Abstract
Patients with the semantic variant of primary progressive aphasia, also known as semantic dementia, and Alzheimer’s disease have deficits in semantic memory. However, few comparative studies have been performed to determine whether these patient groups have distinct semantic memory impairments. We asked 15 patients with semantic variant primary progressive aphasia and 57 patients with Alzheimer’s disease to judge semantic category membership of coloured photos and printed words that are members of familiar natural and manufactured categories, and we related performance to grey matter atrophy. We found that both semantic variant primary progressive aphasia and Alzheimer’s disease are significantly impaired on this task. Moreover, patients with semantic variant primary progressive aphasia had a significantly more prominent deficit for natural objects than their own deficit judging manufactured objects. Both semantic variant primary progressive aphasia and Alzheimer’s disease had atrophy that included portions of the left temporal lobe. Regression analyses related performance in semantic variant primary progressive aphasia to ventral and medial portions of the left temporal lobe, while regression analyses in Alzheimer’s disease related performance to these ventral and medial temporal areas as well as lateral temporal-parietal regions in the left hemisphere. We conclude that both semantic variant primary progressive aphasia and Alzheimer’s disease are significantly impaired in a simple category membership judgement task and the selective impairment for natural kinds in semantic variant primary progressive aphasia is related in part to disease in visual association cortex in ventral–medial portions of the left temporal lobe. We discuss factors that may contribute to the semantic memory deficit in semantic variant primary progressive aphasia.
Keywords: semantic memory, Alzheimer’s disease, semantic dementia, temporal lobe, category-specific
Introduction
Semantic memory is the long-term representation of knowledge about our world, including the meaning of words, objects and actions. There is considerable evidence that semantic memory is impaired in patients suffering from neurodegenerative conditions such as the semantic variant of primary progressive aphasia (PPA), also known as semantic dementia, and Alzheimer’s disease. However, there has been some debate about the nature of this deficit and the neuroanatomical basis for the semantic impairment. Comparative studies of the semantic deficit in these patient groups may be informative, but such work has been rare. In this study, we examine accuracy judging category membership of printed word and coloured photograph exemplars of familiar natural and manufactured semantic categories comparatively in semantic variant PPA and Alzheimer’s disease, and assess the neuroanatomical basis for performance patterns in these patient groups with high-resolution structural MRI studies of grey matter disease.
Observations over the past two decades have emphasized the presence of impaired semantic memory in patients with semantic variant PPA and Alzheimer’s disease. In semantic variant PPA, the core deficit appears to involve semantic memory (Patterson et al., 2007). This interferes with their ability to understand and name objects and single words. Patients with semantic variant PPA are said to have a broad-based, ‘amodal’ deficit in semantic memory (Bozeat et al., 2000, 2002). Yet some have reported a more prominent deficit for natural compared to manufactured objects in patients with semantic variant PPA (Lambon Ralph et al., 2003, 2007; Rogers et al., 2004; Carroll and Garrard, 2005). As the reverse pattern of impairment—greater difficulty with manufactured than natural objects—has also been described in some patients (Sacchett and Humphreys, 1992; Cappa et al., 1998; Moss and Tyler, 2000), it is unlikely that natural objects are simply more difficult than manufactured objects.
A significant impairment in semantic memory also appears to be present in a large proportion of patients with Alzheimer’s disease (Grossman et al., 1996, 2007; Ralph et al., 2001; Adlam et al., 2006; Libon et al., 2007), although they are impaired in several other cognitive domains as well. The semantic deficit in Alzheimer’s disease may contribute to other problems such as confrontation naming difficulty (Lambon Ralph et al., 1997; Garrard et al., 2005; Rogers and Friedman, 2008), for example, although others propose that the naming deficit may be due to impaired lexical retrieval (Nebes et al., 1994) or a visual–perceptual deficit interpreting a target picture (Silveri and Leggio, 1996). A deficit in category-specific semantic memory has been described in some patients with Alzheimer’s diseases (Mauri et al., 1994; Montanes et al., 1996; Garrard et al., 1998, 2005; Grossman et al., 1998, 2013). These patients also may have greater difficulty with natural than manufactured objects.
Recent anatomically-based theories of semantic memory suggest that the pattern of damage to a distributed representation of object knowledge may explain in part the relative deficit for natural compared to manufactured objects in neurodegenerative diseases. One of these approaches, known broadly as the sensory–motor model (Martin, 2007; Barsalou, 2008; Kiefer and Pulvermuller, 2012), suggests that object knowledge as well as word meaning depend at least in part on the sensory/perceptual and motor/action features contributing to the corresponding concepts. For example, an apple is a roundish, often red object that is typically sweet and crunchy. These features are said to be represented in or near regions of modality-specific association cortex corresponding to the areas where these sensory and motor features are processed. For example, the colour and shape of an apple are represented in or near the ventral temporal portions of visual association cortex where colour and shape are processed.
Most support for the sensory–motor approach to semantic memory comes from functional MRI research demonstrating activation of sensory–motor association cortex during processing of sensory–motor feature knowledge associated with an object concept. For example, visual object concepts are associated with activation of visual association cortex in the inferior and ventral temporal regions (Chao et al., 1999; Kan et al., 2003; Simmons et al., 2007; Smith et al., 2012), action concepts appear to be associated with motor association cortex that is responsible in part for motor actions (Hauk et al., 2004), and auditory association cortex is activated in studies evaluating concepts that are enriched in auditory features (Kiefer et al., 2008; Bonner and Grossman, 2012). From this perspective, the representation of object concepts in semantic memory can be viewed in terms of the network of sensory–motor attributes of the object that are located in or near corresponding regions of the brain. It has been asserted, moreover, that natural kinds depend more intimately on visual features than manufactured objects (Saffran et al., 1994). Semantic variant PPA typically includes disease in the ventral and inferolateral temporal lobe, an area that encompasses visual association cortex (Gloor, 1997; Olson et al., 2007). Consistent with this view, some behavioural studies relate disproportionate difficulty with natural kinds in semantic variant PPA to degraded visual feature knowledge (Warrington, 1975; Breedin et al., 1995; Yi et al., 2007; Bonner et al., 2009; Macoir, 2009; Papagno et al., 2009). Recent work has associated semantic deficits in semantic variant PPA directly with visual association cortex in ventral temporal regions including the fusiform gyrus (Mion et al., 2010), and particularly with degraded visual feature knowledge of objects (Bonner et al., 2009). Likewise, if the anatomical distribution of disease compromises visual association cortex in Alzheimer’s disease, as appears to be the found in some cases (Arnold et al., 1991; Braak et al., 1997; Kirby et al., 2010), then natural objects may be more susceptible to impairment than manufactured objects.
A related anatomical model, known as the heteromodal model of semantic memory, builds on the sensory-motor features associated with objects. Heteromodal brain regions are not associated with a specific sensory–motor feature, but receive projections from multiple sensory and motor association cortices (Mesulam et al., 1977; Pandya and Yeterian, 1985). This kind of convergence zone may play an important role in integrating information from many modality-specific inputs (Koenig and Grossman, 2007; Patterson et al., 2007; Binder et al., 2009; Bonner et al., 2013). Heteromodal brain regions also may be important for other aspects of semantic memory. This may include generalization across the wide variety of different sensory and motor features associated with specific exemplars to support formation of a superordinate category. Specific portions of the anterior temporal lobe, the focus of disease in semantic variant PPA, may serve as a potential convergence zone (Davies et al., 2005; Knibb et al., 2006; Grossman, 2010). A potential convergence zone in Alzheimer’s disease is the angular gyrus, a focus of disease in these patients (Arnold et al., 1991; Braak et al., 1997). Previous work has associated disease in the angular gyrus with semantic impairments in Alzheimer’s disease (Desgranges et al., 1998; Grossman, 2003).
