Abstract
The pace of nigrostriatal degeneration, both with regards to striatal denervation and loss of melanin and tyrosine hydroxylase-positive neurons, is poorly understood especially early in the Parkinson’s disease process. This study investigated the extent of nigrostriatal degeneration in patients with Parkinson’s disease at different disease durations from time of diagnosis. Brains of patients with Parkinson’s disease (n = 28) with post-diagnostic intervals of 1–27 years and normal elderly control subjects (n = 9) were examined. Sections of the post-commissural putamen and substantia nigra pars compacta were processed for tyrosine hydroxylase and dopamine transporter immunohistochemistry. The post-commissural putamen was selected due to tissue availability and the fact that dopamine loss in this region is associated with motor disability in Parkinson’s disease. Quantitative assessments of putaminal dopaminergic fibre density and stereological estimates of the number of melanin-containing and tyrosine hydroxylase-immunoreactive neurons in the substantia nigra pars compacta (both in total and in subregions) were performed by blinded investigators in cases where suitable material was available (n = 17). Dopaminergic markers in the dorsal putamen showed a modest loss at 1 year after diagnosis in the single case available for study. There was variable (moderate to marked) loss, at 3 years. At 4 years post-diagnosis and thereafter, there was virtually complete loss of staining in the dorsal putamen with only an occasional abnormal dopaminergic fibre detected. In the substantia nigra pars compacta, there was a 50–90% loss of tyrosine hydroxylase-positive neurons from the earliest time points studied with only marginal additional loss thereafter. There was only a ∼10% loss of melanized neurons in the one case evaluated 1 year post-diagnosis, and variable (30 to 60%) loss during the first several years post-diagnosis with more gradual and subtle loss in the second decade. At all time points, there were more melanin-containing than tyrosine hydroxylase-positive cells. Loss of dopaminergic markers in the dorsal putamen occurs rapidly and is virtually complete by 4 years post-diagnosis. Loss of melanized nigral neurons lags behind the loss of dopamine markers. These findings have important implications for understanding the nature of Parkinson’s disease neurodegeneration and for studies of putative neuroprotective/restorative therapies.
Keywords: Parkinsons disease, human brain, morphometry, substantia nigra, neuroscience
Introduction
Parkinson’s disease is a synucleinopathy characterized by degeneration of the dopaminergic nigrostriatal system, resulting in the classic motor features of the disease (Hughes et al., 2002). In advanced Parkinson’s disease, it is well known that neuronal loss in the substantia nigra pars compacta (SNc) is severe and most pronounced in the caudal and ventrolateral tier (Jellinger, 2012). Information regarding the magnitude and rate of nigral degeneration at different stages of the illness, particularly in the first few years after diagnosis, is limited, even though this information is crucial for understanding the natural history of Parkinson’s disease and for planning clinical trials with putative neuroprotective/restorative agents (Ehringer and Hornykiewicz, 1960; Rajput et al., 2008). Although striatal dopamine levels are reported to be reduced by 44–98% in advanced Parkinson’s disease (Ehringer and Hornykiewicz, 1960; Bernheimer et al., 1973; Rajput et al., 2008) and ∼50% of tyrosine hydroxylase and vesicular monoamine transporter 2 (VMAT2) staining is lost in subjects with incidental Lewy bodies (Beach et al., 2008a; DelleDonne et al., 2008; Dickson et al., 2008), there are no studies to our knowledge that sequentially examined the extent of dopaminergic terminal degeneration at different disease time points in Parkinson’s disease, and little information exists regarding the status of dopamine SNc neurons throughout the full course of the disease. The present study attempts to fill these important information gaps.
Materials and methods
Twenty-eight Parkinson’s disease brains were examined. Twenty two cases (1–27 years post-diagnosis) were obtained from the Arizona Parkinson Disease Consortium/Banner Sun Health Research Institute Brain and Body Donation Program (Arizona cohort) (Beach et al., 2008b) and six cases (3–5 years post-diagnosis) from the Sydney Brain Bank (Australia cohort). In each case, the diagnosis of Parkinson’s disease was made by a movement disorder specialist based on UK brain bank criteria (Lang and Lozano, 1998) and confirmed by a neuropathologist based on the loss of nigral neurons with free neuromelanin, and the presence of alpha synuclein positive Lewy bodies. Age-matched control brains (n = 9) with no clinical or pathological evidence of Parkinson’s disease were obtained from the Rush University Brain Bank (Rush cohort). Brains were fixed in 4% paraformaldehyde (Arizona and Rush cohort) or 10% formaldehyde (Australia cohort), sectioned at 40-μm thickness, and stored in cryoprotectant. Based on the material available and how tissues were fixed and stored, histological investigations were limited to the post-commissural putamen and the SNc, and quantitative assessments were performed on 17 brains from the Arizona cohort.
Immunohistochemistry
Sections containing the putamen and SNc were immunostained for tyrosine hydroxylase or dopamine transporter as previously described (Kordower et al., 2008). In these sections melanin was readily discerned and not obscured by the tyrosine hydroxylase-immunoreactivity. Control sections were processed in the same manner except that an irrelevant IgG was substituted for the primary antibodies. Staining for all cases in each of the cohorts was performed simultaneously. Because the Australian cases had been in fixative for a relatively long period of time, antigen retrieval procedures were performed as previously described (Chu et al., 2002) thereby preventing quantitative assessments.
Quantification of putamenal dopamine fibre density
Quantification of dopaminergic innervation of the post-commissural putamen using optical densities was performed in 17 patients in the Arizona cohort for whom adequate tissue was available (Table 1). All assessments were performed by an investigator blinded to diagnosis and disease duration using a densitometry software program and standardized procedures (Mufson et al., 1995).
Table 1.
