Abstract
Some prominent studies have claimed that the medial temporal lobe is not involved in retention of information over brief intervals of just a few seconds. However, in the last decade several investigations have reported that patients with medial temporal lobe damage exhibit an abnormally large number of errors when required to remember visual information over brief intervals. But the nature of the deficit and the type of error associated with medial temporal lobe lesions remains to be fully established. Voltage-gated potassium channel complex antibody-associated limbic encephalitis has recently been recognized as a form of treatable autoimmune encephalitis, frequently associated with imaging changes in the medial temporal lobe. Here, we tested a group of these patients using two newly developed visual short-term memory tasks with a sensitive, continuous measure of report. These tests enabled us to study the nature of reporting errors, rather than only their frequency. On both paradigms, voltage-gated potassium channel complex antibody patients exhibited larger errors specifically when several items had to be remembered, but not for a single item. Crucially, their errors were strongly associated with an increased tendency to report the property of the wrong item stored in memory, rather than simple degradation of memory precision. Thus, memory for isolated aspects of items was normal, but patients were impaired at binding together the different properties belonging to an item, e.g. spatial location and object identity, or colour and orientation. This occurred regardless of whether objects were shown simultaneously or sequentially. Binding errors support the view that the medial temporal lobe is involved in linking together different types of information, potentially represented in different parts of the brain, regardless of memory duration. Our novel behavioural measures also have the potential to assist in monitoring response to treatment in patients with memory disorders, such as those with voltage-gated potassium channel complex antibody limbic encephalitis.
Keywords: voltage-gated potassium channel antibody; encephalitis; working memory; feature binding; hippocampus, relational memory
Introduction
In their seminal study Scoville and Milner (1957) showed that individuals, including the famous HM, with bilateral damage to their medial temporal lobe (MTL) exhibit complete anterograde amnesia. New incidents in their daily life were forgotten ‘as fast as they occur’. Most interestingly, these patients were still able to retain a three-figure number or a pair of words as long as attention was not diverted to a new topic. Thus, it was considered that short-term memory, typically defined as the retention of information over a few seconds, or working memory, active manipulations on short-term memory representations, remain intact after MTL damage. By contrast long-term memory is severely impaired.
However, more recent imaging studies have shown that the MTL and particularly the hippocampus are active during retention of information over brief retention intervals (Ranganath and D’Esposito, 2001; Piekema et al., 2006; Axmacher et al., 2007; Hannula and Ranganath, 2008). Furthermore, some recent neuropsychological studies have reported that patients with MTL lesions are impaired when the retention interval is as short as a few seconds (Hannula et al., 2006; Olson et al., 2006a, b; Ezzyat and Olson, 2008; Finke et al., 2008) or even when there are no explicit mnemonic requirements (Lee et al., 2005; Romero and Moscovitch, 2012; Warren et al., 2012). Such MTL activations and lesion-related effects were discovered mainly using tasks that required retention of associations, such as object-to-location links (for reviews see Ranganath and Blumenfeld, 2005; Cashdollar et al., 2011). These findings support a view of MTL function, based on ideas originally expressed by Marr (1971), which highlights the role of the MTL and particularly the hippocampus in associating or binding information represented in different parts of the neocortex—critically, regardless of memory duration.
The current study was designed to shed novel light on the nature of memory deficits that result from MTL damage. Unlike previous patient studies, we examined the precision of recall and types of errors (Wilken and Ma, 2004; Bays and Husain, 2008; Bays et al., 2009) rather than the number of errors, as studied in conventional tasks, such as the change detection paradigm, which requires binary decisions (e.g. change or no change). If an individual fails to report a change when it occurs on such a task, it does not necessarily mean that they did not have any memory of the item. Conversely, if they do report change or no change correctly, this does not mean they remembered the item perfectly. Such paradigms may not give us complete insight into the quality or fidelity of memory.
For this purpose we used two tasks in which participants are required to choose the remembered feature of an item from a continuous, analogue space (Wilken and Ma, 2004; Zhang and Luck, 2008; Bays et al., 2009; Gorgoraptis et al., 2011; Pertzov et al., 2012a, b). Such paradigms have two main advantages over the more conventional tasks with binary decisions. First, they provide much more information per trial (several bits versus one bit of information) and therefore are potentially more sensitive and less susceptible to ceiling and floor effects. Second, the continuous space of responses opens a window to investigate the type of errors made by participants, not just the frequency of errors (Bays et al., 2009).
We studied an unusual group of patients with a recently recognized condition typically associated with focal MTL signal change on neuroimaging (Vincent et al., 2011). Vincent et al. (2004) described a series of individuals with a potentially reversible limbic encephalitis associated with antibodies to voltage-gated potassium channels (VGKC-Ab). It has subsequently become clear that most of these antibodies are directed not against the voltage-gated potassium channel itself, but instead against specific components of the channels including the LGI1 (leucine-rich, glioma-inactivated 1) molecule, which seems to be important for synaptic communication (Lai et al., 2010; Vincent et al., 2011; Benarroch, 2012). Although previous studies have investigated some cognitive aspects of patients with VGKC-Ab (Maguire et al., 2006; Chan et al., 2007; Hartley et al., 2007), the full spectrum of cognitive impairment—and specifically performance in short-term memory tasks—in this population is still unknown.
Many previous studies on MTL involvement in memory over brief delays have studied patients suffering from Korsakoff’s syndrome, anoxia or herpes encephalitis, which potentially affect various brain regions outside the MTL. On the other hand, several lines of evidence suggest that VGKC-Ab encephalitis predominantly targets the MTL, mainly the hippocampus. A recent study in a mouse model found Lgi1 gene expression to be most prominent within intrahippocampal circuitry (Herranz-Pérez et al., 2010). Moreover, post-mortem study of a VGKC-Ab patient revealed neural loss restricted to the hippocampus, and amygdala to a lesser extent, but no damage has thus far been evident in other MTL regions or neocortex (Khan et al., 2009). Additional imaging studies have provided further support that the hyperintensity signal (in MRI FLAIR sequences) as a result of VGKC-Ab/acute amnesic encephalopathy, predominantly affects the MTL, specifically the hippocampus (Ances et al., 2005; Harrower et al., 2006; Reid et al., 2009).
The brain dysfunction associated with VGKC-Ab can clearly contribute to our understanding of the role of the MTL in memory and the study reported here also has the potential to aid clinical practice. Among the differential diagnoses of subacute amnesia not due to an infectious cause, VGKC-Ab mediated limbic encephalitis has a poor prognosis, but typically responds well to treatment if it is recognized (Vincent et al., 2011). Developing sensitive tasks for early identification and monitoring of response to treatment in such patients is therefore very important. The computerized behavioural tasks used here are potentially promising; they provide analogue response measures that avoid ceiling or floor effects and use simple visual stimuli that can be easily used in individuals of all cultural and educational backgrounds.
Patients and methods
We report data from seven cases. All patients presented with clinical features of a subacute amnesic encephalopathy compatible with a diagnosis of limbic encephalitis, highly elevated VGKC-Abs levels, no evidence of a tumour and negative results for paraneoplastic antibodies. HSV (herpes simplex virus) encephalitis and other infectious aetiologies were excluded with appropriate serum and CSF tests. Other tests included blood cell count and general chemistry, B12, folic acid, thyroid function tests, thyroglobulin and thyroperoxidase antibodies, syphilis and Lyme serology, antinuclear antibodies, and antibodies to double-stranded DNA. All patients have had clinical follow-up since symptom presentation as well as brain MRI scans. They all gave informed written consent and the studies were approved by the local ethics committee. The studies reported here conform to the Declaration of Helsinki.
