Abstract
Psychopathy is a personality disorder associated with a profound lack of empathy. Neuroscientists have associated empathy and its interindividual variation with how strongly participants activate brain regions involved in their own actions, emotions and sensations while viewing those of others. Here we compared brain activity of 18 psychopathic offenders with 26 control subjects while viewing video clips of emotional hand interactions and while experiencing similar interactions. Brain regions involved in experiencing these interactions were not spontaneously activated as strongly in the patient group while viewing the video clips. However, this group difference was markedly reduced when we specifically instructed participants to feel with the actors in the videos. Our results suggest that psychopathy is not a simple incapacity for vicarious activations but rather reduced spontaneous vicarious activations co-existing with relatively normal deliberate counterparts.
Keywords: vicarious responses, empathy, psychopathy, functional MRI
Introduction
Psychopathy is a personality disorder associated with a profound lack of empathy (Hare, 1991) and elevated reactive and instrumental aggression (Williamson et al., 1987; Serin, 1991; Cornell et al., 1996; Patrick and Zempolich, 1998; Blair, 2007). Dominant models of psychopathy focus on two processes. Some posit that deficits in instrumental learning are central to psychopathy, preventing individuals with psychopathy from associating their antisocial behaviour with the negative affect that should be triggered by the distress of their victims (Blair, 2007). Others posit that deficits in attention might be central, with individuals with psychopathy having relatively preserved deliberate ‘top-down’ attention, but abnormal automatic, ‘bottom-up’ attention (Moul et al., 2012). This imbalance would prevent subjects from adequately processing certain stimuli (e.g. distress cues) that are peripheral to their current goals (e.g. obtaining resources) (Patterson and Newman, 1993).
Here, we advocate considering a third, to date less explored process. Because vicariously experiencing (i.e. empathizing with) the negative emotional reactions of victims may normally inhibit aggression (Miller and Eisenberg, 1988), the increased instrumental aggression in psychopathy might be related to their reduced vicarious experience of the emotions of others (de Vignemont and Singer, 2006; Decety and Moriguchi, 2007).
Functional MRI has shown that witnessing the emotions of others triggers neural activations in brain regions (insula and cingulate cortex) normally associated with feeling similar emotions oneself (Bastiaansen et al., 2009; Lamm et al., 2011), and witnessing what others do and sense recruits one’s own motor and somatosensory cortices (Pineda, 2008; Keysers and Gazzola, 2009; Caspers et al., 2010; Keysers et al., 2010). The strength of these so called vicarious neural activations is predicted by interindividual differences in trait-empathy (Singer et al., 2004; Gazzola et al., 2006; Jabbi et al., 2007) and they are therefore thought to represent a neural marker for empathy (Singer et al., 2004; Iacoboni, 2006; Pineda, 2008; Bastiaansen et al., 2009; Keysers and Gazzola, 2009).
These neural markers for empathy have been investigated in several psychiatric disorders, autism in particular (Dapretto et al., 2006; Decety and Moriguchi, 2007; Dinstein et al., 2008; Minio-Paluello et al., 2009), but surprisingly, not directly in psychopathy. However, a number of studies provide indirect evidence that warrants investigating vicarious activations directly in psychopathy. These studies show that psychopathic individuals display reduced physiological (Aniskiewicz, 1979; Blair et al., 1997; Perkins et al., 2010), blood oxygen level-dependent (BOLD) signal (Müller et al., 2003; but see Harenski et al., 2010) and electroencephalography (Cheng et al., 2012) responses to social emotional stimuli; recognize emotions less accurately from voices or faces (Blair et al., 2002, 2004; Kosson et al., 2002; Hastings et al., 2008); are less likely to attribute guilt to the protagonist in stories (Blair et al., 1995), and have a weaker BOLD response in their premotor and primary somatosensory cortices while they observe faces in a gender discrimination task (Deeley et al., 2006; but see Dolan and Fullam, 2006). Reduced activations in insular, cingulate and secondary somatosensory cortices were also found during fear conditioning (Birbaumer et al., 2005), and in the cingulate cortex and inferior frontal gyrus while memorizing negative words (Kiehl et al., 2001), further pointing to functional deficits in brain regions that are known to be involved in vicarious responses. In addition, grey matter has also been found to be reduced in regions associated with vicarious responses (de Oliveira-Souza et al., 2008; but see Müller et al., 2008; Tiihonen et al., 2008; Yang et al., 2009; Schiffer et al., 2011). However, as these studies did not directly investigate whether regions involved in feeling one’s own emotions are recruited abnormally in psychopathic individuals while viewing the emotions of others, it is impossible to conclude from this body of research that vicarious activations are reduced in individuals with psychopathy.
To directly test this question, we developed short video clips of two hands interacting with each other in a loving, painful, neutral or rejecting way (Fig. 1A). Emotional interactions of hands involve goal-directed actions, sensations and emotions, and can thereby induce vicarious activations in motor, somatosensory and emotional systems at once (Bastiaansen et al., 2009; Keysers and Gazzola, 2009). Different emotions were tested because psychopathic individuals may not be equally impaired at perceiving all types of emotions (Blair et al., 2002; Kosson et al., 2002; Blair et al., 2004; Marsh et al., 2008). Our main hypothesis was that the psychopathic group would show less vicarious activity than controls for at least one stimulus condition. Because previous studies have shown that performance and brain activity in tasks explicitly requiring social cognition are relatively preserved in psychopathy (Blair et al., 1996; Richell et al., 2003; Dolan and Fullam, 2004; Sommer et al., 2010), whereas abnormalities in automatically orienting to salient information might generate the largest group differences in the absence of instruction to attend to specific, emotionally relevant aspects of the stimuli (Patterson and Newman, 1993; Newman et al., 2010; Moul et al., 2012), we further ventured to hypothesize that explicitly encouraging psychopathic individuals to empathize with the actors on the screen might reduce group differences in vicarious activations.