Characteristics of patients with Parkinson’s disease
| Cases | Arizona cohort | Age (years) | Post-mortem interval (hours.min) | Disease duration |
|---|---|---|---|---|
| 1 | 98-15 | 88 | NA | 1 |
| 2 | 96-36* | 88 | NA | 3 |
| 3 | 03-45 | 88 | 1.83 | 4 |
| 4 | 95-19* | 84 | NA | 4 |
| 5 | 99-08 | 64 | 3.75 | 5 |
| 6 | 93-19* | 80 | NA | 5 |
| 7 | 04-01^ | 89 | 6.5 | 7 |
| 8 | 98-38* | 64 | 1 | 11 |
| 9 | 99-26 | 79 | 6 | 11 |
| 10 | 07-40 | 69 | 4.16 | 11 |
| 11 | 01-39 | 85 | 2 | 13 |
| 12 | 04-10^ | 77 | 1.66 | 14 |
| 13 | 04-27 | 79 | 3.5 | 14 |
| 14 | 01-42 | 85 | 4 | 15 |
| 15 | 07-01* ^ | 63 | 18.5 | 15 |
| 16 | 03-20^ | 81 | 4 | 18 |
| 17 | 05-26 | 73 | 7.16 | 18 |
| 18 | 06-62 | 82 | 4.16 | 19 |
| 19 | 99-17^ | 83 | 12 | 21 |
| 20 | 02-18 | 74 | 4 | 21 |
| 21 | 02-17 | 82 | 3 | 22 |
| 22 | 98-03 | 72 | 3 | 27 |
| Mean | 78.59 | 5.01 | ||
| SD | 8.15 | 4.21 | ||
| Cases | Australia cohort | Age (years) | Post-mortem interval (hours.min) | Disease duration |
| 1 | Case#1 | 67 | 8 | 3 |
| 2 | Case#2 | 86 | 13 | 3 |
| 3 | Case#3 | 80 | 12 | 3 |
| 4 | Case#4 | 84 | 4.5 | 4 |
| 5 | Case#5 | 43 | 36 | 4 |
| 6 | Case#6 | 53 | 5 | 5 |
| Mean | 68.83 | 13.08 | ||
| SD | 17.72 | 11.76 | ||
| Cases | Control cohort | Age (years) | Post-mortem interval (hours.min) | Disease duration |
| 1 | B98-57 | 91 | 4 | |
| 2 | B98-115 | 81 | 4 | |
| 3 | B97-39 | 83 | 5.5 | |
| 4 | B98-54 | 91 | 10.7 | |
| 5 | B00-76 | 71 | 4.1 | |
| 6 | B96-50 | 88 | 7 | |
| 7 | B96-98 | 72 | 7.1 | |
| 8 | B98-15 | 81 | 6.1 | |
| 9 | B01-39 | 89 | 4.2 | |
| Mean | 83 | 5.86 | ||
| SD | 8 | 2.21 | ||
Demographic data with age, disease duration and autopsy interval time for all Parkinson’s disease cases as well as control subjects.
*Cases where SNc dopaminergic cell count analysis could not be performed (n = 5).
^Cases from the Arizona cohorts where tyrosine hydroxylase and DAT optical density analysis was not performed as sufficient tissue was not available (n = 5).
Stereological estimates of tyrosine hydroxylase-immunoreactivity and melanin containing nigral neurons
Stereological estimates of tyrosine hydroxylase-immunoreactivity (blue–black reaction product) and melanin-containing neurons (brown melanin granules) in the SNc were performed unilaterally in 17 cases from the Arizona cohort for which sufficient tissue was available. The side chosen was ipsilateral to the putamenal sections evaluated. Estimates were made for the SNc in total and for each subregion as defined by Fearnley and Lees (1991). All quantitative studies were performed by a single blinded investigator using Stereo-Investigator (MicroBrightField, MBFBioscience) using standard optical fractionator principles (West, 1993). Numerical density was measured by the Stereo Investigator software and coefficients of error were calculated (Gundersen and Jensen, 1987). Quantitative analyses were not performed for the Australian cohort as samples were not suitably prepared for stereological analysis.
