role in modifying the disease. Indeed, a 50 base pair deletion in the promoter region of SOD1 has been described to influence SOD1 expression and there have been attempts to correlate this with clinical characteristics in sporadic ALS, although so far results have not been replicated (Broom et al., 2008). To our knowledge no studies analyse the SOD1 trans-allele in SOD1–familial ALS cases for the presence of this variant or other factors influencing the expression of the trans-allele.
Implications of SOD1 loss of function for current therapeutic approaches for amyotrophic lateral sclerosis and other diseases
Therapies are being developed for ALS and other neurodegenerative disease caused by dominant mutations, which entail knockdown of the mutant allele RNA (Smith et al., 2006; Kordasiewicz et al., 2012; Lu and Yang 2012). This approach is showing promise for Huntington’s disease; a recent report has shown suppression of huntingtin in Huntington’s disease mouse models and in the non-human primate brain, and a 75% suppression of huntingtin throughout the CNS appears to be well tolerated (Kordasiewicz et al., 2012).
A number of analogous strategies have been tested for SOD1 (Ralph et al., 2005; Raoul et al., 2005; Saito et al., 2005; Smith et al., 2006; Wang et al., 2010; Towne et al., 2011; Wright et al., 2012) and have shown very encouraging results. Excitingly, a phase 1 clinical trial has been conducted in SOD1-ALS (Fratta, 2013; Miller et al., 2013) using antisense oligonucleotides that silence both mutant and wild-type SOD1, that were previously shown to be effective in a transgenic SOD1-ALS rat model (Smith et al., 2006). The main aim of this study, the first of its kind, was to assess safety and so the treatment was undertaken for periods too brief to obtain a biological effect on SOD1 levels, so although the regime is reported to be well-tolerated, it remains to be determined whether SOD1 downregulation causes unwanted effects.
The Sod1 knockout mouse data presented here are important for these types of studies in illustrating the need to understand the full implications of such strategies. These are not only neuronal; for example, the reason most Sod1 null (and a small percentage of Sod1+/−) mice die is liver cancer. Thus particular attention must be paid to delivery routes, protein levels and distribution.
We note that the null mice lack Sod1 completely, from the earliest time in development, and no appropriate model appears to be available currently for evaluating the effects of long-term endogenous Sod1 knockdown from adulthood. The preclinical trials with RNA interference technologies used tgSOD1-ALS adult models to investigate SOD1 reduction on a very early onset and fast progressing disease, but did not study potential long term effects (Raoul et al., 2005; Smith et al., 2006). Experiments using conditional Sod1 alleles would help clarify the situation. A hopeful note for ALS from the Huntington’s disease study is that transient knockdown ameliorates disease for an extended period (Kordasiewicz et al., 2012).
The data from Sod1+/− animals indicate that in patients with SOD1–ALS there may be a case for epidemiological studies of the known phenotypes that arise with a 50% enzyme loss; for example, is there a greater incidence of cardiovascular disease and stroke in families with SOD1–familial ALS? Do these families have an increased incidence of liver cancer or, protection from tumours such as lung cancer, given that the majority of human lung adenocarcinomas express SOD1 at higher than normal levels (Somwar et al., 2011).
Finally, knowledge of the effects of SOD1 loss of activity clearly indicates that this gene should be screened in cohorts with diseases such as hereditary distal motor neuropathies, age-related macular degeneration and progressive hearing loss, because both homozygous and heterozygous loss of function are compatible with life, at least in mice, but have abnormal phenotypes.
Supplementary Material
Acknowledgements
We thank Dr Peter Joyce and Dr Abraham Acevedo-Arozena for comments and Mr Ray Young for graphics.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- tgSOD1
SOD1 transgenic mice
Funding
P.F is funded by Medical Research Council / Motor Neuron Disease Association Lady Edith Wolfson Fellowship. R.S. and E.M.C.F. are funded by the UK Motor Neuron Disease Association. R.K.A.B.-S., E.M.C.F. are funded by the UK Medical Research Council and the Thierry Latran Foundation.
Supplementary material
Supplementary material is available at Brain online.