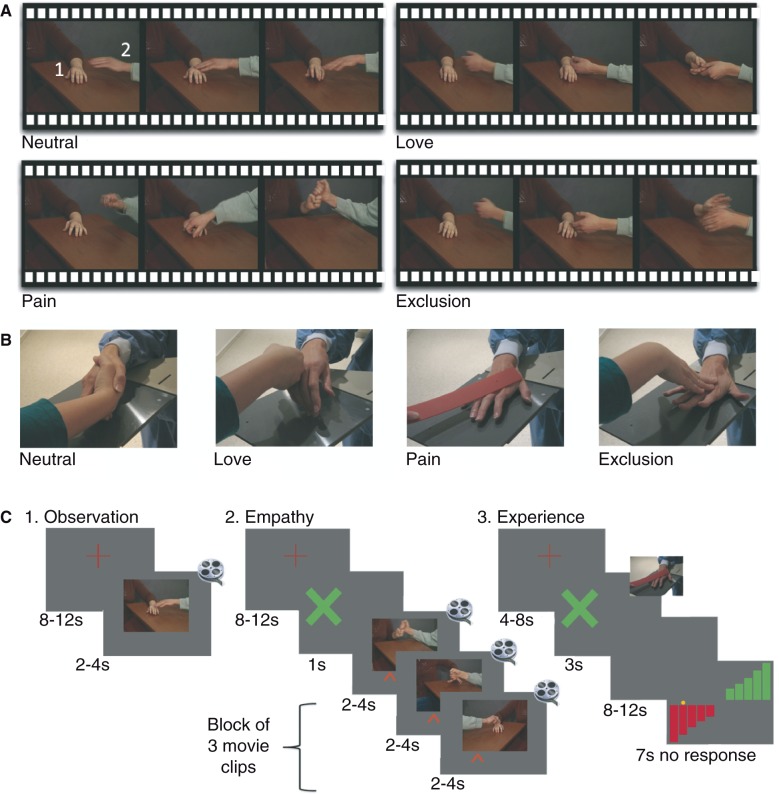
Figure 1.
Experimental paradigm. (A) Three still frames from example videos of each condition. Each video included a receiving (1) and an approaching (2) hand. (B) Photo of hand interactions during the experience. (C) Design of the three experiments (always performed in this order).
Materials and methods
Participants
Twenty male offenders diagnosed with psychopathy (Psychopathy Checklist-Revised ≥ 26) (Hare et al., 2001; Hare, 2003) from Dutch Forensic Psychiatric Clinics, and 26 control subjects (matched at group-level for age, gender, IQ, handedness), without history of neuropsychiatric disorders, participated in the study. Subjects (age 18–60 years) had no previous diagnoses of schizophrenia, other psychotic disorders or autistic spectrum disorders. It was stressed that participation would not influence treatment. Two patients were excluded from analyses, one for technical difficulties and one for drug abuse on the day of scanning. The Research and Ethics Committee of the University Medical Centre Groningen approved the study. All participants signed an informed consent and received €7.50/h for participation.
General assessment
All subjects completed questionnaires about health, age, handedness, education and functional MRI suitability. IQ was measured with the Raven’s Standard Progressive Matrices (RAVEN, Raven et al., 2003), and the abbreviated Groninger Intelligentie Test (GIT-2, Luteijn and Barelds, 2004). All subjects completed the Psychopathic Personality Inventory (Lilienfeld and Andrews, 1996), a 187-item self-report measure for criminal and non-criminal populations, the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 1999) and a list of their medication, which was checked against their official record for the offender group and reviewed for psychoactive properties by J.d.B. (psychiatrist).
Experimental paradigm
Subjects performed three functional MRI experiments.
Observation
Subjects watched videos (2, 3 or 4 s) of hand interactions (Fig. 1A) during which an approaching hand entered the screen to interact with an already present receiving hand. The conditions were love (hands caressing), pain (one hand hitting the other), social exclusion (one hand pushing away the other friendly hand), and neutral videos (approaching hand touching the other and getting a non-emotional response). A duplicate of every video was left-right mirrored (Adobe Premiere) to ensure that hands entered equally often from both sides. Subjects were instructed to watch the videos as if they were watching one of their favourite videos. They were told that a question would be asked later and that they could answer it if they had watched (not memorized) the videos carefully. Two event-related runs (∼8 min) consisted of 36 videos (nine instances of each condition), separated by a fixation cross for 8–12 s (Fig. 1C). Subjects’ left eye-gaze was recorded by an infrared camera (SMI, iView; 50 Hz).
Empathy
Due to time constrains, only the love and pain conditions were presented in a block design (three videos from the same condition per block; 12 love and 12 pain blocks; ∼9 min total; Fig. 1C). Subjects were instructed to feel with the receiving (50%) or the approaching (50%) hand, indicated by a 1 s green cross on the left or right side of the screen. During the videos, a left or right red arrow at the bottom served as a reminder.
Experience
Subjects participated in interactions similar to the ones presented during Observation. Subjects initiated the interaction after the appearance of a green cross (Fig. 1C). The author (H.M.) responded (as instructed by a head-phone) by caressing, hitting, shaking or pushing away the subjects’ hand. Subjects then rated the interaction with the unused hand operating a two-button response box on an 11-point scale (Fig. 1B). The 48 interactions (12 repetitions × four conditions) were split in two runs. Subjects used the left hand in one, the right in the other run (order counterbalanced across subjects). Before the Experience run, every interaction was practiced until smoothly executed.
After the functional MRI experiments, subjects rated all videos by means of an in-house questionnaire (Supplementary material).
Functional magnetic resonance imaging acquisition and analyses
Whole cerebrum activity was measured using a Philips Intera 3 T Quaser, eight-channel head coil, 32 axial gradient-echo slices, 3 mm thickness, no gap, single shot echo planar imaging, echo time: 30 ms, repetition time: 1.5 s. Slices were aligned to the anterior commissure–posterior commissure line. The first five scans were discarded (T1 equilibration). A T1-weighted image was also acquired (repetition time: 7.55 ms, echo time: 3.5 ms, flip angle, field of view: 224; 160; 256, matrix 256 × 229, voxel size: 1 × 1 × 1 mm). The total acquisition lasted ∼47 min.
Images were analysed with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5). For each subject, echo planar images were aligned to each other; the T1 co-registered to the mean echo planar image and normalization parameters (obtained from T1 segmentation) applied to all echo planar images (3 × 3 × 3 mm resolution), followed by smoothing (9 mm Gaussian kernel). Echo planar images were high-pass filtered at 300 s. A separate general linear model was used for each experiment.
At the subject level, observation was modelled with separate predictors for love, pain, exclusion, and neutral videos as boxcar functions convolved with the haemodynamic response function. The same was done for experience, with the addition of a fifth predictor modelling the rating epochs. For empathy, four separate regressors were used for empathizing with the receiving and approaching hand in the love and pain conditions.
Summary volumes per condition, subject and experiment were then brought to the population analysis level to assess whether the groups differed for at least one condition (Pain, Love, Neutral, Exclusion for Experience and Observation; Pain–Receive, Pain–Approach, Love–Receive, Love–Approach for Empathy). This was done in an ANOVA including two groups and four conditions, using the F contrast [1 0 0 0 −1 0 0 0; 0 1 0 0 0 −1 0 0; 0 0 1 0 0 0 −1 0; 0 0 0 1 0 0 0 −1] (first four columns represent conditions of the control group, last four those of the psychopathy group). We then explored the interaction Group × Condition (F-contrast: [1 −1 0 0 −1 1 0 0; 0 1 −1 0 0 −1 1 0; 0 0 1 −1 0 0 −1 1]). Given that this interaction was not significant in observation and empathy, we summed the four conditions to explore the main effect of group (F: [1 1 1 1 −1 −1 −1 −1]). We then performed T-tests to determine which group had stronger activations (T: [−1 −1 −1 −1 1 1 1 1] and the reverse contrast). ANOVAs in SPM use a general linear model approach, which pools the error variance, and are valid for inferences across groups with different n.
