Abstract
Freezing of gait is one of the most debilitating symptoms in Parkinson’s disease as it causes falls and reduces mobility and quality of life. The pedunculopontine nucleus is one of the major nuclei of the mesencephalic locomotor region and has neurons related to anticipatory postural adjustments preceding step initiation as well as to the step itself, thus it may be critical for coupling posture and gait to avoid freezing. Because freezing of gait and postural impairments have been related to frontal lesions and frontal dysfunction such as executive function, we hypothesized that freezing is associated with disrupted connectivity between midbrain locomotor regions and medial frontal cortex. We used diffusion tensor imaging to quantify structural connectivity of the pedunculopontine nucleus in patients with Parkinson’s disease with freezing of gait, without freezing, and healthy age-matched controls. We also included behavioural tasks to gauge severity of freezing of gait, quantify gait metrics, and assess executive cognitive functions to determine whether between-group differences in executive dysfunction were related to pedunculopontine nucleus structural network connectivity. Using seed regions from the pedunculopontine nucleus, we were able to delineate white matter connections between the spinal cord, cerebellum, pedunculopontine nucleus, subcortical and frontal/prefrontal cortical regions. The current study is the first to demonstrate differences in structural connectivity of the identified locomotor pathway in patients with freezing of gait. We report reduced connectivity of the pedunculopontine nucleus with the cerebellum, thalamus and multiple regions of the frontal cortex. Moreover, these structural differences were observed solely in the right hemisphere of patients with freezing of gait. Finally, we show that the more left hemisphere-lateralized the pedunculopontine nucleus tract volume, the poorer the performance on cognitive tasks requiring the initiation of appropriate actions and/or the inhibition of inappropriate actions, specifically within patients with freezing. These results support the notion that freezing of gait is strongly related to structural deficits in the right hemisphere’s locomotor network involving prefrontal cortical areas involved in executive inhibition function.
Keywords: diffusion tensor imaging, inhibition, executive function, falls, balance, white matter, microstructure
Introduction
Freezing of gait is described as a ‘brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk’ (Bloem et al., 2004; Giladi and Nieuwboer, 2008; Nutt et al., 2011). Freezing of gait is one of the most debilitating features of Parkinson’s disease as it causes falls (Kerr et al., 2010) and reduces mobility and quality of life (Moore et al., 2007; Nutt et al., 2011; Tan et al., 2011). Typically, freezing of gait lasts a couple of seconds, but episodes can exceed 30 s (Schaafsma et al., 2003). Freezing of gait is associated with disease severity and longer levodopa treatment (Giladi, 2001a; Macht et al., 2007), but does not affect all patients with Parkinson’s disease (Giladi et al., 2001). It is interesting to note that although freezing of gait is not associated with the cardinal features of Parkinson’s disease (tremor, bradykinesia or rigidity) (Giladi, 2001b; Bartels et al., 2003), it is correlated with postural instability (Giladi et al., 2001) and impaired executive function (Yogev-Seligmann et al., 2008).
Although central pattern generators are involved in stepping at the spinal level, purposeful gait is no longer considered merely an automated motor activity; it requires executive function and attention as well as judgement of the immediate environment and the body’s orientation within that environment (Yogev-Seligmann et al., 2008; Amboni et al., 2012). Supraspinal control is necessary for adapting locomotion: initiating gait, turning, stopping, and avoiding obstacles—the same situations that tend to induce freezing of gait (Jacobs and Horak, 2007a). In fact, we have hypothesized that freezing of gait is because of impaired coupling between postural and locomotor components of locomotion, specifically due to an inability to inhibit postural preparation and initiate stepping (Nutt et al. 2011; Cohen et al., 2013). Normal stepping, whether a voluntary step or a compensatory postural correction, is associated with a single anticipatory postural adjustment that shifts weight off the stepping leg (Jacobs and Horak, 2007b). In contrast, patients with Parkinson’s disease who have freezing of gait show delayed step initiation associated with repetitive anticipatory postural adjustments as if they cannot inhibit their postural preparation and release the stepping programme (Jacobs and Horak, 2007b; Jacobs et al., 2009).
Supraspinal regions involved in locomotion primarily include frontal motor regions (primary motor cortices, supplementary and pre-supplementary motor areas), as well as prefrontal cortices (medial and inferior frontal gyri) and subcortical areas (basal ganglia, pontomedullary reticular formation, mesencephalic locomotor region and cerebellar locomotor region) (Jahn et al., 2008). Recent studies report grey matter atrophy and divergent metabolic changes in fronto-parietal regions in patients with Parkinson’s disease who have freezing of gait compared to those without (Bartels et al., 2006; Bartels and Leenders, 2008; Kostic et al., 2012; Tessitore et al., 2012a, b). Further, functional and structural imaging studies point to structures downstream of the deficient basal ganglia as playing a key role in freezing of gait, specifically the pedunculopontine nucleus in the mesencephalic locomotor region.
The pedunculopontine nucleus is one of the major nuclei of the mesencephalic locomotor region and has neurons related to anticipatory postural adjustments preceding step initiation as well as to the step itself, thus it may be critical for coordinating posture and gait to avoid freezing of gait (Sinnamon et al., 2000). Recent work has shown greater grey matter atrophy of the pedunculopontine nucleus in patients with idiopathic Parkinson’s disease with freezing of gait compared to those without (Snijders et al., 2011). Furthermore, in patients with white matter lesions, reduced white matter microstructural integrity of the pedunculopontine nucleus was observed in patients with freezing of gait compared to individuals with white matter lesions without freezing of gait (Youn et al., 2012). Studies in rodents demonstrate that the pedunculopontine nucleus has ascending supratentorial axonal projections reaching numerous targets including the internal globus pallidus, thalamus and distal frontal structures (Woolf and Butcher, 1986; Hallanger and Wainer, 1988), along with descending projections to the cerebellum and spinal cord (Rye et al., 1988; Spann and Grofova, 1989). Likewise, the pedunculopontine nucleus receives input from many cortical and subcortical (basal ganglia) regions (Nauta and Mehler, 1966).
Increasing evidence suggests that the high level of connectivity of the pedunculopontine nucleus with functionally distinct neuronal systems underlies an integrative function rather than a role as a simple relay nucleus. Recent work from Snijders et al. (2011) reports that patients with freezing of gait showed more activity compared to those without in the mesencephalic locomotor region and decreased responses in mesial frontal regions during motor imagery of gait (relative to visual imagery). Furthermore, patients with freezing of gait have reduced resting-state functional connectivity within the ‘executive-attention’ network of the right hemisphere (middle frontal and angular gyrus) compared with those without freezing of gait (Tessitore et al., 2012b). Finally, recent work suggests that structural connectivity (assessed with diffusion tensor imaging) between the pedunculopontine nucleus and the cerebellum is reduced in patients with freezing of gait (Schweder et al., 2010). These studies suggest that freezing of gait may emerge as a result of an altered supraspinal locomotor neural network comprising the pedunculopontine nucleus, cerebellum, basal ganglia and frontal/executive cortical regions. Two studies specifically implicate alterations isolated to the right hemisphere in subjects with Parkinson’s disease with freezing of gait (Bartels and Leenders, 2008; Tessitore et al., 2012b).
Because freezing of gait and postural impairments have been related to frontal lesions/dysfunction and there is a strong association between executive dysfunction and freezing of gait (Giladi and Hausdorff, 2006; Amboni et al., 2008; Naismith et al., 2010), we hypothesized that freezing of gait is associated with disrupted connectivity between midbrain locomotor regions and medial frontal cortex. We used diffusion tensor imaging to quantify structural connectivity of the pedunculopontine nucleus in participants with Parkinson’s disease with freezing of gait, without freezing of gait, and healthy age-matched controls. We also included executive cognitive tasks to assess inhibitory control to determine whether between-group differences in executive function were related to pedunculopontine nucleus structural network connectivity.