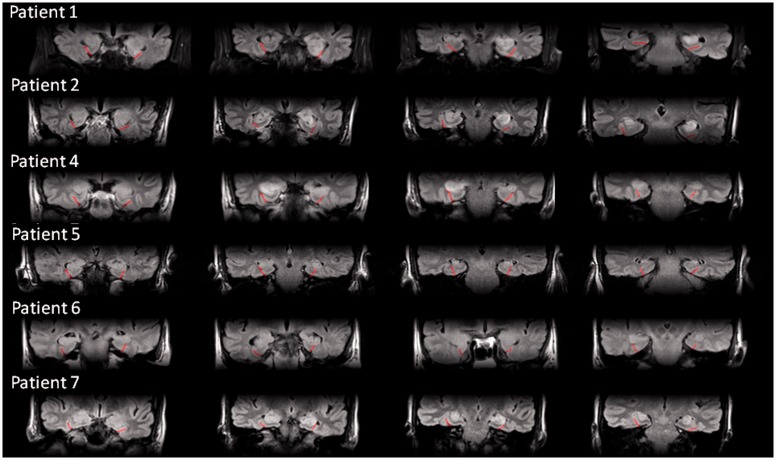
Details of the seven cases are provided in Table 1 and the online Supplementary material. Figure 1 presents MRI scans from the acute stage of six patients (Patient 3’s imaging was also reported to demonstrate abnormal signal bilaterally in the MTL by radiologists at his referring centre but unfortunately these data were not made available to us). No clear imaging abnormality in the MTL was reported in Patients 5 and 6, which has also been observed previously in some patients (Vincent et al., 2011). All seven patients participated in Experiment 1. Patients 1, 2 and 4 also participated in Experiment 2.
Table 1.
Patient demographics, raw scores and percentiles within normal population on a range of standard neuropsychological tests
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | M | M | F |
| Age | 69 | 53 | 77 | 65 | 51 | 62 | 45 |
| Handedness | Left | Right | Right | Right | Right | Right | Right |
| Months from symptoms–onset to test | 5, 10, 25 | 13 | 86 | 29 | 80 | 86 | 55 |
| Education years | 9 | 10 | 10 | 10 | 16 | 11 | 15 |
| RMT Words | 47 (50–75 percentile) | 45 (50 percentile) | 37 (<5 percentile) | 46 (75 percentile) | 41 (10–25 percentile) | 47 (75 percentile) | 49 (>95 percentile) |
| RMT Faces | 36 (5–10 percentile) | 34 (<5 percentile) | 36 (5–10 percentile) | 43(50–75 percentile) | 42 (25–50 percentile) | 39 (25–50 percentile) | 38 (10–25 percentile) |
| AMIPB story 1 | 5 (<10 percentile) | 36 (25–50 percentile) | |||||
| AMIPB story 2 | 2 (<10 percentile) | 34 (25–50 percentile) | |||||
| AMIPB figure 1 | 44 (25–50 percentile) | 72 (75 percentile) | |||||
| AMIPB figure 2 | 30 (10–25 percentile) | 72 (75–90 percentile) | |||||
| Rey copy | 100 (75–100 percentile) | 97.2 (50–75 percentile) | 100 (75–100 percentile) | 97.2 (50–75 percentile) | 97.2 (50–75 percentile) | ||
| Rey immediate | 16.67 (<5 percentile) | 55.56 (25–50 percentile) | 26.39 (<5 percentile) | 20.83 (<5 percentile) | 77.78 (25–50 percentile) | ||
| Rey delayed | 11.11 (<10 percentile) | 63.89 (50–75 percentile) | 26.39 (10–25 percentile) | 23.61 (10–25 percentile) | 70.83 (50–75 percentile) | ||
| WMS3 Logical Memory 1 | 5 (5 percentile) | 16 (>90 percentile) | 1 (<5 percentile) | 7 (10–25 percentile) | 10 (50 percentile) | ||
| WMS3 Logical Memory 2 | 4 (<5 percentile) | 14 (90 percentile) | 3 (<5 percentile) | 2 (<5 percentile) | 5 (5 percentile) | ||
| WMS3 Word List 1 | 7 (10–25 percentile) | 12 (75 percentile) | 2 (<5 percentile) | 8 (25 percentile) | 11 (50–75 percentile) | ||
| WMS3 Word List 2 | 6 (10 percentile) | 13 (75–90 percentile) | 5 (5 percentile) | 11 (50–75 percentile) | 15 (95 percentile) | ||
| WAIS Performance IQ | 79 | 89 | 91 | 105 | 106 | 111 | 116 |
| WAIS Verbal IQ | 79 | 84 | 91 | 106 | 107 | 110 | 115 |
| Trails A | 30 (75 percentile) | 35 (50 percentile) | 75 (10–25 percentile) | 52 (25–50 percentile) | 81 (<5 percentile) | 56 (25%) | 24 (75–90 percentile) |
| Trails B | 122 (25–50 percentile) | 75 (75–90 percentile) | 164 (10–25 percentile) | 144 (25 percentile) | 138 (5 percentile) | 109 (50 percentile) | 42 (75–90 percentile) |
| GNT | 17 (25 percentile) | 23 (75 percentile) | 16 (10–25 percentile) | 24 (75–90 percentile) | 24 (75–90 percentile) | 22 (50–75 percentile) | 24 (75–90 percentile) |
| Stroop | 70 (10–25 percentile) | 93 (25–50 percentile) | 63 (50–75 percentile) | 75 (25–50 percentile) | 55 (75 percentile) | 41 (95 percentile) | 33 (>95 percentile) |
AMIPB = Adult Memory and Information Processing Battery; GNT = Graded Naming Test; Rey = Rey–Osterrieth Complex Figure Test; RMT = Recognition Memory Test; WAIS = Wechsler Adult Intelligence Scale; WMS3 = Wechsler Memory Scale–3rd revision.
Figure 1.
Coronal fluid-attenuation inversion-recovery scans of six VGKC patients during the acute stage of encephalitis. The collateral sulcus, dividing the MTL from the inferior temporal lobe, is marked with a semi-transparent red line.
Experiment 1: What was where?
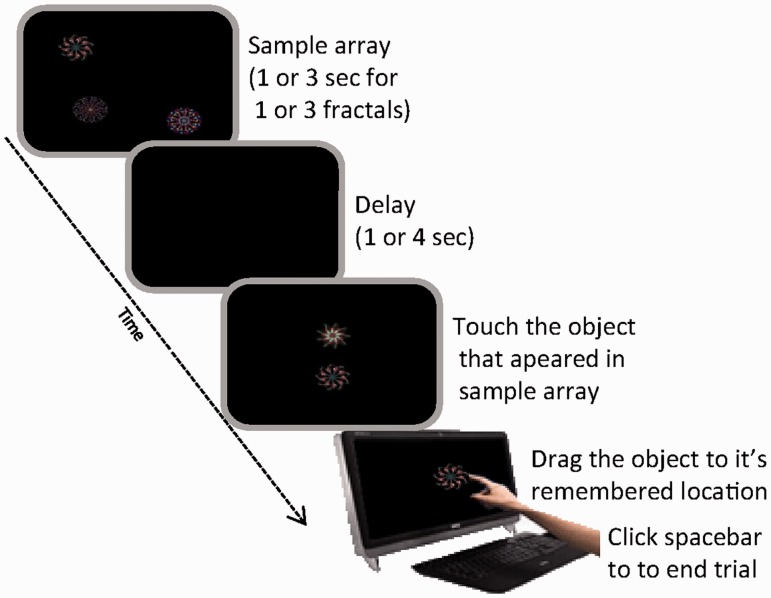
In our first experiment, participants’ ability to remember the identity of objects and their locations was assessed using a recently established paradigm that provides a measure of recall performance on a continuous, analogue scale (Pertzov et al., 2012b). A schematic representation of the task is shown in Fig 2.
