Abstract
The frontal aslant tract is a direct pathway connecting Broca’s region with the anterior cingulate and pre-supplementary motor area. This tract is left lateralized in right-handed subjects, suggesting a possible role in language. However, there are no previous studies that have reported an involvement of this tract in language disorders. In this study we used diffusion tractography to define the anatomy of the frontal aslant tract in relation to verbal fluency and grammar impairment in primary progressive aphasia. Thirty-five patients with primary progressive aphasia and 29 control subjects were recruited. Tractography was used to obtain indirect indices of microstructural organization of the frontal aslant tract. In addition, tractography analysis of the uncinate fasciculus, a tract associated with semantic processing deficits, was performed. Damage to the frontal aslant tract correlated with performance in verbal fluency as assessed by the Cinderella story test. Conversely, damage to the uncinate fasciculus correlated with deficits in semantic processing as assessed by the Peabody Picture Vocabulary Test. Neither tract correlated with grammatical or repetition deficits. Significant group differences were found in the frontal aslant tract of patients with the non-fluent/agrammatic variant and in the uncinate fasciculus of patients with the semantic variant. These findings indicate that degeneration of the frontal aslant tract underlies verbal fluency deficits in primary progressive aphasia and further confirm the role of the uncinate fasciculus in semantic processing. The lack of correlation between damage to the frontal aslant tract and grammar deficits suggests that verbal fluency and grammar processing rely on distinct anatomical networks.
Keywords: aphasia, white matter, language, tractography, dementia, freesurfer, frontal aslant tract, tractography
Introduction
Primary progressive aphasia (PPA) is a clinical dementia syndrome characterized by the progressive breakdown of language functioning, with relative sparing of other cognitive domains (Mesulam, 1982, 2003; Gorno-Tempini et al., 2011). The syndrome is complex and heterogeneous, as patients with PPA manifest different patterns of language impairment as distinguished by three clinical phenotypes (Gorno-Tempini et al., 2011). Patients with the non-fluent/agrammatic variant present with abnormality of syntax or some other aspects of grammar in spoken or written language in the presence of relatively preserved single word comprehension; their fluency is also often impaired. Patients with the semantic variant show abnormality of single word comprehension and naming in the presence of relatively preserved grammar and fluency. Patients with the logopenic form have intermittent word-finding hesitations, phonemic paraphasias and impaired repetition.
In the past two decades, neuroimaging has been used to investigate the neural correlates of the three clinical phenotypes. In agrammatic PPA, atrophy is common in the left posterior frontal areas (Grossman et al., 1996, 2004; Nestor et al., 2003; Josephs et al., 2006; Mesulam et al., 2009), whereas structural damage in the anterior temporal lobes is more frequently associated with the semantic variant (Mummery et al., 2000; Galton et al., 2001; Rosen et al., 2002; Gorno-Tempini et al., 2004). Atrophy in the left posterior temporal lobe and inferior parietal lobule are characteristic of the logopenic variant (Gorno-Tempini et al., 2004). However, clinical-anatomical correlations in PPA reflect group-wide probabilities and often, individual patients show atrophy that extends to regions characteristically affected in other variants, suggesting that there may be no rigid boundaries between clinical variants.
Recent studies have examined the relationship between the distribution of cortical atrophy and specific language dimensions across PPA variants. MRI-derived estimates of cortical atrophy, for example, have shown distinct patterns of correlations for verbal fluency and grammatical processing (Rogalski et al., 2011). Poor fluency was associated with atrophy in the posterior inferior and middle frontal gyrus, whereas grammatical processing was associated with more widespread atrophy, including other regions of the inferior frontal gyrus and supramarginal gyrus. Furthermore, the association between specific symptoms and distant but anatomically connected regions suggests that vulnerability of specific networks might be correlated with domain-specific deficits (Catani et al., 2012a). This hypothesis has been recently tested with diffusion tensor imaging (DTI) tractography (Agosta et al., 2010; Galantucci et al., 2011; Wilson et al., 2011).
In particular tractography has been used to probe the microstructural properties of white matter along specific tracts and to identify correlations between tract-specific degeneration and language deficit severity (Wilson et al., 2011) or with specific subtypes of PPA (Galantucci et al., 2011). Using this approach, microstructural damage to the left arcuate fasciculus has been associated with deficits in comprehension and production of grammar (Wilson et al., 2011), whereas more ventral tracts (e.g. uncinate fasciculus) are greatly damaged in patients with PPA with semantic deficits (Agosta et al., 2010; Galantucci et al., 2011). Although the function of the arcuate and uncinate fasciculi in language has been well documented in PPA, the role of other tracts connecting to language areas remains to be determined.
In this study we used tractography in patients with PPA to study the white matter integrity of the frontal aslant tract, a newly described pathway connecting posterior Broca’s region with medial frontal areas (Lawes et al., 2008; Oishi et al., 2008; Ford et al., 2010), and its relationship to neuropsychological performance. This tract is left lateralized in most right-handed subjects, suggesting a role in language (Catani et al., 2012b). Furthermore it connects regions of the frontal lobe that have been associated with impaired fluency and mutism in patients with stroke (Kinkingnéhun et al., 2007) and brain tumours (Bizzi et al., 2012). In addition to the frontal aslant tract, we dissected the uncinate fasciculus to confirm previous reports of its association with semantic processing. The frontal aslant tract and uncinate fasciculus of the left hemisphere were dissected and the number of streamlines for each tract used as a surrogate measure of tract volume. We also extracted the fractional anisotropy and radial diffusivities as possible indirect measures of white matter spatial organization, myelination and axonal integrity (Beaulieu, 2011). Based on previous imaging findings of distinct areas within the inferior frontal gyrus reported to be specialized for different language functions (Bookheimer, 2002; Anwander et al., 2007), our hypothesis was that damage to the frontal aslant connections to the posterior inferior frontal gyrus (e.g. pars opercularis) would be associated with verbal fluency and syntax impairment, whereas abnormalities in the uncinate connections of more anterior regions (e.g. pars orbitalis) would be associated with semantic deficits.