Materials and methods
Participants
Twenty-six mild to moderate patients with Parkinson’s disease (four female and 22 male) were recruited through the Parkinson’s Centre of Oregon clinics at Oregon Health and Science University (OHSU). Fifteen age-matched healthy controls (10 female) were also recruited from the surrounding Portland, OR area. Participants were excluded if they could not safely walk 20 ft without walking aids, or if they had a joint replacement, musculoskeletal or vestibular disorder, white matter disease, dementia, claustrophobia, severe tremor, or had metal in their body. The Unified Parkinson’s Disease Rating Scale (UPDRS) – III assessed Parkinsonian motor disability and the Montreal Cognitive Assessment assessed overall cognitive function. The new freezing of gait questionnaire is a self-report measure of freezing of gait. It begins with the presentation of a short (30 s) video to illustrate freezing, and then follows with a few simple questions. Patients were classified as exhibiting freezing of gait based on a score >3 on the new freezing of gait questionnaire (Nieuwboer and Giladi, 2008). Patients scoring ≤3 were classified as not exhibiting freezing of gait. Functional MRI data were also collected within this cohort (not reported here), thus parkinsonian patients were tested in the morning OFF medication, after 12–18 h withdrawal from anti-parkinsonian medications to mitigate the pharmacological effects on neural activity. This study was approved by OHSU’s Institutional Review Board and all participants gave their informed written consent before beginning the experiment.
Behavioural assessments of executive/frontal function
Stroop task
The Stroop task (Stroop, 1935) assesses inhibitory control and response competition management. Our version of the task was implemented in MATLAB and Psychophysics Toolbox-3. The task used three separate conditions of 50 stimuli each in which participants were instructed to: (i) read colour words (Stroop Colour); (ii) name colour bars (Stroop Word); and (iii) name the colour of colour words while inhibiting the prepotent response of reading the word (Stroop Conflict). The dependent measures collected were number of errors, and mean time to complete 50 trials for each condition.
Eriksen Flanker task
The Flankers task (Eriksen and Schultz, 1979) is a common paradigm used to assess the ability to respond with inhibition of surrounding distractors. The task was implemented in MATLAB and Psychophysics Toolbox-3, similar to previous work (Wylie et al., 2009). In each trial, an array of five side-by-side stimuli was displayed on a computer screen. The target was the central stimuli, and is a 4-cm wide × 3.5-cm tall arrow pointing either left or right (with a probability 0.5 of either direction). The two stimuli on either side of the target were identical and randomly selected on each trial from three experimental conditions: (i) Congruent: each flanker was identical to the central stimuli; (ii) Incongruent: each flanker was an arrow facing the opposite direction of the central stimuli; and (iii) Neutral: each flanker was a diamond. Each trial began with a central fixation cross (500 ms), followed by the stimulus (1000 ms) and ended with a black screen (1000 ms). Participants were instructed to press a button with their left thumb if the central stimuli pointed to the left, and to press a different button with their right thumb if the central stimuli pointed to the right. The dependent measures collected were percentage of correct responses, and median reaction time of correct responses.
Behavioural assessments of mobility
Instrumented Timed Up and Go test
All participants performed quantitative assessments of balance and gait using Opal inertial sensors, Mobility Lab, the clinical user interface and automated algorithms by Ambulatory Parkinson’s Disease Monitoring (APDM Inc.). Specifically, all participants performed three trials of the extended Instrumented Timed Up and Go test, which involved standing up from a chair, walking 7 m, turning around to walk back to the chair, and sitting down (Salarian et al., 2010). Subjects wore seven Opal sensors (APDM Inc.) composed of 3D accelerometers, 3D gyroscopes and 3D magnetometers. The sensors were positioned with Velcro belts on the posterior trunk, near the body centre of mass, on the anterior shank of each leg, on each wrist, on the sternum, and on the forehead. Data were acquired and automatically analysed with MobilityLab (Mancini and Horak, 2010). We provide measurements of stride time, stride length, stride velocity, stride asymmetry, double support time, turning duration and cadence.
Turning in place
Because freezing of gait is notoriously difficult to elicit in the laboratory or clinic setting, we also included a trial in which participants performed tight clockwise and counter-clockwise turns, a task known to provoke freezing. Participants continuously turned in place by turning one 360° revolution to their right, and then turning one 360° revolution to their left at their own comfortable pace for 2 min. Turning trials were video recorded and three neurologists from the OHSU Department of Neurology’s Parkinson Centre of Oregon subsequently reviewed and scored Parkinson’s disease participants’ freezing severity. Scores were based upon on ordinal scale from 0–4 where 0 = absent, 1 = mild, 2 = moderate, 3 = significant interference with movement, and 4 = severe with risk for falls. The raters were blinded to whether the patients with Parkinson’s disease were identified as having freezing of gait or not based upon their self-assessed new freezing of gait questionnaire score.
Magnetic resonance imaging acquisition protocol
All images were acquired using a 3.0 T Siemens Magnetom Tim Trio scanner with a 12-channel headcoil at the OHSU Advanced Imaging Research Centre. One whole brain high-resolution structural T1-weighted MP-RAGE sequence (orientation = sagittal, echo time = 3.58 ms, repetition time = 2300 ms, 256 × 256 matrix, resolution = 1 mm3, 1 average, total scan time = 9 min 14 s) was collected. Three sets of diffusion-weighted images were collected using a 30-gradient direction, whole-brain echoplanar imaging sequence (repetition time = 9100 ms, echo time = 88 ms, field of view = 240 mm2, b value = 1000 s/mm2, isotropic voxel dimensions = 2 mm3) and three images in which the b-value was equal to zero. A static magnetic field map was also acquired using the same parameters as the diffusion-weighted image sequence.
Image analysis
Image processing
Diffusion data were processed using the tools implemented in FSL (Version 4.1.9; www.fmrib.ox.ac.uk/fslwww.fmrib.ox.ac.uk/fsl). The three raw data sets were first corrected for eddy current distortions and motion artefacts using the correction tool (FDT 1.0) and then averaged to improve signal-to-noise ratio (Eickhoff et al., 2010) and subsequently skull-stripped (using BET). The principal diffusion direction was estimated for each voxel as a probability density function, using Bayes’ rules in order to account for noise and uncertainty in the measured data. As described elsewhere (Behrens et al., 2003), the implicit modelling of noise in a probabilistic model enables a fibre-tracking procedure without externally added constraints such as fractional anisotropy threshold or fibre angle. Thus, fibre tracking in or near cortical areas becomes more sensitive. The use of a 2-fibre model (Behrens et al., 2007) also improved the modelling of crossing fibres. By sending out 25 000 streamline samples per seed voxel, we mapped the probabilistic connectivity distributions for each voxel in the region of interest identified (see below). For each individual, the fractional anistropy images were normalized into Montreal Neurological Institute (MNI) space by using a linear (affine) registration and Fourier interpolation through the FMRIB linear image registration tool.
Identification of regions of interest
The pedunculopontine nucleus was identified in the current study through an amalgamation of previous work in the animal and human literature. The pedunculopontine nucleus is located in the mesencephalic reticular formation of the upper pons and has an irregular shape delimited by the borders of its population of cholinergic neurons (Martinez-Gonzalez et al., 2011). Previous work using choline acetyltransferase immunohistochemical staining in humans has shown the mean (range) location of the pedunculopontine nucleus to be 7.1 mm (4.6–9 mm) from the midline, 5.8 mm (4.1–7.4 mm) ventrodorsal from the floor of the fourth ventricle and −5.3 mm (−2.2 to −8.0 mm) rostro-caudal from a pontomesencephalic line connecting the pontomesencephalic junction to the inferior colliculi caudal margin (Ferraye et al., 2010). In MNI coordinates the centroid of the pedunculopontine nucleus is located at x = ±7.1 mm, y = −32 mm, z = −22 mm with respect to the anterior commissure (0, 0, 0 in MNI coordinates) (Thevathasan et al., 2012a). This is further supported by work using local field potentials (Thevathasan et al., 2012b) and immunohistochemistry (Mesulam et al., 1989) demonstrating that the pedunculopontine nucleus extends from 2 mm above and 4 mm below the pontomesencephalic junction. Thus in the current study the pedunculopontine nucleus region of interest is centred at x = ±7, y = −32, z = −22 and ranges from x = ±6 to 9, y = −30 to −35, z = −17 to −26 (Fig. 1). The pedunculopontine nucleus regions of interest were also constrained by each individual’s fractional anisotropy map to ensure that only voxels with a fractional anistropy of <0.2 were included in the region of interest, thus excluding white matter from the initial regions of interest. To demonstrate the specificity of the pedunculopontine nucleus tractography we also created regions of interest of the right and left inferior colliculi. The inferior colliculi regions of interest comprised the same x and y dimensions, but spanned −9 to −13 in the z dimension.