Results
Patient demographics and post-mortem intervals are provided in Table 1. The number of specimens at different time points after diagnosis were: 1 year: n = 1; 3 years: n = 4; 4 years: n = 4; 5 years: n = 3; 6–11 years: n = 4; 12–16 years: n = 5; 17–21 years: n = 5; ≥ 22 years: n = 2.
Post-commissural putamen: tyrosine hydroxylase and dopamine transporter immunostaining as a function of time from diagnosis
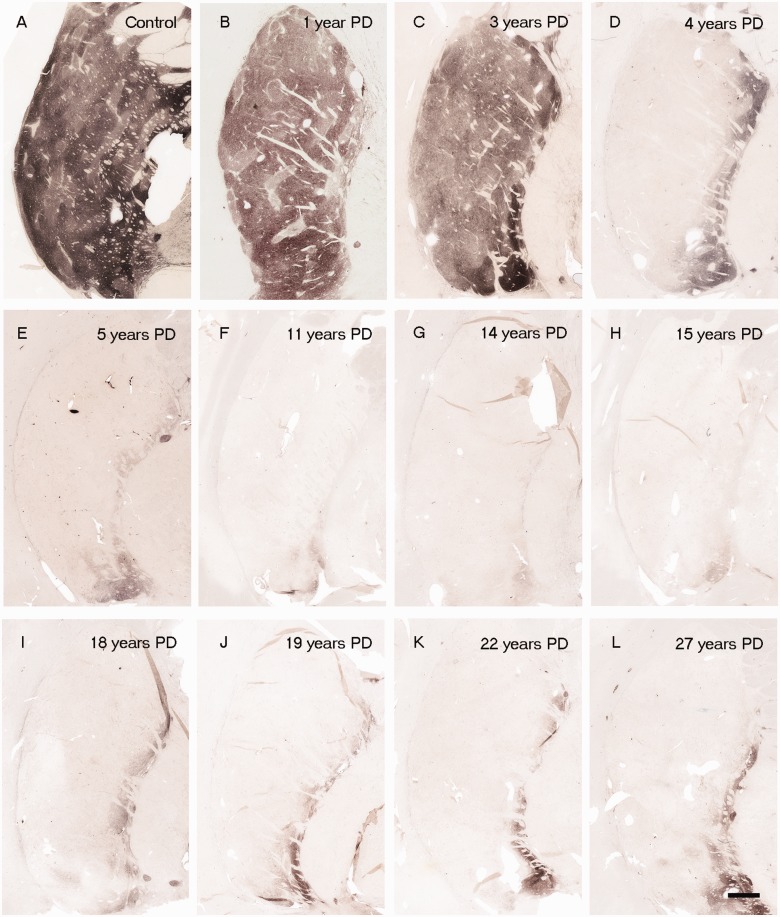
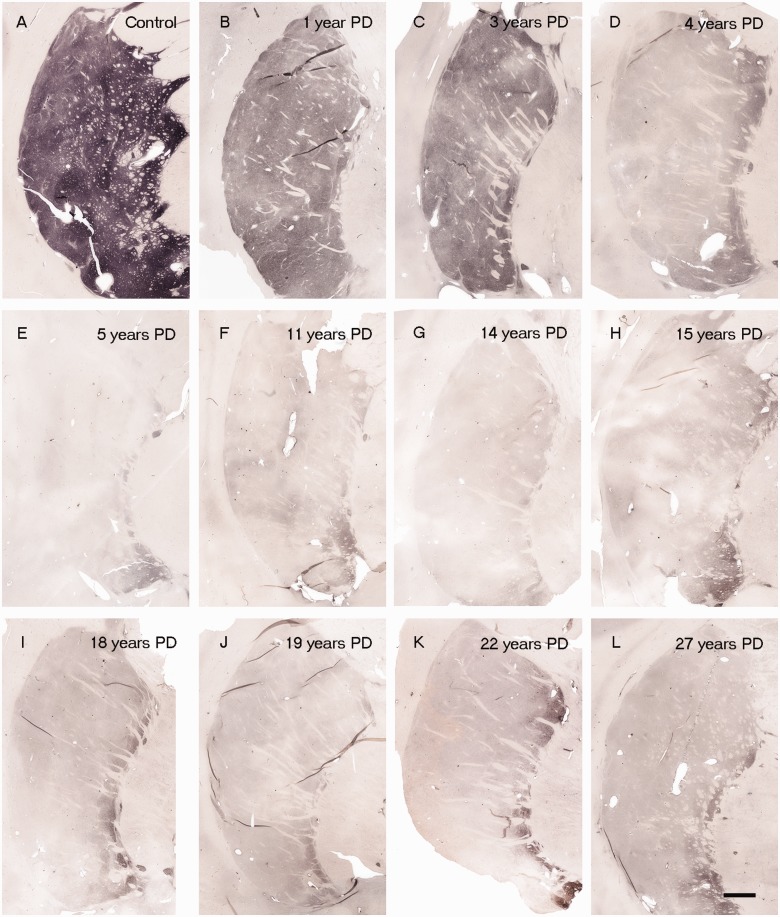
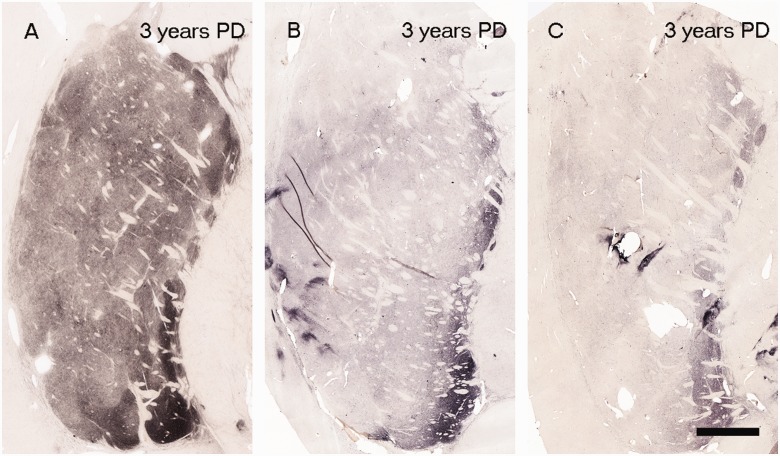
In each age-matched control, there was robust tyrosine hydroxylase and dopamine transporter fibre and terminal network staining throughout the dorsal and ventral putamen (Figs 1A and 2A). In contrast, there was a clear diminution in tyrosine hydroxylase and dopamine transporter staining in all patients with Parkinson’s disease (Figs 1 and 2). In the single case with disease duration of 1 year, there was only a mild reduction in tyrosine hydroxylase and dopamine transporter staining. In the four cases with disease duration of 3 years, reductions in staining were variable ranging from moderate to marked (Fig. 3). By 4 years following diagnosis and thereafter, minimal, if any, tyrosine hydroxylase or dopamine transporter staining remained throughout the dorsal and mid-to-lateral aspects of the putamen (Figs 1D–L and 2D–L). In all cases, a relatively high density band of tyrosine hydroxylase and dopamine transporter stained fibres was observed in the ventral putamen, which coursed dorsally across the medial aspect of the putamen likely en route to the caudate nucleus. This fibre pathway gradually diminished in size with increasing disease duration becoming a thin band by Year 12, but was still present through to Year 27 (Figs 1 and 2). Under high power, morphologically intact dopaminergic fibres were seen in the dorsal portion of the striatum in Parkinson’s disease cases with disease duration of 3 years or less (Fig. 4). By 4 years, only a few remaining tyrosine hydroxylase or dopamine transporter fibres could be detected in the dorsal putamen, and morphologically they had thick, enlarged, swollen varicosities suggesting ongoing degeneration (Fig. 4 insets). The comprehensive loss of dopamine markers in the dorsal putamen by Years 4–5 was observed in both younger and older patients in both the Arizona and Australia cohorts (Fig. 4).