Materials and methods
Participants
Thirty-five patients with PPA and 29 age- and gender-matched healthy control subjects were recruited for this study (Table 1). All subjects were recruited from the Primary Progressive Aphasia Program, at the Cognitive Neurology and Alzheimer’s Disease Centre of the Northwestern University Feinberg School of Medicine. The diagnosis of PPA was made by an experienced clinician (M.M.M.) on the basis of isolated and progressive language impairment. In addition to the root diagnosis of PPA, all patients received a descriptive diagnosis of logopenic PPA (n = 9), agrammatic PPA (n = 14), semantic variant PPA (n = 8), mixed (n = 2) or unclassified/severe (n = 2) PPA according to established guidelines (Table 2) (Mesulam et al., 2009, 2012; Gorno-Tempini et al., 2011).
Table 1.
Subject demographics and neuropsychological scores
| Variable | Patients with PPA (n = 35) | Healthy controls (n = 29) | Group comparisons |
|---|---|---|---|
| Age, years | 63.2 ± 8.3 | 62.4 ± 6 | t = −0.525, P = 0.62 |
| Duration of illness, years | 3.9 ± 1.8 | – | – |
| Sex, n | |||
| Male | 16 | 15 | |
| Female | 19 | 14 | χ2 = 0.322; P = 0.57 |
| Handedness (EHI score) | 95.2 ± 8.9 | 93.2 ± 9.7 | t = −0.925, P = 0.359 |
| WAB AQ (%) | 79.9 ± 13.6 | – | – |
| WAB repetition (%) | 78.7 ± 17.8 | 98.9 ± 2 | t = 7.132, P < 0.001 |
| NAT 10 (%) | 76.9 ± 26.6 | 98.4 ± 4.6 | t = 4.206, P < 0.001 |
| BNT (%) | 60 ± 34.1 | 97 ± 3 | t = 6.761, P < 0.001 |
| PPVT (%) | 77.8 ± 24.4 | 98.1 ± 3.6 | t = 4.964, P < 0.001 |
| MLU words (%) | 8.4 ± 2.6 | 11 ± 2 | t = 3.701, P < 0.001 |
| WPM | 84.9 ± 42.8 | 131 ± 19 | t = 4.751, P < 0.001 |
Numbers are means with standard deviations. Group comparisons were by t-test or Chi-squared. BNT = Boston Naming Test; EHI = Edinburgh Handedness Inventory; NAT = Northwestern Anagram Test; PPVT = Peabody Picture Vocabulary Test; MLU = mean length of utterance; WAB AQ = Western Aphasia Battery Aphasia Quotient; WPM = words per minute.
Table 2.
Neuropsychological scores for the three PPA variants
| Variable | PPA-L | PPA-G | PPA-S |
|---|---|---|---|
| (n = 9) | (n = 14) | (n = 8) | |
| Age, years | 65 ± 7 | 63.5 ± 8.8 | 57 ± 4.3 |
| Duration of illness, years | 3.6 ± 1.9 | 3.9 ± 1.3 | 4.1 ± 1.7 |
| WAB AQ (%) | 87.7 ± 13.4 | 76.5 ± 13.4 | 80.2 ± 10 |
| WAB repetition (%) | 83.1 ± 17.6 | 72.5 ± 17.6 | 91 ± 8.7 |
| NAT 10 (%) | 93.7 ± 10.6 | 54.5 ± 24.6* | 94.2 ± 15.1 |
| BNT (%) | 80.5 ± 20.6 | 75.5 ± 25.2 | 16 ± 19.4* |
| PPVT (%) | 93.5 ± 9.4 | 92.5 ± 6.2 | 38.4 ± 10.2* |
| MLU words (%) | 9.5 ± 2.3 | 7.3 ± 2.1 | 10.2 ± 2.1 |
| WPM | 104.3 ± 38 | 58.5 ± 19.5* | 146.9 ± 25.4 |
Numbers are means with standard deviations.
BNT = Boston Naming Test; MLU = mean length of utterance, NAT = Northwestern Anagram Test; PPA-G = non-fluent/agrammatic; PPA-L = logopenic variant; PPA-S = semantic variant; PPVT = Peabody Picture Vocabulary Test; WAB AQ = Western Aphasia Battery Aphasia Quotient; WPM = words per minute.
*Statistically different versus other two variants (P < 0.001).
The Western Aphasia Battery Aphasia Quotient was used to measure aphasia severity (Kertesz, 2006). The Western Aphasia Battery Aphasia Quotient represents a summary of test scores from the auditory comprehension, naming, repetition, and spontaneous speech subtests.
The 10-item version of the Northwestern Anagram Test (Weintraub et al., 2009; Thompson et al., 2012a), which measures object- and subject-extracted ‘who’ questions (e.g. Who is the groom carrying? Who is carrying the bride?, respectively), was used as an offline measure of grammatical processing. In this test, the patient is asked to order single words, each printed on a separate card, to correctly depict the action in a target picture.
The Boston Naming Test was used as a measure of naming ability (Goodglass and Kaplan, 2001). For this test, the patient is asked to name 60 line drawings of increasing difficulty.