The current research examined patterns of impairment in semantic memory comparatively in patients with semantic variant PPA and Alzheimer’s disease. We used a simple comprehension test involving printed words and colour photographs of familiar natural and manufactured objects. By examining two semantic categories matched for frequency and other properties we sought to determine whether the deficit in Alzheimer’s disease and semantic variant PPA is category-specific or equally affects multiple semantic categories. With the use of pictures and words, we could dissociate visual perception of features of an object from the representation of visual feature knowledge associated with object concepts regardless of access from a word or a picture. Moreover, performance was related to the anatomical distribution of reduced grey matter density. This allowed us to establish whether the anatomic region related to poor performance on this task was associated with modality-specific association cortex or heteromodal association cortex.
We expected that both patient groups would have difficulty performing this task. Moreover, we expected disproportionate difficulty with natural kinds compared to manufactured objects, consistent with a category-specific deficit. Equal difficulty across semantic categories would be consistent instead with a broad-based semantic deficit. As performance was anticipated to reflect a central deficit in the representation of object concepts, we did not expect any effects for the material used to present the stimuli. A material-specific deficit would implicate a visual–perceptual impairment or difficulty accessing object concepts from lexical representations. In addition, we related the pattern of semantic impairment to the anatomical distribution of disease. If performance was associated with disease in the visual association cortex, this would implicate degraded visual feature knowledge of objects in the semantic deficits of these patients, particularly if there was a deficit for natural kinds and no evidence for disproportionate difficulty with picture stimuli. By comparison, if performance was related to disease in heteromodal cortical regions, then this would associate semantic deficits with a modality-neutral mechanism.
Materials and methods
Participants
One hundred and seven participants took part in this research. This included 57 patients who met published criteria for Alzheimer’s disease (McKhann et al., 2011). This group was characterized by differential impairment that included poor episodic memory. Fifteen patients with semantic variant PPA also were recruited (Gorno-Tempini et al., 2011). These patients presented with fluent speech and deficits in naming and comprehension. All dementia patients were evaluated and recruited by experienced behavioural neurologists (D.J.I., M.G.). At least two trained reviewers of a consensus committee confirmed the presence of a specific dementia phenotype based on an independent review of the semi-structured history obtained from patients and their families and a comprehensive neurological examination that included a detailed mental status evaluation. Finally, a group of 35 healthy seniors was tested. Healthy seniors were all living in the community. Table 1 summarizes the demographic features of these patients. Patients with Alzheimer’s disease were somewhat older than the healthy control subjects [F(2,104) = 4.28, P < 0.018], although there was no correlation between age and performance accuracy (r = −0.036; not significant). There was no difference between Alzheimer’s disease and semantic variant PPA groups for education and Mini-Mental State Examination (MMSE), although healthy seniors scored higher on the MMSE [F(2,104) = 30.65, P < 0.001] compared with both dementia groups (P < 0.001 for both pairwise group comparisons). Performance on neuropsychological tests is also provided in Table 1. Both patients with semantic variant PPA and Alzheimer’s disease differed from controls in their neuropsychological performance related to semantic memory. Patients with Alzheimer’s disease were worse than patients with semantic variant PPA on verbal episodic memory free recall, but these groups otherwise did not differ on other measures.
Table 1.
Mean (SD) demographic features in Alzheimer’s disease, semantic variant PPA and healthy seniors
| Alzheimer’s disease (n = 57) | Semantic variant PPA (n = 15) | Healthy seniors (n = 35) | |
|---|---|---|---|
| Age | 72.04 (8.28)^ | 67.87 (9.69) | 66.77 (8.26) |
| Education | 14.56 (2.94) | 15.67 (3.37) | 15.20 (2.13) |
| MMSE | 21.40 (5.63)* | 23.07 (5.40)* | 29.11 (1.05) |
| Boston Naming Test (Z-score)a | −3.53 (3.2)* (n = 56) | −5.52 (4.3)*+ (n = 15) | 0.03 (0.8) (n = 34) |
| Semantic category fluency (words/min) | 8.61 (5.3)* (n = 57) | 8.20 (5.6)* (n = 15) | 21.12 (5.9) (n = 34) |
| FAS category fluency (words/min)b | 7.64 (3.8)* (n = 51) | 6.59 (4.9)* (n = 14) | 13.98 (4.0) (n = 35) |
| Pyramid and Palm Tree (% correct) | 86.75 (10.5)* (n = 36) | 88.36 (7.6)* (n = 10) | 97.45 (2.4) (n = 20) |
| Verbal episodic memory free recall (Z-score)c | −3.49 (1.1)*# (n = 56) | −2.07 (1.7)^ (n = 14) | −0.19 (1.1) (n = 35) |
Not all patients performed all neuropsychological tests because of a variety of circumstances and technical errors, and the n for each task for each group is provided in the corresponding cell of the table summarizing performance. All ANOVAs for neuropsychological tests significant at P < 0.001.
^Alzheimer’s disease differing from semantic variant PPA and controls at P < 0.01.
*Differing from controls at P < 0.001.
+Semantic variant PPA worse than Alzheimer’s disease at P < 0.020.
#Alzheimer’s disease worse than semantic variant PPA at P < 0.001.
aFifteen-item and 30-item versions of Boston Naming test were administered, and Z-scores relative to controls performing the same versions were computed to combine the results of these versions into a single measure.
bWe calculated words/minute by dividing total performance for three categories by three.
cParticipants performed one of two verbal list-learning episodic memory tasks—a 10-word list presented for three learning trials (n = 77) or a 9-word list presented for five learning trials (n = 28). Following a filled period of several minutes, we assessed free recall. Performance on each task was converted to Z-scores based on healthy seniors’ performance on the same task, and these Z-scores were combined.
FAS = this refers to the letters “F”, “A” and “S”.
The initial clinical diagnosis of a neurodegenerative disease was consistent with the results of serum studies, structural imaging studies such as MRI or CT, and functional neuroimaging studies such as SPECT or PET (these studies were not available to the consensus committee). Exclusion criteria included the presence of other neurological conditions such as stroke or hydrocephalus (consistent with imaging studies reviewed by a neurologist), primary psychiatric disorders (depression, psychosis), or a systemic illness that can interfere with cognitive functioning. Some patients were taking a cholinesterase inhibitor (e.g. donepezil, rivastigmine, galantamine), memantine, a small dosage of a non-sedating antidepressant (e.g. serotonin-specific reuptake inhibitors such as sertraline), or a small dosage of an atypical neuroleptic agent (e.g. quetiapine) consistent with clinical care, but no patient demonstrated evidence of sedation. This research was approved by the University of Pennsylvania Institutional Review Board and consent was obtained consistent with the Declaration of Helsinki.
Behavioural materials
Category Membership Task: word judgement
Patients were shown 24 single words printed in black, lower-case letters on white cards one at a time, and judged whether each was an instance of a familiar superordinate category that was provided orally by the examiner and was available on a printed card during the task. Participants provided a ‘yes’ or ‘no’ response. The stimuli were presented in a blocked fashion for each of two superordinate categories (i.e. vegetables, tools) to minimize any risk that executive functioning for category-switching could interfere with performance. Half of the stimuli for each superordinate were targets and half were foils; half of the foils were related to the target because they overlapped with the target category in many semantic and perceptual features (e.g. apple – for the target category ‘vegetable’), and half were unrelated to the target category because they overlapped with the target category in relatively few semantic and perceptual features (e.g. chair – for the target category ‘vegetable’). Stimuli were matched across semantic category for frequency of occurrence (t = 1.421; not significant) and representativeness (t = 0.40; not significant) (Rosch, 1975). Within each category, the stimuli were presented in a fixed, pseudo-randomized order.
Category Membership Task: pictures judgement
This task was similar in structure to the category membership judgements for words, but the stimuli consisted of 24 colour photographs of objects. The target object was photographed in isolation and presented in a typical view to maximize its informativeness. The names of the pictured objects were matched in frequency across categories, and matched the frequency of the printed words in each category.
Correlation studies showed that performance on this category membership judgement task did not correlate with age, disease duration at the time of testing, or MMSE score (all P-values > 0.20 in each group). Overall accuracy performance on this task in patients correlated with overall accuracy performance on the Pyramid and Palm Tree test (r = 0.35; P = 0.019).