To assess whether the instructions to empathize significantly changed the group differences, we performed an interaction analysis between Group (psychopathic versus control participants) and Experiment (observation and empathy). Only pain and love were tested in both experiments. Given that we found no interactions between Group and Condition during observation or empathy, we averaged parameter estimates for pain and love videos for observation and Pain–Receive, Pain–Approach, Love–Receive and Love-Approach videos for empathy. This resulted in one summary parameter estimate y per subject i for observation (yi,obs) and one for ympathy (yi,emp). We then performed a full-factorial repeated-measures ANOVA with factors Group (patient, control) and Experiment (observation, empathy) on Y.
Reporting of effects
All analyses were reported using a false-discovery correction at qfdr < 0.05 to control the overall false positive rate. A minimum voxel-wise threshold was additionally imposed at P < 0.001 (uncorrected). This means that the more stringent the T/F corresponding to qfdr < 0.05 or P < 0.001 was used. Only clusters of >10 voxels are reported. For null-findings, we indicate qfdr > 0.05, and then the smallest T/F value at which there was no effect. All analyses were confined to the grey matter, as defined by averaging the normalized grey matter segment of all participants and thresholding at >0.3. The qfdr < 0.05 threshold was determined for a search volume including the entire grey matter irrespective of whether results are restricted to the region of interest or not, i.e. we did not use a small volume correction for the region of interest. There is only one exception, the interaction analysis between Group × Experiment, where a small volume correction was used to maximize sensitivity.
Region of interest
To explore if group differences were located in regions known to exhibit vicarious activations, we additionally inclusively masked the whole-brain results (thresholded at the whole-brain threshold determined above) with a region of interest defined as follows:
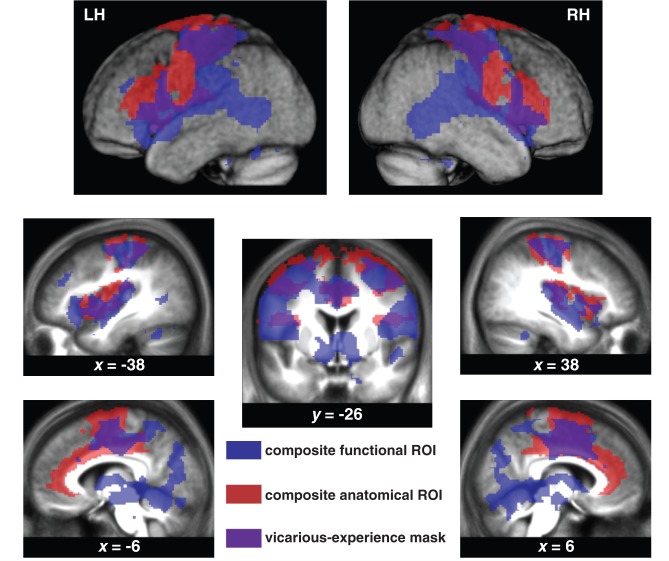
We selected studies and meta-analyses that included an experience and an observation condition of actions, sensations or emotions and selected the regions most consistently activated by experience and observation. Whenever possible we then used the Anatomy ToolBox (Eickhoff et al., 2007) or the AAL atlas of the WFU Pickatlas (Maldjian et al., 2003) for regions not yet included in the Anatomy ToolBox and then combined them into a single composite anatomical region of interest using a logical ‘OR’ (Fig. 2, blue transparent regions). These anatomical regions, defined using Anatomy ToolBox included the premotor cortices (Brodmann area 6 in Anatomy ToolBox) (Gazzola and Keysers, 2009; Caspers et al., 2010), the primary and secondary somatosensory cortices (SI = BA3a + b + BA1 + BA2 and SII = OP1 + OP2 + OP3 + OP4) (Keysers et al., 2010). Those defined using the AAL included the insula and the anterior/mid cingulate (Wicker et al., 2003; Singer et al., 2004, 2006; Jabbi et al., 2007; Bastiaansen et al., 2009; Lamm et al., 2011).
We used the results from our localizer experiment (i.e. experience) to select voxels that were significantly activated by at least one of our stimuli by either one of the groups (Fig. 2, red transparent regions). Eight one-sample random effect t-tests, comparing the four summary volumes (love, pain, neutral, exclusion) of the controls and those of the patients against zero (Punc < 0.001), were combined by a logical ‘OR’ (Marsbar; http://marsbar.sourceforge.net/) to generate a composite functional region of interest. We combined all hand interactions into a single region of interest, because empathic responses in the viewer are not always equal to the emotion in the actor; seeing pain can trigger tender feelings in addition to, or instead of, pain. Using a composite functional region of interest that included voxels triggered by any of the experiences (love, pain, exclusion or neutral), ensured an inclusive analysis that also captured empathic responses that are not equal to the viewed emotions: tender feelings triggered by a pain video would be captured by such a composite functional region of interest, because similar tender feelings will have been triggered by the love interactions also included in the region of interest definition. The same holds for pain triggered by the sight of exclusion etc.
A logical ‘AND’ was used to combine the composite functional and anatomical regions of interest (Fig. 2, purple transparent regions) leading to our final region of interest.
Figure 2.
Region of interest definition. A voxel was included in the region of interest (ROI, purple) if and only if it was significantly activated (Punc < 0.001 and qfdr < 0.05) during one of the interactions performed by subjects (controls or patients) during experience (blue) and if it belonged to anatomically defined brain regions the literature has associated with vicarious responses (red). LH = left hemisphere; RH = right hemisphere.
Results
Groups did not differ on age, IQ and handedness (Table 1). The patient group was significantly lower educated (Table 1 and Supplementary material). Mean Psychopathy Checklist-Revised score for the patient group was 32.3 (standard error 0.85; Supplementary Fig. 2). As expected, the control group scored lower (P < 0.05) on the Psychopathic Personality Inventory. Diagnoses of the included patients are summarized in Supplementary Table 1 (see Supplementary material for assessment details). None of the participants scored above the cut-off for autistic spectrum disorder on the Autism Diagnostic Observation Schedule. Two patients were taking psychoactive medication (citalopram 20 mg/day; amitriptyline 175 mg/day). None of the control subjects reported using psychoactive medication.
Table 1.