Figure 2.
‘What was where?’ task. One or three fractals were simultaneously presented in pseudo-random locations. Following a delay of 1 or 4 s, a two alternative forced choice between one of the displayed fractals and a foil was presented. Participants were required to ‘drag’ the previously presented fractal on the touch screen to its remembered, original location on the screen.
In different trials, participants viewed one or three fractal objects, each located randomly on the screen. They were asked to remember both the objects and their locations. After a delay of 1 or 4 s, two fractals were presented on the vertical meridian. One of these had appeared in the memory array (target) whereas the other one was a foil that had not. Participants were required to touch the remembered object to indicate which fractal they thought had appeared in the memory array, and then ‘drag’ it on the touch screen to its remembered location. Thus we could measure memory for object identity separately of object location. Localization performance was analysed only on trials in which an object was correctly identified.
Participants sat ∼42 cm in front of an interactive touch-sensitive screen (Inspiron all-in-one 2320, Dell) with a 1920 × 1080 pixel matrix corresponding to ∼62 × 35° of the visual angle. Stimuli were drawn from a library of 60 fractals (Supplementary material; taken from Sprott’s Fractal Gallery; http://sprott.physics.wisc.edu/fractals.htm), randomly selected without repetitions for every trial. Each fractal had a maximum width and height of 120 pixels (∼4° of visual angle). Stimuli were presented on a black background.
The location of fractals was determined by a Matlab script (MathWorks, Inc.) in a random manner, with several restrictions. Objects were never located within 9° of each other. Moreover they were positioned with a minimum of 3.9° from the edges of the screen and 6.5° from the centre of screen. Participants performed two test blocks of 50 trials each. A block consisted of 10 trials with one fractal and 40 trials with three fractals. A different number of trials per condition was determined in pilot studies to maximize the power of the analysis while keeping it as short as possible. Fractals were repeated between three to four times in different trials within a block. The blank maintenance interval was 1 s in half of the trials and 4 s in the other half, in random order. Following this, the object identification part of the task was introduced: two objects were presented above and below central fixation, one of them was a foil and the other one had appeared in the memory array. The foil was not an unfamiliar object, but taken from the library of fractals used across the experiment.
Localization memory was computed by taking the distance between the centre of the target object after it had been dragged to its remembered location from the actual centre of the object in the initial memory array. For convenience, we converted this value to a visual angle for a viewing distance of 42 cm. As discussed below, we also computed the distance between the remembered location of the target object to that of non-target (unprobed) items that had appeared in the original memory array. Progress was self-paced and termination of each trial was signalled by pressing the space bar.
Patients who performed worse than two standard deviations below healthy controls on the identification task were excluded from further analysis because the focus of this study was to examine the distribution of errors of correctly remembered items. In total, seven VGKC patients were able to perform the identification task well enough for us to analyse the distribution of their localization recall errors. Their performance was compared to 12 aged and education-matched control subjects (mean age: 63 years, standard deviation 7.2; mean education level: 12 years, standard deviation 1.9) using mixed repeated measures ANOVA with number-of-items (fractals) as within subject factor, and group (patients versus controls) as between subjects factor (SPSS v. 18). Further analysis was performed using two-tailed t-tests. Percentages and proportions were normalized before statistical comparisons using natural log transformation.
Experiment 2: Memory for stimuli presented sequentially at one location
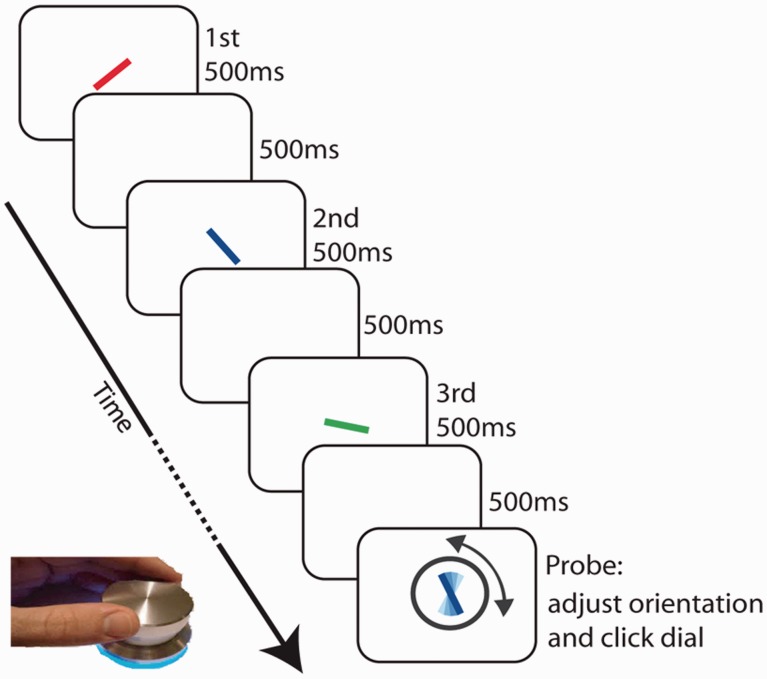
Object location is often treated as a privileged property and might behave unlike other visual properties such as colour (Treisman, 1988; Tsal and Lavie, 1993; Huang and Pashler, 2007). In our second experiment, we therefore eliminated the requirement to remember different locations, using a serial order task. Our second experiment used a new paradigm that also provides a measure of recall on a continuous, analogue scale (Gorgoraptis et al., 2011), rather than a binary response. A schematic representation of the task is shown in Fig. 4.
Figure 4.
Sequential task. Participants were presented with a sequence of one to three coloured bars, each with a different orientation. A probe item of a randomly chosen colour (in this case, blue) was then presented and participants adjusted the orientation of the probe item to that of the orientation of the target item of the same colour shown in the sequence (in this case, the second item).
In this task, participants viewed a series of one to three coloured bars, each of different orientation, at screen centre, presented one at a time. At the end of the sequence, recall for one of the items was probed by redisplaying a bar of the same colour, but with a random orientation. Participants were asked to rotate the probe on the screen using a response dial (PowerMate, Griffin Technology) to match the remembered orientation of the bar of the same colour in the sequence. Pushing the dial signalled the termination of the trial and initiated the next sequence.
Each trial consisted of a sequence of one to three coloured bars (2° × 0.2° of visual angle) consecutively presented on a grey background on a laptop screen (32° × 19°) at a viewing distance of ∼52 cm. In each trial, participants did not know in advance how many objects they would have to remember. Each bar had a different colour and orientation and all were presented at fixation, at the centre of the display. The orientation of stimuli within a sequence differed by at least 10°, but was otherwise randomly selected. The sequence of colours in each trial was produced by permutation of a random selection of five easily distinguishable colours. Each stimulus was shown for 500 ms, followed by a blank screen for 500 ms.
At the end of a sequence, the probe item appeared with a circle surrounding it (radius of 3.9°) making it easily distinguishable from the to-be-remembered items in the sequence. Participants used the response dial to match the remembered orientation of the item of the same colour in the sequence—henceforth termed the target. Note the term ‘target’ is used here simply to distinguish it from other objects in the sequence, or non-targets, that were not probed. We emphasize that, due to randomization, participants did not know which item would be tested or how long each sequence would be.
Patients 1, 2 and 4 took part in at least one session of the experiment. Patient 1 performed this experiment in four different time points. Every session consisted of a total of 120 interleaved trials: 20 trials with one item, 40 with two items and 60 with three items in the sequence. The performance of each patient was compared with eight age- and education-matched control subjects (mean age: 69, standard deviation 6.6; mean education level: 12 year, standard deviation 1.9) using mixed ANOVA with number-of-items as a within subjects factor and group (patients versus controls) as between subjects factor. Further statistics were performed using one-tailed t-tests. The size of effect at the individual level was also reported with respect to the controls’ performance using normalized z-scores.