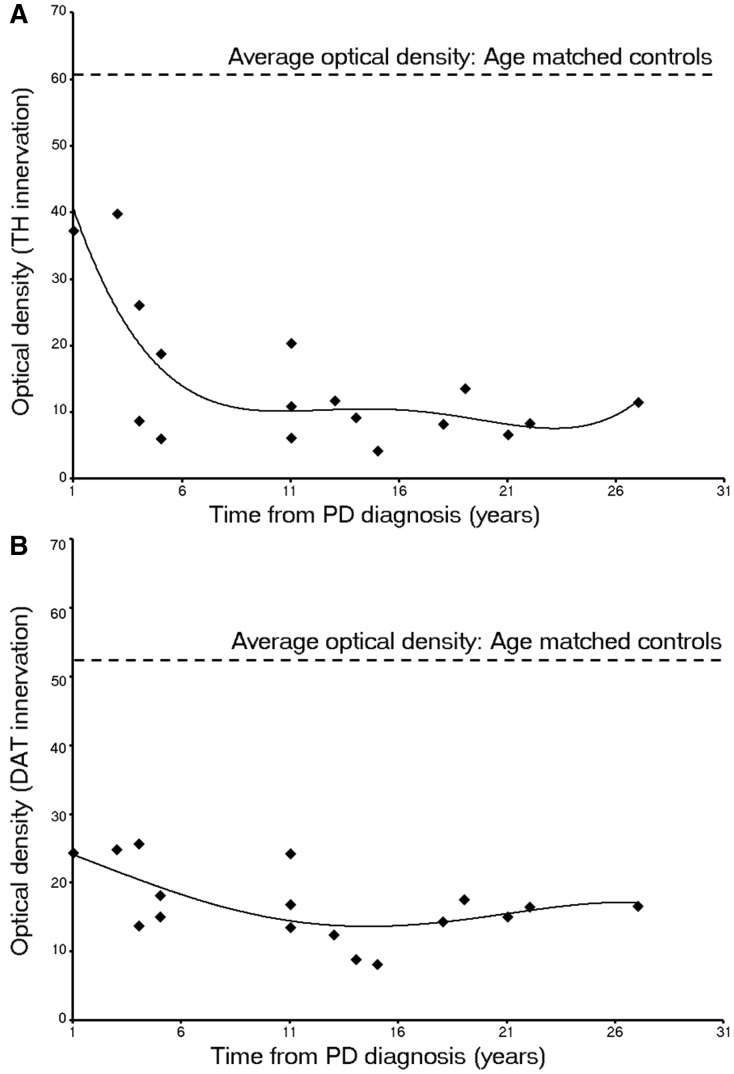
Figure 5.
Optical density of putaminal (A) tyrosine hydroxylase and (B) dopamine transporter immunohistochemistry with the progression of Parkinson’s disease (PD; Arizona cohort, n = 17). The data illustrate the modest loss at Year 1–3 after diagnosis, the rapid decline through Years 4–5 and the relatively stable numbers thereafter.
Figure 1.
(A) Tyrosine hydroxylase immunoreactivity in putamen region in control subjects and patients with Parkinson’s disease with varying disease duration from time of diagnosis (n = 28). Representative tyrosine hydroxylase-stained post-commissural putamen sections from a control (A) and patients with Parkinson’s disease, with 1 year (B), 3 years (C), 4 years (D), 5 years (E), 11 years (F), 14 years (G), 15 years (H), 18 years (I), 19 years (J), 22 years (K) and 27 years (L) disease duration. Note the distinct intact tyrosine hydroxylase stained fibre band pattern in the medial putamen. Scale bar in L = 500 µm.
Figure 2.
Dopamine transporter immunoreactivity in the putamen region in control subjects and patients with Parkinson’s disease with varying disease duration from time of diagnosis (n = 28). Representative dopamine transporter-stained post-commissural putamen sections from a control (A) and patients with Parkinson’s disease, with 1 year (B), 3 years (C), 4 years (D), 5 years (E), 11 years (F), 14 years (G), 15 years (H), 18 years (I), 19 years (J), 22 years (K) and 27 years (L) disease durations. Scale bar in L = 500 µm.
Figure 3.
Tyrosine hydroxylase staining from three cases with disease duration of 3 years following diagnosis: Scale bar in C = 500 µm. PD = Parkinson’s disease.
Figure 4.
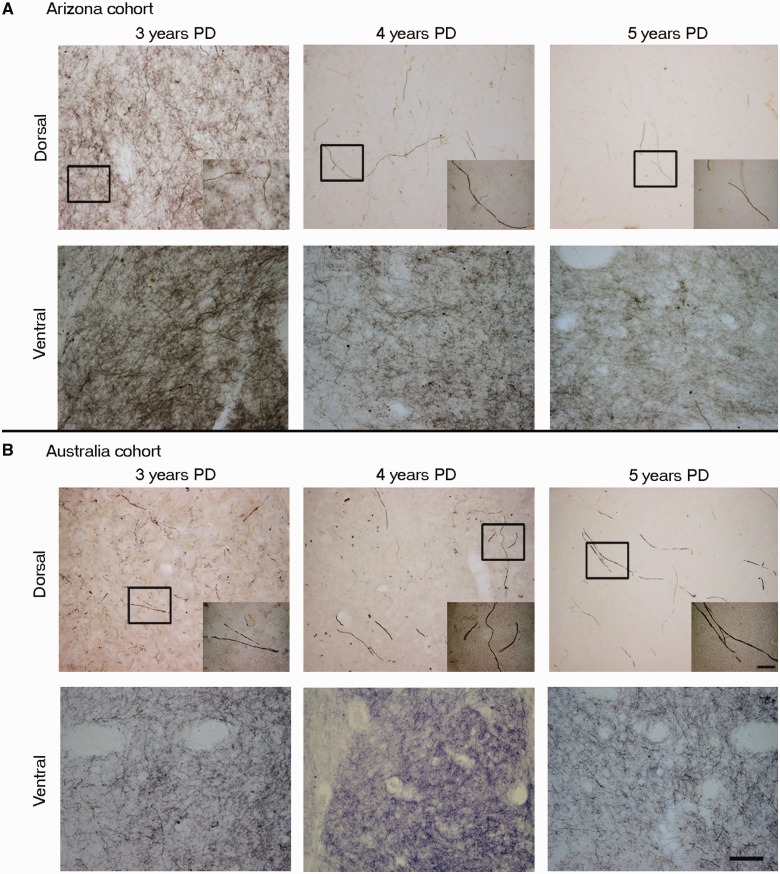
Comparison between the tyrosine hydroxylase immunoreactivity in the putamen of Arizona versus Australia cases. High magnification photomicrographs of representative dorsal and ventral post-commissural putamen sections comparing Arizona cohort Parkinson’s disease (PD) cases (A) of diseasedurations 3 years, 4 years and 5 years with Australia cohort Parkinson’s disease cases (B) of disease durations 3 years, 4 years and 5 years. Note that the reduction in tyrosine hydroxylase staining at Years 4 and 5 is comparable in the two cohorts, with Year 3 showing more variable results as described in text. Inset demonstrates a single but relatively normal dopamine fibre at Year 3 and isolated morphologically abnormal fibres at Years 4 and 5. Note the relative preservation of the ventral versus dorsal putamen. Scale bars: A and B = 100 µm; insets = 20 µm.