Assessment data
| Questionnaire | Mean control | Mean patient | P-value t-test |
|---|---|---|---|
| (n = 26) | (n = 18) | ||
| Age | 36.96 (1.62) | 39.17 (2.41) | P2-tailed = 0.434 |
| GIT2-IQ (Groninger Intelligentie Test) | 98.77 (2.79) | 93.65 (4.00) | P2-tailed = 0.283 |
| Raven-IQ (Raven’s Standard Progressive matrices) | 115.08 (2.05) | 106.62 (5.30) | P2-tailed = 0.101 |
| Handedness | 10.08 (0.38) | 9.67 (0.83) | P2-tailed = 0.623 |
| Years of education | 11.96 (0.34) | 7.39 (0.40) | P2-tailed < 0.001 |
| Education level | 4.88 (0.54) | 2.00 (0.47) | P2-tailed < 0.001 |
| PCL-R | n/a | 32.3 (0.85) | n/a |
| F1 | n/a | 13.5 (0.62) | n/a |
| F1 | n/a | 14.9 (0.67) | n/a |
| Psychopathic Personality Inventory Total | 398.92 (7.61) | 422.39 (9.48) | P1-tailed = 0.023 |
Numbers in brackets represent standard errors of the means. T-tests were used to compare the means of the groups; P-values are based on two-tailed testing except where directed hypotheses existed. Data for the Psychopathy Checklist-Revised (PCL-R) are not available for the control group. See Supplementary Fig. 1 for the histogram of the Psychopathy Checklist-Revised scores for the patient group and Supplementary Table 2 for a list of the clinical diagnosis for each patient.
Experience
We tested the null hypothesis that for each condition, the two groups had the same BOLD signal using an ANOVA. No significant group differences were detected [qfdr > 0.05; F(4,168) < 9.17]. Except for a (14-voxel) cluster in the pars orbitalis of the inferior frontal gyrus [MNI: −42, −18, 14; qfdr < 0.05; F(3,168) > 8.24], there was no significant Group × Experience interaction.
Observation
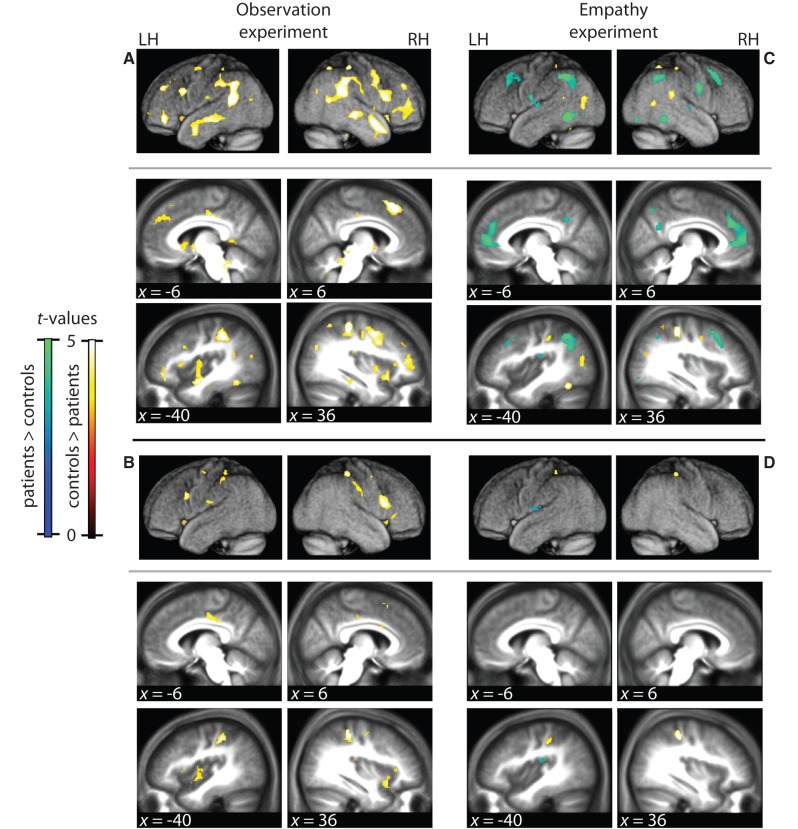
The ANOVA testing the null hypothesis that for each condition, the two groups had the same BOLD response revealed a large network of regions where this hypothesis was violated [Punc < 0.001, F(3,168) = 4.85; qfdr < 0.05]. A repeated measures ANOVA revealed no significant Group × Video type interaction [qfdr > 0.05, F(3,83,2) < 8.58]. We therefore summed the activations of all videos to examine the main effect of Group. This revealed an extended network of brain regions with BOLD signal differing between the groups [Punc < 0.001, F(1,168) = 11.22; qfdr < 0.05] (Fig. 3A and Supplementary Table 2). All of these regions showed higher BOLD for the control subjects. To explore if these group differences could include vicarious activations, we masked the analysis inclusively with our region of interest (i.e. removed all significant voxels outside of the region of interest), at the same statistical threshold as the whole brain analysis. Group differences during observation indeed include a number of regions within this region of interest [Punc < 0.001, F(1,168) = 11.22; qfdr < 0.05] (Fig. 3B and Table 2), including the dorsal and ventral premotor cortex, primary and secondary somatosensory cortices, the anterior insula and the cingulate cortex.
Figure 3.
Observation and empathy results. (A and B) Group differences during observation (Punc < 0.001,qfdr < 0.05) (Supplementary Table 3). (C and D) Group differences during empathy (Punc < 0.001, qfdr < 0.05) (Supplementary Table 4). (B and D) Same as A and C, but masked inclusively with the region of interest (Tables 2 and 3). Hot colours: BOLD response control > psychopaths; cold colours: psychopaths > controls. RH = right hemisphere; LH = left hemisphere; x = MNI coordinate of sagittal plane. Slices and renders are taken from the mean normalized anatomy of all subjects.
Table 2.