Results
Experiment 1: What was where?
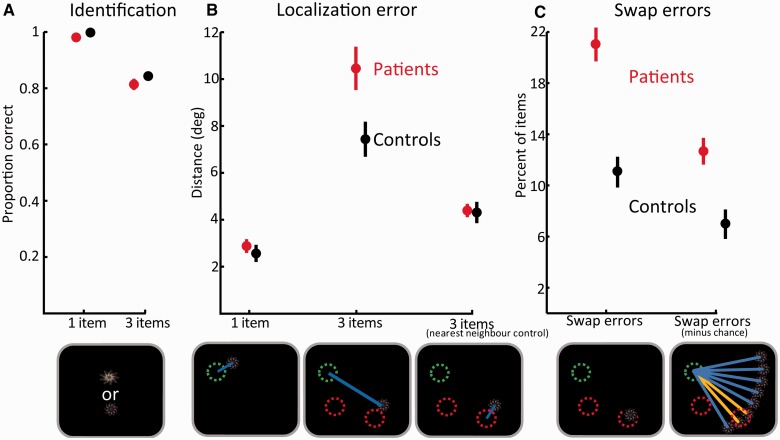
In this experiment, one or three fractals were presented at random locations. Following a brief delay, participants had to touch the fractal they remembered from the display (but not the foil) and drag it to its memorized location (Fig. 2). First, we analysed identification performance (Fig. 3A; for numerical values see Supplementary Table 1), i.e. the frequency with which participants touched the correct fractal in the two alternative forced choice. We used repeated measures ANOVA on identification performance with number-of-items (one versus three fractals) and group (controls versus patients) as within and between subjects factors, respectively. Identification performance was worse when three fractals were presented compared to one [F(1,17) = 70, P < 0.001, 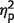 = 0.8]. However, performance was similar for control subjects and patients, as implied by insignificant effect of group [F(1,17) = 1.7, P = 0.21,
= 0.8]. However, performance was similar for control subjects and patients, as implied by insignificant effect of group [F(1,17) = 1.7, P = 0.21, 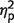 = 0.09] and interaction [F(1,17) = 0.5, P = 0.45,
= 0.09] and interaction [F(1,17) = 0.5, P = 0.45, 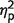 = 0.03].
= 0.03].
Figure 3.
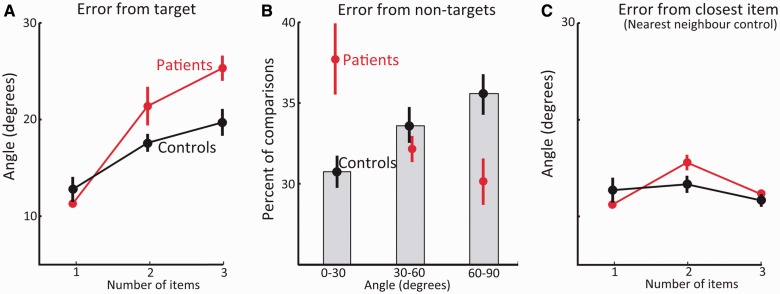
Results of ‘What was where?’ task. (A) Identification performance: the proportion of times subjects touched the correct object in the two alternative forced choice. Performance in trials with one and three fractals for healthy controls (black) and patients (red). (B) Localization errors for trials with one and three fractals for healthy controls (black) and patients (red). The ‘nearest neighbour’ control error (right) was calculated as the minimal distance between a reported location and any one of the previously presented items for three-item displays. Bottom images depict a specific example relevant to the above plots. Circles represent the original locations of the target fractal (green) and two others (red); blue lines illustrate the localization errors used in the above plots. (C) Swap errors are the number of times target objects were localized in proximity to the remembered locations of non-target (unprobed) items in the original display (here illustrated as red circles). The image below shows how a target item might be misplaced at the location of a non-target item generating a swap error. On the right is the number of swap errors after subtraction of errors that could be predicted by chance, assuming the same amplitude—but different directions—of error from the correct target location. The image shows how the direction of a non-target relative to a target (orange lines) is highly demarcated from all other possible locations at the same amplitude of error, but at different directions (blue lines).
Next, we analysed the distance between the reported location and the true, original location of a fractal (Fig. 3B; Supplementary Table 1). Trials with incorrect identifications were excluded from further analysis. Similarly to identification performance, we used ANOVA with the factors of number-of-items and group to analyse localization performance. The main effect of number-of-items was significant [F(1,17) = 246, P < 0.001, 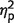 = 0.93] reflecting larger errors when three fractals were presented. Unlike identification performance, patients had larger localization errors relative to control subjects, as reflected in the main effect of group [F(1,17) = 4.5, P = 0.05,
= 0.93] reflecting larger errors when three fractals were presented. Unlike identification performance, patients had larger localization errors relative to control subjects, as reflected in the main effect of group [F(1,17) = 4.5, P = 0.05, 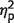 = 0.21]. But this difference seems to arise mainly from impaired localization performance when three fractals were presented, as suggested by the significant interaction [F(1,17) = 11.6, P = 0.003,
= 0.21]. But this difference seems to arise mainly from impaired localization performance when three fractals were presented, as suggested by the significant interaction [F(1,17) = 11.6, P = 0.003, 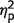 = 0.41] and by further t-test comparisons between groups [one item: t(17) = 0.6 , P = 0.54; three items: t(17) = 2.7, P = 0.017]. This finding raises a critical question: is the patients’ increased error for multiple items simply a result of degraded memory of a fractal’s location, or could it be that patients localized a fractal at the location of another object they had seen in the original memory array?
= 0.41] and by further t-test comparisons between groups [one item: t(17) = 0.6 , P = 0.54; three items: t(17) = 2.7, P = 0.017]. This finding raises a critical question: is the patients’ increased error for multiple items simply a result of degraded memory of a fractal’s location, or could it be that patients localized a fractal at the location of another object they had seen in the original memory array?
To examine this issue we counted the frequency with which fractals were localized within a circumference of 4.5° eccentricity from the location of other fractals presented in the original memory array. We term any errors within this perimeter as ‘swapped objects’ or ‘swap errors’ because they arise from swapping the location of an object with that of another item in the array. We used a threshold of 4.5° because objects were never presented <9° from each other. Using a 4.5° cut-off means that the reported location of an object could never be attributed (‘swapped’) to more than one object since the reported location could never be within 4.5° of two original locations. Because of the jitter or variability in localization errors, using a stricter threshold might lead to erroneous exclusion of some trials in which participants reported the location of another object but in a relatively imprecise manner. In any event, using a threshold of 4° for determining ‘swap’ errors did not alter the qualitative nature of the results. A 4.5° threshold is also well above basic localization precision as measured with a single object (Fig. 3B).
Strikingly, patients exhibited almost twice as many swap errors compared with control subjects [Fig. 3C; controls: 11%; patients: 21%; t(17) = 5.7 P < 0.001]. However, it might be argued that the increased number of swap errors resulted simply from increased distance of errors in patients’ responses; objects localized further away from their original location might generate more (apparent) swap errors simply by chance. To control for this, we used the fact that if participants localized fractals particularly near the original locations of other (non-target) items in the memory array, their responses would have a specific vector of error (absolute distance and angular deviation) with respect to the correct location of the target. In contrast, if their memory for the original target location was randomly corrupted, they would drag the object to locations that are not systematically related to the relative direction of non-targets from the target. This principle is illustrated in Fig. 3C.