Figure 1.

Location of region of interest for the pedunculopontine nucleus overlaid on MNI template in radiologic convention. (A) x = −7, y = −22, z = −22. pedunculopontine nucleus location mean (range) in MNI coordinates: ±7 (6–9), −32 (−30 to −35), −22 (−17 to −26). L = left; R = right.
Probabilistic tractography
To analyse the pedunculopontine nucleus network we identified unrestricted connectivity maps for regions of interest in both hemispheres. Probabilistic fibre tracking (using FDT 1.0; see Behrens et al., 2007) was initiated from every voxel within the binarized region of interest sphere in each participant’s native diffusion space. Streamline samples (25 000) were sent out from each voxel, with a step length of 0.5 mm and a curvature threshold of 0.2. For group analyses, the probabilistic connectivity distribution maps from individual participants were thresholded at a 5% level (thus selecting all connections where >1250 of 25 000 samples passed) (Gschwind et al., 2012). To ensure that this threshold did not influence results we also performed the same analyses thresholded at 15%. Tracts were then binarized, transferred into MNI space and summed up across participants to obtain the connectivity probability map of the group. Note that no restriction was used in order to explore all brain regions to which the white matter pathways were directed. Tractography from the inferior colliculi was also performed in order to demonstrate the specificity of the structural network identified for the pedunculopontine nucleus. This served as a control to ensure that fibre tracking was indeed possible and selective.
We assessed ‘tract quality’ by computing the average fractional anistropy value (an index of microstructural integrity) along the identified tracts of interest. We assessed ‘tract quantity’ by computing the volume comprising the identified tracts that exceeded a fractional anistropy threshold of 0.2, a standardized value used to differentiate between grey and white matter (Davis et al., 2009; Fling et al., 2013). To ensure that this threshold did not influence results we also performed the same analyses with fractional anistropy thresholds of 0.25 and 0.3. Finally, for tracts that demonstrated significant volumetric differences between the right and left hemisphere we calculated a laterality index as follows: laterality index = (Left − Right) / (Left + Right) where a positive number indicates more fibre tracts in the left hemisphere (Schacher et al., 2006). This allows for a quantitative metric of hemispheric differences within an individual.
Statistical analyses
A one-way analysis of covariance (ANCOVA) was performed to assess group differences in performance on cognitive tasks as well as all metrics of gait derived from the Opal sensors. All comparisons were controlled for participant’s UPDRS motor score (OFF medication). A 2 × 3 repeated measures ANCOVA was also performed (region of interest × group) to identify hemispheric and group differences in pedunculopontine nucleus fibre tract microstructural integrity and volume while controlling for the UPDRS motor score. Significant main effects were subjected to post hoc Student’s t-tests and Bonferroni corrected for multiple comparisons. We also performed linear regression between task performance (solely on tasks where group differences were observed) and pedunculopontine nucleus fibre tract laterality index to identify relationships between task-related performance and pedunculopontine nucleus network asymmetry while controlling for the UPDRS motor score.
Results
Participant demographics can be viewed in Table 1. Briefly, patients with freezing of gait had more severe parkinsonism as assessed by the Hoehn and Yahr scale and UPDRS motor scores (particularly within the postural instability and gait disorder subsection) as well as poorer general cognition as assessed by the Montreal Cognitive Assessment. Poorer postural instability and gait disorder subtype was primarily due to more severe abnormalities in the postural stepping response for the Pull test in the group with freezing of gait compared to the group without freezing of gait (0.75 versus 0.08, respectively).
Table 1.
Demographic and clinical feature of all participants
| Healthy control subjects (n = 15) | Patients without freezing of gait (n = 12) | Patients with freezing of gait (n = 14) | P-value | |
|---|---|---|---|---|
| Age, years | 66.7 (7.6) | 65.5 (7.0) | 67.1 (5.4) | 0.5 |
| Gender (F/M) | 5/10 | 3/9 | 1/13 | 0.3 |
| Montreal Cognitive Assessment | 26.9 (2.5) | 27.8 (1.9) | 25.5 (3.1) | 0.04 |
| Disease duration | n.a. | 6.4 (4.2) | 10.4 (6.1) | 0.07 |
| Hoehn and Yahr | n.a. | 2.0 (0.7) | 2.7 (0.6) | 0.007 |
| Symptom-onset side (right/left/bilateral) | n.a. | 5/6/1 | 4/8/2 | |
| Symptom-dominant side (R/L/B) | n.a. | 5/6/1 | 5/7/2 | |
| New freezing of gait questionnaire score | 0 (0.0) | 0.3 (0.9) | 17.3 (5.2) | 0.001 |
| UPDRS Motor | 5.7 (3.9) | 29.3 (7.1) | 37.1 (9.3) | 0.03 |
| Postural instability and gait disorder subtype | n.a. | 1.8 (1.2) | 3.4 (1.9) | 0.02 |
| l-DOPA, mg/day | n.a. | 485 (71.6) | 646 (95.3) | 0.2 |
P-values given are for comparisons between patients with Parkinson’s disease with freezing compared to those without freezing. Data are mean (±SD). R = right, L = left, B = bilateral (i.e. no dominant side). UPDRS refers to part III of the UPDRS assessment. All Parkinson’s disease participants were tested OFF medication. PIGD = postural instability and gait disorder subtype.
Behavioural results
Cognitive performance
Descriptive statistics of performance on the Stroop and Flanker’s tasks can be viewed in Table 2. As expected, a significant main effect of group was noted for speed on the Stroop task (F > 3.4; P < 0.05 for all conditions). However, when controlling for UPDRS, significance only survives for the Stroop conflict condition (F = 4.0; P = 0.03). For the Stroop Conflict condition, post hoc t-tests show that participants with freezing of gait were significantly slower than healthy control subjects (t = 3.4; P < 0.01) and particiaants without freezing of gait (t = 2.5; P = 0.02). No difference was noted between those patients without freezing of gait and healthy controls. We also observed a group main effect of errors on the Stroop conflict condition (F = 4.3; P = 0.02), but this effect did not meet our significance threshold when controlling for UPDRS (F = 2.4; P = 0.11).
Table 2.