References
- Acevedo-Arozena A, Kalmar B, Essa S, Ricketts T, Joyce P, Kent R, et al. A comprehensive assessment of the SOD1G93A low-copy transgenic mouse, which models human amyotrophic lateral sclerosis. Dis Model Mech. 2011;4:686–700. doi: 10.1242/dmm.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acsadi G, Lee I, Li X, Khaidakov M, Pecinova A, Parker GC, et al. Mitochondrial dysfunction in a neural cell model of spinal muscular atrophy. J Neurosci Res. 2009;87:2748–56. doi: 10.1002/jnr.22106. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Al Chalabi A. Clinical genetics of amyotrophic lateral sclerosis: what do we really know? Nat Rev Neurol. 2011;7:603–15. doi: 10.1038/nrneurol.2011.150. [DOI] [PubMed] [Google Scholar]
- Andersen PM, Nilsson P, Ala-Hurula V, Keranen ML, Tarvainen I, Haltia T, et al. Amyotrophic lateral sclerosis associated with homozygosity for an Asp90Ala mutation in CuZn-superoxide dismutase. Nat Genet. 1995;10:61–6. doi: 10.1038/ng0595-61. [DOI] [PubMed] [Google Scholar]
- Andrus PK, Fleck TJ, Gurney ME, Hall ED. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1998;71:2041–8. doi: 10.1046/j.1471-4159.1998.71052041.x. [DOI] [PubMed] [Google Scholar]
- Apostolski S, Nikolic J, Bugarski-Prokopljevic C, Miletic V, Pavlovic S, Filipovic S. Serum and CSF immunological findings in ALS. Acta Neurol Scand. 1991;83:96–8. doi: 10.1111/j.1600-0404.1991.tb04656.x. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Rakowicz W, Gardner R, Pestronk A. Frequent atrophic groups with mixed-type myofibers is distinctive to motor neuron syndromes. Muscle Nerve. 2007a;36:107–10. doi: 10.1002/mus.20755. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Schmidt RE, Pestronk A, Milbrandt J. Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J Neurosci. 2007b;27:422–30. doi: 10.1523/JNEUROSCI.4798-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48:629–41. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Beal MF. Role of excitotoxicity in human neurological disease. Curr Opin Neurobiol. 1992;2:657–62. doi: 10.1016/0959-4388(92)90035-j. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Bhusari SS, Dobosy JR, Fu V, Almassi N, Oberley T, Jarrard DF. Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics. 2010;5:402–9. doi: 10.4161/epi.5.5.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birve A, Neuwirth C, Weber M, Marklund SL, Nilsson AC, Jonsson PA, et al. A novel SOD1 splice site mutation associated with familial ALS revealed by SOD activity analysis. Hum Mol Genet. 2010;19:4201–6. doi: 10.1093/hmg/ddq338. [DOI] [PubMed] [Google Scholar]
- Bodendorf U, Danner S, Fischer F, Stefani M, Sturchler-Pierrat C, Wiederhold KH, et al. Expression of human beta-secretase in the mouse brain increases the steady-state level of beta-amyloid. J Neurochem. 2002;80:799–806. doi: 10.1046/j.0022-3042.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci USA. 1994;91:8292–6. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukaftane Y, Khoris J, Moulard B, Salachas F, Meininger V, Malafosse A, et al. Identification of six novel SOD1 gene mutations in familial amyotrophic lateral sclerosis. Can J Neurol Sci. 1998;25:192–6. doi: 10.1017/s0317167100034004. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Schulz JB, Brown RH, Jr., Beal MF. Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1993;61:2322–5. doi: 10.1111/j.1471-4159.1993.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Broom WJ, Greenway M, Sadri-Vakili G, Russ C, Auwarter KE, Glajch KE, et al. 50bp deletion in the promoter for superoxide dismutase 1 (SOD1) reduces SOD1 expression in vitro and may correlate with increased age of onset of sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:229–37. doi: 10.1080/17482960802103107. [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, Baik MJ, Gurney M, Brown RH, Jr., Beal MF. Metabolic dysfunction in familial, but not sporadic, amyotrophic lateral sclerosis. J Neurochem. 1998;71:281–7. doi: 10.1046/j.1471-4159.1998.71010281.x. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–38. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, et al. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281:1851–4. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- Casaril M, Corso F, Bassi A, Capra F, Gabrielli GB, Stanzial AM, et al. Decreased activity of scavenger enzymes in human hepatocellular carcinoma, but not in liver metastases. International Journal of Clinical and Laboratory research. 1994;24:94–97. doi: 10.1007/BF02593907. [DOI] [PubMed] [Google Scholar]