First we calculated the baseline probability of obtaining swap errors by chance. For each trial, we took the absolute distance of error from the target. Then we computed all potential locations with that distance from the correct location, but crucially at all possible angular deviations (using steps of 1°) from the true location. There was one proviso: a simulated location had to be within screen dimensions and the invisible margins used for generating the display. The chance probability of obtaining a swap error is therefore the number of simulated locations within our 4.5° threshold perimeter around non-targets (orange lines in Fig. 3C), divided by all possible valid, simulated locations (all lines in Fig. 3C).
We performed this calculation for every trial using its specific distance of error from the target item. Next, we subtracted the number of swap errors predicted by chance from the measured number of swap errors (Fig. 3C). Importantly, even in this analysis, in which the distance of error from target was controlled, patients exhibited almost twice as many swap errors compared to control subjects [Fig. 3C; patients 13% versus 7% for controls; t(17) = 3.5 P = 0.003]. Both patients [t(6) = 13.2 P < 0.001] and healthy controls [t(11) = 6.4 P < 0.001] exhibited significantly more swap errors than could be predicted by chance. The latter finding replicates the results of our previous study in healthy participants (Pertzov et al., 2012b), but this time in an older group. The results indicate that some mislocalizations in memory are indeed clustered around locations of other objects in the array. Thus, memory reports are biased by other items simultaneously held in memory, but this effect is significantly worse for VGKC patients.
How critical are these swap errors for the increased mislocalization error measured in patients? To answer this question we calculated localization error in a slightly different manner. In this control analysis, whenever an object was localized far from its original location but closer to the original location of another object, the closest location was treated as if it was the object’s original location. In other words, localization error was now measured as the distance between the reported location of the object and the nearest original location of any object in the memory array, not exclusively to the original location of the probed object. This analysis controls for swap errors because whenever a swap occurs, it is treated as if the swapped location is in fact the object’s original location. This manipulation, which we term ‘nearest neighbour’ control (Fig. 3B), revealed that when swap errors were controlled, the difference in localization error between patients and controls was no longer evident [t(17) = 0.14; P = 0.89]. Accordingly, using the controlled errors in the ANOVA instead of the standard three items localization errors abolishes the previously significant main effect of group [F(1,17) = 0.1, P = 0.71, 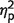 = 0.01] and interaction [F(1,17) = 0.4, P = 0.52,
= 0.01] and interaction [F(1,17) = 0.4, P = 0.52, 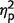 = 0.02].
= 0.02].
Importantly, the nearest neighbour control also provides a window for measuring ‘isolated’ localization performance as object identity is now rendered irrelevant. The result implies that patients’ localization performance, similarly to identification performance, was in fact comparable with healthy control subjects even when three items had to be memorized. Thus, the above analysis strongly suggest that spatial memory per se, similarly to identification performance, was not impaired in the patient group but rather the impairment patients exhibit arises from increased tendency to report the location of a non-target item from the memory array.
It is important to bear in mind that locating objects to their correct locations would be expected to be more difficult than remembering object identity or location alone (Postma and De Haan, 1996; Dent and Smyth, 2005). Therefore ceiling effects could theoretically lead to identification and localization performance being similar in patients and controls. However, identity and location performance per se were clearly far from ceiling in our participants. Precision of recall in the nearest neighbour control, our proxy for ‘isolated’ localization errors (controlling for swap errors and relevance of object identity), is much worse than localization precision for one item, strongly suggesting it is not at ceiling. Similar logic might be applied to identification performance; this was worse when three items were memorized compared with one, so it is unlikely that similar performance for patients and control subjects could be attributed to ceiling effects.
As mentioned previously, spatial position is often treated as a privileged property of an object and might behave unlike other visual properties such as colour (Treisman, 1988; Tsal and Lavie, 1993; Huang and Pashler, 2007), especially with respect to MTL function (O’Keefe and Speakman, 1987; Piekema et al., 2006; Burgess, 2008). Therefore, we designed the next experiment to investigate if the abnormal level of swap errors is specific to object localization, or if this impairment could be generalized to non-spatial object attributes.
Experiment 2: Memory for stimuli presented sequentially at one location
In this experiment we measured how participants reproduced—from memory—the orientation of an item. In this task a series of one to three coloured bars, each of different orientation, was presented sequentially at the centre of screen. Memory for one of the items was then probed by redisplaying a bar of the same colour with a random orientation. Participants rotated the probe on the screen until it matched the remembered orientation of the bar of the same colour in the sequence (Fig. 4). Thus, similar to the task in Experiment 1, this task provided analogue measure of error, rather than binary measure.
First, we analysed the difference between the reported orientation and the orientation of the target item (Fig. 5A). Replicating previous reports (Gorgoraptis et al., 2011), the precision of recall deteriorated as more items were included in the sequence [F(2,18) = 38, P < 0.001, 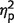 = 0.81]. Overall, patients’ performance was not significantly different to control subjects [F(1,9) = 2, P = 0.2,
= 0.81]. Overall, patients’ performance was not significantly different to control subjects [F(1,9) = 2, P = 0.2, 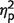 = 0.18] but crucially the group effect significantly interacted with the number-of-items [F(2,18) = 4, P = 0.03,
= 0.18] but crucially the group effect significantly interacted with the number-of-items [F(2,18) = 4, P = 0.03, 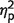 = 0.33]. Further statistical tests (group level and individual) show that the significant interaction reflects increased errors in patients specifically when multiple items were presented.
= 0.33]. Further statistical tests (group level and individual) show that the significant interaction reflects increased errors in patients specifically when multiple items were presented.
Figure 5.
Results of sequential task. (A) Mean angular error between the target item and the reported angle, for sequences of one to three items for healthy controls (black) and patients (red). (B) Percentage of comparisons (between reported orientation to non-target item) in which the angle of the reported orientation was within 0–30o, 30–60o and 60–90o of a non-target (an item that was in the original sequence but not probed). Note how patients’ responses were biased by non-target items. (C) ‘Nearest neighbour’ control: minimal angle between the reported orientation and any one of the displayed items. Error bars represent standard error of the mean across participants.
When one item was presented, patients were actually slightly—but not significantly—better than the averaged control, with all patients exhibiting slightly smaller angular error compared with the mean control result. However, their performance was worse than control subjects when they had to retain two [t(9) = 1.8, P = 0.056] and especially three items [t(9) = 2.1, P = 0.03]. These findings were also typically reflected at the individual level [for two items, Patient 1: Z = 2.5; Patient 2: Z = 1.9; Patient 4: Z = −0.35; and for three items, Patient 1: Z = 1.9; Patient 2: Z = 0.6; Patient 4: Z = 1.5].
What is the origin of this increased error? Is it a result of random guessing or, similar to the localization deficit, does it represents a systematic bias to report the values of other items in memory? To investigate this question we analysed the angle of error between the reported orientation and the orientation of the non-target (non-probed) items in the memory array (Fig. 5B). If indeed reports were biased towards other items in memory, we would expect to see a higher number of reports close to the non-target items and fewer reports farther away.
This analysis revealed that patients had significantly larger number of reports within 30° of the non-target orientations compared to healthy control subjects [t(9) = 3.0; P = 0.016], as also observed at the individual level [Patient 1: Z = 4.1; Patients 2 and 4: Z = 1.4]. There were correspondingly similar frequency of reports at 30–60° [t(9) = −0.7, P = 0.48], and fewer reports between 60 to 90° from the target orientation [t(9) = −2.2, P = 0.055]. These results strongly suggest that patients, more often than controls, misreported the orientation of other stimuli in the sequence. Similarly to the localization experiment, we term such errors as ‘swap errors’ because they presumably arise from swapping the orientation of one item with that of another.