At all time points, greater numbers of tyrosine hydroxylase and dopamine transporter stained fibres were observed in the ventral compared to the dorsal putamen, consistent with the relative preservation of the medial portion of the nigra/ventral tegmental area which projects to this region (Hirsch et al., 1988; McRitchie et al., 1997) (Fig. 4). With disease progression, there was a gradual reduction in staining in the ventral putamen as well, but not to the extent observed in the dorsal putamen, and even 27 years post-diagnosis some fibres remained. The relative preservation of dopamine staining in the ventral putamen (Fig. 4) confirms the integrity of the staining procedure.
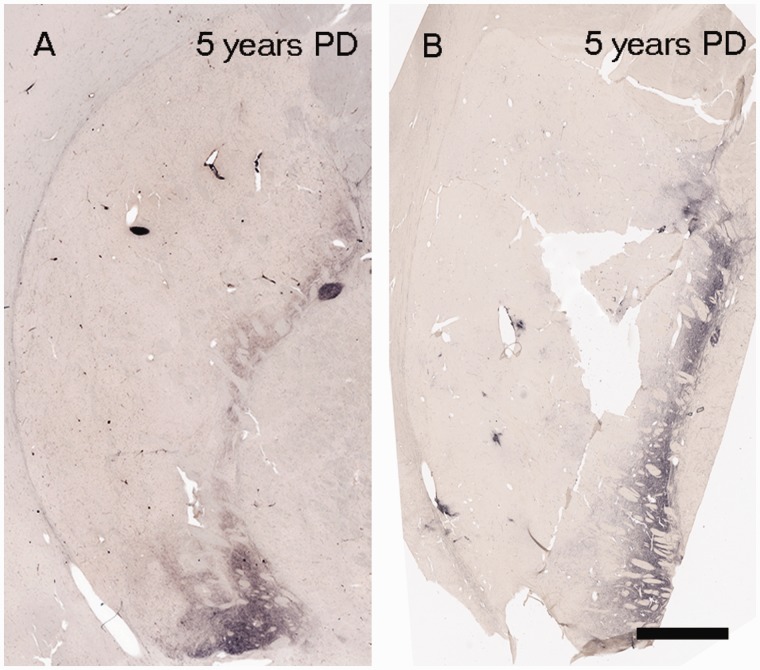
Quantitative analyses revealed a 35–75% loss of tyrosine hydroxylase and dopamine transporter optical fibre density at Years 1–3 after diagnosis, ∼70–90% reduction by Year 5, and relatively little change thereafter. The relatively stable levels of tyrosine hydroxylase and dopamine transporter optical densities for cases with disease durations >4–5 years suggest that loss of putamenal dopaminergic function, at least as evidenced by these markers, is largely complete by this time point. The pattern and magnitude of degeneration seen with tyrosine hydroxylase and dopamine transporter in the Australian cohort was identical to that observed in the Arizona cohort (Figs 3, 4 and 6). These cases provide samples from a different brain bank and include younger patients with relatively early disease (3–5 years post- diagnosis) when nigrostriatal degeneration appears to occur most rapidly and where few autopsy studies have previously been reported (Table 1). Specifically, the Australia cohort included two patients aged 43 and 53 years who died within 5 years following diagnosis and showed a marked reduction in dopamine transporter and tyrosine hydroxylase staining identical to that seen in cases from the Arizona cohort with short post-diagnosis durations (Fig. 6). Importantly the dramatic reductions observed in the dorsal putamen at these time points in these younger patients indicate that these changes do not simply reflect the effects of age, but rather the pathogenic pattern seen in Parkinson’s disease.
Figure 6.
Parkinson’s disease cases 5 years after disease diagnosis from Arizona cohort (82 years) (A) and Australia cohort (53 years) (B).