A 36-item subset of moderately difficult items (items 157–192) from the fourth edition of the Peabody Picture Vocabulary Test was used as a test of auditory single-word lexical-semantic processing (Dunn and Dunn, 2006). For this test, the patient is required to match an auditory word representing an object, action, or attribute to one of four picture choices.
Speech samples were recorded from each participant while they told the story of Cinderella from a wordless picture book. Each sample was transcribed and segmented into utterances based on syntactic, prosodic and semantic criteria. Each utterance was coded and analysed using a previously published procedure that quantifies both lexical and structural detail (Thompson et al., 1995, 1997, 2012b). Mean length of utterance (number of words per sentence) and words per minute were used as the metrics of fluency (Supplementary material).
Diffusion tensor imaging and T1 acquisition and data processing
DTI acquisition was carried out at the Centre for Translational Imaging at Northwestern University, Chicago. A total of 72 contiguous near-axial slices were acquired on a 3 T Siemens Trio MRI system, using an acquisition sequence fully optimized for DTI, providing isotropic (2 × 2 × 2 mm) resolution and whole head coverage. Sixty diffusion-weighted images (b-value of 1000 s/mm2) were acquired together with eight images with no diffusion gradient applied. DTI processing was performed using Explore DTI (http://www.exploredti.com). Subject motion and geometrical distortions were corrected simultaneously with re-orientation of the b-matrix. Remaining outliers due to subject motion and cardiac pulsation were excluded using the RESTORE function (Chang et al., 2005). The tensor model was fitted to the data using a non-linear least square fitting procedure. DTI scalar maps, including fractional anisotropy and radial diffusivity were calculated and exported. Whole brain tractography was performed using a b-spline interpolated streamline algorithm (stepsize 0.5 mm; fractional anisotropy threshold 0.15; angle threshold 35°). The whole brain tractography was imported in TrackVis (http://www.trackvis.org) using homemade software written in Matlab 2009b (http://www.matworks.com) (Thiebaut de Schotten et al., 2011).
T1-weighted MPRAGE sequences (repetition time 2300 ms; echo time 2.86 ms; flip angle, 9°; field of view, 256 mm; 60 slices; slice thickness 1.0 mm) were acquired. Magnetic resonance images were processed using the image analysis suite FreeSurfer (version 4.5.0) (http://surfer.nmr.mgh.harvard.edu/). Cortical thickness estimates were calculated by measuring the distance between representations of the white–grey and pial–CSF boundaries across each point of the cortical surface (Fischl and Dale, 2000). Statistical surface maps were generated using a general linear model that displayed differences in cortical thickness between the 34 PPA patients and 27 healthy controls for each vertex along surface representations of the entire neocortex using an FDR of 0.001 (Rogalski et al., 2011) (Fig. 1).
Figure 1.
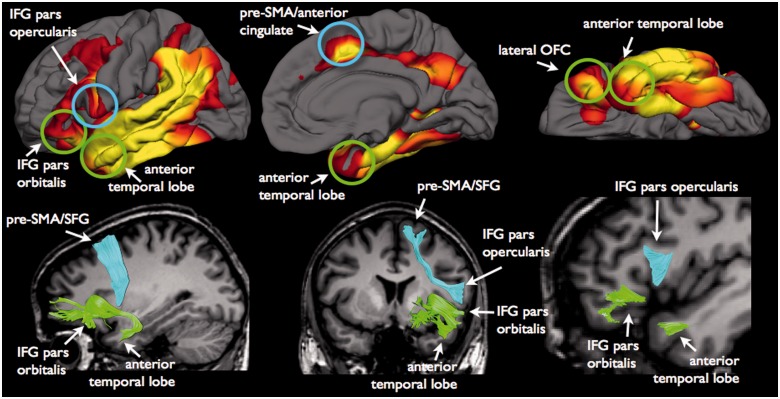
The frontal aslant tract (cyan) and the uncinate fasciculus (green) connect regions of the frontal and temporal lobes that show atrophic changes in patients with PPA. In particular the frontal aslant tract connects the pars opercularis of the inferior frontal gyrus (IFG) to the pre-supplementary motor area (pre-SMA) and anterior cingulate cortex. The uncinate connects anterior temporal lobe regions, including amygdala and temporal pole cortex, to the pars orbitalis of the inferior frontal gyrus and the orbitofrontal cortex (OFC). These left hemisphere regions also show significant cortical thinning in patients with PPA compared to control subjects, where yellow represents the most significant atrophy (FDR < .001; min/max -log10(p) = 2.81/5.63).
Virtual dissections and tract-specific measurements
Virtual in vivo dissections of the tracts of interest were performed using TrackVis in 35 PPA patients and 23 controls. These include the frontal aslant tract and the uncinate fasciculus of the left hemisphere (Fig. 1). Based on previous tractography work (Craig et al., 2009; Catani and Thiebaut de Schotten 2008; Catani et al., 2012b), regions of interest were defined manually on the axial, coronal, and sagittal fractional anisotropy images of each participant, and were used as seed regions for tracking. The dissectors (E.J., F.M.) were trained by an expert tractographer (M.C.) on 10 practice data sets and dissections for this study began only when high reliability was achieved. Each dissector was blind to the results of the cortical atrophy analysis and to the identity of the individual data sets. E.J. dissected the uncinate fasciculus in all subjects whereas F.M. dissected the frontal aslant tract in all subjects. Reliability between M.C. and the trained dissectors was calculated on a sample of 10 data sets (five controls and five PPA) using intraclass correlation analysis (>0.90).
To dissect the frontal aslant tract, the first region of interest was located in the white matter of the inferior frontal gyrus and the second region of interest in the white matter of the superior frontal gyrus (Lawes et al., 2008; Oishi et al., 2008; Ford et al., 2010; Catani et al., 2012b; Thiebaut de Schotten et al., 2012a).