How critical are these additional swap errors to the increased error measured in patients? To answer this question we recalculated the angle of error using the ‘nearest neighbour’ procedure—the minimal angle between the reported orientation and any one of the items in the memory array (Fig. 5C). In this control analysis, whenever the reported orientation was distant from the target orientation and closer to the orientation of another (non-target) item from the display, the most similar item was treated as if it was the target. Controlling for swap errors effectively diminished the patients’ impairment when two items were presented [t(9) = 1.3, P = 0.11 ; individual level: Patient 1 versus controls: Z = 0.7; Patient 2: Z = 1.5; Patient 4: Z = 0.3]. It completely abolished the deficit in the case of three items [t(9) = 0.5, P = 0.30; individual level: Patient 1 Z = 0.28; Patient 2: Z = 0.0; Patient 4: Z = 0.7].
We conclude that patients were normal when the orientation of a single item had to be remembered, but were typically impaired when two or three oriented bars had to be memorized (Fig. 5A). Similar to the ‘What was where?’ task, much of the additional error in patients’ performance arose from reporting the orientation of the wrong item i.e. swap errors. Note that a slight impairment could be observed in two-item sequences even in the nearest item control (Fig. 5C). This raises the possibility that there might be other deficits—in addition to the abnormally high frequency of swap errors—that might contribute to some patients’ performance.
Monitoring performance with treatment interventions
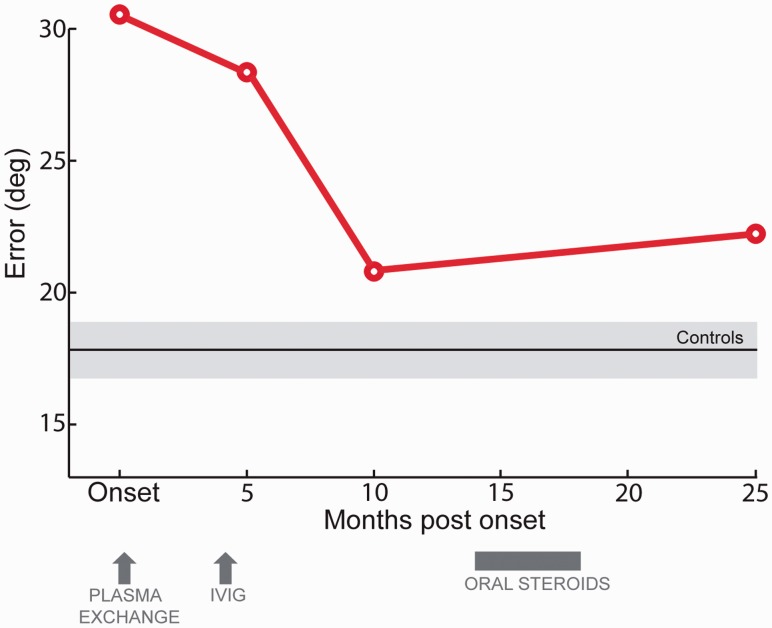
It is possible that these kinds of tasks might provide sensitive methods for monitoring response to treatment. Patient 1 performed the sequential paradigm four different times after his initial clinical assessment (Fig. 6). The first assessment was just before plasma exchange, his initial treatment, commenced. This did not appear to lead to any substantial improvement in the overall precision of recall. By contrast, the next treatment, intravenous immunoglubulin, was followed by a considerable improvement in memory performance as well as clinical state, not immediately but over the next few months.
Figure 6.
Averaged angular error of Patient 1 at several time points. Angular error in the oriented-bars task as a function of the time the test was performed. Normal range is illustrated by black and grey horizontal lines that represent controls average performance and SEM, respectively. IVIG = intravenous immunoglobulin.
Because of concerns over subsequent elevation of VGKC-Ab titre, further treatment with daily doses of oral prednisolone (steroids) was introduced. This led to improvement in antibody titre but did not lead to any more improvement in memory performance. Further studies with more frequent testing would be needed to validate this approach but this example provides proof-of-concept that memory performance can be tracked and the effects of treatment monitored using such continuous-report measures.
Discussion
We used two different continuous report memory tasks to investigate the nature of errors in short-term memory tasks associated with VGKC-Ab limbic encephalitis, which affects mainly the MTL and hippocampus (Khan et al., 2009; Vincent et al., 2011). Two key findings were consistent across tasks and patients in our studies. First, memory impairment was evident when several objects had to be remembered over short durations, but not when only one item had to be retained. Secondly, the impairment was reflected by increased number of swap errors, whereby participants reported in a fairly precise manner a feature of another item held in memory that was not probed. Indeed, when the data were analysed as if participant responses were related to unprobed items in the memory array (i.e. agnostic to swap errors), patients’ performance was within the normal range (Figs 3B and 5C).
These findings suggest that there is a specific deficit of keeping together—binding—different properties of an item (identity and location, colour and orientation, respectively in our two studies) when there is more than one object retained in memory. In the next section, we discuss the nature of memory impairment implied by these findings and how it relates to current theories on MTL functions.
Medial temporal lobe is necessary for binding different stimulus properties in memory
It has been claimed previously, on the basis of behavioural evidence, that the representation of a single object in ‘the focus of attention’ might be distinct from other representations in memory (Cowan, 1988; Oberauer, 2002). A single representation was suggested to have a privileged status in terms of accessibility and manipulability (Oberauer, 2009) and the ability to guide attention (Olivers et al., 2011). Recent imaging studies have also suggested that a similar distinction should be applied to the role MTL has in memory (Axmacher et al., 2007; Öztekin et al., 2010; Lewis-Peacock et al., 2011). For example, the MTL was found to be active in retention of multiple items over brief delays but not in the case of a single item (Axmacher et al., 2007). A recent series of studies has also shown that MTL patients exhibit impairment only when several items need to be remembered over few seconds, but not when a single item is memorized (Jeneson et al., 2010, 2012). However, both these brain activation and lesion impairments could arise simply because maintaining multiple items in memory is more difficult rather than due to a true difference in neural mechanisms responsible for remembering single versus multiple items in memory. In other words, the deficit exhibited by patients might relate to general task difficulty and not specifically to the process of maintaining several items in memory.
Here, we introduced two novel analogue response tasks and showed that memory for isolated properties of the stimuli was in fact intact, even when multiple items were in memory, ruling out any general effect of task difficulty. Note that identification performance for three items in Experiment 1 was not at ceiling, excluding the explanation that a ceiling effect might lead to similar identification performance in patients and controls. The key problem revealed by our analysis of the type of errors made by patients is that they reported features of other, unprobed items retained in memory. Such a result suggests that items were retained well in memory. However, the properties belonging to each item—location or identity, colour or orientation—were associated with the wrong object. Note also that performance on standard neuropsychological tests (e.g. Stroop task, trail making) demonstrate that our patients’ executive functions were not consistently impaired (Table 1), implying that their deficit is unlikely to originate from executive dysfunction such as inhibiting responses.
Consequently, we would argue that our results are consistent with a true distinction between the neural mechanisms involved in remembering one versus many items. Because different features of a visual stimulus are represented in separate neural structures (Zeki et al., 1991), a problem arises when multiple items are maintained simultaneously. How does the brain represent the fact that a set of specific properties belongs to Object A, whereas others, although active at the same time, belong to Object B? This ‘binding problem’ has been traditionally discussed with regard to visual perception (Treisman, 1996) but is very much relevant also to memory (for an extensive review see Zimmer et al., 2006). Thus, whenever more than one item has to be remembered, a putative binding mechanism is required to distinguish between the various properties that comprise distinct items, and to allow later access to one of them (Burgess and Hitch, 2005). Accordingly, a distinction should be made between the neural processes required for maintaining faithfully multiple features that belong to several items in memory, and the processes needed for retaining a single item.
We have shown here that when a single item was tested, our patients exhibited normal performance in both tasks. However, when multiple items had to be memorized, accessing an attribute of a specific item, even following a brief delay, requires associative or binding mechanisms that are likely to involve the MTL. Thus retaining several items led to clear impairments associated with an increased number of swap errors, evident in both experiments. These swap errors are reminiscent of the common transposition or intrusion errors made during serial recall tasks (Burgess and Hitch, 1992; Henson, 1996) in which participants recall stimuli from the wrong lists or positions rather than making arbitrary errors.