Substantia nigra pars compacta
Dopaminergic staining and stereological neuronal counts as a function of time from diagnosis
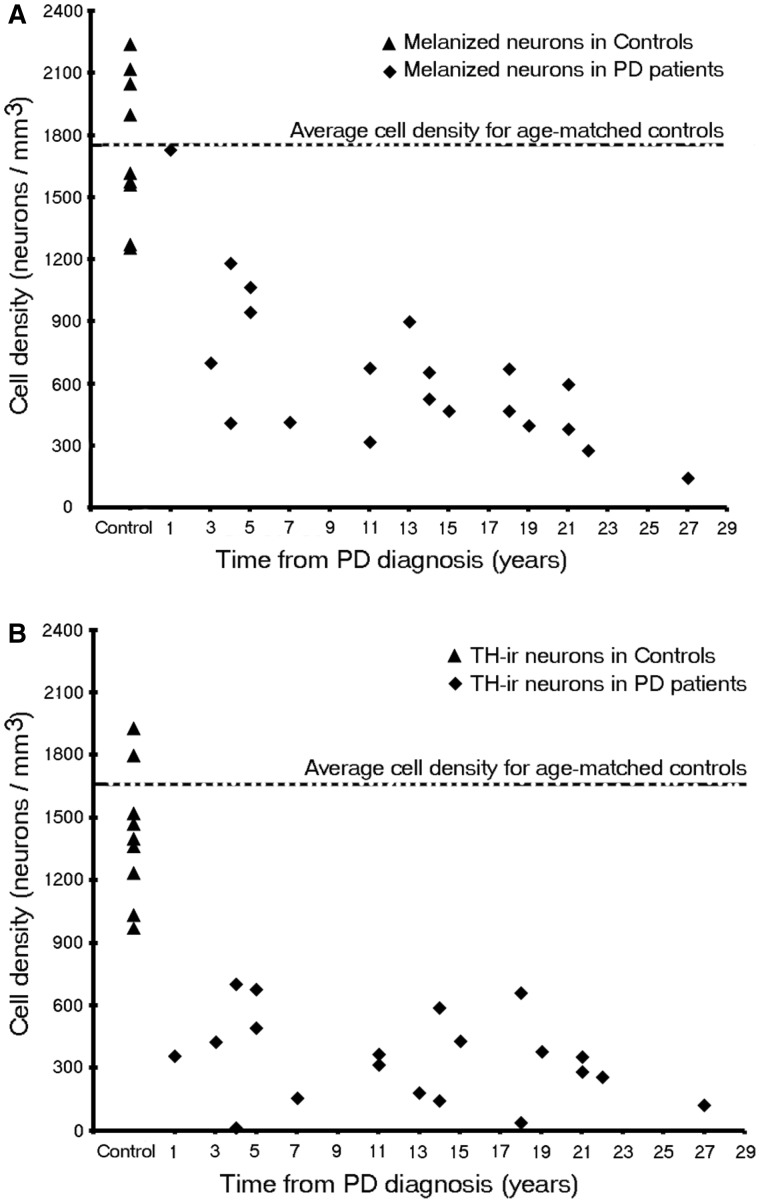
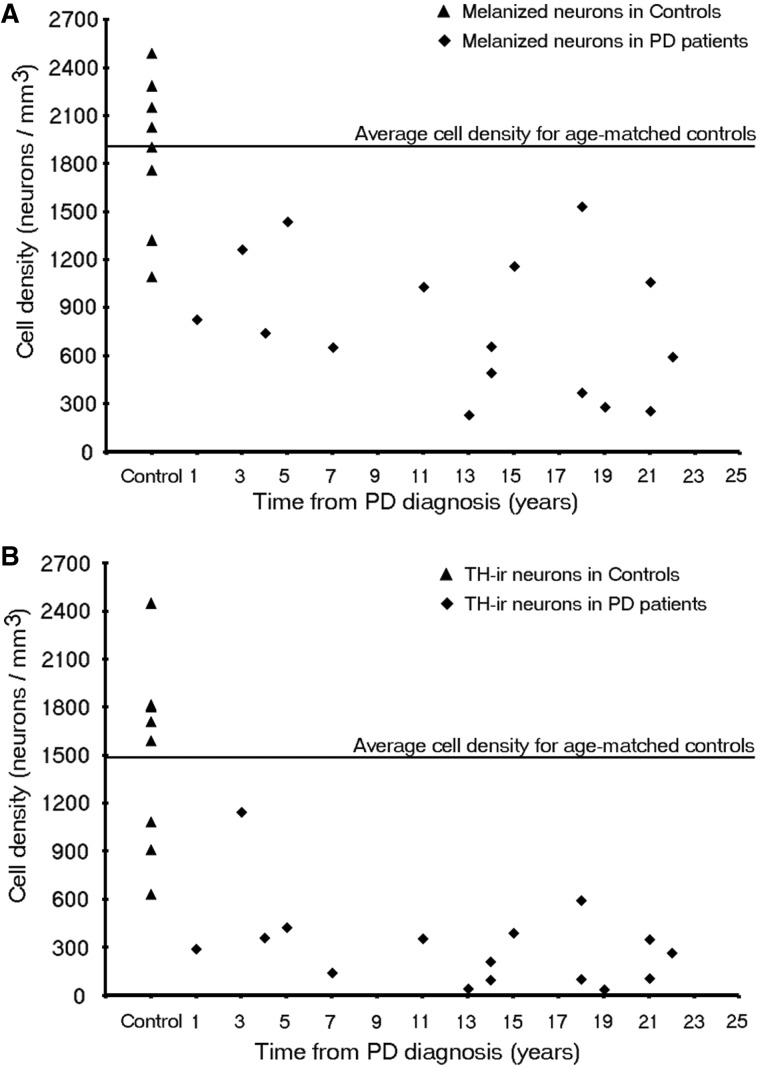
In age-matched control subjects, numerous neurons were detected in the SNc. In each case there were far more melanin-containing than tyrosine hydroxylase-positive (Figs 7 and 8a) or dopamine transporter-positive (data not shown) cells. In patients with Parkinson’s disease, there were reductions in neurons expressing all three markers, relative to age-matched control subjects, although residual SNc dopamine neurons and fibres were still detected even at 27 years post diagnosis. Stereological estimates of SNc dopamine neurons were performed in the nine control subjects and 17 patients with Parkinson’s disease from the Arizona cohort (Table 1). In control subjects, the mean density (±SEM) of SNc melanin-containing neurons was 1730.53 ± 120.3/mm3 and that of tyrosine hydroxylase-immunoreactivity neurons was 1412.32 ± 105.4/mm3 (Fig. 8b), reflecting an 18% decrease in phenotypic expression of tyrosine hydroxylase in SNc melanized dopamine neurons in normal control subjects (P < 0.01), likely as a consequence of ageing (Chu and Kordower, 2007).
Figure 7.