To dissect the uncinate fasciculus, a temporal region of interest was defined around the white matter of the anterior temporal lobe and a second region of interest was defined around the white matter of the anterior floor of the extreme capsule (Catani et al., 2002; Craig et al., 2009).
The tractography procedure used here offers some advantages compared to previous publications (Catani et al., 2002) and probabilistic approaches. First, whole brain tractography is performed by seeding from each voxel of the brain. Other methods seed only from the region of interest selected by the operator, which make tractography reconstructions more dependent on the dissector’s anatomical knowledge or the quality of the cortical masks used to identify specific cortical regions. The other advantage of performing whole-brain tractography is the possibility of selecting streamlines that visually appear anatomically correct and belong to the tract of interest. Hence, even when surface anatomical landmarks differ among the subjects or the tracts of interest are distorted by the pathology, the operator is able to select streamlines that are consistent among subjects. In other words the dissector is guided by the anatomy of the tract itself rather than a priori anatomical knowledge or atlas-based constraints that may not apply to each single subject.
Outcome variable and statistical analysis
For each tract the number of streamlines, fractional anisotropy and radial diffusivity were calculated. In neurodegenerative disorders the number of streamlines reduces with the severity of the pathology and clinical symptoms (Catani, 2006). For this reason the number of streamlines are generally considered as a surrogate measure of tract volume and atrophy.
Fractional anisotropy varies from 0 to 1 and represents a quantitative index of the degree of anisotropy of biological tissues. Fractional anisotropy is usually considered as an indirect measure of white matter spatial organization and integrity, although its relationship with pathological alterations of white matter fibres requires careful interpretation in voxels containing multiple fibre orientations (Dell’Acqua and Catani, 2012; Dell'Acqua et al., 2012).
Radial diffusivity measures the diffusivity along directions orthogonal to the eigenvector of the diffusion tensor. Radial diffusivity is generally considered a sensitive measure for axonal/myelin damage, although interpretation of their changes in regions with crossing fibres is not always straightforward (Dell'Acqua et al., 2012).
Statistical comparisons were performed using SPSS18.0. A student’s t-test (two-tailed) for independent samples or Chi-squared test was used to investigate differences between controls and patients with PPA. One-way ANOVA for independent samples was used to investigate differences between PPA subtypes and controls. Bonferroni correction was applied for the post hoc analysis. Pearson bivariate correlation analysis was used to detect the strength of the correlation between tract-specific measurements and language scores in the PPA group. Our DTI and neuropsychology variables were highly correlated. In particular among the neuropsychological tests we found a high correlation between Western Aphasia Battery Aphasia Quotient, Northwestern Anagram Test and Western Aphasia Battery repetition (Cluster 1), Peabody Picture Vocabulary Test and Boston Naming Test (Cluster 2), and mean length of utterance and words per minute (Cluster 3). Similarly, there was a high correlation between fractional anisotropy, perpendicular diffusivity and number of streamlines for the uncinate fasciculus and fractional anisotropy and perpendicular diffusivity for the frontal aslant tract. In this case a Bonferroni correction for all seven neuropsychological tests and the six diffusion variables would be too conservative being the actual family-wise error rate much less than the prescribed level α. For this reason we have used a Bonferroni correction of 3 × 3 (corrected threshold P < 0.005) for the correlation analysis.
Additional operator-independent analysis
To confirm the validity of the tractography measurements and reduce any operator-dependent bias an atlas-based approach was used to obtain fractional anisotropy and radial diffusivity measurements of the frontal aslant tract and uncinate fasciculus. Automatic tractography was performed on 80 healthy subjects. For the automatic dissection approach predefined regions of interest normalized to a common reference space and applied to individual DTI templates were used. An expert anatomist (M.C.) checked the anatomical validity of the generated streamlines and 2D masks were created for each tract and each subject. Masks from all individuals were overlapped after normalization. Average percentage maps of the frontal aslant and uncinate tract were created using a threshold of 75% overlap (Thiebaut de Schotten et al., 2011). The 75% percentage masks were applied to the data sets (native space) of the patients with PPA and control subjects. Fractional anisotropy and perpendicular diffusivity were extracted from the individualized mask applied to each subject. Full details of this atlas-based analysis are reported in Thiebaut de Schotten et al., 2012b. This analysis was intended to replicate the results from the first tractography procedure (Supplementary material). However, one inconvenience of this approach is the lack of proxy measures of volume atrophy. For this measurement tractography dissections are the only method available.
Results
Table 1 and 2 show the demographic characteristics and neuropsychological performance of the subjects recruited for the study. Patients with PPA and healthy control subjects were matched for age, gender and handedness.
Frontal aslant tract
The frontal aslant tract connects the inferior frontal gyrus (pars opercularis) to the anterior cingulate cortex and medial regions of the superior frontal gyrus such as pre-supplementary motor and anterior cingulate areas. These cortical projection zones of the frontal aslant tract show reduced cortical thickness in patients with PPA (Fig. 1).