Descriptive statistics of performance data
| Healthy control subjects | Patients without freezing of gait | Patients with freezing of gait | P-value (not controlling for UPDRS) | P-value (controlling for UPDRS) | |
|---|---|---|---|---|---|
| Stroop Task | |||||
| Time to completion (s) | |||||
| Colour | 32.5 (1.2) | 36.3 (1.2) | 40.8 (2.9) | 0.02 | 0.07 |
| Word | 22.4 (0.8) | 26.7 (1.2) | 28.0 (2.3) | 0.04 | 0.23 |
| Conflict | 62.5 (8.0) | 70.3 (7.0) | 94.2 (40.3) | 0.005 | 0.03 |
| Error (total # out of 50) | |||||
| Colour | 0.07 (0.07) | 0.7 (0.3) | 0.9 (0.4) | 0.19 | 0.62 |
| Word | 0.1 (0.1) | 0.08 (0.08) | 0.07 (0.07) | 0.81 | 0.69 |
| Conflict | 1.3 (0.3) | 3.0 (1.1) | 6.3 (1.9) | 0.02 | 0.11 |
| Flankers Task | |||||
| Reaction time (ms) | |||||
| Congruent | 481.5 (18.6) | 471.4 (24.9) | 539.6 (122.3) | 0.17 | 0.14 |
| Incongruent | 523.4 (71.2) | 512.9 (88.7) | 572.0 (135.4) | 0.32 | 0.30 |
| Neutral | 497.6 (16.5) | 490.2 (27.1) | 715.7 (157.6) | 0.16 | 0.22 |
| Accuracy (%) | |||||
| Congruent | 99.6 (0.2) | 98.8 (0.5) | 95.9 (1.6) | 0.02 | 0.08 |
| Incongruent | 96.8 (1.1) | 92.9 (2.2) | 92.3 (2.2) | 0.16 | 0.38 |
| Neutral | 98.7 (0.5) | 96.3 (0.9) | 93.5 (2.6) | 0.08 | 0.26 |
Data are all mean (±SEM) except for the flanker’s task reaction time, which is median (±SEM).
A significant main effect of group was noted for accuracy on the flanker’s congruent condition (F = 4.0; P = 0.03); however, this group effect did not meet our significance threshold when controlling for UPDRS (F = 2.7; P < 0.08). Despite slower performance for the group with freezing of gait, no significant group differences were noted for reaction time in any of the flanker’s conditions (P > 0.16), likely due to the large variability in the group with freezing of gait.
Mobility performance
Descriptive statistics of the motor performances during the Instrumented Timed Up and Go are shown in Table 3. Briefly, we report no differences among groups on stride time, stride velocity, double support time, cadence, or stride asymmetry. A significant group main effect was noted for stride length and turning duration (F = 5.63; P = 0.007, F = 5.02; P = 0.01, respectively). Post hoc t-tests revealed that patients with freezing of gait had significantly reduced stride length compared with both healthy control subjects (P < 0.01) and patients without freezing of gait (P < 0.05), with no difference observed between healthy control subjects and those without freezing of gait. Turning duration was significantly higher in both patients with and without freezing of gait compared with healthy control subjects (P < 0.01), but not different between the two Parkinson’s disease groups. Whereas only mild group differences were noted on objective gait measures during the Instrumented Timed Up and Go, the mean severity of freezing score during turning, as rated by three blinded movement disorders neurologists (inter-rater reliability = 0.97), was significantly higher in patients with freezing of gait compared with those without.
Table 3.
Descriptive statistics of gait and freezing severity
| Healthy control subjects | Patients without freezing of gait | Patients with freezing of gait | P-value (not controlling for UPDRS) | P-value (controlling for UPDRS) | |
|---|---|---|---|---|---|
| Instrumented Timed Up and Go | |||||
| Stride length (% stature) | 81.72 (1.12) | 80.04 (1.35) | 74.11 (2.30) | 0.007 | 0.13 |
| Stride time (s) | 1.07 (0.02) | 1.09 (0.02) | 1.07 (0.03) | 0.73 | 0.73 |
| Stride velocity (% stature/s) | 77.61 (2.41) | 73.62 (2.15) | 70.79 (3.44) | 0.22 | 0.66 |
| Double support (% gait cycle) | 21.59 (1.08) | 21.81 (0.60) | 20.01 (1.04) | 0.37 | 0.53 |
| Cadence (steps/minute) | 113.60 (2.46) | 110.47 (2.38) | 113.92 (3.16) | 0.64 | 0.65 |
| Stride asymmetry | 1.55 (0.09) | 1.43 (0.22) | 1.78 (0.19) | 0.37 | 0.45 |
| Turning duration (s) | 2.13 (0.11) | 2.69 (0.15) | 3.06 (0.31) | 0.01 | 0.50 |
| Turning in place | |||||
| Clinical freezing rating | n.a. | 0.11 (0.06) | 1.62 (0.38) | 0.001 | 0.001 |
Data are mean (±SEM).
Fibre tractography
Fibre tracts were identifiable in both hemispheres for all participants (Fig. 2A and B). Consistent with previous work (Muthusamy et al., 2007; Martinez-Gonzalez et al., 2011) the pedunculopontine nucleus showed inferior connections to cerebellar mid-line structures including the cerebellar locomotor region, fastigial nuclei and the spinal cord (relayed through the ponto-medullary reticular formation) as well as superior projections that connect to subcortical structures including the thalamus, subthalamic nucleus, internal globus pallidus, and midline cortical targets including the superior and middle frontal gyri, anterior cingulate, pre-supplementary and supplementary motor areas, and pre- and postcentral gyri. The descending fibre tracts were primarily located within the inferior cerebellar peduncle and the cortico-ponto-cerebellar tracts, whereas the ascending fibre tracts were primarily confined to the cortico-ponto-cerebellar pathways and the internal capsule.
Figure 2.
Summed group images of fibre tracts passing through the right pedunculopontine nucleus region of interest in the coronal view (A) and the axial view (B). Data are displayed in MNI space on the 1 mm MNI template and are in radiologic convention. The colour bar describes the percentage of participants with overlap in the given region; warmer areas indicate regions of greater fibre tract overlap. FOG+ = patients with freezing of gait; FOG− = patients without freezing of gait; L = left; R = right.
Tractography from seeds in the inferior coliculi confirmed the specificity of the pedunculopontine nucleus network. Representing known anatomical connections, fibre tracts from the inferior colliculus passed through the lateral geniculate body and projected more posteriorly to the temporal gyri, as opposed to the anterior fibre tracts identified from the pedunculopontine nucleus seeds (Supplementary Fig. 1).
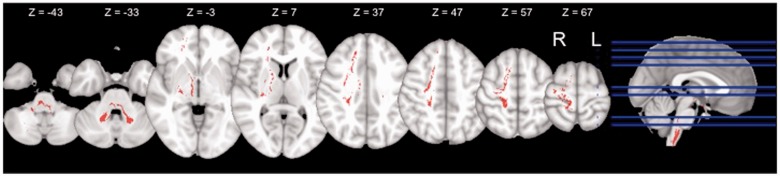
Statistical results remained the same regardless of the fractional anistropy threshold (0.2, 0.25 or 0.3), or the exclusion threshold (5% or 15%). Therefore, results discussed are those using a fractional anistropy threshold of 0.2 and an exclusion threshold of 5% and all comparisons were controlled for UPDRS motor score. Fibre quality, as expressed by the average fractional anistropy values, was found to be equal between all groups (P > 0.25). Additionally, no significant difference was found for pedunculopontine nucleus tract volume between hemispheres (for all participants combined). A significant main effect of pedunculopontine nucleus tract volume was found for group (F = 3.6, P = 0.02), and a group by hemisphere interaction (F = 3.1, P = 0.02) was observed. Post hoc t-tests revealed that individuals with freezing of gait had significantly reduced right hemisphere pedunculopontine nucleus fibre tract volume compared with healthy controls (P < 0.01) and patients without freezing of gait (P < 0.05; Fig. 3). No difference was observed between the healthy control subjects and patients without freezing of gait (P > 0.18). Within the right hemisphere pedunculopontine nucleus network, patients with freezing of gait had reduced fibre tracts in areas including the putamen, internal globus pallidus, cingulate, thalamus, precentral and postcentral gryus, superior and middle frontal gyrus, supplementary and pre-supplementary motor areas and bilateral cerebellar locomotor regions (Fig. 4). There were no areas in which participants with freezing of gait demonstrated greater fibre tract volume than either healthy control subjects or participants without freezing of gait.
Figure 3.
Microstructural integrity and tract volume for locomotor network fibre tracts passing through the right and left pedunculopontine nucleus (PPN) regions of interest. No group or hemispheric differences were observed for microstructural integrity. Patients with freezing of gait (FOG+) had significantly reduced volume of fibre tracts solely in the right hemisphere compared to both healthy controls (HC) and patients without freezing of gait (FOG−). No differences were found between healthy control subjects and patients without freezing of gait. Data are mean ± SD, *P < 0.05, **P < 0.01. FA = fractional anisotropy; WM = white matter.