Intriguingly, an early study of MTL patients using serial recall also ‘surprisingly’ found an elevated number of intrusion errors from prior lists that could not be reconciled with a pure long-term memory impairment (Baddeley and Warrington, 1970). Such errors led computational models of memory to highlight the contribution of binding each item to its context for successful memory retrieval, regardless of retention duration (Burgess and Hitch, 2005). Impairment of this binding process, which might involve phase synchronization among different brain regions (Fell and Axmacher, 2011), would be expected to generate exactly the pattern of errors we report here: increased frequency of misreporting the wrong item but preserved memory of the isolated attributes of the items on their own.
Indeed, when our analysis controlled for swap errors—and therefore correct binding was rendered irrelevant—patients’ performance was effectively normal (Figs 3B and 5C). This strongly suggests that memory for isolated aspects of each item (locations and orientations, respectively in our experiments) were unimpaired. Thus, we would conclude that these patients with MTL damage are impaired at maintaining multiple items as a result of a failure to bind the different aspects of a coherent item to a distinguishable context signal. When this binding mechanism is impaired, ‘competitive cueing’ mechanisms during recall (Nairne, 2002; Bullock, 2004; Burgess and Hitch, 2005) are expected to lead to reporting the wrong item in memory—a swap error.
This conceptualization of the nature of the memory deficit in VGKC-Ab patients converges with several independent lines of research regarding MTL dysfunction and normal function. A recent study found that individuals at risk of developing familial Alzheimer’s disease have intact performance when required to maintain isolated features over a few seconds. However, they were impaired when the task required retaining bound features (Parra et al., 2010). And indeed, consistent with our findings, the MTL seems to be one of the earliest neural structures to be affected in this disease (Fox et al., 1996).
The refined view proposed here predicts that MTL activation should only be observed when associative mechanisms are required to retrieve a property of a specific item from memory, even following brief delays. Thus, in a sequential presentation of several items, the MTL should be involved in reporting any item in the sequence except the last one, which is still under the focus of attention. Indeed, some recent imaging studies that used a probe-recognition task with sequential presentation of lists of items have reported that the main difference in MTL activation during retrieval was between the last item of the sequence and all other items, regardless of whether they were within the presumed span of short-term memory or above it (Öztekin et al., 2009, 2010; Lewis-Peacock et al., 2011).
The involvement of the MTL in binding together separate aspects of memory is generally congruent with the associative ‘binding’, or the ‘relational’ hypotheses (for recent reviews see Davachi, 2006; Eichenbaum, 2006; Konkel and Cohen, 2009). These proposals consider the hippocampus as the neural structure responsible for maintaining links relating separate aspects of memory and enabling flexible recombination of memory parts. Critically, the role of the hippocampus in associative binding has been traditionally viewed as relevant only for declarative long-term memory (Eichenbaum et al., 1994; Fernández et al., 1999; Fernández and Tendolkar, 2001; Davachi and Wagner, 2002; Davachi, 2006; Eichenbaum, 2006). However, as we pointed out earlier, recent studies have shown that MTL patients exhibit an abnormally large number of errors in tasks that require maintenance of associations and feature conjunctions over even brief delays (Hannula et al., 2006; Olson et al., 2006a, b; Ezzyat and Olson, 2008; Finke et al., 2008).
MTL patients were also impaired when associative processes were required for discriminating visible complex stimuli (Lee et al., 2005; Graham et al., 2010; Warren et al., 2012), and generated abnormally low number of relations between sub-components when required to imagine detailed events in the future (Race et al., 2013; Romero and Moscovitch, 2012). These recent studies, among others (for a review see Graham et al., 2010), highlight MTL contribution to binding and associative processes in general, and not exclusively to declarative long-term memory. Our results support this view and extend it by showing that following brief delays, the nature of errors of MTL patients, not only the frequency, reflects binding or relational impairment.
Our results also have potentially important clinical implications. Patients presenting with subacute amnesia are not uncommonly seen in neurological practice. Amongst the differential diagnoses, infectious conditions (such as herpes simplex encephalitis), Korsakoff’s syndrome and autoimmune limbic encephalitis are generally considered. Antibody-related limbic encephalitis is not always easy to diagnose but often responds well to treatment. However, tracking response to treatment, particularly in the cognitive domain, is not always straightforward. Traditional neuropsychological tests might not always be sensitive to minor changes in patients’ symptoms. The analogue report tasks used here provide a precise, objective behavioural marker, without ceiling and floor effects, that can potentially help in monitoring response to treatment.
Conclusion
We found that memory performance of VGKC-Ab limbic encephalitis patients is comparable with control subjects when one item has to be retained over brief intervals. However, a significant impairment emerged when additional items had to be maintained in memory. This deficit was strongly associated with an increased tendency to report the wrong item in memory, strongly supporting the role MTL has in binding distinct visual aspects of an item across brief retention intervals. Our novel experimental design also provides a promising technique for assessing the integrity of MTL function.
Funding
This research was supported by The Wellcome Trust. B.C. is supported by the NIHR Academic Clinical Lectureship. T.D.M. is supported by an Entry Scholarship from the Guarantors of Brain. Aspects of this work were supported by the NIHR Queen Square Dementia BRU and the Wolfson Foundation.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- MTL
medial temporal lobe
- VGKC-Ab
voltage-gated potassium channel complex antibody
References
- Ances BM, Vitaliani R, Taylor RA, Liebeskind DS, Voloschin A, Houghton DJ, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–77. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Cohen MX, Elger Christian E, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 2007;27:7807–16. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD, Warrington EK. Amnesia and the distinction between long-and short-term memory1. J Verb Learn Verb Behav. 1970;9:176–89. [Google Scholar]
- Bays PM, Catalao RF, Husain M. The precision of visual working memory is set by allocation of a shared resource. J Vis. 2009;9:1–11. doi: 10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays PM, Husain M. Dynamic shifts of limited working memory resources in human vision. Science. 2008;321:851–4. doi: 10.1126/science.1158023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. ADAM proteins, their ligands, and clinical implications. Neurology. 2012;78:914–20. doi: 10.1212/WNL.0b013e31824c4728. [DOI] [PubMed] [Google Scholar]
- Bullock D. Adaptive neural models of queuing and timing in fluent action. Trends Cogn Sci. 2004;8:426–33. doi: 10.1016/j.tics.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Burgess N, Hitch GJ. Toward a network model of the articulatory loop. J Mem Lang. 1992;31:429–60. [Google Scholar]
- Burgess N, Hitch GJ. Computational models of working memory: putting long-term memory into context. Trends Cogn Sci. 2005;9:535–41. doi: 10.1016/j.tics.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Ann N Y Acad Sci. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Cashdollar N, Duncan JS, Duzel E. Challenging the classical distinction between long-term and short-term memory: reconsidering the role of the hippocampus. Future Neurol. 2011;6:351–62. [Google Scholar]
- Chan D, Henley S, Rossor MN, Warrington EK. Extensive and temporally ungraded retrograde amnesia in encephalitis associated with antibodies to voltage-gated potassium channels. Arch Neurol. 2007;64:404. doi: 10.1001/archneur.64.3.404. [DOI] [PubMed] [Google Scholar]
- Cowan N. Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol Bull. 1988;104:163–91. doi: 10.1037/0033-2909.104.2.163. [DOI] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neurophysiol. 2002;88:982–90. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Dent K, Smyth MM. Verbal coding and the storage of form-position associations in visual-spatial short-term memory. Acta Psychol. 2005;120:113–40. doi: 10.1016/j.actpsy.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Otto T, Cohen NJ. Two functional components of the hippocampal memory system. Behav Brain Sci. 1994;17:449–72. [Google Scholar]