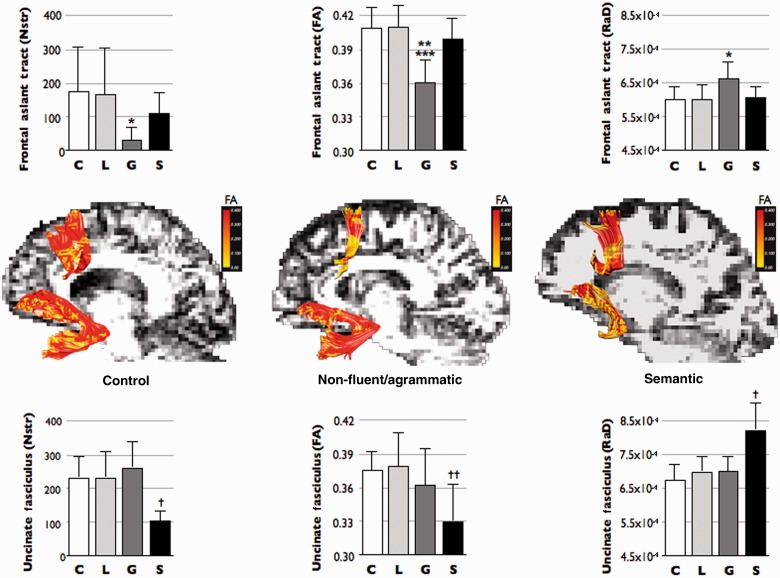
ANOVA between PPA subtypes and controls showed statistically significant differences in the number of streamlines (F = 4.601; P = 0.007), fractional anisotropy (F = 7.931; P < 0.001) and radial diffusivity (F = 3.686; P < 0.01) of the frontal aslant tract. Abnormalities in the frontal aslant tract were particularly evident for the agrammatic PPA group (Fig. 2). These findings are indicative of macro- and microstructural abnormalities in the frontal aslant tract of patients with agrammatic PPA.
Figure 2.
Differences in tract-specific measurements of the frontal aslant tract and uncinate fasciculus between control subjects (C) and patients with logopenic (L) non-fluent/agrammatic (G), and semantic (S) variants of PPA. Measurements of the number of streamlines (Nstr), fractional anisotropy (FA) and radial diffusivity (RaD) are reported for the frontal aslant tract (upper row) and the uncinate fasciculus (lower row). *statistically significant different versus control group (P < 0.01); **statistically significant different versus semantic group (P < 0.05); ***statistically significant different versus controls and logopenic group (P < 0.001); †statistically significant different versus all other groups (P < 0.001); ††statistically significant different versus controls and logopenic group (P < 0.001). Coronal images in the middle row show the fractional anisotropy values mapped onto the streamlines of the frontal aslant tract and uncinate fasciculus of a control subjects and two representative patients with PPA with non-fluent/agrammatic and semantic variant.
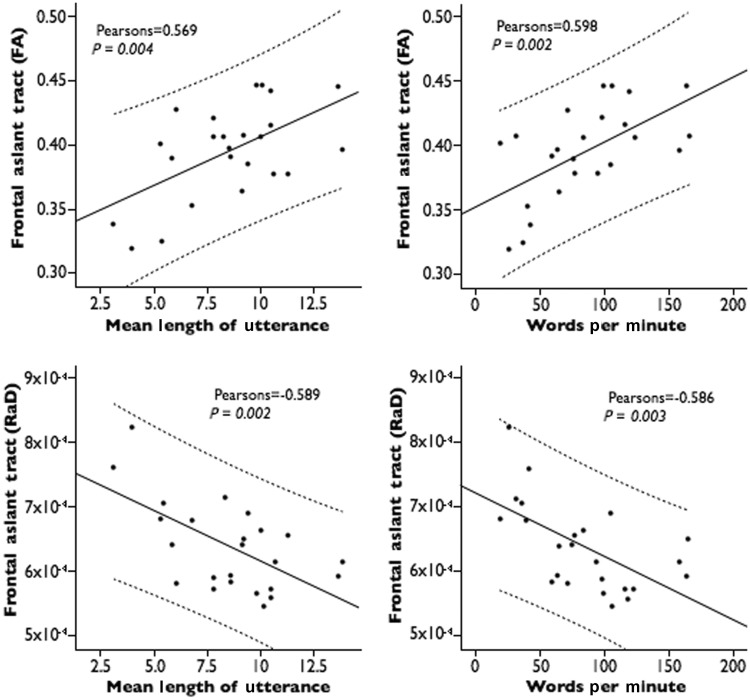
Fractional anisotropy measurements in the frontal aslant tract showed a positive correlation with mean length of utterance (P = 0.004) and words per minute (P = 0.002) scores, whereas radial diffusivity measurements correlated inversely with mean length of utterance (P = 0.002) and words per minute (P = 0.003) scores (Fig. 3 and Table 3). No correlations were found between the frontal aslant tract and measures of overall language impairment (Western Aphasia Battery Aphasia Quotient), grammar deficits (Northwestern Anagram Test), repetition (Western Aphasia Battery—repetition) or single words comprehension (Peabody Picture Vocabulary Test). These findings suggest that microstructural abnormalities of the frontal aslant underlie verbal fluency deficits in patients with PPA.
Figure 3.
Correlation between tract-specific measurements of the frontal aslant tract and performances on verbal fluency tests. FA = fractional anisotropy; RaD = radial diffusivity. Mean length of utterance is measured as number of words per sentence.
Table 3.