Group differences in observation within the region of interest
| Cluster size | T | MNI coordinates |
Hemi-sphere | Macro anatomical location of peak voxel | Overlap with cytoarchitectonic regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controls larger than patients | |||||||
| 173 | 6,26 | 36 | −33 | 54 | R | Postcentral gyrus | BA2, BA3ab |
| 4,25 | 51 | −24 | 42 | R | Supramarginal gyrus | ||
| 3,87 | 60 | −15 | 27 | R | Supramarginal gyrus | ||
| 242 | 6,20 | −33 | −36 | 45 | L | Postcentral gyrus | BA2, BA3ab |
| 4,68 | −9 | −18 | 42 | L | Midcingulate gyrus | ||
| 4,33 | −12 | −42 | 48 | L | Precuneus | ||
| 35 | 5,64 | 21 | −3 | 54 | R | Superior frontal gyrus | BA6 |
| 4,80 | 6 | 18 | 54 | R | Pre-SMA | ||
| 4,04 | 12 | 6 | 57 | R | Pre-SMA | ||
| 84 | 5,41 | 60 | 15 | 18 | R | Inferior frontal gyrus (p. oper.) | BA44, BA45 |
| 3,87 | 48 | 12 | 24 | R | Inferior frontal gyrus (p. oper.) | ||
| 85 | 5,15 | −36 | −6 | −12 | L | Insula | Insula (Id) |
| 3,89 | −36 | −3 | 12 | L | Insula | ||
| 72 | 5,08 | 27 | −12 | 48 | R | Precentral gyrus (dPM) | BA6 |
| 20 | 4,87 | −51 | 15 | 27 | L | Inferior frontal gyrus (p. oper.) | BA44 |
| 45 | 4,86 | 30 | 15 | −18 | R | Anterior insula | |
| 4,72 | 36 | 18 | −12 | R | Anterior insula | ||
| 67 | 4,80 | −27 | −21 | 51 | L | Precentral gyrus (dPM) | BA 6 |
| 4,24 | −27 | −9 | 54 | L | Precentral gyrus (dPM) | ||
| 4,06 | −33 | −6 | 60 | L | Precentral gyrus (dPM) | ||
| 72 | 4,38 | −33 | 15 | 3 | L | Anterior insula | |
| 4,07 | −27 | 21 | 6 | L | Anterior insula | ||
| 35 | 4,25 | 9 | −21 | 39 | R | Midcingulate gyrus | |
| 30 | 4,14 | 42 | −33 | 15 | R | Superior temporal gyrus | |
| 4,00 | 45 | −24 | 18 | R | Rolandic operculum | OP1, OP2 | |
| 49 | 4,07 | 45 | −3 | −9 | R | Superior temporal gyrus | |
| 3,99 | 42 | 3 | 0 | R | Middle insula | ||
| 14 | 4,03 | 54 | 30 | 0 | R | Inferior frontal gyrus (p. tri.) | BA45 |
| 27 | 3,86 | −54 | −15 | 21 | L | Postcentral gyrus | OP1, OP4 |
| 3,25 | −54 | −15 | 33 | L | Postcentral gyrus | BA3b | |
| 16 | 3,83 | 9 | 9 | 27 | R | Midcingulate gyrus | |
| 3,52 | 3 | 15 | 21 | R | Midcingulate gyrus | ||
| 14 | 3,82 | 36 | 30 | 6 | R | Anterior insula | |
Differences (Punc < 0.001 and qfdr < 0.05) in brain activity between the psychopathy and control group within the region of interest (see Supplementary Table 2 for the results outside the region of interest). No regions were more active for the psychopathy than the control group. The table specifies the size of each cluster, then, for its peak, the T-value, MNI coordinates, hemisphere and macro-anatomical location based on the groups’ mean anatomy. Finally, the table specifies cytoarchitectonic brain regions that the cluster overlaps with according to the Anatomy Toolbox.
BA = Brodmann area; SMA = supplementary motor area; dPM = dorsal premotor cortex; p.tri = pars triangularis; p.oper = pars opercularis.
Empathy
The null hypothesis that the two groups generated the same BOLD response for all four stimuli was rejected using an ANOVA [qfdr < 0.05, F(3,168) = 4.89]. There was no Group × Video type interaction [qfdr > 0.05, F(1,168) < 11.22, Punc > 0.001] and there was no Group × Empathized hand interaction [qfdr > 0.05, Punc > 0.1, F(1,168) < 2.74]. Accordingly, we examined the main effect of Group by summing over video type and empathized hand (Fig. 3C) [Punc < 0.001 at F(1,168) > 11.22; qfdr < 0.05]. Some areas were more activated for the control subjects, but fewer than during the observation experiment. Importantly, the instruction to feel with the hands in the videos led to a number of brain regions being more activated in the patients, including the medial and dorsolateral prefrontal cortex and the angular gyrus (Fig. 3C and Supplementary Table 3). Masking the analysis with the region of interest revealed very limited group differences in the region of interest; the control group only had higher BOLD in the primary somatosensory cortex; the patient group in the secondary somatosensory cortex (both Punc < 0.001, T = 3.14; qfdr < 0.05) (Fig. 3D and Table 3).
Table 3.
Group differences in empathy within the region of interest
| Cluster size | T | MNI coordinates |
Hemi-sphere | Macro anatomical location of peak voxel | Overlap with cytoarchitectonic regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Controls larger than patients | |||||||
| 53 | 6,64 | 36 | −33 | 51 | R | Postcentral gyrus | BA2, BA3 |
| 48 | 4,87 | −33 | −36 | 45 | L | Postcentral gyrus | BA2, BA3 |
| Patients larger than controls | |||||||
| 33 | 4,63 | 42 | −15 | 18 | R | Parietal operculum | OP1–4 |
| 48 | 3,89 | −57 | −12 | 9 | L | Parietal operculum | OP1–4 |
Table lists areas that were more activated (Punc < 0.001 and qfdr < 0.05) for the controls (positive effect of controls) and areas that were more activated for the patients (positive effect of patients) within the region of interest (see Supplementary Table 3 for differences between the two groups outside the region of interest). Conventions as in Table 2.
BA = Brodmann area.
Effect of conditions
In all experiments, comparing the different stimulus categories recruited distinct and meaningful neural circuitries (Supplementary material).
Interaction analysis between Group and Experiment
To test if group differences were significantly reduced by the instruction to empathize, we examined the interaction between Experiment (observation, empathy) and Group, considering only the conditions common to both experiments (i.e. pain and love). We found the group differences to be smaller in the empathy compared to the observation experiment in a number of brain regions (Punc < 0.001, T = 3.19, qfdr < 0.05 for the entire brain), including the medial and anterior left insula, left hippocampus, bilateral thalamus, left caudate, left anterior cingulate cortex, and bilateral angular gyrus (Table 4). No regions showed the opposite effect. Within the region of interest, the left anterior insula showed reduced group differences under the instruction to empathize (Table 4, asterisk, small volume correction). A similar analysis with z-transformed data led to identical results (Supplementary material). To graphically illustrate this interaction analysis containing pain and love only, we also calculated the group differences during observation, only considering pain and love trials (Supplementary Fig. 3), and plotted activation during empathy in regions showing group differences during observation (Supplementary Fig. 4). Results are very similar to those considering all conditions (see also Fig. 3A for the group differences using all four conditions).
Table 4.