Statistical procedures
Demographic and neuropsychological characteristics were normally distributed. All test performance was assessed using one-way ANOVA and Tukey post hoc tests, with significance set at P < 0.050. In addition to assessing overall accuracy judging targets and foils, we performed a d’ analysis (z-score hits minus z-score false alarms, consisting of correct target judgements minus errors judging foils, normalized to healthy controls’ performance). We also performed an error analysis examining performance with related and unrelated foils. This error analysis provided specific information about the basis for patients’ impaired performance. Thus, there is an important qualitative distinction between related foils that involve object concepts with shared features (e.g. judging ‘apple’ as a kind of vegetable) compared to unrelated foils that involve object concepts that share very few features (e.g. judging ‘chair’ as a kind of vegetable). Whereas deficits with related foils implicate judgements of subtle distinctions for specific features that may depend on perceptual or semantic processing, difficulty judging unrelated foils instead reveals gross deficits that are likely to be associated with degraded semantic feature knowledge and much less likely to involve a perceptual confusion.
Imaging methods
Sixteen patients (Alzheimer’s disease: n = 11; semantic variant PPA: n = 5) had a volumetric MRI scan obtained on average within 2 months of neuropsychological testing. These cases matched the entire group demographically (Supplementary Tables 1 and 2). Briefly, nine images (Alzheimer’s disease: n = 6; semantic variant PPA: n = 3) were collected using a Siemens Trio 3.0 T scanner with a T1-weighted 3D spoiled gradient-echo sequence, at repetition time = 1620 ms, echo time = 3 ms, flip angle = 15o, matrix = 195 × 256, slice thickness = 1 mm, and in-plane resolution = 0.9 × 0.9 mm. The remaining seven images (Alzheimer’s disease: n = 5; semantic variant PPA: n = 2) were obtained with a GE 1.5 T Horizon Echospeed scanner. This 3D spoiled gradient echo sequence was acquired with repetition time = 35 ms, echo time = 6 ms, flip angle = 30°, matrix = 128 × 256, slice thickness = 1.3 mm, and in plane resolution = 0.9 × 0.9 mm. We minimized potential scanner and sequence bias by including a nuisance covariate in all grey matter analyses.
Voxel-based morphometry was used to identify areas of grey matter atrophy. T1 images were normalized to a standard space and segmented using PipeDream, a structural image processing pipeline (http://sourceforge.net/projects/neuropipedream/). Registration was performed using the ANTS toolkit (http://www.picsl.upenn.edu/ANTS/), which implements a diffeomorphic and symmetric registration and normalization method that is the most reliable tool available (Klein et al., 2009). A local T1 template of 1 mm3 resolution was built using ANTS. After registering the subject image to the local template, three-tissue (grey matter, white matter and CSF) segmentation was performed with the Atropos tool in ANTS. Post-segmentation grey matter probability images were transformed into MNI space for statistical analysis. Images were downsampled to 2 mm3 resolution and smoothed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) using a 5-mm full-width at half-maximum Gaussian kernel to minimize individual gyral variations. Statistical analyses were conducted in SPM8. T-tests were used to identify areas of significant grey matter atrophy between patients and a group of 36 controls matched for age, sex and education. A probability mask restricted analyses to areas of grey matter. We identified clusters consisting of at least 50 adjacent voxels at a false-discovery-rate (FDR)-corrected height threshold of q < 0.01.
For regression analyses relating behaviour to grey matter imaging results, we identified the grey matter voxels that were significantly atrophic in Alzheimer’s disease or semantic variant PPA, and used this as a mask to search for voxels that were significantly related to overall semantic category membership judgement accuracy in the patients with imaging. Then we identified the voxels that corresponded to the anatomical distribution of atrophic voxels in each group’s volumetric atrophy analysis. This allowed us to specify the grey matter regions of disease contributing to each patient group’s semantic performance. In this way, we minimized the risk of over-interpreting the meaningfulness of voxels not associated with a disease process and that instead might simply reflect a healthy ageing process independent of disease. Linear regression analyses were performed using SPM8. We report an uncorrected P < 0.05 threshold with clusters containing a peak-voxel with P < 0.001 and a minimum of 10 adjacent voxels.
Results
Behavioural results
Both patient groups were impaired in their category membership judgement performance relative to healthy seniors. The effect of group for overall accuracy judging targets and foils was significant [F(2,104) = 11.77, P < 0.001]. As summarized in Table 2, follow-up analyses demonstrated significantly worse performance in semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.001) compared with healthy control subjects, although the two patient groups did not differ from each other in overall performance. The d’ signal detection analysis yielded identical results [F(2,104) = 12.21, P < 0.001], with patients with semantic variant PPA and Alzheimer’s disease obtaining lower scores compared to healthy adults (P < 0.001 for both comparisons), and the patient groups not differing from each other. We examined subsets of the stimuli below.
Table 2.
Mean (SD) per cent accuracy judging stimulus semantic categories and modalities of presentation in Alzheimer’s disease, semantic variant PPA, and healthy senior control subjects
| Alzheimer’s disease | Semantic variant PPA | Healthy seniors | |
|---|---|---|---|
| Overall | 86.18 (9.54) | 83.06 (13.01) | 94.17 (4.84) |
| Natural | 85.31 (10.33) | 78.61 (14.67)* | 92.98 (6.53) |
| Manufactured | 87.06 (12.21) | 87.50 (13.99) | 95.36 (6.37) |
| Words | 86.33 (10.67) | 83.89 (12.87) | 94.76 (6.17) |
| Pictures | 86.04 (10.19) | 82.22 (14.21) | 93.57 (4.66) |
*Semantic variant PPA differs from Alzheimer’s disease at P < 0.010.
Semantic category
We found a different pattern of category-specific difficulty in semantic variant PPA compared with Alzheimer’s disease. Table 2 shows that significant group effects were found for both natural [F(2,104) = 12.28, P < 0.001] and manufactured [F(2,104) = 6.61, P < 0.002] categories. Follow-up pair-wise group comparisons for natural stimuli found worse performance for both semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.002) compared to healthy control subjects. For manufactured stimuli, follow-up comparisons found worse performance compared to controls for semantic variant PPA (P < 0.050) and Alzheimer’s disease (P < 0.002). A signal detection analysis for the natural category [F(2,104) = 10.30, P < 0.001] also showed deficits in semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.001) relative to healthy seniors; and similar deficits were seen for the manufactured category [F(2,104) = 7.63; P < 0.005] in semantic variant PPA (P < 0.020) and Alzheimer’s disease (P < 0.001) relative to healthy seniors. Moreover, within-group comparisons using a paired t-test showed that patients with semantic variant PPA are more impaired for natural than manufactured stimuli [t(14) = 2.84; P < 0.020]. Inspection of individual patient profiles demonstrated that 14 (93.3%) of 15 patients with semantic variant PPA showed an equal or greater impairment for natural than manufactured objects. This effect was not found in Alzheimer’s disease, where relative difficulty with the two categories of knowledge was balanced [27 (47%) had greater difficulty with natural kinds, 16 (28%) had greater difficulty with manufactured objects, and 14 (25%) had equal difficulty on both categories]. Both patients with semantic variant PPA and patients with Alzheimer’s disease thus were impaired for natural and manufactured categories, and patients with semantic variant PPA were particularly compromised for natural objects.
Stimulus material
Significant groups effects were obtained for both picture [F(2,104) = 10.00, P < 0.001] and word [F(2,104) = 10.15, P < 0.001] stimuli. For picture stimuli, follow-up comparisons found worse performance in semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.001) compared to healthy control subjects. An identical profile was obtained for word stimuli, where semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.001) were worse than healthy seniors. A signal detection analysis also showed deficits for pictures [F(2,104) = 10.86, P < 0.001] in semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.01) relative to controls; and similar deficits were present for word stimuli [F(2,104) = 10.19, P < 0.001] for semantic variant PPA (P < 0.007) and Alzheimer’s disease (P < 0.001). The material used for stimulus presentation thus had no effect on performance.