Correlations between neuropsychological performances and tract-specific measurements of the frontal aslant tract and uncinate fasciculus in the PPA group
| Tract | Diffusion tensor imaging | WAB AQ | NAT 10 | BNT | PPVT | WAB rep | MLU | WPM |
|---|---|---|---|---|---|---|---|---|
| Frontal aslant tract | N streamlines | 0.121 | 0.298 | 0.045 | −0.161 | 0.096 | 0.211 | 0.312 |
| P = 0.488 | P = 0.139 | P = 0.797 | P = 0.388 | P = 0.585 | P = 0.272 | P = 0.099 | ||
| Fractional anisotropy | 0.289 | 0.488 | −0.238 | −0.256 | 0.266 | −0.569* | 0.598* | |
| P = 0.122 | P = 0.025 | P = 0.205 | P = 0.207 | P = 0.156 | P = 0.004 | P = 0.004 | ||
| Radial diffusivity | −0.305 | −0.423 | 0.110 | 0.175 | −0.267 | −0.589* | −0.586* | |
| P = 0.101 | P = 0.056 | P = 0.563 | P = 0.393 | P = 0.153 | P = 0.002 | P = 0.003 | ||
| Uncinate fasciculus | N streamlines | 0.093 | −0.052 | 0.495* | 0.653* | −0.120 | 0.085 | −0.310 |
| P = 0.594 | P = 0.800 | P = 0.003 | P < 0.001 | P = 0.493 | P = 0.661 | P = 0.101 | ||
| Fractional anisotropy | −0.03 | 0.070 | 0.265 | 0.385 | −0.226 | 0.279 | −0.029 | |
| P = 0.862 | P = 0.733 | P = 0.124 | P = 0.033 | P = 0.191 | P = 0.143 | P = 0.882 | ||
| Radial diffusivity | 0.038 | −0.183 | −0.413 | −0.605* | 0.326 | −0.020 | 0.313 | |
| P = 0.827 | P = 0.372 | P = 0.014 | P < 0.001 | P = 0.056 | P = 0.918 | P = 0.098 |
*Values survive Bonferroni correction for multiple comparisons.
BNT = Boston Naming Test; NAT = Northwestern Anagram Test; PPVT = Peabody Picture Vocabulary Test; MLU = mean length of utterance; WAB AQ = Western Aphasia Battery Aphasia Quotient; WAB rep = Western Aphasia Battery repetition; WPM = words per minute.
Uncinate fasciculus
The uncinate fasciculus connects the inferior frontal gyrus (pars orbitalis) and orbitofrontal cortex to the anterior temporal lobe. The cortical projection regions of the uncinate fasciculus show reduced cortical thickness in patients with PPA compared with control subjects (Fig. 1).
ANOVA between PPA subtypes and controls showed statistically significant differences in the number of streamlines (F = 7.777; P < 0.001), fractional anisotropy (F = 7.068; P < 0.001) and radial diffusivity (F = 15.911; P = 0.001) in the uncinate fasciculus. In particular the semantic variant group had statistically significant reduction of the number of streamlines and increased radial diffusivity tract-specific differences compared with controls and both logopenic and agrammatic groups (Fig. 2). Differences in the fractional anisotropy were not significant between the semantic and non-fluent/agrammatic group. Overall these findings suggest both microstructural and volume abnormalities in the uncinate fasciculus of patients with semantic variant.
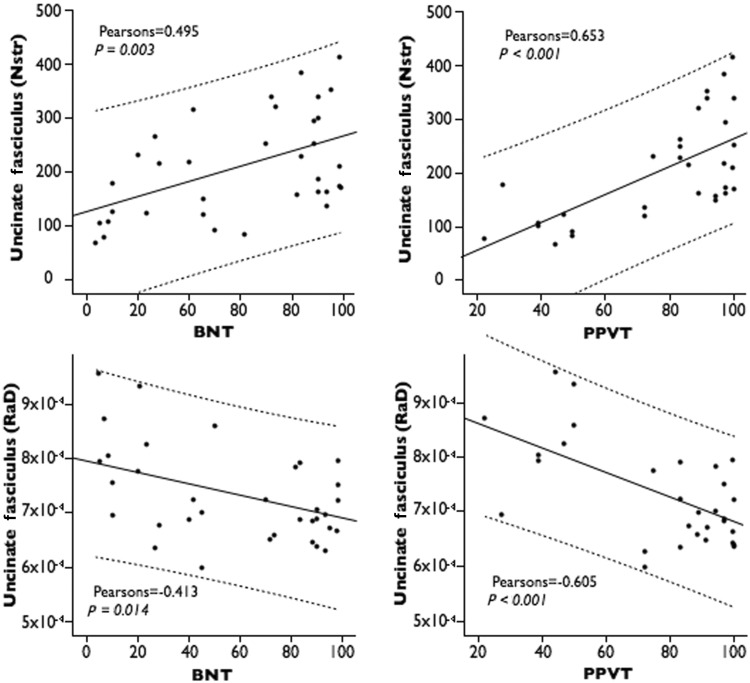
The number of streamlines of the uncinate fasciculus showed a positive correlation with Boston Naming Test (P = 0.003) and Peabody Picture Vocabulary Test (P < 0.001) scores, whereas radial diffusivity measurements correlated inversely with Peabody Picture Vocabulary Test scores (P < 0.001) (Fig. 4 and Table 3). Correlations between radial diffusivity and Boston Naming Test were also significant (P = 0.014) but did not survive Bonferroni correction. No correlations were found between the uncinate fasciculus and measures of overall language impairment (Western Aphasia Battery Aphasia Quotient), grammar deficits (Northwestern Anagram Test), repetition (Western Aphasia Battery—repetition) or verbal fluency (mean length of utterance, words per minute). These findings suggest that macro- and microstructural abnormalities of the uncinate fasciculus underlie deficits in single word comprehension and naming in patients with PPA.
Figure 4.
Correlation between tract-specific measurements of the uncinate fasciculus and performances on semantic processing tests. Nstr = number of streamlines; RaD = radial diffusivity; BNT = Boston Naming Test, PPVT = Peabody Picture Vocabulary Test.
The analysis of the fractional anisotropy and radial diffusivity of the frontal aslant tract and uncinate fasciculus was repeated using and atlas-based approach. This analysis replicated the main findings of the tractography method (Supplementary material).