- Cassereau J, Chevrollier A, Gueguen N, Desquiret V, Verny C, Nicolas G, et al. Mitochondrial dysfunction and pathophysiology of Charcot-Marie-Tooth disease involving GDAP1 mutations. Exp Neurol. 2011;227:31–41. doi: 10.1016/j.expneurol.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Chang LY, Slot JW, Geuze HJ, Crapo JD. Molecular immunocytochemistry of the CuZn superoxide dismutase in rat hepatocytes. J Cell Biol. 1988;107:2169–79. doi: 10.1083/jcb.107.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestnut BA, Chang Q, Price A, Lesuisse C, Wong M, Martin LJ. Epigenetic regulation of motor neuron cell death through DNA methylation. J Neurosci. 2011;31:16619–36. doi: 10.1523/JNEUROSCI.1639-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS One. 2010;5:e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Rees HD, Weintraub ST, Levey AI, Chin LS, Li L. Oxidative modifications and aggregation of Cu,Zn-superoxide dismutase associated with Alzheimer and Parkinson diseases. J Biol Chem. 2005;280:11648–55. doi: 10.1074/jbc.M414327200. [DOI] [PubMed] [Google Scholar]
- Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci USA. 1992;89:10405–9. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Barreto J, Fryer JD, Shaw CA, Orr HT, Zoghbi HY. Partial loss of ataxin–1 function contributes to transcriptional dysregulation in spinocerebellar ataxia type 1 pathogenesis. PLoS Genet. 2010;6:e1001021. doi: 10.1371/journal.pgen.1001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J Biol Chem. 1997;272:23469–72. doi: 10.1074/jbc.272.38.23469. [DOI] [PubMed] [Google Scholar]
- Culotta VC, Lin SJ, Schmidt P, Klomp LW, Casareno RL, Gitlin J. Intracellular pathways of copper trafficking in yeast and humans. Adv Exp Med Biol. 1999;448:247–54. doi: 10.1007/978-1-4615-4859-1_22. [DOI] [PubMed] [Google Scholar]
- de Lustig ES, Serra JA, Kohan S, Canziani GA, Famulari AL, Dominguez RO. Copper-zinc superoxide dismutase activity in red blood cells and serum in demented patients and in aging. J Neurol Sci. 1993;115:18–25. doi: 10.1016/0022-510x(93)90062-4. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–8. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, et al. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993;261:1047–51. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci USA. 2006;103:7142–7. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Zhai H, Bigio EH, Yan J, Fecto F, Ajroud K, et al. FUS-immunoreactive inclusions are a common feature in sporadic and non-SOD1 familial amyotrophic lateral sclerosis. Ann Neurol. 2010;67:739–48. doi: 10.1002/ana.22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler R, Konstanzer A, Kuther G, Hesse S, Wolf W, Struppler A. Amyotrophic lateral sclerosis: macro-EMG and twitch forces of single motor units. Muscle Nerve. 1990;13:545–50. doi: 10.1002/mus.880130612. [DOI] [PubMed] [Google Scholar]
- Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–9. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson RL, Van Remmen H, et al. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. doi: 10.1038/sj.onc.1208207. [DOI] [PubMed] [Google Scholar]
- Ezzi SA, Urushitani M, Julien JP. Wild-type superoxide dismutase acquires binding and toxic properties of ALS-linked mutant forms through oxidation. J Neurochem. 2007;102:170–8. doi: 10.1111/j.1471-4159.2007.04531.x. [DOI] [PubMed] [Google Scholar]
- Faes L, Callewaert G. Mitochondrial dysfunction in familial amyotrophic lateral sclerosis. J Bioenerg Biomembr. 2011;43:587–92. doi: 10.1007/s10863-011-9393-0. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–40. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Glass JD. Oxidative stress induced by loss of Cu,Zn-superoxide dismutase (SOD1) or superoxide-generating herbicides causes axonal degeneration in mouse DRG cultures. Acta Neuropathol. 2010;119:249–59. doi: 10.1007/s00401-009-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Igoudjil A, Magrané J, Li Y, Hansen JM, Manfredi G, et al. SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse. Brain. 2011;134:196–209. doi: 10.1093/brain/awq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Li Y, Asress SA, Jones DP, Glass JD. Absence of SOD1 leads to oxidative stress in peripheral nerve and causes a progressive distal motor axonopathy. Exp Neurol. 2012;233:163–71. doi: 10.1016/j.expneurol.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice PS, Shaw IC, Kleiner HE, Miller RT, Monks TJ, Lau SS, et al. Evidence for DNA damage in amyotrophic lateral sclerosis. Muscle Nerve. 1996;19:797–8. [PubMed] [Google Scholar]