Foil judgements
We performed an error analysis for the different types of foils. This emphasized the profound impairment for natural kinds in semantic variant PPA. First, we examined foils that were unrelated to the target category. As summarized in Table 3, the effect of group in unrelated foils was significant [F(2,104) = 3.34, P < 0.039]. Follow-up comparisons found worse performance in semantic variant PPA relative to healthy seniors (P < 0.032) and a trend suggesting worse performance relative to patients with Alzheimer’s disease (P = 0.079); patients with Alzheimer’s disease did not differ from control subjects (P > 0.5). Thus, patients with semantic variant PPA were impaired at judging a foil that is unrelated to the target category.
Table 3.
Mean (SD) per cent accuracy judging foils from natural and manufactured categories presented as pictures and words in Alzheimer’s disease, semantic variant PPA and healthy seniors
| Alzheimer’s disease | Semantic variant-PPA | Healthy seniors | |
|---|---|---|---|
| Unrelated foils | 97.37 (10.92) | 89.44 (25.87) | 99.29 (2.36) |
| Related foils | 69.30 (28.39) | 70.00 (24.15) | 93.81 (12.99) |
| Natural unrelated | 98.25 (9.28) | 87.78 (26.32) | 99.52 (2.81) |
| Natural related | 62.28 (37.20) | 62.22 (35.89) | 91.43 (22.27) |
| Manufactured unrelated | 96.49 (14.34) | 91.11 (25.87) | 99.05 (3.92) |
| Manufactured related | 76.32 (30.69) | 77.78 (22.42) | 96.19 (8.17) |
| Words unrelated | 96.49 (13.26) | 85.56 (28.07) | 99.52 (2.81) |
| Words related | 67.54 (30.76) | 70.00 (25.35) | 91.90 (16.84) |
| Pictures unrelated | 98.25 (9.28) | 93.33 (25.82) | 99.05 (3.92) |
| Pictures related | 71.05 (31.73) | 70.00 (29.68) | 95.71 (11.67) |
Moreover, we found that this effect for unrelated foils in semantic variant PPA was significant for natural objects but not manufactured objects. Thus, there was a significant group effect for natural-unrelated foils [F(2,104) = 5.56, P < 0.005]. Follow-up comparisons demonstrated that patients with semantic variant PPA performed worse than both healthy seniors (P < 0.005) and patients with Alzheimer’s disease (P < 0.009) for natural-unrelated foils. This effect for natural-unrelated foils was particularly evident for word stimuli (semantic variant PPA versus healthy seniors: words P < 0.005, pictures P > 0.2; semantic variant PPA versus patients with Alzheimer’s disease: words P < 0.030, pictures P > 0.3), and inspection of individual stimuli suggested that this could not be attributed to surface dyslexia. This emphasizes that the difficulty encountered by patients with semantic variant PPA for unrelated natural foils could not be attributed easily to a visual-perceptual deficit perceiving the photographs of unrelated stimuli. There was no difference between healthy seniors and patients with Alzheimer’s disease for natural-unrelated foils (P > 0.5). The effect for group for manufactured-unrelated foils was not significant. Thus, the effect for unrelated foils in semantic variant PPA was due specifically to their difficulty with natural objects, such as deciding whether a chair is a kind of vegetable.
The findings for related foils showed significant deficits for both groups relative to controls, emphasizing the overall difficulty judging these stimuli for Alzheimer’s disease and semantic variant PPA alike. The effect for group for related foils thus was significant [F(2,104) = 12.31, P < 0.001]. Follow-up comparisons demonstrated worse performance in both semantic variant PPA (P < 0.005) and Alzheimer’s disease (P < 0.001) compared to healthy seniors. For natural-related foils, there was a significant group effect [F(2,104) = 9.26, P < 0.001], and both semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.020) performed worse than healthy seniors. The effect of group for manufactured-related foils also was significant [F(2,104) = 7.58, P < 0.001], with follow-up comparison showing worse performance in both semantic variant PPA (P < 0.001) and Alzheimer’s disease (P < 0.040) compared to healthy seniors.
Imaging
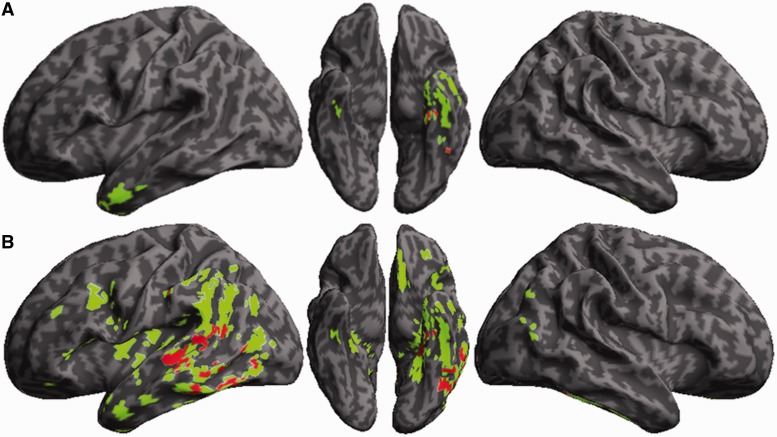
Table 4 summarizes the anatomical distribution of reduced grey matter density in semantic variant PPA and Alzheimer’s disease. As illustrated in Fig. 1A, semantic variant PPA had reduced grey matter density in the anterior and ventral portions of the temporal lobe that was more prominent on the left than the right. This is similar to the anatomical distribution of disease reported in other studies of semantic variant PPA. In Fig. 1B, a fairly extensive anatomical distribution of reduced grey matter density was seen in temporal, parietal and frontal brain regions in Alzheimer’s disease that was more prominent on the left than the right.
Table 4.
Grey matter atrophy in patients with Alzheimer’s disease and semantic variant PPA relative to healthy seniors
| Anatomical locus (Brodmann area) | MNI coordinates |
Z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Alzheimer’s disease < healthy seniors | |||||
| L fusiform gyrus (20) | −44 | −28 | −22 | 7.52 | 5935 |
| L middle temporal gyrus (21) | −50 | −8 | −18 | 5.20 | 198 |
| L superior temporal gyrus (38) | −52 | 14 | −22 | 4.01 | 64 |
| L inferior parietal lobule (40) | −40 | −16 | 20 | 4.90 | 448 |
| L inferior parietal lobule (40) | −46 | −24 | 32 | 3.98 | 52 |
| L inferior parietal lobule (40) | −42 | −36 | 22 | 4.53 | 97 |
| L angular gyrus (39) | −40 | −72 | 28 | 4.59 | 52 |
| L precuneus (7) | −10 | −62 | 40 | 4.79 | 60 |
| L precuneus (31) | −12 | −48 | 34 | 4.66 | 95 |
| L inferior frontal gyrus (45) | −38 | 30 | 6 | 4.91 | 272 |
| L inferior frontal gyrus (11) | −28 | 38 | −14 | 4.70 | 101 |
| L middle frontal gyrus (9) | −32 | 12 | 34 | 4.71 | 255 |
| L middle frontal gyrus (46) | −50 | 30 | 26 | 4.30 | 91 |
| L medial frontal gyrus (9) | −10 | 48 | 16 | 4.72 | 70 |
| L subcallosal gyrus (25) | −10 | 20 | −18 | 5.01 | 273 |
| R parahippocampal gyrus (36) | 26 | −34 | −16 | 5.11 | 232 |
| R fusiform gyrus (36) | 40 | −28 | −26 | 4.32 | 66 |
| R parahippocampal gyrus (30) | 12 | −50 | 2 | 3.93 | 158 |
| R superior occipital gyrus (19) | 44 | −76 | 32 | 3.94 | 95 |
| R anterior cingulate gyrus (32) | 14 | 38 | 22 | 4.55 | 71 |
| Semantic variant PPA < healthy seniors | |||||
| L inferior temporal gyrus (20) | −50 | 0 | −32 | 7.41 | 570 |
| L fusiform gyrus (20) | −46 | −30 | −24 | 6.14 | 524 |
| R fusiform gyrus (20) | 40 | −20 | −30 | 5.49 | 63 |
L = left; R = right.