Discussion
This study used tractography to show that patients with PPA have a significant reduction in the structural integrity of the frontal lobe connections. An important dissociation was observed in the functional anatomy of tracts underlying verbal fluency and semantic processing. Abnormalities of the frontal aslant tract were correlated with measurements of verbal fluency but not semantic processing or naming. Conversely, tract-specific measurements of the uncinate fasciculus correlated with semantic processing scores but not with verbal fluency. These findings suggest that verbal fluency and semantic processing depend on distinct tracts, both projecting to adjacent regions of the inferior frontal gyrus.
The frontal aslant tract is a white matter bundle recently described using post-mortem dissections and diffusion tractography. In humans, stimulation of the pre-supplementary motor area and anterior cingulate cortex produces both vocalization and arrest of speech (Penfield and Rasmussen, 1950). Patients with lesions of the pre-supplementary motor area present with various degrees of speech impairment from a total inability to initiate speech (i.e. mutism) to mild altered fluency (Ackermann and Riecker, 2011). Our findings suggest that these medial regions of the frontal lobe could facilitate speech initiation through direct connection to the pars opercularis of the inferior frontal gyrus. Indirect support of this interpretation comes from the frequent observation of impaired fluency in patients with deep lesions in the frontal periventricular white matter (Naeser et al., 1989). In these cases, a disconnection of the frontal aslant could explain the emergence of symptoms usually associated with frontal cortical damage.
For the first time, our study provides evidence of the involvement of the frontal aslant tract in PPA. The damage along the fibres of the frontal aslant tract was particularly evident for patients with agrammatic PPA compared with controls and other PPA variants. This is in line with previous studies showing cortical atrophy in the posterior frontal regions in this subtype of PPA. However, our group-wide analysis showed that the severity of the diffusion abnormalities correlated with scores in verbal fluency, but not with grammar tests, supporting a dissociation between verbal fluency and grammar (Rogalski et al., 2011; Thompson et al., 2012). A similar dissociation has been described in patients with aphemia, a clinical syndrome associated with cortical abnormalities of the medial frontal and lateral opercular cortex (Gallassi et al., 2011). Thus, in light of our findings and previous studies reporting a correlation between grammar and white matter abnormalities of the arcuate fasciculus, we conclude that verbal fluency and grammatical deficits in patients with PPA have a different anatomical substrate. In future studies we aim to understand whether the anatomical substrate of fluency and grammar is limited to these two tracts or extends to other tracts (e.g. fronto-striatal or fronto-insular tracts) (Catani et al., 2012b). We also recognize the limits of the Northwestern Anagram Test for testing grammatical ability and the need to use additional measures (e.g. tests of complex sentence comprehension, tests of grammatical morphology) as well as on-line measures of sentence processing for this purpose.
In addition to the frontal aslant tract we were able to demonstrate that the uncinate fasciculus is altered in PPA, especially in the semantic variant. Diffusivity changes in the uncinate fasciculus were correlated with performances in single word comprehension and naming. These results confirm previous studies highlighting the importance of the anterior temporal pole and its connections to the frontal lobe for verbal representation (Grossman et al., 1996; Mummery et al., 2000; Mesulam et al., 2009; Agosta et al., 2010).
Overall, our results suggest that the anatomical changes in PPA extend to those white matter tracts connecting regions affected by the pathology. These changes have significant correlations with the clinical manifestations, although the exact correspondence to the underlying neuropathology is difficult to determine without post-mortem analysis. Differences in fractional anisotropy and radial diffusivity have been associated with abnormalities in the axonal microstructure and myelination (Agosta et al., 2010; Beaulieu 2011). Atrophic changes, gliosis and demyelination are common findings in post-mortem studies of patients with PPA (Mesulam 2003; Gorno-Tempini et al., 2011). These pathological changes can affect the diffusivity measurements and therefore, explain our findings of decreased fractional anisotropy and increased radial diffusivity. In early PPA, white matter changes are often not detectable with conventional MRI despite the evidence of cortical atrophy or reduced metabolism. Hence, the most likely mechanism for white matter abnormalities in PPA is a secondary axonal degeneration of white matter fibres connecting cortical regions affected by the pathology. Future studies addressing the correlation between white matter changes and cortical atrophy may provide information on the exact link between cortical atrophy and altered white matter integrity in PPA.
Finally, our results also add further credence to the division of Broca’s area into functionally distinct regions, each displaying slightly different specializations for fluency, grammar and verbal associations (Bookheimer 2002; Anwander et al., 2007). Selective degeneration of different parts of Broca’s area or its connections through the arcuate fasciculus, frontal aslant tract and uncinate fasciculus could explain the heterogeneity of PPA presentation and specific-domain dissociation among patients classified within the same subtype.
In conclusion, our results complement recent DTI studies and suggest that in patients with PPA, deficits in verbal fluency are associated with degeneration of a Broca pre-supplementary motor area network (i.e. the frontal aslant tract), whereas semantic and grammar deficits are correlated with degeneration of the uncinate and arcuate fasciculus, respectively. These findings prompt a re-evaluation of the classical anatomy of language, where, in addition to the arcuate fasciculus, other tracts such as the frontal aslant tract and the uncinate fasciculus, should be included in a broader model of language networks.