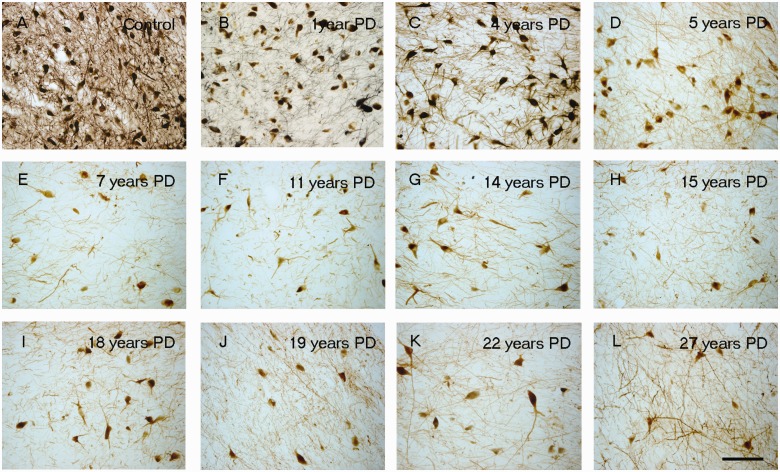
Tyrosine hydroxylase immunoreactivity (TH-ir) decreases in SN region with the progression of disease. Representative nigra sections from a control (A) and patients with Parkinson’s disease, with a disease duration of 1 year (B), 4 years (C), 5 years (D), 7 years (E), 11 years (F), 14 years (G), 15 years (H), 18 years (I), 19 years (J), 22 years (K) and 27 years (L). Scale bar in L = 100 µm.
Figure 8.
Stereologic Estimates of (A) melanin-containing and (B) tyrosine hydroxylase-immunoreactivity (TH-ir) neurons in patients with Parkinson’s disease (PD) of the whole SNc at different disease durations (n = 20).
In Parkinson’s disease cases where the entire nigra was considered, (Fig. 8b) the number of tyrosine hydroxylase stained neurons in the SNc was reduced at all post-diagnosis time points. This reduction was marked but highly variable (50–90%) in comparison to control subjects, even from the earliest time points examined (with several of the short-duration subjects showing comparable, severe loss of tyrosine hydroxylase-positive neurons to that seen in subjects 20 years, post-diagnosis). There was relatively little additional loss of tyrosine hydroxylase-positive neurons (i.e. <20%) over the remaining course of the illness, and a residual population of tyrosine hydroxylase-positive neurons still was observed decades after diagnosis. In contrast there was minimal reduction in the number of melanized neurons 1-year post-diagnosis case (albeit one case) and a highly variable 33–80% reduction over the next several years, post-diagnosis. Beyond the first decade, there was relatively little further loss, and there remained a population of residual SNc melanin-containing neurons. At all time points in patients with Parkinson’s disease and control subjects, the number of SNc melanin-containing neurons was greater than the number of tyrosine hydroxylase-stained neurons until more than two decades post-diagnosis was reached; Fig. 8). A similar histological pattern of tyrosine hydroxylase-staining in the SNc was observed in the Australia and Arizona cases.
We also stereologically examined cell loss in subregions of the SNc as delineated by Fearley and Lees (1991) (Table 2). The ventrolateral tier was the most devastated subregion (Table 2; Fig. 9) as has been previously reported (Fearnley and Lees, 1991; Ma et al., 1997; Greffard et al., 2006). Indeed, these papers have shown previously an inverse association between nigral cell number and clinical function (Ma, 1997; Greffard et al., 2006) but an attempt to find a correlation of nigral cell counts with disease duration were confounded by outliers (Ma, 1997; Greffard et al., 2006) and now appreciated flawed counting procedures (Fearnley and Lees, 1991). In each subregion there was a greater loss of tyrosine hydroxylase-immunoreactivity neurons relative to melanin-containing neurons (Table 2 and Fig. 9). Interestingly, when the nigra was considered as a whole, or when the dorsomedial, dorsolateral or ventromedial subdivisions were considered, there was little to no loss of melanin-containing cells in the case that was 1 year post-diagnosis. In contrast, this case showed extensive melanin positive cell loss in the ventrolateral tier and the pars lateralis region of the nigra. In the ventrolateral tier (Fig. 9), there was considerable variability in melanin-positive cell loss across disease duration with no general pattern emerging. In contrast, this subregion displayed an almost comprehensive loss of tyrosine hydroxylase-immunoreactivity neurons beginning at 1 year disease duration and continuing through the time from diagnosis assessment period (Fig. 9).
Table 2.
Subregional analysis of neuromelanin and tyrosine hydroxylase-immunoreactive neurons as a function of disease duration
| DM |
DL |
VM |
VL |
PL |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Disease durations | NM | TH | NM | TH | NM | TH | NM | TH | NM | TH |
| 1 | Controls (n = 8) | 984 ± 95.40 | 748 ± 71.51 | 813 ± 70.02 | 688 ± 67.33 | 1368 ± 87.68 | 1115 ± 93.49 | 1879 ± 167.87 | 1499 ± 207.70 | 1645 ± 383.42 | 932 ± 341.97 |
| Cells/mm3 ± SEM | |||||||||||
| 2 | 1 | 1292 | 386 | 1104 | 431 | 1463 | 484 | 828 | 291 | 598 | 322 |
| 3 | 3 | 1065 | 1022 | 962 | 612 | 2323 | 1971 | 1267 | 1145 | 408 | 272 |
| 4 | 4 | 703 | 595 | 696 | 562 | 1261 | 705 | 746 | 361 | 263 | n/a |
| 5 | 5 | 946 | 398 | 510 | 185 | 2109 | 465 | 1439 | 428 | 607 | 303 |
| 6 | 7 | 602 | 212 | 277 | 126 | 586 | 247 | 657 | 143 | 318 | n/a |
| 7 | 11 | 974 | 299 | 799 | 439 | 1321 | 237 | 1031 | 356 | 578 | n/a |
| 8 | 13 | 269 | 134 | 642 | 84 | 371 | 50 | 232 | 46 | 603 | 133 |
| 9 | 14 | 435 | 14 | 423 | 13 | 801 | 59 | 659 | 98 | 513 | 125 |
| 10 | 14 | 434 | 334 | 474 | 297 | 502 | 188 | 497 | 213 | 379 | n/a |
| 11 | 15 | 640 | 471 | 490 | 453 | 1294 | 388 | 1160 | 394 | 333 | 190 |
| 12 | 18 | 601 | 95 | 337 | 61 | 437 | 26 | 372 | 106 | 169 | 84 |
| 13 | 18 | 885 | 531 | 545 | 385 | 1148 | 395 | 1775 | 596 | 439 | 293 |
| 14 | 19 | 713 | 475 | 832 | 185 | 422 | 162 | 283 | 40 | 628 | 523 |
| 15 | 21 | 516 | 196 | 370 | 148 | 614 | 116 | 255 | 108 | 681 | 143 |
| 16 | 21 | 677 | 150 | 489 | 139 | 777 | 104 | 1062 | 354 | 495 | 123 |
| 17 | 22 | 454 | 319 | 248 | 198 | 619 | 364 | 592 | 269 | 305 | 85 |
DM = dorsomedial; DL = dorsolateral; VM = ventromedial; VL = ventrolateral; PL = pars lateralis.