Figure 1.
Grey matter atrophy, and regression analyses relating category membership judgement performance to atrophy. The coloured areas correspond to regions of significantly reduced grey matter density relative to healthy seniors. Red areas are regions of significantly reduced grey matter density related to impaired category membership judgement accuracy. (A) Semantic variant PPA; (B) Alzheimer’s disease.
We examined the areas of reduced grey matter density that were directly related to overall semantic judgement accuracy in the behavioural test. This is illustrated in red in the figures and summarized in Table 5. As indicated in Supplementary Table 2, semantic judgement performance in the subgroup of patients with imaging paralleled that found in the entire group. In semantic variant PPA, Fig. 1A and Table 5 show that areas of atrophy in the left ventral temporal lobe were associated with semantic judgement accuracy. As shown in Fig. 1B, the areas associated with semantic judgement accuracy in Alzheimer’s disease included left ventral as well as left lateral temporal regions. Category judgement accuracy in both semantic variant PPA and Alzheimer’s disease thus appeared to be related to disease in the left fusiform gyrus. An area of atrophy in a ventral-medial temporal distribution was associated with semantic category judgement accuracy only in semantic variant PPA, and this distinct area may have contributed to the difficulty these patients encountered with natural objects. By comparison, an area of atrophy in posterolateral temporal-parietal regions encompassing an area of heteromodal cortex was seen only in Alzheimer’s disease, and this may explain the absence of a category-specific effect in these patients.
Table 5.
Regression analysis relating performance to grey matter atrophy in patients with Alzheimer’s disease and semantic variant PPA
| Anatomical locus (Brodmann area) | MNI coordinates |
Cluster size (voxels) | ||
|---|---|---|---|---|
| x | y | z | ||
| Regression in Alzheimer’s disease | ||||
| L fusiform gyrus (37) | −42 | −52 | −14 | 586 |
| L parahippocampal gyrus (36) | −26 | −32 | −20 | 156 |
| L superior temporal gyrus (22) | −54 | −32 | −8 | 474 |
| L inferior parietal lobule (40) | −54 | −24 | 34 | 40 |
| Regression in semantic variant PPA | ||||
| L fusiform gyrus (37) | −42 | −52 | −14 | 36 |
| L inferior temporal gyrus (20) | −34 | −22 | −28 | 36 |
L = left; R = right.
Discussion
Patients with semantic variant PPA and Alzheimer’s disease have semantic memory difficulty. This study investigated the nature of this impairment comparatively. Using a simple category membership judgement task, we found that patients with semantic variant PPA and Alzheimer’s disease are significantly impaired relative to healthy seniors. Moreover, we found a distinguishing pattern of impairment in semantic variant PPA. These patients were significantly more impaired in their judgements of natural objects compared to their own judgements of manufactured objects. The severity of this deficit in semantic variant PPA is exemplified by their significant difficulty judging foils unrelated to the natural target category. Patients with Alzheimer’s disease were impaired for both natural and manufactured categories. Anatomical studies associated atrophy in the left temporal lobe with impaired semantic judgements in both patient groups. However, the pattern of atrophy associated with impaired semantic category judgements in semantic variant PPA was restricted to the left ventral-medial temporal region, while patients with Alzheimer’s disease also had atrophy affecting the left posterolateral temporal-parietal region. We discuss these findings in greater detail below.
In large cohorts of patients with semantic variant PPA and Alzheimer’s disease, we found significant deficits on a very simple measure requiring a semantic category membership judgement. This confirms previous observations demonstrating an impairment in semantic memory in these patient groups (Grossman et al., 1996, 2007; Bozeat et al., 2000, 2002; Ralph et al., 2001; Adlam et al., 2006; Libon et al., 2007). Moreover, we observed disproportionate difficulty with natural objects in semantic variant PPA. One possible account is related to the observation that knowledge of natural objects tends to depend more on visual feature knowledge than manufactured objects, and visual feature knowledge of objects tends to be impaired in semantic variant PPA. One study examining colour knowledge of animals reported that patients with semantic variant PPA are impaired at judging objects with an unnatural colour (Rogers et al., 2007). The vocabulary of patients with semantic variant PPA loses high imageability words and increasingly consists of abstract words (Hoffman et al., 2013b). Several reports have described so-called reversal of the concreteness effect in many patients with semantic variant PPA, where there is relative difficulty with visual feature knowledge of objects compared with abstract concepts (Warrington, 1975; Breedin et al., 1995; Yi et al., 2007; Bonner et al., 2009; Macoir, 2009; Papagno et al., 2009).
There has been considerable debate about the contribution of visual feature knowledge to the semantic memory deficits in patients with semantic variant PPA. One recent survey study investigated performance in seven patients with semantic variant PPA on seven tasks, and claimed that visual feature knowledge was not compromised in their object concepts (Hoffman and Lambon Ralph, 2011). Close inspection raises some questions about this study. One task, a synonym judgement task designed to include both target words and choices that are concrete or abstract, demonstrated a strong concreteness effect. However, the data were averaged across high, middle and low word frequency levels. Considering the high frequency stimuli that these patients are likely to know, an assessment of individual patient performance profiles in the original publication of this task (Jefferies et al., 2009) demonstrated that six (67%) of nine patients (two additional patients had 100% accuracy) had the greatest difficulty with high image ability stimuli, thus showing sensitivity to degraded visual feature knowledge. There is also concern about the informativeness of three additional tasks. The results of these multiple-choice measures were difficult to interpret because the target word was selected to be concrete or abstract, but the four choices were concrete concepts even for supposedly abstract trials. Finally, two additional tasks used verbal descriptions of concrete and abstract stimuli in a multiple-choice paradigm, thereby eliminating potential confounds with the concreteness of pictured multiple choices. These studies showed that five (72%) of seven cases had greater difficulty with concrete than abstract stimuli. Other studies claiming to disprove a role for degraded visual feature knowledge in semantic variant PPA have been limited by methodological issues such as ceiling effects that cloud the interpretation of results (Hoffman et al., 2013a). More recently, these researchers have acknowledged the relative importance of a deficit for visual feature knowledge in semantic variant PPA (Hoffman et al., 2012). In our view, many individuals with semantic variant PPA begin with disease in the visual association cortex that compromises the representation of visual feature knowledge of objects, and this proves devastating for many object concepts that depend so heavily on visual feature knowledge. Over time, disease progresses dorsally and posteriorly in the temporal lobe (Avants et al., 2007; Rohrer et al., 2008; Brambati et al., 2009), and connectivity with other brain regions becomes compromised (Duda et al., 2008; Agosta et al., 2010), eventually resulting in a multimodal semantic memory deficit in semantic variant PPA.
In this context, it is important to distinguish between visual features that contribute to an object concept, and the particular material used to access the mental representation of the concept such as a photograph. We found that the stimulus material used to present a test item played little role in performance. Thus, the relative deficit for natural objects in semantic variant PPA was equally present for colour photos and printed words. This also suggests that the deficits seen in these patients were unlikely to be due to a visual-perceptual deficit perceiving a pictured stimulus or an alexia interfering with reading a printed stimulus.
Patients with semantic variant PPA were selectively impaired in their judgements of foils that are unrelated to natural objects. Thus, these patients were uniquely impaired at judging whether a chair or a lamp could be a natural object. Findings such as this emphasize the profound degradation of knowledge of natural objects in semantic variant PPA. This deficit was more prominent for printed words, again emphasizing that the deficit is unlikely to be explained by difficulty visually perceiving the photographic stimuli. By comparison, both patients with semantic variant PPA and patients with Alzheimer’s disease were significantly impaired in their judgements of related foils. Moreover, both groups were impaired at judging this ‘related foil’ class of stimuli regardless of the semantic category; they were equally impaired at deciding whether an apple is a kind of vegetable and whether a chair is a kind of tool. We speculate that these stimuli may have been so challenging for semantic variant PPA and Alzheimer’s disease alike because the related foils share so many features with other members of the target category.