Figure 4.
Axial view of right hemispheric pedunculopontine nucleus tracts observed in healthy control subjects and patients without freezing of gait, but not in patients with freezing of gait. Prominent areas include putamen, internal globus pallidum, thalamus, precentral gyrus, postcentral gyrus, superior frontal gyrus, middle frontal gyrus, supplementary motor area, pre-supplementary motor area, and cerebellar locomotor region. Data are displayed in MNI space on the 1 mm MNI template and are in radiologic convention. L = left; R = right.
Laterality index
We also calculated a laterality index based on pedunculopontine nucleus tract volume. A positive number indicates left-lateralized fibre tracts (see Methods). The mean laterality index in healthy control subjects was −0.01 (±0.03). In participants without freezing of gait the mean laterality index was 0.02 (±0.03), whereas in participants with freezing of gait the mean laterality index was 0.1 (±0.03). There was a significant main effect of group (F = 4.2, P = 0.02). Post hoc t-tests demonstrated that pedunculopontine nucleus tract laterality was significantly more left-hemisphere lateralized in participants with freezing of gait compared with both healthy control subjects (P < 0.01) and participants without freezing of gait (P < 0.05) (Fig. 5A). No difference in laterality index was found between participants without freezing of gait and healthy control subjects (P > 0.45).
Figure 5.
(A) Significant group differences in laterality of fibre tract volume of locomotor network fibre tracts passing through the pedunculopontine nucleus (PPN). Pedunculopontine nucleus tract volume was significantly more left lateralized in patients with freezing of gait (FOG+) compared with patients without freezing of gait (FOG−) or healthy control subjects (HC). No differences were found between healthy control subjects and patients without freezing of gait. A value of zero would indicate equal pedunculopontine nucleus tract volume in the left and right hemisphere. Data are mean ± SD. (B) Linear regression between pedunculopontine nucleus tract laterality index and time to complete the Stroop conflict task as well as accuracy on the Flanker’s congruent task. Significant relationships exist solely for the group with freezing of gait, such that the more left-lateralized the pedunculopontine nucleus tract volume, the poorer the performance. Data are mean ± SD, *P < 0.05, **P < 0.01.
It is worth noting that no differences in quantity or quality of fibre tracts originating from the inferior colliculus were noted between groups (Supplementary Fig. 2). Furthermore, no hemispheric asymmetries were found for fibre tracts from the inferior colliculus. Thus, the structural connectivity differences observed for patients with freezing of gait in the current study were specific to fibre connections with the pedunculopontine nucleus.
Relationships between pedunculopontine nucleus tract structure and behavioural performance
For behavioural tasks where significant group differences were observed, we performed linear regression between pedunculopontine nucleus tract laterality and performance. All correlations were Bonferroni-corrected for multiple comparisons (0.05/6; α = 0.008) and controlled for UPDRS. Specific to the group with freezing of gait, pedunculopontine nucleus laterality index was significantly related to speed on the Stroop conflict condition (Table 4). Asymmetric pedunculopontine nucleus tract volume was also associated with total number of errors on the Stroop conflict task as well as accuracy on the Flanker’s congruent task in the group with freezing of gait. Thus, the more lateralized the pedunculopontine nucleus tract volume, the slower and less accurate the performance on cognitive tasks requiring action inhibition, solely in the group with freezing of gait (Fig. 5B). Finally, no association was found between pedunculopontine nucleus laterality index and clinical ratings of freezing severity assessed by three neurologists’ ratings.
Table 4.
Linear regression between pedunculopontine nucleus laterality index and behavioural performance
| Healthy control subjects R2 | Patients without freezing of gait R2 | Patients with freezing of gait R2 | |
|---|---|---|---|
| Stroop Task | |||
| Time to completion | |||
| Colour | 0.0001 | 0.04 | 0.25 |
| Word | 0.0004 | 0.001 | 0.12 |
| Conflict | 0.0001 | 0.005 | 0.35* |
| Error | |||
| Conflict | 0.23 | 0.28 | 0.45** |
| Flanker’s Task | |||
| Accuracy | |||
| Congruent | 0.02 | 0.06 | 0.43** |
| Turning in place | |||
| Clinical freezing rating | n.a. | 0.01 | 0.06 |
*P < 0.01, **P < 0.001. Correlations were adjusted for UPDRS Part III Motor score.
Discussion
The current study is the first to report differences in structural connectivity of the locomotor pathway in patients with Parkinson’s disease with freezing of gait, with reduced connectivity from the pedunculopontine nucleus to the cerebellar locomotor regions, thalamus, and multiple regions of the frontal and prefrontal cortex. These structural differences were observed solely in the right hemisphere of patients with freezing of gait. Additionally, we show that the more left hemisphere-lateralized the pedunculopontine nucleus tract volume, the poorer the performance (both with regards to speed and accuracy) on tasks requiring the initiation of appropriate actions and/or the inhibition of inappropriate actions, specifically within patients with freezing of gait. Our data demonstrate the feasibility of identifying widespread connections between regions thought to comprise the locomotor network in humans with diffusion tensor imaging. These multi-synaptic anatomical connections are strongly supported by anatomical tracer work performed in animals (Kuypers and Lawrence, 1967; Carpenter et al., 1981; Hylden et al., 1985; Grunwerg et al., 1992; Hazrati and Parent, 1992; Matsumura et al., 1997; Chivileva and Gorbachevskaya, 2005), and diffusion imaging in a small sample of humans (Muthusamy et al., 2007).
Recent neuroimaging work suggests a complex supraspinal locomotor network (Jahn et al., 2008). It has been proposed that this widespread network controls modulated gait (changing direction, modulating speed, starting and stopping) whereby the locomotor command runs from the prefrontal cortex via the basal ganglia to the subthalamic nucleus and the mesencephalic locomotor region. Information from the mesencephalic locomotor region then converges with output from the cerebellar locomotor region and travels inferiorly to the spinal pattern generators via the pontomedullary reticular nuclei (Bakker et al., 2008; Iseki et al., 2008; Jahn et al., 2008). If there is an interruption of the connectivity of this network at any of these points, initiation, maintenance, and modulation of posture and gait is likely to be impaired. In patients with freezing of gait we found significant reduction of the right hemisphere’s locomotor pathway implicating disruption of the ability to communicate information throughout this network. Recent work in patients with progressive supranuclear palsy demonstrates significantly reduced activation of this pathway during locomotion, resulting in problems of initiation of locomotion and control of step length (Zwergal et al., 2013). The authors suggest that although these deficits occur early in progressive supranuclear palsy, they may not occur until relatively late in Parkinson’s disease. In accord with this suggestion, freezing of gait does not typically occur until late in Parkinson’s disease progression. Thus, it is possible that early in Parkinson’s disease the locomotor pathway is not affected (as in the participants without freezing of gait), whereas with disease progression structural connections between these pathways begin to degrade, resulting in impaired communication and freezing of gait. Indeed, within our current cohort of patients with freezing of gait, pedunculopontine nucleus laterality index was positively correlated with UPDRS Motor score (r = 0.33), but not in the group without freezing of gait (r = −0.10). Conversely, we report no relationship between freezing severity and pedunculopontine nucleus laterality within the current cohort. Thus, the observed pedunculopontine nucleus laterality may help differentiate between patients with Parkinson’s disease with or without freezing, however, it does not appear to be associated with the severity of freezing.