Regions normalized after empathy instruction
| Cluster size | T | MNI coordinates |
Hemi-sphere | Macro anatomical location of peak voxel | Overlap with cytoarchitectonic regions | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| 59 | 5.69 | −21 | −33 | −3 | L | Thalamus, hippocampus | Hipp (FD, SUB, CA) |
| 30 | 5.19 | −42 | 42 | −12 | L | Inferior frontal gyrus (p. orb.) | |
| 59 | 4.91 | −48 | 0 | −27 | L | Middle temporal gyrus, temporal pole | |
| 137 | 4.53 | −48 | −54 | 39 | L | Inferior parietal lobe | hIP1, IPC (PGa, PF, PFm) |
| 11 | 4.49 | 36 | 39 | −12 | R | Inferior frontal gyrus, middle orbital gyrus | |
| 207 | 4.39 | 33 | 15 | 45 | R | Middle frontal gyrus | |
| 49 | 4.36 | 9 | 30 | 48 | R | Superior medial gyrus, anterior cingulate gyrus | |
| 49 | 4.33 | −9 | 39 | 33 | L | Superior medial gyrus, anterior cingulate gyrus | |
| 36 | 4.24 | −48 | 21 | 30 | L | Inferior frontal gyrus | BA44, BA45 |
| 12 | 4.16 | −21 | 57 | −3 | L | Superior orbital gyrus | |
| 31 | 4.04 | −30 | 24 | 45 | L | Medial frontal gyrus | |
| 63* | 3.99 | −36 | 3 | −6 | L | Insula lobe | |
| 45 | 3.99 | 45 | −42 | 24 | R | Inferior parietal cortex | IPC (PFm, PGa) |
| 16 | 3.87 | −21 | 36 | 33 | L | Superior frontal gyrus | |
| 13 | 3.87 | −15 | 15 | 0 | L | Caudate | |
| 13 | 3.83 | 21 | −33 | 3 | R | Hippocampus | Hipp (FD) |
| 12 | 3.48 | −33 | −15 | −24 | L | Hippocampus | Hipp (CA, SUB, EC) |
Analysis is based on raw values.
*The insula cluster, 31 voxels that are also significant when restricting the analysis to the region of interest (small volume correction, q < 0.05 for the entire region of interest).
Conventions as in Table 2. See Supplementary Table 4 for the results of the same analysis on z-transformed values.
BA = Brodmann area; Hipp = hippocampus; p. orbitalis = pars hIP1 = Cytoarchitectonic subdivisions of the intraparietal sulcus according to Choi et al., 2006; Scheperjans, 2008a,b; IPC = inferior parietal cortex; PGa., PF., PFm = Cytoarchitectonic subdivisions of the inferior parietal cortex according to Caspers et al., 2006, 2008; FD., SUB., CA., EC. = Cytoarchitectonic subdivisions of the hippocampus according to Amunts et al., 2005.
Additional analyses to exclude confounds
Eye track data indicated no significant differences in spatial eye gaze pattern or in time spent looking at the videos (Supplementary material); baseline activation did not significantly differ between the groups during observation, empathy or experience, nor in the interaction of Group and Experiment (observation versus empathy) (Supplementary material). Additionally, if empathy normalized after instructions to empathize, we would expect that both groups would report similar experiences while viewing the videos after the end of the experiment. A debriefing questionnaire confirmed this prediction (Supplementary material). Finally, group differences during observation could not be explained entirely by variance during experience (Supplementary material).
Discussion
In this study, we analysed vicarious activations in a group of incarcerated subjects with a diagnosis of psychopathy, by having them experience and observe emotional hand interactions, while being scanned. We compared these activations with those of age- and IQ-matched healthy controls. During observation, individuals with psychopathy showed, compared to controls, a wide network with reduced activations, including regions of the temporal, insular, parietal and frontal lobe. In addition to agnostically reporting all brain regions showing group differences, we aimed to critically examine if these group differences provide robust evidence for reduced vicarious activations. To do so, we used a dual, conservative approach, in which we excluded visually triggered group differences (i.e. during observation) as evidence of abnormal vicarious activation if they occurred in regions (i) that were not activated by the experience of hand interactions (i.e. during experience); and (ii) that were not consistently associated with vicarious activations in the literature. Doing so, we show that areas associated with vicarious activations to hand actions (premotor cortices), sensations (primary and secondary somatosensory cortices), and emotions (anterior cingulate and insula cortices) were indeed hypo-activated in the psychopathy compared to the control group during observation. However, group differences attributable to vicarious activations were significantly reduced when specifically instructing participants to empathize with the actors in the videos. Despite the fact that the different types of hand actions induced different brain activations during experience and observation, including the expected recruitment of dorsal anterior cingulate cortex for painful and the ventromedial prefrontal cortex for loving interactions (Supplementary material), none of the experiments indicated that the patients were more different from the control subjects for a particular type of hand action.
Our study has a number of limitations. First, because there was a security risk considered unacceptable associated with bringing non-psychopathic incarcerated offenders to our scanning facility, we lack such a control group. Secondary factors linked to criminal lifestyle (e.g. lower level of education, history of drug abuse and length of incarceration) could thus contribute to our group differences. Unfortunately, as these variables were highly correlated with the factor Group [e.g. r(Group, Substance abuse) = 0.86], disentangling their contribution through inclusion of nuisance covariates in our analyses is impossible (Miller and Chapman, 2001). Secondly, although we included one of the largest group of psychopathic offenders ever scanned at 3 T, it is difficult to ascertain that our findings generalize to all incarcerated psychopathic offenders, making a replication study in a different country particularly important. Third, incarcerated psychopaths are a subgroup of psychopaths. It is unclear whether those psychopaths that have never been incarcerated would show the same pattern of brain activity.
With these limitations in mind, our results shed new light on the neural basis of psychopathy in two ways. First, they point to reduced vicarious activity in regions involved in performing actions, feeling touch and experiencing emotions, that are considered functional markers of empathy, as a possible neural basis for the reduced empathy and antisocial behaviour, central to psychopathy (Hare et al., 2001; Hare, 2003; Blair et al., 2006; Hare and Neumann, 2009). For most of us, seeing someone get hurt triggers vicarious activity in pain areas. This vicarious pain gives us an ‘egoistic’ reason to refrain from antisocial behaviour; do not hurt others because it (vicariously) hurts you (Miller and Eisenberg, 1988). In psychopathy, reduced somatosensory, insula and anterior cingulate vicarious activations could disinhibit antisocial behaviour. Because we found these deficits to be independent of video type, they suggest reduced empathy even for the pleasure of others.
Second, our results show that explicit instructions to empathize significantly reduced the group differences within regions associated with vicarious activations. In particular, instructions led to a partial ‘normalization’ (i.e. significantly reduced quantitative group differences) of brain activity in the insula within our region of interest according to the Experiment × Group interaction. Because stimulation of the insula can inhibit aggression in monkeys (Caruana et al., 2011), this instruction-triggered increase of insular activations in psychopathy could have important violence-inhibiting behavioural effects. Future experiments should directly investigate the brain–behaviour link we propose, by creating conditions in which for instance psychopathic and control participants are given a choice to harm a confederate to gain money, while measuring the participants’ brain activity. Our data would predict that, particularly in participants with psychopathy, instructions to empathize with the confederate will increase vicarious pain activations in the insula and reduce the propensity to harm the confederate. The effect of instruction is also apparent in the brain activity in the psychopathy group outside the vicarious activation mask, where brain activity changed from never being more active compared to control subjects during the observation experiment (Table 2 and Supplementary Table 3, and even when taking only love and pain into account Supplementary Fig. 3), to being more active in half the voxels during the empathy experiment (Table 3 and Supplementary Table 4). Altogether, combining the findings of the observation and empathy experiment, our data suggest a profound reconceptualization of psychopathy more as a reduced propensity, rather than an incapacity, to generate vicarious activations. Indeed, in self-report measures of empathy, our psychopathy group reported normal levels of trait empathy (data not shown) and, after instructions to empathize, to have their own feelings affected as much by the different video types than our control group (Supplementary material), in line with our finding of relatively preserved capacity for empathy, at least under certain instructions.