Funding
This work was supported by the following Grants: DC008552 from the National Institute on Deafness and Communication Disorders (NIDCD), AG13854 (the Alzheimer Disease Centre) from the National Institute on Aging (NIA), NS075075 from the National Institute of Neurological Disorders and Stroke (NINDS), and 5KL2RR025740 from the National Centre for Research Resources. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Centre for Research Resources or the National Institutes of Health. This study was also in part supported by Guy’s and St Thomas’ Charity, and the Biomedical Research Centre for Mental Health at South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviation
- PPA
primary progressive aphasia
References
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2011;214:419–33. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain. 2010;133:286–99. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knösche TR. Connectivity-based parcellation of Broca’s area. Cereb Cortex. 2007;17:816–25. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. What makes diffusion anisotropic in the nervous system? In: Jones D, editor. Diffusion MRI. Oxford: Oxford University Press; 2011. pp. 92–109. [Google Scholar]
- Bizzi A, Nava S, Ferrè F, Castelli G, Aquino D, Ciaraffa F, et al. Aphasia induced by gliomas growing in the ventrolateral frontal region: assessment with diffusion MR tractography, functional MR imaging and neuropsychology. Cortex. 2012;48:255–72. doi: 10.1016/j.cortex.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–88. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19:599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Bizzi A, Forkel SJ, Williams SC, Simmons A, et al. Beyond cortical localization in clinico-anatomical correlation. Cortex. 2012a;48:1262–87. doi: 10.1016/j.cortex.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. Short frontal lobe connections of the human brain. Cortex. 2012b;48:273–91. doi: 10.1016/j.cortex.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for in vivo dissections. Cortex. 2008;44:1105–32. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Chang LC, Jones DK, Pierpaoli C. Restore: robust estimation of tensors by outlier rejection. Mag Res Med. 2005;53:1088–95. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, et al. Altered connections on the road to psychopathy. Mol Psychiatry. 2009;14:946–53. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Dell'acqua F, Simmons A, Williams SC, Catani M. Can spherical deconvolution provide more information than fiber orientations? Hindrance modulated orientational anisotropy, a true-tract specific index to characterize white matter diffusion. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22080. doi: 10.1002/hbm.22080 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Acqua F, Catani M. Structural human brain networks: hot topics in diffusion tractography. Curr Opin Neurol. 2012;25:375–83. doi: 10.1097/WCO.0b013e328355d544. [DOI] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody picture vocabulary test. Toronto, Ontario: Pearson Canada Assessment; 2006. [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford A, McGregor KM, Case K, Crosson B, White KD. Structural connectivity of Broca’s area and medial frontal cortex. NeuroImage. 2010;52:1230–7. doi: 10.1016/j.neuroimage.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, et al. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011;134:3011–29. doi: 10.1093/brain/awr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallassi R, Sambati L, Poda R, Oppi F, Stanzani Maserati M, Cevolani D, et al. Slowly progressive aphemia: a neuropsychological, conventional, and functional MRI study. Neurol Sci. 2011;32:1179–86. doi: 10.1007/s10072-011-0625-1. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon-Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Boston Diagnostic Aphasia Examination. Austin Texas: Pro-Ed; 2001. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–49. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D'Esposito M, Ding X-S, et al. Progressive non-fluent aphasia: language, cognitive and PET measures contrasted with probable Alzheimer's disease. J Cogn Neurosci. 1996;8:135–54. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–98. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. Western Aphasia Battery–Revised (WAB-R) Austin, TX: Pro-Ed; 2006. [Google Scholar]
- Kinkingnéhun S, Volle E, Pélégrini-Issac M, Golmard JL, Lehéricy S, du Boisguéheneuc F, et al. A novel approach to clinical-radiological correlations: Anatomo-Clinical Overlapping Maps (AnaCOM): method and validation. Neuroimage. 2007;37:1237–49. doi: 10.1016/j.neuroimage.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Lawes INC, Barrick TR, Murugam V, Spierings N, Evans DR, Song M, et al. Atlas-based segmentation of white matter tracts of the human brain using diffusion tensor tractography and comparison with classical dissection. NeuroImage. 2008;39:62–79. doi: 10.1016/j.neuroimage.2007.06.041. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia-a language-based dementia. N Engl J Med. 2003;349:1535–42. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–8. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson C, et al. Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009;132:2553–65. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–53. doi: 10.1093/brain/aws080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Naeser MA, Palumbo CL, Helm-Estabrooks N, Stiassny-Eder D, Albert ML. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR, et al. Progressive non-fluent aphasia is associated with hypometabolism centered on the left anterior insula. Brain. 2003;126:2406–18. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. NeuroImage. 2008;43:447–57. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Rasmussen R. The Cerebral Cortex of Man. New York: The Macmillan Company; 1950. [Google Scholar]
- Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, et al. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–50. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. Patterns of cerebral atrophy in primary progressive aphasia. Am J Geriatr Psychiatry. 2002;10:89–97. [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Forkel S, Simmons A, Vergani F, Murphy DG, et al. A lateralized brain network for visuo-spatial attention. Nat Neurosci. 2011;14:1245–6. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012a;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S. Learning to Read Improves the Structure of the Arcuate Fasciculus. Cereb Cortex. 2012b doi: 10.1093/cercor/bhs383. doi:10.1093/cercor/bhs383 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- Thompson CK, Cho S, Hsu CJ, Wieneke C, Rademaker A, Weitner BB, et al. Dissociations between fluency and agrammatism in primary progressive aphasia. Aphasiology. 2012a;26:20–43. doi: 10.1080/02687038.2011.584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CK, Shapiro LP, Tait ME, Jacobs B, Schneider S, Ballard K. A system for the linguistic analysis of agrammatic language production. Brain Lang. 1995;51:124–9. [Google Scholar]
- Thompson CK, Weintraub S, Mesulam MM. The Northwestern Anagram Test. Evanston, Illinois: Northwestern University; 2012b. https://flintbox.com/public/project/19927 (4 May 2012, date last accessed) [Google Scholar]
- Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24:408–16. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML, et al. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.