A growing body of literature demonstrates that gait is not simply an automated motor activity, but one that requires attention and executive function (Yogev-Seligmann et al., 2008; Amboni et al., 2012). Early disturbances in these cognitive processes are associated with slower gait and gait instability and these cognitive disruptions assist in the prediction of future mobility loss and falls in healthy ageing (cf. Monetro-Odasso et al., 2012). Moreover, recent work reveals an age-related shift from automatic (subcortical) to attentional (cortical) control during locomotion and stance, likely reflecting a higher cognitive input to locomotion and stance in older adults (Zwergal et al., 2012). This shift to stronger cortical control of locomotion may be significantly hampered in patients with Parkinson’s disease due to cognitive impairment and deficits in executive function (Owen, 2004). A recent review by Vandenbossche et al. (2012) further suggests that freezing of gait is characterized by impairments in both the execution and acquisition of automatic behaviours as well as deficits in executive functioning and point to deficits in fronto-striatal circuitry. Our current data fit well with the notion of a frontal lobe disconnection from subcortical structures critical for gait. The frontal cortex may play a crucial role in controlling gait patterns when environmental conditions change (Nutt et al., 1993), thus when frontal areas are dysfunctional, this could lead to a decreased ability to focus attention to a motor programme and continue this programme when other stimuli need to be integrated (e.g. passing through a doorway). The disruption of the right hemisphere’s locomotor pathway, primarily showing a lack of connection between frontal/prefrontal cortices and the pedunculopontine nucleus, implicates that declines in communication between these regions likely serve a role in the gait disruptions seen in freezing of gait.
We note that these network disruptions were solely observed in the right hemisphere, which is in agreement with a growing body of literature demonstrating that the right hemispheric circuitry of the brain appears to be selectively affected in freezing of gait. The brain is organized with certain specialized functions lateralized to each hemisphere. For example, the left hemisphere is preferentially involved in verbal processing (Ivry and Robertson, 1998) and motor control (Serrien et al., 2006), whereas the right hemisphere plays a stronger role in spatial cognition (Ivry and Robertson, 1998), body schema (Cummings, 1997) and action inhibition (Aron and Poldrack, 2006). A growing body of imaging studies suggests that right-hemisphere circuitry seems to be more affected than the left in patients with freezing of gait (Bartels et al., 2006; Helmich et al., 2007; Snijders et al., 2011; Tessitore et al., 2012b; for a review see Bartels and Leenders, 2008). Primarily left-sided symptoms in Parkinson’s disease (right hemisphere) have been associated with worse cognitive performance on verbal recall, word retrieval, semantic verbal fluency, visuospatial analysis, abstract reasoning, attention span, and mental tracking (Tomer et al., 1993; Katzen et al., 2006), slower gait and poorer judgement of narrow doorways (Lee et al., 2001; van der Hoorn et al., 2012) and poorer axial kinaesthesia (Wright et al., 2010). In agreement with the structural differences noted here, Tessitore et al. (2012b) report reduced functional connectivity within the executive-attention network of patients with freezing of gait, particularly within the right middle frontal gyrus. Further supporting our findings, the authors report no such difference between participants without freezing of gait and healthy control subjects. Collectively, these works point to alterations in both the structural and functional network connectivity within the right hemisphere in patients with Parkinson’s disease with freezing of gait.
Our current study points to specific losses of fibres within known nodes of the right hemisphere’s inhibitory network. The right ventrolateral prefrontal cortex and pre-supplementary motor area are often implicated for a range of tasks that require response inhibition and switching from automatic to controlled behaviour (Aron and Poldrack, 2006; Zandbelt and Vink, 2010) and are thought to enact inhibitory control through the subthalamic nucleus (Aron and Poldrack, 2006; Isoda and Hikosaka, 2008). Activity in the subthalamic nucleus suppresses the motor system by inhibiting output from the basal ganglia (Alexander and Crutcher, 1990; Parent and Hazrati, 1995). The subthalamic nucleus receives input from the frontal cortex through the hyperdirect pathway (Nambu et al., 2002), and it has been suggested that the subthalamic nucleus lies at the heart of the system controlling response inhibition (Aron and Poldrack, 2006; Frank, 2006; Hikosaka and Isoda, 2010). Further, Coxon et al. (2012) report that age-related declines in inhibitory control are strongly related to the structural decline of right hemisphere subthalamic nucleus projections.
In the current study we report significantly poorer performance on behavioural tasks that require response inhibition solely in patients with freezing of gait, compared to either those without freezing of gait or healthy control subjects. Our results further strengthen the relationship between inhibitory executive function and locomotion in patients with freezing of gait (Vandenbossche et al. 2011). Participants with freezing of gait made more errors on the Stroop conflict task, and even on correct trials they still performed slower. Additionally, patients with freezing of gait made more errors on the congruent condition of the Flanker’s task. Pedunculopontine nucleus tract laterality was strongly associated with performance on these response inhibition tasks, but only in participants with freezing of gait and not in healthy control subjects or participants without freezing of gait. That is to say, in patients who experience freezing of gait, the more asymmetrical the pedunculopontine nucleus fibre tract volume, the poorer the performance. We suggest that the breakdown in both structural and functional connectivity of the right hemisphere’s response inhibition pathway may partially contribute to freezing of gait. This is supported by work demonstrating medial frontal cortex activity during the Stroop task (Mayer et al., 2012). Thus, the observed decrease in structural connectivity between the right hemisphere’s pedunculopontine nucleus and the medial frontal gyrus in patients with freezing of gait likely contributes to a difficulty with inhibiting competing postural preparation and stepping during locomotion (Nutt et al., 2011; Cohen et al., under review).
We observed significantly reduced structural connectivity between the pedunculopontine nucleus nuclei and the cerebellar locomotor region in participants with freezing of gait. These results are comparable to those reported by Schweder et al. (2010) in a small sample of patients (n = 2). The cerebellar locomotor region has been proposed as a pacemaker, providing rhythmic output to control temporal components of gait (Zwergal et al., 2013). Recent work demonstrates that patients with freezing of gait are capable of adapting stride length to compensate for differences in leg speed imposed by a split-belt treadmill; however, these individuals were unable to adapt stride time (Nanhoe-Mahabier et al., 2013). In combination with the current study, these results suggest that impaired structural connectivity between the pedunculopontine nucleus and the cerebellar locomotor region may result in a reduced capability for the cerebellum to adequately control temporal parameters of gait.
It is worth noting that pedunculopontine nucleus fibre tract microstructural integrity was not different between the patient groups with and without freezing of gait within the current study, nor was it different between either the Parkinson’s disease group and age-matched control subjects. Although a variety of studies have identified white matter differences in patients with Parkinson’s disease compared with control subjects, there is limited overlap between these studies. We refer the reader to a recent meta-anlaysis by Cochrane and Ebmeier (2013) showing that reduction in fractional anistropy of the substantia nigra is repeatedly demonstrated in Parkinson’s disease, whereas results in other areas of the brain are not consistently demonstrated within this population. Our current results are further supported by a recent study reporting no white matter hyperintensity differences between patients with Parkinson’s disease classified as either (i) postural instability and gait difficulty sub-type; or (ii) tremor dominant sub-type (Herman et al., 2013). Furthermore, there was only a mild relationship between these hyperintensities and the postural instability and gait disorder symptoms. Thus, the authors note that while a strong association exists between white matter hyperintensities and gait, balance, mobility and cognitive deficits in healthy older adults, these relationships are not demonstrated among patients with Parkinson’s disease.
There are some limitations to the current study. Diffusion tensor imaging offers a quantitative method to reduce anatomical information to a tensor and then subsequently to a scalar value. Due to this fact, when differences are found using this metric, it is difficult to draw specific conclusions about the underlying causes at the cellular level (Tournier et al., 2011). For example, fractional anistropy can be modulated by changes in myelination, axon diameter and axon density (Chepuri et al., 2002). As described above, probabilistic tractography includes direct and indirect white matter connections, thus our data cannot be construed as direct connections from the pedunculopontine nucleus to the cortical targets observed.