- Eichenbaum H. Memory binding in hippocampal relational networks. In: Zimmer HD, Mecklinger A, Lindenberger U, editors. Handbook of binding and memory. New York: Oxford University Press; 2006. pp. 25–52. [Google Scholar]
- Ezzyat Y, Olson IR. The medial temporal lobe and visual working memory: comparisons across tasks, delays, and visual similarity. Cogn Affect Behav Neurosci. 2008;8:32–40. doi: 10.3758/cabn.8.1.32. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–18. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fernández G, Effern A, Grunwald T, Pezer N, Lehnertz K, Dümpelmann M, et al. Real-time tracking of memory formation in the human rhinal cortex and hippocampus. Science. 1999;285:1582–5. doi: 10.1126/science.285.5433.1582. [DOI] [PubMed] [Google Scholar]
- Fernández G, Tendolkar I. Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: human declarative memory formation at the system level. Brain Res Bull. 2001;55:1–9. doi: 10.1016/s0361-9230(01)00494-4. [DOI] [PubMed] [Google Scholar]
- Finke C, Braun M, Ostendorf F, Lehmann TN, Hoffmann KT, Kopp U, et al. The human hippocampal formation mediates short-term memory of colour-location associations. Neuropsychologia. 2008;46:614–23. doi: 10.1016/j.neuropsychologia.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain. 1996;119:2001–7. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Gorgoraptis N, Catalao RF, Bays PM, Husain M. Dynamic updating of working memory resources for visual objects. J Neurosci. 2011;31:8502–11. doi: 10.1523/JNEUROSCI.0208-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–53. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial temporal lobe activity predicts successful relational memory binding. J Neurosci. 2008;28:116–24. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–9. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrower T, Foltynie T, Kartsounis L, De Silva RN, Hodges JR. A case of voltage-gated potassium channel antibody-related limbic encephalitis. Nat Clin Pract Neurol. 2006;2:339–43. doi: 10.1038/ncpneuro0194. [DOI] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, et al. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Unchained memory: Error patterns rule out chaining models of immediate serial recall. Q J Exp Psychol A. 1996;49:80–115. [Google Scholar]
- Herranz-Pérez V, Olucha-Bordonau FE, Morante-Redolat JM, Pérez-Tur J. Regional distribution of the leucine-rich glioma inactivated (LGI) gene family transcripts in the adult mouse brain. Brain Res. 2010;1307:177–94. doi: 10.1016/j.brainres.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Huang L, Pashler H. A Boolean map theory of visual attention. Psychol Rev. 2007;114:599–631. doi: 10.1037/0033-295X.114.3.599. [DOI] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR. Intact working memory for relational information after medial temporal lobe damage. J Neurosci. 2010;30:13624. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Wixted JT, Hopkins RO, Squire LR. Visual working memory capacity and the medial temporal lobe. J Neurosci. 2012;32:3584–9. doi: 10.1523/JNEUROSCI.6444-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NL, Jeffree MA, Good C, Macleod W, Al-Sarraj S. Histopathology of VGKC antibody–associated limbic encephalitis. Neurology. 2009;72:1703–5. doi: 10.1212/WNL.0b013e3181a55eb3. [DOI] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3:166–74. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–85. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, et al. Perceptual deficits in amnesia: challenging the medial temporal lobe ‘mnemonic’view. Neuropsychologia. 2005;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lewis-Peacock JA, Drysdale AT, Oberauer K, Postle BR. Neural evidence for a distinction between short-term memory and the focus of attention. J Cogn Neurosci. 2011;24:61–79. doi: 10.1162/jocn_a_00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129:2894–2907. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Nairne JS. Remembering over the short-term: The case against the standard model. Annu Rev Psychol. 2002;53:53–81. doi: 10.1146/annurev.psych.53.100901.135131. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Oberauer K. Access to information in working memory: exploring the focus of attention. J Exp Psychol Learn Mem Cogn. 2002;28:411–21. [PubMed] [Google Scholar]
- Oberauer K. Design for a working memory. Psychol Learn Motiv. 2009;51:45–100. [Google Scholar]
- Olivers CN, Peters J, Houtkamp R, Roelfsema PR. Different states in visual working memory: when it guides attention and when it does not. Trends Cogn Sci. 2011;15:327–34. doi: 10.1016/j.tics.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci. 2006a;18:1087–97. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci. 2006b;26:4596–601. doi: 10.1523/JNEUROSCI.1923-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztekin I, Davachi L, McElree B. Are representations in working memory distinct from representations in long-term memory? Psychol Sci. 2010;21:1123–33. doi: 10.1177/0956797610376651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztekin I, McElree B, Staresina BP, Davachi L. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. J Cogn Neurosci. 2009;21:581–93. doi: 10.1162/jocn.2008.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra MA, Abrahams S, Logie RH, Méndez LG, Lopera F, Della Sala S. Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain. 2010;133:2702–13. doi: 10.1093/brain/awq148. [DOI] [PubMed] [Google Scholar]
- Pertzov Y, Bays PM, Joseph S, Husain M. Rapid Forgetting Prevented by Retrospective Attention Cues. Journal of Experimental Psychology: Human Perception and Performance. 2012a doi: 10.1037/a0030947. Advance online publication. doi: 10.1037/a0030947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertzov Y, Dong MY, Peich MC, Husain M. Forgetting what was where: the fragility of object-location binding. PLoS One. 2012b;7:e48214. doi: 10.1371/journal.pone.0048214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, Petersson KM, Fernández G. The right hippocampus participates in short-term memory maintenance of object-location associations. Neuroimage. 2006;33:374–82. doi: 10.1016/j.neuroimage.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Postma A, De Haan E. What was where? Memory for object locations. Q J Exp Psychol. 1996;49:178–199. doi: 10.1080/713755605. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus. 2013;23:268–277. doi: 10.1002/hipo.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci. 2005;9:374–80. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–73. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Reid JM, Foley P, Willison HJ. Voltage-gated potassium channel-associated limbic encephalitis in the West of Scotland: case reports and literature review. Scott Med J. 2009;54:27–31. doi: 10.1258/rsmsmj.54.4.27. [DOI] [PubMed] [Google Scholar]
- Romero K, Moscovitch M. Episodic memory and event construction in aging and amnesia. J Mem Lang. 2012;67:270–284. [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurology, Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman A. Features and objects: The fourteenth Bartlett memorial lecture. Q J Exp Psychol. 1988;40:201–37. doi: 10.1080/02724988843000104. [DOI] [PubMed] [Google Scholar]
- Treisman A. The binding problem. Curr Opin Neurobiol. 1996;6:171–8. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- Tsal Y, Lavie N. Location dominance in attending to color and shape. J Exp Psychol Hum Percept Perform. 1993;19:131–9. doi: 10.1037//0096-1523.19.1.131. [DOI] [PubMed] [Google Scholar]
- Vincent A, Bien Christian G, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–72. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- Vincent A, Buckley C, Schott JM, Baker I, Dewar BK, Detert N, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12. doi: 10.1093/brain/awh077. [DOI] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: Lesions of the medial temporal lobe impair online representation. Hippocampus. 2012;22:1577–88. doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilken P, Ma W. A detection theory account of change detection. J Vis. 2004;4:1120–35. doi: 10.1167/4.12.11. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. J Neurosci. 1991;11:641–9. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Luck SJ. Discrete fixed-resolution representations in visual working memory. Nature. 2008;453:233–5. doi: 10.1038/nature06860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HD, Mecklinger A, Lindenberger U. Handbook of binding and memory. USA: Oxford University Press; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.