Whereas patients with semantic variant PPA were relatively more impaired with natural compared to manufactured objects, they were nevertheless impaired relative to control subjects with manufactured objects as well. From this perspective, it is reasonable to consider the contribution of additional factors to the deficit in semantic variant PPA. To be sure, performance on this task cannot distinguish between loss of knowledge of a category member or loss of knowledge of the superordinate category. Nevertheless, one possibility is that impaired performance on this category membership judgement task may be due in part to difficulty with the mental representation of the superordinate category. It has been proposed that knowledge of a superordinate category may be relatively robust to neurodegenerative disease because of the redundant contribution of features from many overlapping instances of the category (Gonnerman et al., 1997; Devlin et al., 1998). In semantic variant PPA, for example, analyses of naming errors suggest that these patients use superordinate terms when basic level names of objects become difficult to access (Hodges et al., 1995; Patterson, 2007). On the other hand, there is no specific representation of a superordinate like ‘vegetable’ or ‘tool,’ and superordinates of this sort must be constructed from a range of exemplars that have a variety of sensory-motor features. From this perspective, we speculate that knowledge of the meaning of a superordinate may prove fragile in patients with semantic memory deficits, and this may have contributed to difficulty on this task regardless of semantic category.
Some work has addressed the role of superordinate knowledge in the semantic memory deficits of neurodegenerative patients. In one critical study, patients with semantic variant PPA and Alzheimer’s disease matched on explicit semantic memory measures showed different patterns of semantic priming (Rogers and Friedman, 2008). Specifically, only patients with semantic variant PPA did not prime for superordinate semantic relationships. This is consistent with the finding in the present study using an explicit task—semantic category membership judgements were impaired in semantic variant PPA, and this may have been due in part to difficulty with the representation of superordinates. By comparison, others have claimed that patients with semantic variant PPA have better performance with superordinates than basic object level names (Rogers and Patterson, 2007). This effect was related to disease severity. The authors reasoned that progressive disease resulted in greater degradation of object concept knowledge, leaving redundant features that are sufficient to support the representation of a superordinate category. It is possible that patients with semantic variant PPA in the present study were more demented overall, despite matching patients with Alzheimer’s disease in overall dementia severity using the MMSE, because the MMSE is not very sensitive to the deficits found in semantic variant PPA. There is some reason to discount this account in the present study. We did not find an empirical relationship between performance on our measure of category membership judgements and measures of disease severity such as MMSE and disease duration at the time of test. Moreover, these patient groups were matched in their performance on semantic measures like the Pyramid and Palm Tree test and semantically-guided category naming fluency, and were matched on their overall accuracy on the category membership judgement task used in the present study.
Few studies have compared performance on semantic memory measures in Alzheimer’s disease and semantic variant PPA. Previous work has generally shown qualitatively similar patterns of impairment in direct comparisons of these patient groups, although patients with semantic variant PPA are often quantitatively more impaired than patients with Alzheimer’s disease (Cross et al., 2008). These assessments frequently depended on associativity judgements such as indicating that a pyramid is associated more with a palm tree than a pine tree because a pyramid and a palm tree are both found in Egypt (Rogers, 2004; Rogers et al., 2006). Patients with Alzheimer’s disease also have been reported to have a relative deficit for natural objects (Grossman et al., 1998, 2013; Garrard et al., 2005). However, we did not observe this in the present study. Several possibilities may account for this discrepancy. One possibility may be related to the specific category of natural objects that we probed. We used vegetables rather than the category used in most previous reports—animals. We speculate that patients with Alzheimer’s disease may be able to judge vegetables because they are somewhat object-like in nature—they are inanimate like manufactured objects and do not move or have a head and sensory organs, like eyes as in animals, for example. Many previous reports of a category-specific deficit in Alzheimer’s disease used animal stimuli, and patients with Alzheimer’s disease may be particularly sensitive to motion features associated with animals. A related possibility concerns the task we used. Some work demonstrating a category-specific deficit in Alzheimer’s disease specifically probed visual feature knowledge of objects (Grossman et al., 2013). In this context, it is possible that patients with Alzheimer’s disease may show the same pattern of impairment as patients with semantic variant PPA on tasks where performance depends only on the integrity of representations of visual feature knowledge of objects in visual association cortex. Tasks that do not emphasize visual feature knowledge, such as the one used in the present study, may not reveal a category-specific effect in Alzheimer’s disease. In this context, it is possible to speculate that there has been broader degradation of feature knowledge associated with objects in Alzheimer’s disease than in semantic variant PPA. This would be consistent with the more extensive disease seen in the participants with Alzheimer’s disease, specifically involving heteromodal association cortex. This would have obscured the observation of a category-specific effect linked to the specific degradation of visual feature knowledge seen in semantic variant PPA. Another possibility is that the representation of object concepts is relatively preserved in Alzheimer’s disease, and that there may be difficulty explicitly accessing these representations (Nebes and Brady, 1990; Cronin-Golomb et al., 1992; Ober et al., 1995).
Both Alzheimer’s disease and semantic variant PPA have considerable disease in the temporal lobe, an area critical for object knowledge. Previous work in neurodegenerative disease has related semantic difficulties directly to disease in the temporal lobe (Desgranges et al., 1998; Grossman, 2003; Bonner et al., 2009; Mion et al., 2010). This brain–behaviour correlation is not unique to neurodegenerative disease, as patients with stroke in the temporal lobe also have semantic deficits (Hillis et al., 2001; Newhart et al., 2007).
In the present study, we found that only an area in left ventral-medial temporal grey matter was associated with impaired category membership judgements in semantic variant PPA. This included portions of fusiform and parahippocampal gyri. We speculate that ventral medial temporal cortex contributes to the category-specific deficit in semantic variant PPA. This ventral-medial area of the temporal lobe is thought to be part of the visual association cortex (Gloor, 1997; Olson et al., 2007), and previous functional MRI work has suggested that medial regions may be more closely related to natural objects and more lateral portions of the ventral temporal lobe may play a role in manufactured objects (Chao et al., 1999). As natural objects such as vegetables, are associated with consistent visual-perceptual features, we speculate that these concepts also may have a relatively stable anatomic representation in visual association cortex, and category membership judgements of natural objects thus may be at greater risk for impairment following disease in this area of visual association cortex. Moreover, others suggest that fusiform activation is related to the process of semantic judgement rather than the content of a concept (Rogers et al., 2005). In the present study, fusiform disease was seen in both patient groups, although two different patterns of semantic category judgement performance were observed—one group with a category-specific semantic deficit and one not. This raises the possibility that, in addition to the content of the category, disease in these patients may also impact the semantic judgement process. Inspection of individual patient MRI studies suggests that not all patients with semantic variant PPA have disease extending to this area, and we speculate that this may contribute to some of the variability seen in patients with semantic variant PPA profile of impaired semantic memory. Transcranial magnetic stimulation of the left anterior temporal lobe has been used to assess the concreteness effect in semantic variant PPA (Pobric et al., 2009). Unfortunately, the fusiform gyrus and other, more medial portions of the temporal lobe implicated in studies of semantic variant PPA are somewhat distant from the lateral surface of the temporal lobe where the stimulator is located. The neuroanatomical extent of tissue that is affected by transcranial magnetic stimulation is relatively imprecise, particularly in areas of abundant atrophy where there is also excess CSF, which is an excellent conductor.