- Flood DG, Reaume AG, Gruner JA, Hoffman EK, Hirsch JD, Lin YG, et al. Hindlimb motor neurons require Cu/Zn superoxide dismutase for maintenance of neuromuscular junctions. Am J Pathol. 1999;155:663–72. doi: 10.1016/S0002-9440(10)65162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K, Jonsson PA, Andersen PM, Bergemalm D, Graffmo KS, Hultdin M, et al. Novel antibodies reveal inclusions containing non-native SOD1 in sporadic ALS patients. PLoS One. 2010;5:e11552. doi: 10.1371/journal.pone.0011552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratta P. Antisense makes sense for ALS. Lancet Neurol. 2013;12:416–417. doi: 10.1016/S1474-4422(13)70059-0. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–42. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986;247:1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fukada K, Nagano S, Satoh M, Tohyama C, Nakanishi T, Shimizu A, et al. Stabilization of mutant Cu/Zn superoxide dismutase (SOD1) protein by coexpressed wild SOD1 protein accelerates the disease progression in familial amyotrophic lateral sclerosis mice. Eur J Neurosci. 2001;14:2032–6. doi: 10.1046/j.0953-816x.2001.01828.x. [DOI] [PubMed] [Google Scholar]
- Ge WW, Leystra-Lantz C, Sanelli TR, McLean J, Wen W, Strong W, et al. Neuronal tissue-specific ribonucleoprotein complex formation on SOD1 mRNA: alterations by ALS SOD1 mutations. Neurobiol Dis. 2006;23:342–50. doi: 10.1016/j.nbd.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Graffmo KS, Forsberg K, Bergh J, Birve A, Zetterstrom P, Andersen P, et al. Expression of wild-type human superoxide dismutase-1 in mice causes amyotrophic lateral sclerosis. Human Mol Genet. 2013;22:51–60. doi: 10.1093/hmg/dds399. [DOI] [PubMed] [Google Scholar]
- Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, et al. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA. 2012;109:5074–9. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Chen Y, Zhou X, Kar A, Ray P, Chen X, et al. An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity. Nat Struct Mol Biol. 2011;18:822–30. doi: 10.1038/nsmb.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–5. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- Hayward C, Brock DJH, Minns RA, Swingler RJ. Homozygosity for Asn86Ser mutation in the CuZn superoxide dismutase gene produces a severe clinical phenotype in a juvenile onset case of familial amyotrophic lateral sclerosis. J Med Genet. 1998;35:174. doi: 10.1136/jmg.35.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273:7765–9. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- Hu J, Chen K, Ni B, Li L, Chen G, Shi S. A novel SOD1 mutation in amyotrophic lateral sclerosis with a distinct clinical phenotype. Amyotroph Lateral Scler. 2012;13:149–54. doi: 10.3109/17482968.2011.621437. [DOI] [PubMed] [Google Scholar]
- Huang TT, Yasunami M, Carlson EJ, Gillespie AM, Reaume AG, Hoffman EK, et al. Superoxide-mediated cytotoxicity in superoxide dismutase-deficient fetal fibroblasts. Arch Biochem Biophys. 1997;344:424–32. doi: 10.1006/abbi.1997.0237. [DOI] [PubMed] [Google Scholar]
- Huppke P, Brendel C, Korenke GC, Marquardt I, Donsante A, Yi L, et al. Molecular and biochemical characterization of a unique mutation in CCS, the human copper chaperone to superoxide dismutase. Hum Mutat. 2012;33:1207–15. doi: 10.1002/humu.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–72. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, et al. Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1. Neurobiol Dis. 2000;7:623–43. doi: 10.1006/nbdi.2000.0299. [DOI] [PubMed] [Google Scholar]
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–90. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson PA, Bergemalm D, Andersen PM, Gredal O, Brannstrom T, Marklund SL. Inclusions of amyotrophic lateral sclerosis-linked superoxide dismutase in ventral horns, liver, and kidney. Ann Neurol. 2008;63:671–5. doi: 10.1002/ana.21356. [DOI] [PubMed] [Google Scholar]
- Jonsson PA, Ernhill K, Andersen PM, Bergemalm D, Brannstrom T, Gredal O, et al. Minute quantities of misfolded mutant superoxide dismutase-1 cause amyotrophic lateral sclerosis. Brain. 2004;127:73–88. doi: 10.1093/brain/awh005. [DOI] [PubMed] [Google Scholar]