It is also important to note that there were differences in disease severity between participants with and without freezing of gait. Although we attempted to match disease severity between groups, non-freezers with higher UPDRS scores usually had significant tremor and therefore had to be excluded for the MRI protocol used in the current study. However, we controlled for UPDRS in our statistical analyses to ensure that the observed differences were not solely due to disease severity. The higher motor UPDRS scores in the group with freezing of gait was related to worse postural responses in the Pull test, consistent with a difficulty coupling postural preparation with compensatory stepping, just as in voluntary turning (Jacobs et al., 2009). Furthermore, objective measures of gait reveal similar movement performance between all groups, in absentia of freezing episodes. Dopaminergic denervation is not distributed evenly in the striatum in Parkinson’s disease; it begins asymmetrically and then becomes bilateral later in the disease (Hornykiewicz, 1966). Though not statistically different, the group with freezing of gait did trend towards longer disease duration, therefore the asymmetric pedunculopontine nucleus tract volume noted in this group may be the result of an increased bilateral involvement with disease progression. On the other hand, while the differences in structural connectivity were observed solely in the right hemisphere of patients with freezing of gait, the symptom-dominant side was equally dispersed within both Parkinson’s disease groups, thus this is unlikely to affect the observed results.
In conclusion, the current study demonstrates differences in structural connectivity of the locomotor pathway in patients with Parkinson’s disease with freezing of gait, with reduced connectivity between the pedunculopontine nucleus and cerebellum, thalamus, and multiple regions of the frontal cortex, solely in the right hemisphere. We also report that the more left hemisphere-lateralized the pedunculopontine nucleus tract volume, the poorer the performance on tasks requiring the cognitive initiation of appropriate actions and/or the inhibition of inappropriate actions, specifically within patients with freezing of gait. In combination with recent work by Tessitore et al. (2012b) these results suggest that breakdowns in structural and functional connectivity of the right hemisphere’s locomotor pathway may contribute to freezing of gait.
Supplementary Material
Acknowledgements
We thank the patients and volunteers for participating in this study and the Parkinson’s Centre of Oregon for providing subjects. We are grateful to Patricia Carlson-Kuhta for administrative oversight and Mari Nomura, Michael Fleming, and Krystal Klein for assistance in data collection and analysis. We are also grateful to the Oregon Clinical and Translational Research Institute for providing facilities to conduct our studies. We would like to thank the anonymous reviewers for their constructive suggestions to improve this work, and neurologists B. Schoenberg, M.D., N. Giladi, M.D., and J. Nutt, M.D. (co-author) for their assistance in providing clinical ratings of freezing severity.
Glossary
Abbreviation
- UPDRS
Unified Parkinson’s Disease Rating Scale
Funding
This work was supported by a pilot award from the Pacific Northwest Udall Center (Cohen, PI) and a MERIT award from the National Institute on Aging (Horak, PI: R37 AG006457).
Supplementary material
Supplementary material is available at Brain online.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–71. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Amboni M, Barone P, Iuppariello L, Lista I, Tranfaglia R, Fasano A, et al. Gait patterns in Parkinsonian patients with or without mild cognitive impairment. Mov Disord. 2012;27:1536–43. doi: 10.1002/mds.25165. [DOI] [PubMed] [Google Scholar]
- Amboni M, Cozzolino A, Longo K, Picillo M, Barone P. Freezing of gait and executive functions in patients with Parkinson's disease. Mov Disord. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci. 2006;26:2424–33. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41:998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Balash Y, Gurevich T, Schaafsma JD, Hausdorff JM, Giladi N. Relationship between freezing of gait (FOG) and other features of Parkinson's FOG is not correlated with bradykinesia. J Clin Neurosci. 2003;10:584–8. doi: 10.1016/s0967-5868(03)00192-9. [DOI] [PubMed] [Google Scholar]
- Bartels AL, De Jong BM, Giladi N, Schaafsma JD, Maguire RP, Veenma L, et al. Striatal dopa and glucose metabolism in PD patients with freezing of gait. Mov Disord. 2006;21:1326–32. doi: 10.1002/mds.20952. [DOI] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord. 2008;23(Suppl 2):S461–7. doi: 10.1002/mds.21912. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser J, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:1–14. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Carpenter MB, Baton RR, Carleton SC, Keller JT. Interconnections and organization of pallidal and subthalamic nucleus neurons in the monkey. J Comp Neurol. 1981;197:579–603. doi: 10.1002/cne.901970404. [DOI] [PubMed] [Google Scholar]
- Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA. Diffusion anisotropy in the corpus callosum. Am J Neuroradiol. 2002;23:803–8. [PMC free article] [PubMed] [Google Scholar]
- Chivileva OG, Gorbachevskaya AI. Organization of efferent projections of the pedunculopontine nucleus of the tegmentum of the striatum in the dog brain. Neurosci Behav Physiol. 2005;35:881–5. doi: 10.1007/s11055-005-0139-5. [DOI] [PubMed] [Google Scholar]
- Cochrane CJ, Ebmeier KP. Diffusion tensor imaging in parkinsonian syndromes. Neurology. 2013;80:857–64. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Nomura M, Klein K, Fleming M, Mancini M, Nutt JG, et al. Inhibitory deficits are associated with freezing of gait in Parkinson’s disease. 2nd Joint World Congress of the International Society for Posture and Gait Research and Gait and Mental function. 2013 [Google Scholar]
- Coxon JP, Van Impe A, Wenderoth N, Swinnen SP. Aging and inhibitory control of action: cortico-subthalamic connection strength predicts stopping performance. J Neurosci. 2012;32:8401–12. doi: 10.1523/JNEUROSCI.6360-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Neuropsychiatric manifestations of right hemisphere lesions. Brain Lang. 1997;57:22–37. doi: 10.1006/brln.1997.1832. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–41. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010;30:6409–21. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen CW, Schultz DW. Information processing in visual search: a continuous flow conception and experimental results. Percept Psychophys. 1979;25:249–63. doi: 10.3758/bf03198804. [DOI] [PubMed] [Google Scholar]
- Ferraye MU, Debû B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedunculopontine nucleus area stimulation on gait disorders in Parkinson's disease. Brain. 2010;133:205–14. doi: 10.1093/brain/awp229. [DOI] [PubMed] [Google Scholar]
- Fling BW, Benson BL, Seidler RD. Transcallosal sensorimotor fiber tract structure-function relationships. Hum Brain Mapp. 2013;34:384–95. doi: 10.1002/hbm.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw. 2006;19:1120–36. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Giladi N. Freezing of gait. Clinical overview. Adv Neurol. 2001a;87:191–7. [PubMed] [Google Scholar]
- Giladi N. Gait disturbances in advanced stages of Parkinson's disease. Adv Neurol. 2001b;86:273–8. [PubMed] [Google Scholar]
- Giladi N, Hausdorff JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci. 2006;248:173–6. doi: 10.1016/j.jns.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Giladi N, Mcdermott MP, Fahn S, Przedborski S, Jankovic J, Stern M, et al. Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology. 2001;56:1712–21. doi: 10.1212/wnl.56.12.1712. [DOI] [PubMed] [Google Scholar]
- Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. 2008;23(Suppl 2):S423–5. doi: 10.1002/mds.21927. [DOI] [PubMed] [Google Scholar]
- Grunwerg BS, Krein H, Krauthamer GM. Somatosensory input and thalamic projection of pedunculopontine tegmental neurons. Neuroreport. 1992;3:673–5. doi: 10.1097/00001756-199208000-00004. [DOI] [PubMed] [Google Scholar]
- Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cereb Cortex. 2012;22:1564–76. doi: 10.1093/cercor/bhr226. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Wainer BH. Ascending projections from the pedunculopontine tegmental nucleus and the adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;274:483–515. doi: 10.1002/cne.902740403. [DOI] [PubMed] [Google Scholar]
- Hazrati LN, Parent A. Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res. 1992;585:267–71. doi: 10.1016/0006-8993(92)91216-2. [DOI] [PubMed] [Google Scholar]