What mechanisms might mediate this effect of instruction? The interaction analysis revealed that normalization of brain activity in the region of interest following instructions to empathize was accompanied by increases relative to control subjects in regions (outside the region of interest), associated with conscious mentalizing (including the medial prefrontal cortex and the temporal pole) (Amodio and Frith, 2006; Van Overwalle and Baetens, 2009), and deliberate reappraisal (dorsolateral prefrontal cortex and angular gyrus, Ochsner et al., 2004). A recent functional MRI study in healthy volunteers showed that the anterior insula could be activated through the abovementioned mentalizing and reappraisal circuits while participants deliberately try to imagine what a character in a written story would feel (Jabbi et al., 2008). Taken together, this suggests that under instructions to empathize, our psychopathic participants relied more than our control participants on deliberate mechanisms to boost the activation of their own actions, emotions and sensations. Accordingly, we do not find that the overall brain activity is ‘normal’ in psychopathic individuals while deliberately empathizing with others, but rather, that they manage to recruit vicarious activations to a similar level. In the context of many residual group differences outside the vicarious activation region of interest, future research will need to use other methods (e.g. physiological recordings—galvanic skin response, pupillary dilation, heart rate, respiration or blood pressure—or qualitative analyses of verbal reports) to further explore how the subjective experience of psychopathic individuals compares with that of controls during deliberate empathy. This is particularly true because many qualitatively different emotions trigger quantitatively similar, overlapping patterns of activation (Jabbi et al., 2007), and quantitatively similar activations in the two groups within the vicarious activation region of interest could thus coexist with qualitative differences in the actual emotions triggered.
So far, we focused on hypo-activations within our region of interest that indicate reduced vicarious activations during observation. However, in the main effect of Group during observation, we also measured reduced activations in the bilateral amygdala. Because the amygdala is central to learning (Blair, 2007) and attention (Patterson and Newman, 1993; Moul et al., 2012) centred theories of psychopathy, this reduced amygdala activation invites us to consider how our findings relate to these dominant theories of psychopathy. Our hypo-vicarious view of psychopathy integrates well with models of moral development emphasizing deficits in learning, where the amygdala of children normally helps them associate their instrumental aggressive behaviour, which is originally neutral, with negative emotions triggered by the distress of their victims (Blair, 2007). This is because our reduced spontaneous vicarious activations (within our region of interest) could lead to reduced personal distress while harming others and our reduced bilateral amygdala activity (outside the region of interest) may then lead to reduced associations between aggression and the (reduced) personal distress in psychopathic individuals. Our hypo-vicarious view of psychopathy, however, also overlaps with attention-based theories. As the amygdala is increasingly conceived as an automatic detector of salient features in the environment (Adolphs, 2010), our finding that deliberate attention can reduce vicarious activation deficits in psychopathy dovetails with theories of psychopathy emphasizing abnormal amygdale-based attention (Moul et al., 2012), by suggesting that reduced empathy is a downstream result of impaired stimulus driven, bottom-up attention to the emotions of others, which can under certain conditions (here through instructions) be compensated by deliberate, bottom-up attention. Hence, our work on vicarious activations is no alternative to work on instrumental learning or attention. Instead it complements these efforts by adding a third protagonist, vicarious activations, to a complex scenario in which abnormal bottom-up attention might fail to trigger normal empathy, which in turn prevents the instrumental learning so central to moral development. In this scenario, deliberate, top-down modulation of attention may restore vicarious activations. Understanding how the amygdala mechanistically interacts with regions involved in vicarious activations to direct this scenario remains to be understood.
In many circumstances, empathy can be counterproductive: if you want to take the Rolex off an old man’s arm, empathizing with the man will not serve your greed. Only if empathy is a spontaneous response, will you feel empathy in this circumstance, and reduce your violence (Miller and Eisenberg, 1988). Accordingly, the lack of spontaneous vicarious activation we measured in our observation experiment will have severe violence-disinhibiting consequences even in the face of a preserved deliberate empathy. For therapists requesting a scientific understanding of psychopathy to tailor their therapies (Salekin et al., 2010), our finding that instructions to empathize can reduce group differences indicates that therapeutic efforts may actually not need to create a capacity for empathy, for such a capacity may already exist (at least in our sample of incarcerated psychopathic offenders). Instead, therapies may need to focus on making the existing capacity more automatic, so that it will come into play even when inhibiting goal-directed behaviour. Such therapies could harvest the patients’ potential to normalize vicarious activations through deliberately focusing attention on empathizing with others. The fact that psychopathic individuals often display low motivation for change and comply less with therapy than other criminals (Salekin et al., 2010) will certainly represent an unfortunate challenge in such efforts.
Supplementary Material
Acknowledgements
We thank A.J. Sibeijn-Kuiper for her help in setting up the specific safety protocol and scanning the subjects, R. Renken for his help with data analysis, S. Hwang for proofreading the manuscript. We also thank the participants for their cooperation.
Glossary
Abbreviation
- BOLD
blood oxygen level-dependent
Funding
The work was supported by the Dutch Science Foundation (NWO) [grant number 452-04-305, 451-09-006]. The Forensic Psychiatric Clinic Dr. S van Mesdag paid for transport and security costs and partially funded H.M. None of the authors have conflicts of interest regarding this work.
Supplementary material
Supplementary material is available at Brain online.