- Joyce PI, Fratta P, Fisher EM, Acevedo-Arozena A. SOD1 and TDP-43 animal models of amyotrophic lateral sclerosis: recent advances in understanding disease toward the development of clinical treatments. Mamm Genome. 2011;22:420–48. doi: 10.1007/s00335-011-9339-1. [DOI] [PubMed] [Google Scholar]
- Kato M, Aoki M, Ohta M, Nagai M, Ishizaki F, Nakamura S, et al. Marked reduction of the Cu/Zn superoxide dismutase polypeptide in a case of familial amyotrophic lateral sclerosis with the homozygous mutation. Neurosci Lett. 2001;312:165–8. doi: 10.1016/s0304-3940(01)02212-1. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005;209:76–85. doi: 10.1016/j.heares.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GA, Warner TG, Steimer KS, Hallewell RA. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci USA. 1991;88:7381–5. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel PF, Finiels F, Revah F, Mallet J. Neuromuscular function impairment is not caused by motor neurone loss in FALS mice: an electromyographic study. Neuroreport. 1996;7:1427–31. doi: 10.1097/00001756-199605310-00021. [DOI] [PubMed] [Google Scholar]
- Khare SD, Caplow M, Dokholyan NV. The rate and equilibrium constants for a multistep reaction sequence for the aggregation of superoxide dismutase in amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2004;101:15094–9. doi: 10.1073/pnas.0406650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi J, Kuroda M, Kawata A, Mochizuki Y, Mizutani T, Komori T, et al. Novel G37V mutation of SOD1 gene in autopsied patient with familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13:570–72. doi: 10.3109/17482968.2012.686512. [DOI] [PubMed] [Google Scholar]
- Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, et al. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–9. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–44. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrominova TY. Advanced age-related denervation and fiber-type grouping in skeletal muscle of SOD1 knockout mice. Free Radic Biol Med. 2010;49:1582–93. doi: 10.1016/j.freeradbiomed.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–4. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Remmen HV, Richardson A, et al. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1400–7. doi: 10.1152/ajpregu.00093.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi A, Abbruzzese G, Arata L, Cocito L, Vische M. Cerebrospinal fluid (CSF) findings in amyotrophic lateral sclerosis. J Neurol. 1984;231:75–8. doi: 10.1007/BF00313720. [DOI] [PubMed] [Google Scholar]
- Liaw KY, Lee PH, Wu FC, Tsai JS, Lin-Shiau SY. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am J Gastroenterol. 1997;92:2260–2263. [PubMed] [Google Scholar]
- Lim J, Crespo-Barreto J, Jafar-Nejad P, Bowman AB, Richman R, Hill DE, et al. Opposing effects of polyglutamine expansion on native protein complexes contribute to SCA1. Nature. 2008;452:713–8. doi: 10.1038/nature06731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Wang MY, Liaw KY, Lee PH, Chien SF, Tsai JS, et al. Superoxide dismutase in hepatocellular carcinoma affects patient prognosis. Hepatogastroenterology. 2001;48:1102–1105. [PubMed] [Google Scholar]
- Lobsiger CS, Boillee S, McAlonis-Downes M, Khan AM, Feltri ML, Yamanaka K, et al. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci USA. 2009;106:4465–70. doi: 10.1073/pnas.0813339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XH, Yang XW. "Huntingtin Holiday": Progress toward an antisense therapy for Huntington's disease. Neuron. 2012;74:964–6. doi: 10.1016/j.neuron.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa S, Leigh PN, King A, Jones E, Steele JC, Bodi I, et al. TDP-43 is consistently co-localized with ubiquitinated inclusions in sporadic and Guam amyotrophic lateral sclerosis but not in familial amyotrophic lateral sclerosis with and without SOD1 mutations. Neuropathology. 2009;29:672–83. doi: 10.1111/j.1440-1789.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Ziv V, Nevo N, Harris-Cerruti C, Mahler O. Cu/Zn superoxide dismutase plays important role in immune response. J Immunol. 2003;170:2993–3001. doi: 10.4049/jimmunol.170.6.2993. [DOI] [PubMed] [Google Scholar]