In Alzheimer’s disease, semantic deficits have been linked to more extensive portions of the temporal lobe, including both lateral and ventral temporal regions (Desgranges et al., 1998; Grossman, 2003; Grossman et al., 2013). In this comparative study, we observed that portions of the lateral temporal lobe are related to semantic judgements in Alzheimer’s disease but not semantic variant PPA. There are at least two possible accounts linking lateral temporal-parietal disease in Alzheimer’s disease to their broad semantic deficit without category specificity. First, posterolateral temporal and inferior parietal cortex includes heteromodal association cortex. This is a multimodal area thought to play an important role in integrating feature knowledge from multiple modalities into an object concept (Bonner et al., 2013). Disease in this area thus may result in multimodal impairments with all categories of object concepts. A second possibility is related to a variant of Alzheimer’s disease known as logopenic variant PPA. These patients have prominent disease in auditory association cortex in dorsal portions of the lateral temporal lobe, and they have particular difficulty with concepts dependent on auditory feature knowledge (Bonner and Grossman, 2012). The anatomical correlate of category membership judgement performance in Alzheimer’s disease in the present study also encompassed this lateral temporal anatomic distribution of disease, consistent with the possibility of degradation of both visual and auditory feature knowledge in Alzheimer’s disease. Indeed, the anatomical distribution of disease in Alzheimer’s disease is highly variable, resulting in specific syndromes depending on the anatomical distribution of disease such as logopenic variant PPA, posterior cortical atrophy, and others (Alladi et al., 2007). From this perspective, the emergence of a category-specific deficit for natural objects may be seen in some studies of Alzheimer’s disease if patients participating in a particular study have the appropriate anatomical distribution of disease.
In summary, our findings are consistent with the hypothesis that many patients with semantic variant PPA have a category-specific deficit characterized by disproportionate difficulty with natural objects, although this pattern was less evident in Alzheimer’s disease. This category-specific deficit in semantic variant PPA appears to be related to disease in ventral-medial portions of left temporal cortex. This is an area of visual association cortex, and we speculate that disease in this area interferes with the representation of visual feature knowledge that is crucial to natural object concepts. We found that semantic memory deficits for multiple semantic categories were related to more extensive disease in Alzheimer’s disease, including temporal-parietal regions of the lateral temporal lobe.
Funding
This work was supported by NIH Grants AG017586, NS044266, AG015116, AG032953, NS053488, AG038490, HD060406 and the Wyncote Foundation.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- MMSE
Mini-Mental State Examination
- PPA
primary progressive aphasia
References
- Adlam A-LR, Bozeat S, Arnold R, Watson PC, Hodges JR. Semantic knowledge in mild cognitive impairment and mild Alzheimer's disease. Cortex. 2006;42:675–84. doi: 10.1016/s0010-9452(08)70404-0. [DOI] [PubMed] [Google Scholar]
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, et al. Language networks in semantic dementia. Brain. 2010;133:286–99. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alladi S, Xuereb J, Bak T, Nestor P, Knibb JA, Patterson K, et al. Focal cortical presentations of Alzheimer's disease. Brain. 2007;130:2636–45. doi: 10.1093/brain/awm213. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW. The topographic and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Avants B, Anderson C, Grossman M, Gee JC. Spatiotemporal normalization for longitudinal analysis of gray matter atrophy in frontotemporal dementia. Med Image Comput Comput Assist Interv. 2007;10:309–10. doi: 10.1007/978-3-540-75759-7_37. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–45. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Grossman M. Gray matter density of auditory association cortex relates to knowledge of sound concepts in orimary progressive aphasia. J Neurosci. 2012;32:7986–91. doi: 10.1523/JNEUROSCI.6241-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Peelle JE, Cook PA, Grossman M. Heteromodal conceptual processing in the angular gyrus. Neuroimage. 2013;71:175–86. doi: 10.1016/j.neuroimage.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner MF, Vesely L, Price C, Anderson C, Richmond L, Farag C, et al. Reversal of the concreteness effect in semantic dementia. Cogn Neuropsychol. 2009;26:568–79. doi: 10.1080/02643290903512305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR. When objects lose their meaning: what happens to their use? Cogn Affect Behav Neurosci. 2002;2:236–51. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E, Iqbal K, Winblad B, Nishimura T, Takeda M, et al. Patterns of cortical lesions in Alzheimer's disease: biology, diagnosis, and therapeutics. New York: Wiley; 1997. pp. 227–37. [Google Scholar]
- Brambati SM, Rankin KP, Narvid J, Seeley WW, Dean D, Rosen HJ, et al. Atrophy progression in semantic dementia with asymmetric temporal involvement: a tensor-based morphometry study. Neurobiol Aging. 2009;30:103–11. doi: 10.1016/j.neurobiolaging.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of a concreteness effect in a patient with semantic dementia. Cogn Neuropsychol. 1995;11:617–60. [Google Scholar]
- Cappa S, Frugoni M, Pasquali P, Perani D, Zorat F. Category-specific naming impairment for artefacts: a new case. Neurocase. 1998;4:391–7. [Google Scholar]
- Carroll E, Garrard P. Knowledge of living, nonliving and “sensory quality” categories in semantic dementia. Neurocase. 2005;11:338–50. doi: 10.1080/13554790591006339. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby J, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–19. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Cronin-Golomb A, Keane MM, Kokodis A, Corkin S, Growdon JH. Category knowledge in Alzheimer's disease: Normal organization and a general retrieval deficit. Psychol Aging. 1992;7:359–66. doi: 10.1037//0882-7974.7.3.359. [DOI] [PubMed] [Google Scholar]
- Cross K, Smith EE, Grossman M. Knowledge of natural kinds in semantic dementia and Alzheimer’s disease. Brain Lang. 2008;105:32–40. doi: 10.1016/j.bandl.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–95. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, de la Sayette V, Petit-Taboue MC, Benali K, Landeau B, et al. The neural substrates of memory systems impairment in Alzheimer's disease: a PET study of resting brain glucose utilization. Brain. 1998;121:611–31. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Gonnerman LM, Andersen ES, Seidenberg M. Category-specific semantic deficits in focal and widespread brain damage: a computational account. J Cogn Neurosci. 1998;10:77–94. doi: 10.1162/089892998563798. [DOI] [PubMed] [Google Scholar]
- Duda JT, Avants B, Asmuth JA, Zhang H, Grossman M, Gee JC. A fiber tractography-based examination of neurodegeneration on language-network neuroanatomy. Med Image Comput Comput Assist Analysis. 2008:191–8. [Google Scholar]
- Garrard P, Lambon Ralph MA, Patterson K, Pratt KH, Hodges JR. Semantic feature knowledge and picture naming in dementia of the Alzheimer's type. Brain Lang. 2005;93:79–94. doi: 10.1016/j.bandl.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Garrard P, Patterson K, Watson PC, Hodges JR. Category-specific semantic loss in dementia of Alzheimer's type: functional-anatomical correlations from cross-sectional analyses. Brain. 1998;121(Pt 4):633. doi: 10.1093/brain/121.4.633. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and Limbic System. Oxford University Press. NY: Nature Publishing Group; 1997. [Google Scholar]
- Gonnerman LM, Andersen ES, Devlin JT, Kempler D, Seidenberg MS. Double dissociation of semantic categories in Alzheimer's disease. Brain Lang. 1997;57:254–79. doi: 10.1006/brln.1997.1752. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hills AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Neural basis for semantic memory difficulty in Alzheimer's disease: an fMRI study. Brain. 2003;126:292–311. doi: 10.1093/brain/awg027. [DOI] [PubMed] [Google Scholar]
- Grossman M. Primary progressive aphasia: clinical-pathological correlations. Nat Rev Neurol. 2010;6:88–97. doi: 10.1038/nrneurol.2009.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, D'Esposito M, Hughes E, Onishi K, Biassou N, White-Devine T, et al. Language comprehension difficulty in Alzheimer's disease, vascular dementia, and fronto-temporal degeneration. Neurology. 1996;47:183–9. doi: 10.1212/wnl.47.1.183. [DOI] [PubMed] [Google Scholar]
- Grossman M, Libon DJ, Forman MS, Massimo L, Wood E, Moore P, et al. Distinct antemortem profiles in patients with pathologically defined frontotemporal dementia. Arch Neurol. 2007;64:1601–9. doi: 10.1001/archneur.64.11.1601. [DOI] [PubMed] [Google Scholar]