- Helmich RC, De Lange FP, Bloem BR, Toni I. Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia. 2007;45:2201–15. doi: 10.1016/j.neuropsychologia.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Herman T, Rosenberg-Katz K, Jacob Y, Auriel E, Gurevich T, Giladi N, et al. White matter hyperintensities in Parkinson's disease: do they explain the disparity bwetween the postural instability gait difficulty and tremor dominant subtypes? PLoS One. 2013;8:e55193. doi: 10.1371/journal.pone.0055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends Cogn Sci. 2010;14:154–61. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966;18:925–64. [PubMed] [Google Scholar]
- Hylden JL, Hayashi H, Bennett GJ, Dubner R. Spinal lamina I neurons projecting to the parabrachial area of the cat midbrain. Brain Res. 1985;336:195–8. doi: 10.1016/0006-8993(85)90436-6. [DOI] [PubMed] [Google Scholar]
- Iseki K, Hanakawa T, Shinozaki J, Nankaku M, Fukuyama H. Neural mechanisms involved in mental imagery and observation of gait. Neuroimage. 2008;41:1021–31. doi: 10.1016/j.neuroimage.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–18. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Robertson LC. The two sides of perception. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007a;114:1339–48. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res. 2007b;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–41. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, Deutschländer A, Stephan T, Kalla R, Wiesmann M, Strupp M, et al. Imaging human supraspinal locomotor centers in brainstem and cerebellum. Neuroimage. 2008;39:786–92. doi: 10.1016/j.neuroimage.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Katzen HL, Levin BE, Weiner W. Side and type of motor symptom influence cognition in Parkinson's disease. Mov Disord. 2006;21:1947–53. doi: 10.1002/mds.21105. [DOI] [PubMed] [Google Scholar]
- Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–24. doi: 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- Kostic VS, Agosta F, Pievani M, Stefanova E, Jecmenica-Lukic M, Scarale A, et al. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78:409–16. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Lawrence DG. Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 1967;4:151–88. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- Lee AC, Harris JP, Atkinson EA, Fowler MS. Disruption of estimation of body-scaled aperture width in Hemiparkinson's disease. Neuropsychologia. 2001;39:1097–104. doi: 10.1016/s0028-3932(01)00032-x. [DOI] [PubMed] [Google Scholar]
- Macht M, Kaussner Y, Möller JC, Stiasny-Kolster K, Eggert KM, Krüger HP, et al. Predictors of freezing in Parkinson's disease: a survey of 6,620 patients. Mov Disord. 2007;22:953–6. doi: 10.1002/mds.21458. [DOI] [PubMed] [Google Scholar]
- Mancini M, Horak FB. The relevance of clinical balance assessment tools to differentiate balance deficits. Eur J Phys Rehabil Med. 2010;46:239–48. [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gonzalez C, Bolam JP, Mena-Segovia J. Topographical organization of the pedunculopontine nucleus. Front Neuroanat. 2011;5:22. doi: 10.3389/fnana.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Watanabe K, Ohye C. Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci Res. 1997;28:155–65. doi: 10.1016/s0168-0102(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane MS, Stephen JM, et al. Modeling conflict and error in the medial frontal cortex. Hum Brain Mapp. 2012;33:2843–55. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Geula C, Bothwell MA, Hersh LB. Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J Comp Neurol. 1989;283:611–33. doi: 10.1002/cne.902830414. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain funciton and the risk of falling. J Am Geriatr Soc. 2012;60:2127–36. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Mov Disord. 2007;22:2192–5. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- Muthusamy KA, Aravamuthan BR, Kringelbach ML, Jenkinson N, Voets NL, Johansen-Berg H, et al. Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg. 2007;107:814–20. doi: 10.3171/JNS-07/10/0814. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Shine JM, Lewis SJ. The specific contributions of set-shifting to freezing of gait in Parkinson's disease. Mov Disord. 2010;25:1000–4. doi: 10.1002/mds.23005. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal ‘hyperdirect' pathway. Neurosci Res. 2002;43:111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Snijders AH, Delval A, Weerdesteyn V, Duysens J, Overeem S, et al. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience. 2013;236:110–6. doi: 10.1016/j.neuroscience.2013.01.038. [DOI] [PubMed] [Google Scholar]
- Nauta H, Mehler WR. Projections of the lentiform nucleus in the monkey. Brain Res. 1966;1:3–42. doi: 10.1016/0006-8993(66)90103-x. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Giladi N. The challenge of evaluating freezing of gait in patients with Parkinson's disease. Br J Neurosurg. 2008;22(Suppl 1):S16–8. doi: 10.1080/02688690802448376. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particulary in the elderly. Neurology. 1993;43:268–79. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive dysfunction in Parkinson's disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–41. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng. 2010;18:303–10. doi: 10.1109/TNSRE.2010.2047606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol. 2003;10:391–8. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Schacher M, Haemmerle B, Woermann FG, Okujava M, Huber D, Grunwald T, et al. Amygdala fMRI lateralizes temporal lobe epilepsy. Neurology. 2006;66:81–7. doi: 10.1212/01.wnl.0000191303.91188.00. [DOI] [PubMed] [Google Scholar]
- Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ. Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait. Neuroreport. 2010;21:914–6. doi: 10.1097/WNR.0b013e32833ce5f1. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nat Rev Neurosci. 2006;7:160–6. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Jassen AK, Vita LA. Brainstem regions with neuronal activity patterns correlated with priming of locomotor stepping in the anesthetized rat. Neuroscience. 2000;99:77–91. doi: 10.1016/s0306-4522(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, et al. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Spann BM, Grofova I. Origin of ascending and spinal pathways from the nucleus tegmenti peduculopontinus in the rat. J Comp Neurol. 1989;283:13–27. doi: 10.1002/cne.902830103. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- Tan DM, McGinley JL, Danoudis ME, Iansek R, Morris ME. Freezing of gait and activity limitations in people with Parkinson's disease. Arch Phys Med Rehabil. 2011;92:1159–65. doi: 10.1016/j.apmr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, et al. Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. Am J Neuroradiol. 2012a;33:1804–9. doi: 10.3174/ajnr.A3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, et al. Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord. 2012b;18:781–7. doi: 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, et al. A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. 2012a;135:1446–54. doi: 10.1093/brain/aws039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain. 2012b;135:148–60. doi: 10.1093/brain/awr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson's disease. Ann Neurol. 1993;34:579–84. doi: 10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–56. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoorn A, Burger H, Leenders KL, De Jong BM. Handedness correlates with the dominant Parkinson side: a systematic review and meta-analysis. Mov Disord. 2012;27:206–10. doi: 10.1002/mds.24007. [DOI] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, et al. Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Front Hum Neurosci. 2012;6:356. doi: 10.3389/fnhum.2012.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Spildooren J, Vercruysse S, Nieuwboer A, et al. Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabil Neural Repair. 2011;25:765–73. doi: 10.1177/1545968311403493. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–37. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson's disease: effects of levodopa. Exp Neurol. 2010;225:202–9. doi: 10.1016/j.expneurol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, et al. The effect of speed-accuracy strategy on response interference control in Parkinson's disease. Neuropsychologia. 2009;47:1844–53. doi: 10.1016/j.neuropsychologia.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–42; quiz 472. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J, Cho JW, Lee WY, Kim GM, Kim ST, Kim HT. Diffusion tensor imaging of freezing of gait in patients with white matter changes. Mov Disord. 2012;27:760–4. doi: 10.1002/mds.24034. [DOI] [PubMed] [Google Scholar]
- Zandbelt BB, Vink M. On the role of the striatum in response inhibition. PLoS One. 2010;5:e13848. doi: 10.1371/journal.pone.0013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwergal A, La Fougère C, Lorenzl S, Rominger A, Xiong G, Deutschenbaur L, et al. Functional disturbance of the locomotor network in progressive supranuclear palsy. Neurology. 2013;80:634–41. doi: 10.1212/WNL.0b013e318281cc43. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2012;33:1073–84. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.