- Marklund SL, Holme E, Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982;126:41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Fujii N, Kondo A, Iwaki A, Hokonohara T, Honda H, et al. An autopsied case of sporadic adult-onset amyotrophic lateral sclerosis with FUS-positive basophilic inclusions. Neuropathology. 2011;31:71–6. doi: 10.1111/j.1440-1789.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–11. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Miller T, Pestronk A, David W, Rothstein J, Simpson E, Appel SH, et al. A Phase I, randomised, first-in-human study of an antisense oligonucleotide directed against SOD1 delivered intrathecally in SOD1–familial ALS patients. Lancet Neurol. 2013;12:435–442. doi: 10.1016/S1474-4422(13)70061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JJ, Trakadis YJ, Scriver CR. Phenylalanine hydroxylase deficiency. Genet Med. 2011;13:697–707. doi: 10.1097/GIM.0b013e3182141b48. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Santella RM, Liu X, Bogdanov M, Zipprich J, Wu HC, et al. Oxidative stress biomarkers in sporadic ALS. Amyotroph Lateral Scler. 2008;9:177–83. doi: 10.1080/17482960801933942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–73. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006;40:1993–2004. doi: 10.1016/j.freeradbiomed.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Munch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci USA. 2011;108:3548–53. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray LM, Talbot K, Gillingwater TH. Review: neuromuscular synaptic vulnerability in motor neurone disease: amyotrophic lateral sclerosis and spinal muscular atrophy. Neuropathol Appl Neurobiol. 2010;36:133–56. doi: 10.1111/j.1365-2990.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–23. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pokrishevsky E, Grad LI, Yousefi M, Wang J, Mackenzie IR, Cashman NR. Aberrant localization of FUS and TDP43 is associated with misfolding of SOD1 in amyotrophic lateral sclerosis. PLoS One. 2012;7:e35050. doi: 10.1371/journal.pone.0035050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramatarova A, Figlewicz DA, Krizus A, Han FY, Ceballos-Picot I, Nicole A, et al. Identification of new mutations in the Cu/Zn superoxide dismutase gene of patients with familial amyotrophic lateral sclerosis. Am J Genet. 1995;56:592–6. [PMC free article] [PubMed] [Google Scholar]
- Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum Mol Genet. 2008;17:1728–37. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Durazo A, Whitelegge JP, Borchelt DR. An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Hum Mol Genet. 2010;19:4774–89. doi: 10.1093/hmg/ddq408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–19. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Pupillo E, Messina P, Logroscino G, Zoccolella S, Chio A, Calvo A, et al. Trauma and amyotrophic lateral sclerosis: a case-control study from a population-based registry. Eur J Neurol. 2012;19:1509–17. doi: 10.1111/j.1468-1331.2012.03723.x. [DOI] [PubMed] [Google Scholar]
- Rakhit R, Crow JP, Lepock JR, Kondejewski LH, Cashman NR, Chakrabartty A. Monomeric Cu,Zn-superoxide dismutase is a common misfolding intermediate in the oxidation models of sporadic and familial amyotrophic lateral sclerosis. J Biol Chem. 2004;279:15499–504. doi: 10.1074/jbc.M313295200. [DOI] [PubMed] [Google Scholar]
- Ralph GS, Radcliffe PA, Day DM, Carthy JM, Leroux MA, Lee DC, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–33. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- Raoul C, Abbas-Terki T, Bensadoun JC, Guillot S, Haase G, Szulc J, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–8. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- Ratovitski T, Corson LB, Strain J, Wong P, Cleveland DW, Culotta VC, et al. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum Mol Genet. 1999;8:1451–60. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68:1576–82. doi: 10.1212/01.wnl.0000261045.57095.56. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, et al. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13:43–7. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Reddi AR, Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell. 2013;152:224–35. doi: 10.1016/j.cell.2012.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht W, Sapp P, Viaene MK, Rosen D, McKenna-Yasek D, Haines J, et al. Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J Neurochem. 1994;62:384–7. doi: 10.1046/j.1471-4159.1994.62010384.x. [DOI] [PubMed] [Google Scholar]