- Grossman M, Peelle JE, Smith EE, McMillan CT, Cook PA, Powers JM, et al. Category-specific semantic memory: converging evidence from bold fMRI and Alzheimer's disease. Neuroimage. 2013;68:263–74. doi: 10.1016/j.neuroimage.2012.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, White-Devine T, Robinson KM, Biassou N, D'Esposito M. Semantic memory in Alzheimer's disease: ontologic category, representativeness, and material. Neuropsychology. 1998;12:34–42. doi: 10.1037//0894-4105.12.1.34. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–7. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, et al. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561–6. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organisation of semantic memory. Memory. 1995;3:463–95. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Lambon Ralph MA. The degraded concept representation system in semantic dementia: damage to pan-modal hub, then visual spoke. Brain. 2012;135:3770–80. doi: 10.1093/brain/aws282. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Jones RW, Lambon Ralph MA. Be concrete to be comprehended: consistent imageability effects in semantic dementia for nouns, verbs, synonyms and associates. Cortex. 2013a;49:1206–18. doi: 10.1016/j.cortex.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Lambon Ralph MA. Reverse concreteness effects are not a typical feature of semantic dementia: evidence for the hub-and-spoke model of conceptual representation. Cereb Cortex. 2011;21:2103–12. doi: 10.1093/cercor/bhq288. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Meteyard L, Patterson K. Broadly speaking: vocabulary in semantic dementia shifts towards general, semantically diverse words. Cortex. 2013b doi: 10.1016/j.cortex.2012.11.004. doi: 10.1016/j.cortex.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23:492–9. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon K, Thompson-Schill SL. Role of mental imagery in a property-verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cogn Neuropsychol. 2003;20:525–40. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Pulvermulle F. Conceptual representations in mind and brain: theoretical developments, current evidence and future directions. Cortex. 2012;48:805–25. doi: 10.1016/j.cortex.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Kiefer M, Sim E-J, Herrnberger B, Grothe J, Hoenig K. The sound of concepts: four markers for a link between auditory and conceptual brain systems. J. Neurosci. 2008;28:12224–30. doi: 10.1523/JNEUROSCI.3579-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby E, Bandelow S, Hogervorst E. Visual impairment in Alzheimer's disease: a critical review. J Alzheimer's Dis. 2010;21:15–34. doi: 10.3233/JAD-2010-080785. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson JLR, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibb JA, Xuereb J, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- Koenig P, Grossman M. Process and content in semantic memory Neural Basis of Semantic Memory. Cambridge, UK: Cambridge University Press; 2007. pp. 247–64. [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–37. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Semantic dementia with category specificity: a comparative case-series study. Cogn Neuropsychol. 2003;20:307–26. doi: 10.1080/02643290244000301. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K, Hodges JR. The relationship between naming and semantic knowledge for different categories in dementia of Alzheimer's type. Neuropsychologia. 1997;35:1251–60. doi: 10.1016/s0028-3932(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farme J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–75. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- Macoir J. Is a plum a memory problem?: Longitudinal study of the reversal of concreteness effect in a patient with semantic dementia. Neuropsychologia. 2009;47:518–35. doi: 10.1016/j.neuropsychologia.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Mauri A, Daum I, Sartori G, Riesch G, Birbaumer N. Category-specific semantic impairment in Alzheimer's disease and temporal lobe dysfunction: a comparative study. J Clin Exp Neuropsychol. 1994;16:689–701. doi: 10.1080/01688639408402682. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (Area PG) in the rhesus monkey: a study with a new method of horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson KE, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–68. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- Montanes P, Goldblum MC, Boller F. Classification deficits in Alzheimer's disease with special reference to living and nonliving things. Brain Lang. 1996;54:335–58. doi: 10.1006/brln.1996.0079. [DOI] [PubMed] [Google Scholar]
- Moss HE, Tyler LK. A progressive category-specific deficit for non-living things. Neuropsychologia. 2000;38:60–82. doi: 10.1016/s0028-3932(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Brady CB. Preserved organization of semantic attributes in Alzheimer's disease. Psychol Aging. 1990;5:574–9. doi: 10.1037//0882-7974.5.4.574. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Craik FIM, Salthouse TA. Cognitive dysfunction in Alzheimer's disease The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 1994. pp. 373–446. [Google Scholar]
- Newhart M, Ken L, Kleinman JT, Heidler-Gary J, Hillis AE. Neural networks essential for naming and word comprehension. Cogn Behav Neurol. 2007;20:25–30. doi: 10.1097/WNN.0b013e31802dc4a7. [DOI] [PubMed] [Google Scholar]
- Ober BA, Shenaut GK, Allen PA, Bashore TR. Semantic priming in Alzheimer's disease: meta-analysis and theoretical evaluation age differences in word and language processing. Vol. 1. Amsterdam: North Holland Press; 1995. pp. 247–71. [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 4. Association and Auditory Cortex. New York: Plenum Press; 1985. pp. 3–61. [Google Scholar]
- Papagno C, Capasso R, Miceli G. Reversed concreteness effect for nouns in a subject with semantic dementia. Neuropsychologia. 2009;47:1138–48. doi: 10.1016/j.neuropsychologia.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Patterson K. The reign of typicality in semantic memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:813–21. doi: 10.1098/rstb.2007.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Roger TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Pobric G, Lambon Ralph MA, Jefferies E. The role of the anterior temporal lobes in the comprehension of concrete and abstract words: rTMS evidence. Cortex. 2009;45:1104–10. doi: 10.1016/j.cortex.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MAL, Powel J, Howard D, Whitworth AB, Garrard P, Hodges JR. Semantic memory is impaired in both dementia with Lewy bodies and dementia of Alzheimer's type: a comparative neuropsychological study and literature review. J Neurol Neurosurg Psychiatry. 2001;70:149–56. doi: 10.1136/jnnp.70.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Friedman RB. The underlying mechanisms of semantic memory loss in Alzheimer's disease and semantic dementia. Neuropsychologia. 2008;46:12–21. doi: 10.1016/j.neuropsychologia.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Mechelli A, Patterson K, Price C. Fusiform activation to animals is driven by the process, not the stimulus. J Cogn Neurosci. 2005;17:434–45. doi: 10.1162/0898929053279531. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClellan JL, Hodges JR, et al. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K. Object categorization: reversals and explanations of the basic-level advantage. J Exp Psychol Gen. 2007;136:451–69. doi: 10.1037/0096-3445.136.3.451. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K, Graham K. Colour knowledge in semantic dementia: it is not all black and white. Neuropsychologia. 2007;45:3285–98. doi: 10.1016/j.neuropsychologia.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Patterson K, Hodges JR, Ivanoiu A. Semantic memory in Alzheimer's disease and the frontotemporal dementias: a longitudinal study of 236 patients. Neuropsychology. 2006;20:319–35. doi: 10.1037/0894-4105.20.3.319. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, McNaught E, Foster J, Clegg SL, Barnes J, Omar R, et al. Tracking progression in frontotemporal lobar degeneration: serial MRI in semantic dementia. Neurology. 2008;71:1445–51. doi: 10.1212/01.wnl.0000327889.13734.cd. [DOI] [PubMed] [Google Scholar]
- Rosch E. Cognitive representation of semantic categories. J Exp Psychol Gen. 1975;104:192–233. [Google Scholar]
- Sacchett C, Humphreys GW. Calling a squirrel a squirrel but a canoe a wigwam: a category-specific deficit for artefactual objects and body parts. Cogn Neuropsychol. 1992;4:131–85. [Google Scholar]
- Saffran E, Schwartz MF, Umilta C, Moscovitch M. Of cabbages and things: semantic memory from a neuropsychological perspective–a tutorial review attention and performance XV: conscious and nonconscious information processing. Cambridge: MIT Press; 1994. pp. 507–36. [Google Scholar]
- Silveri MC, Leggio MG. Influence of disorders of visual perception in word-to-picture matching tasks in patients with Alzheimer's disease. Brain Lang. 1996;54:326–34. doi: 10.1006/brln.1996.0078. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–10. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Myers N, Sethi U, Pantazatos S, Yanagihara T, Hirsch J. Conceptual representations of perceptual knowledge. Cogn Neuropsychol. 2012;29:237–48. doi: 10.1080/02643294.2012.706218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quart J Exp Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Yi HA, Moore P, Grossman M. Reversal of the concreteness effect for verbs in semantic dementia. Neuropsychology. 2007;21:9–19. doi: 10.1037/0894-4105.21.1.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.