References
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Aniskiewicz AS. Autonomic components of vicarious conditioning and psychopathy. J Clin Psychol. 1979;35:60–7. doi: 10.1002/1097-4679(197901)35:1<60::aid-jclp2270350106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philos Trans R Soc London B Biol Sci. 2009;364:2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, Erb M, Hermann C, Grodd W, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair J, Sellars C, Strickland I, Clark F, Williams A, Smith M, et al. Theory of Mind in the psychopath. J Forens Psychiatry Psychol. 1996;7:15–25. [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11:387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–8. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DGV, Peschardt KS, Colledge E, Leonard RA, Shine JH, et al. Reduced sensitivity to others' fearful expressions in psychopathic individuals. Pers Individ Diff. 2004;37:1111–22. [Google Scholar]
- Blair RJ, Mitchell DGV, Richell RA, Kelly S, Leonard A, Newman C, et al. Turning a deaf ear to fear: Impaired recognition of vocal affect in psychopathic individuals. J Abnorm Psychol. 2002;111:682–6. doi: 10.1037//0021-843x.111.4.682. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DGV, Pine DS. The development of psychopathy. J Child Psychol Psychiatry. 2006;47:262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Sellars C, Strickland I, Clark F, Williams AO, Smith M, et al. Emotion attributions in the psychopath. Pers and Individ Diff. 1995;19:431–7. [Google Scholar]
- Caruana F, Jezzini A, Sbriscia-Fioretti B, Rizzolatti G, Gallese V. Emotional and social behaviors elicited by electrical stimulation of the insula in the macaque monkey. Curr Biol. 2011;21:195–9. doi: 10.1016/j.cub.2010.12.042. [DOI] [PubMed] [Google Scholar]
- Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, Zilles K, et al. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–95. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–48. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev Psychopathol. 2012;24:623–36. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Choi H-J, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, et al. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell DG, Warren J, Hawk G, Stafford E, Oram G, Pine D. Psychopathy in instrumental and reactive violent offenders. J Consult Clin Psychol. 1996;64:783–90. doi: 10.1037//0022-006x.64.4.783. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Azevedo Ignácio F, Tovar-Moll F, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–13. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Decety J, Moriguchi Y. The empathic brain and its dysfunction in psychiatric populations: implications for intervention across different clinical conditions. BioPsychoSocial Medicine. 2007;1:22. doi: 10.1186/1751-0759-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, et al. Facial emotion processing in criminal psychopathy: Preliminary functional magnetic resonance imaging study. Br J Psychiatry. 2006;189:533–9. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Curr Biol. 2008;18:R13–8. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Theory of mind and mentalizing ability in antisocial personality disorders with and without psychopathy. Psychol Med. 2004;34:1093–102. doi: 10.1017/s0033291704002028. [DOI] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychol Med. 2006;36:1563–9. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Curr Biol. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–55. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare psychopathy checklist-revised. Toronto: Multi-Health Systems; 1991. [Google Scholar]
- Hare RD. Manual for the Revised Psychopathy Checklist. 2nd edn. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Hare RD, Neumann CS. Psychopathy: Assessment and forensic implications. Can J Psychiatry. 2009;54:791–802. doi: 10.1177/070674370905401202. [DOI] [PubMed] [Google Scholar]
- Hare RD, Vertommen H, Brink WVD, Ruiter CD. De Psychopathie Checklist revised. Handleiding. Lisse: Swets & Zeitlinger; 2001. [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol. 2010;119:863–74. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings ME, Tangney JP, Stuewig J. Psychopathy and identification of facial expressions of emotion. Pers Individ Diff. 2008;44:1474–83. doi: 10.1016/j.paid.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M. Failure to deactivate in autism: the co-constitution of self and other. Trends Cogn Sci. 2006;10:431–3. doi: 10.1016/j.tics.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen JA, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3:8. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Cur Opin Neurobiol. 2009;19:666–71. doi: 10.1016/j.conb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol Psychiatry. 2001;50:677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Kosson DS, Suchy Y, Mayer AR, Libby J. Facial affect recognition in criminal psychopaths. Emotion. 2002;2:398–411. doi: 10.1037/1528-3542.2.4.398. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal population. J Pers Assessment. 1996;66:488. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule. Manual. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Luteijn F, Barelds DPF. GIT 2: Groninger intelligentie test 2. Harcourt; 2004. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165:712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110:40–8. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller PA, Eisenberg N. The relation of empathy to aggressive and externalizing/antisocial behavior. Psychol Bull. 1988;103:324–44. doi: 10.1037/0033-2909.103.3.324. [DOI] [PubMed] [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in asperger syndrome. Biol Psychiatry. 2009;65:55–62. doi: 10.1016/j.biopsych.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Moul C, Killcross S, Dadds MR. A model of differential amygdala activation in psychopathy. Psychol Rev. 2012;119:789–806. doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Müller JL, Gänssbauer S, Sommer M, Döhnel K, Weber T, Schmidt-Wilcke T, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Res. 2008;163:213–22. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Röder CH, et al. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: Evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biol Psychiatry. 2003;54:152–62. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry. 2010;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Zempolich KA. Emotion and aggression in the psychopathic personality. Aggression Violent Behav. 1998;3:303–38. [Google Scholar]
- Patterson CM, Newman JP. Reflectivity and learning from aversive events: Toward a psychological mechanism for the syndromes of disinhibition. Psychol Rev. 1993;100:716–36. doi: 10.1037/0033-295x.100.4.716. [DOI] [PubMed] [Google Scholar]
- Perkins T, Stokes M, McGillivray J, Bittar R. Mirror neuron dysfunction in autism spectrum disorders. J Clin Neurosci. 2010;17:1239–43. doi: 10.1016/j.jocn.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Pineda JA. Sensorimotor cortex as a critical component of an ‘extended' mirror neuron system: does it solve the development, correspondence, and control problems in mirroring? Behav and Brain Funct. 2008;4:47. doi: 10.1186/1744-9081-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court JH. Manual for Raven's Progressive Matrices and Vocabulary Scales. Section 1: General Overview. San Antonio: TX: Harcourt Assessment; 2003. [Google Scholar]
- Richell RA, Mitchell DGV, Newman C, Leonard A, Baron-Cohen S, Blair RJR. Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes'? Neuropsychologia. 2003;41:523–6. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- Salekin RT, Worley C, Grimes RD. Treatment of psychopathy: a review and brief introduction to the mental model approach for psychopathy. Behav Sci Law. 2010;28:235–66. doi: 10.1002/bsl.928. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, et al. Probabilistic Maps, Morphometry, and Variability of Cytoarchitectonic Areas in the Human Superior Parietal Cortex. Cereb Cortex. 2008b;18:2141–57. doi: 10.1093/cercor/bhm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K, et al. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 2008a;18:846–67. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Müller BW, Scherbaum N, Hodgins S, Forsting M, Wiltfang J, et al. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry. 2011;68:1039–49. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- Serin RC. Psychopathy and violence in criminals. J Interpers Violence. 1991;6:423–31. [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Sodian B, Döhnel K, Schwerdtner J, Meinhardt J, Hajak G. In psychopathic patients emotion attribution modulates activity in outcome-related brain areas. Psychiatry Res Neuroimag. 2010;182:88–95. doi: 10.1016/j.pscychresns.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, Hodgins S, Testa C, Perez J, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res. 2008;163:201–12. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williamson S, Hare RD, Wong S. Violence—Criminal Psychopaths and Their Victims. Can J Behav Sci. 1987;19:454–62. [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–2. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.