- Roe JA, Butler A, Scholler DM, Valentine JS, Marky L, Breslauer KJ. Differential scanning calorimetry of Cu,Zn-superoxide dismutase, the apoprotein, and its zinc-substituted derivatives. Biochemistry. 1988;27:950–8. doi: 10.1021/bi00403a017. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Bowling AC, Patterson D, Usdin TB, Sapp P, Mezey E, et al. A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3:981–7. doi: 10.1093/hmg/3.6.981. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 (Suppl 1):S3–9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yokota T, Mitani T, Ito K, Anzai M, Miyagishi M, et al. Transgenic small interfering RNA halts amyotrophic lateral sclerosis in a mouse model. J Biol Chem. 2005;280:42826–30. doi: 10.1074/jbc.M507685200. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Aoki M, Nagai M, Kobayashi M, Itoyama Y. Mitochondrial alterations in transgenic mice with an H46R mutant Cu/Zn superoxide dismutase gene. J Neuropathol Exp Neurol. 2009;68:365–73. doi: 10.1097/NEN.0b013e31819ba185. [DOI] [PubMed] [Google Scholar]
- Sato T, Nakanishi T, Yamamoto Y, Andersen PM, Ogawa Y, Fukada K, et al. Rapid disease progression correlates with instability of mutant SOD1 in familial ALS. Neurology. 2005;65:1954–7. doi: 10.1212/01.wnl.0000188760.53922.05. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Reaume A, Scott R, Coyle JT. Effects of over- and under-expression of Cu,Zn-superoxide dismutase on the toxicity of glutamate analogs in transgenic mouse striatum. Brain Res. 1998;789:32–39. doi: 10.1016/s0006-8993(97)01469-8. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995;38:691–5. doi: 10.1002/ana.410380424. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Reaume AG, Flood DG, Scott RW, Kowall NW, Ferrante RJ, et al. Mice lacking cytosolic copper/zinc superoxide dismutase display a distinctive motor axonopathy. Neurology. 1999;53:1239–46. doi: 10.1212/wnl.53.6.1239. [DOI] [PubMed] [Google Scholar]
- Shibata N, Nagai R, Uchida K, Horiuchi S, Yamada S, Hirano A, et al. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res. 2001;917:97–104. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- Simpson EP, Henry YK, Henkel JS, Smith RG, Appel SH. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62:1758–65. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Miller TM, Yamanaka K, Monia BP, Condon TP, Hung G, et al. Antisense oligonucleotide therapy for neurodegenerative disease. J Clin Invest. 2006;116:2290–6. doi: 10.1172/JCI25424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RG, Henry YK, Mattson MP, Appel SH. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1998;44:696–9. doi: 10.1002/ana.410440419. [DOI] [PubMed] [Google Scholar]
- Somwar R, Erdjument-Bromage H, Larsson E, Shum D, Lockwood WW, Yang G, et al. Superoxide dismutase 1 (SOD1) is a target for a small molecule identified in a screen for inhibitors of the growth of lung adenocarcinoma cell lines. Proc Natl Acad Sci USA. 2011;108:16375–80. doi: 10.1073/pnas.1113554108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Fu Q, Puttaparthi K, Matthews CM, Elliott JL. Redox susceptibility of SOD1 mutants is associated with the differential response to CCS over-expression in vivo. Neurobiol Dis. 2009;34:155–62. doi: 10.1016/j.nbd.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–4. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol. 1997;10:485–94. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276:38084–9. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- Subramaniam JR, Lyons WE, Liu J, Bartnikas TB, Rothstein J, Price DL, et al. Mutant SOD1 causes motor neuron disease independent of copper chaperone-mediated copper loading. Nat Neurosci. 2002;5:301–7. doi: 10.1038/nn823. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Ronchi D, Keskin I, Basak AN, Wilhelm C, Gobbi C, et al. Mutant superoxide dismutase-1 indistinguishable from wild-type causes ALS. Hum Mol Genet. 2012;21:3568–74. doi: 10.1093/hmg/dds188. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Dinse GE, Foley JF, Hardisty JF, Maronpot RR. Comparative prevalence, multiplicity, and progression of spontaneous and vinyl carbamate-induced liver lesions in five strains of male mice. Toxicol Pathology. 2002;30:599–605. doi: 10.1080/01926230290105776. [DOI] [PubMed] [Google Scholar]
- Towne C, Setola V, Schneider BL, Aebischer P. Neuroprotection by gene therapy targeting mutant SOD1 in individual pools of motor neurons does not translate into therapeutic benefit in fALS mice. Mol Ther. 2011;19:274–83. doi: 10.1038/mt.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Ackerley S, Davies KE, Talbot K. Dismutase-competent SOD1 mutant accumulation in myelinating Schwann cells is not detrimental to normal or transgenic ALS model mice. Hum Mol Genet. 2010;19:815–24. doi: 10.1093/hmg/ddp550. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Borchelt DR. Mapping superoxide dismutase 1 domains of non-native interaction: roles of intra- and intermolecular disulfide bonding in aggregation. J Neurochem. 2006;96:1277–88. doi: 10.1111/j.1471-4159.2005.03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Deng HX, Grisotti G, Zhai H, Siddique T, Roos RP. Wild-type SOD1 overexpression accelerates disease onset of a G85R SOD1 mouse. Hum Mol Genet. 2009a;18:1642–51. doi: 10.1093/hmg/ddp085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Grisotti G, Roos RP. Mutant SOD1 knockdown in all cell types ameliorates disease in G85R SOD1 mice with a limited additional effect over knockdown restricted to motor neurons. J Neurochem. 2010;113:166–74. doi: 10.1111/j.1471-4159.2010.06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gutmann DH, Roos RP. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum Mol Genet. 2011;20:286–93. doi: 10.1093/hmg/ddq463. [DOI] [PubMed] [Google Scholar]
- Wang L, Pytel P, Feltri ML, Wrabetz L, Roos RP. Selective knockdown of mutant SOD1 in Schwann cells ameliorates disease in G85R mutant SOD1 transgenic mice. Neurobiol Dis. 2012;48:52–7. doi: 10.1016/j.nbd.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Wang L, Sharma K, Grisotti G, Roos RP. The effect of mutant SOD1 dismutase activity on non-cell autonomous degeneration in familial amyotrophic lateral sclerosis. Neurobiol Dis. 2009b;35:234–0. doi: 10.1016/j.nbd.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–7. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kuno N, Kono Y, Nanba E, Ohama E, Nakashima K, et al. Absence of the mutant SOD1 in familial amyotrophic lateral sclerosis (FALS) with two base pair deletion in the SOD1 gene. Acta Neurol Scand. 1997;95:167–72. doi: 10.1111/j.1600-0404.1997.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Wei JP, Srinivasan C, Han H, Valentine JS, Gralla EB. Evidence for a novel role of copper-zinc superoxide dismutase in zinc metabolism. J Biol Chem. 2001;276:44798–803. doi: 10.1074/jbc.M104708200. [DOI] [PubMed] [Google Scholar]
- Wen HL, Lin YT, Ting CH, Lin-Chao S, Li H, Hsieh-Li HM. Stathmin, a microtubule-destabilizing protein, is dysregulated in spinal muscular atrophy. Hum Mol Genet. 2010;19:1766–78. doi: 10.1093/hmg/ddq058. [DOI] [PubMed] [Google Scholar]
- Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5:51–66. doi: 10.1038/nprot.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox KC, Zhou L, Jordon JK, Huang Y, Yu Y, Redler RL, et al. Modifications of superoxide dismutase (SOD1) in human erythrocytes: a possible role in amyotrophic lateral sclerosis. J Biol Chem. 2009;284:13940–7. doi: 10.1074/jbc.M809687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain- and loss-of-function in neurodegenerative diseases. EMBO J. 2008;27:336–49. doi: 10.1038/sj.emboj.7601930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Waggoner D, Subramaniam JR, Tessarollo L, Bartnikas TB, Culotta VC, et al. Copper chaperone for superoxide dismutase is essential to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA. 2000;97:2886–91. doi: 10.1073/pnas.040461197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PD, Wightman N, Huang M, Weiss A, Sapp PC, Cuny GD, et al. A high-throughput screen to identify inhibitors of SOD1 transcription. Front Biosci (Elite Ed) 2012;4:2801–8. doi: 10.2741/e584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip PK, Malaspina A. Spinal cord trauma and the molecular point of no return. Mol Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon EJ, Park HJ, Kim GY, Cho HM, Choi JH, Park HY, et al. Intracellular amyloid beta interacts with SOD1 and impairs the enzymatic activity of SOD1: implications for the pathogenesis of amyotrophic lateral sclerosis. Exp Mol Med. 2009;41:611–7. doi: 10.3858/emm.2009.41.9.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Maulik N, Engelman RM, Ho YS, Das DK. Targeted disruption of the mouse Sod I gene makes the hearts vulnerable to ischemic reperfusion injury. Circ Res. 2000;86:264–9. doi: 10.1161/01.res.86.3.264. [DOI] [PubMed] [Google Scholar]
- Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- Zinman L, Liu HN, Sato C, Wakutani Y, Marvelle AF, Moreno D, et al. A mechanism for low penetrance in an ALS family with a novel SOD1 deletion. Neurology. 2009;72:1153–9. doi: 10.1212/01.wnl.0000345363.65799.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington's disease. Physiol Rev. 2010;90:905–81. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.