Figure 9.
Stereological estimates of (A) melanin-containing and (B) tyrosine hydroxylase-immunoreactivity (TH-ir) neurons in patients with Parkinson’s disease in the ventrolateral tier of the SNc at different disease durations (n = 16).
Discussion
Our study attempts to fill an important knowledge gap involving the status of the nigrostriatal system in Parkinson’s disease at varying time points after diagnosis. We found that dopamine markers in the fibres of the Parkinson’s disease dorsal putamen are variably reduced (moderate to marked) in the initial 4 years, post-diagnosis, and virtually absent by Years 4 and thereafter. It should be noted that all patients had been diagnosed by a movement disorders expert and the diagnosis confirmed at post-mortem. These findings were confirmed by optical density measurements; however, the presence of a small optical density signal at these time-points reflects a general background signal (even with subtracting out the optical density from the corpus callosum) and does not reflect preservation of genuine fibre staining. Although some ventral and medial putaminal fibres are relatively preserved, they are most likely traversing the putamen en route to the caudate nucleus. The observations in the present paper provide a unique glimpse into the degenerative changes that occur in the putamen soon after Parkinson’s disease diagnosis, offering a potentially important observation of an early and substantial reduction in dopamine function in the terminal areas that heretofore has been unrecognized. The same pattern was seen in both Arizona and Australia cohorts and was not influenced by age. Furthermore, our patterns of striatal changes are similar to those reported when specific dopamine transporter ligands are employed (Nandhagopal et al., 2009; Piccini, personal communication) or through neurochemical measures in more advanced cases (Riederer and Wuketich, 1976; Kish et al., 1988; Piggott et al., 1999). Replication and extension of our novel findings will be important as additional rare, early-stage cases become available for study.
Quantitative optical density was reduced ∼50% at Years 1–3 suggesting that this level of dopamine reduction is required to produce clinical symptoms sufficient to make a diagnosis of Parkinson’s disease. Reductions in optical density were maximal by 4–5 years post-diagnosis and tyrosine hydroxylase and dopamine transporter staining at this time point suggests there are negligible fibres efficiently synthesizing dopamine in the dorsal putamen. However, it remains uncertain whether the loss of tyrosine hydroxylase and dopamine transporter staining in the dorsal striatum is due to the fibre degeneration or dopaminergic dysfunction that could be reflected by a profound loss of dopaminergic phenotype in those fibres. It is impossible using post-mortem material to distinguish between these two possibilities as generalized markers of axons would be influenced by the massive innervation to the striatum from non-nigral regions such as the cortex, thalamus, and raphe as well as efferents emanating from striatal projections neurons. Answers to this fundamental question require that additional tools be developed to help distinguish these two alternatives, as well as that far more early-stage tissue becomes available than can currently be found.
Several previous studies reported a marked reduction in melanized and tyrosine hydroxylase-immunoreactive neurons in the SNc in patients with advanced Parkinson’s disease (Bernheimer et al., 1973; Hirsch et al., 1988; Halliday et al., 1996; McRitchie et al., 1997). Although consistent with these general observations, our results provide far more information than has heretofore been available. Most reviews estimate a loss of 50–70% of SNc dopamine neurons at the time when symptoms emerge (Marsden, 1990; Damier et al., 1999; Rajput et al., 2008), though little primary data are presented to support these estimates. Fearnley and Lees (1991) used logistic regression analysis to estimate a 31% reduction in the number of melanized SNc neurons at the time of symptom onset (Fearnley and Lees, 1991). However, only cells in single sections through the caudal nigra were counted and modern unbiased stereological counting techniques were not yet available. Ma et al. (1997) calculated similar estimates using a quantitative morphometric approach and a linear regression analysis, but also examined few cases, especially in the early stages of the disease. In their study, Ma et al. (1997) performed stereological estimates of pigmented cells in the substantia nigra of control subjects and patients with Parkinson’s disease. As seen in other studies, they found a 51% reduction in melanin containing nigral neurons in Parkinson’s disease. In contrast to our findings, they found a statistically significant correlation between disease duration and the number of melanin-containing neurons. However, examination of their data reveals that this correlation was due to two cases with ∼17 years disease duration. If these cases were removed from the analysis, the loss of melanin-positive cells as a function of disease duration would occur in a manner virtually identical to what is seen in the present study. Greffard et al. (2006) also examined nigral cell loss as a function of disease duration. They claim—based upon a linear regression analysis—that motor symptom onset occurs after a 30% loss of nigral neurons. However, this figure is based upon control values and one Parkinson’s disease case with 1 year disease duration that displayed a 70% loss of nigral neurons. We quantified both tyrosine hydroxylase-positive and melanin-containing neurons in the SNc in a relatively large number of subjects over a multi-decade post-diagnostic time frame, including several rare cases where death occurred relatively soon after diagnosis. We observed a profound but variable loss of tyrosine hydroxylase-positive neurons in the earliest cases with modest-to-negligible loss for decades thereafter. This was true whether we assessed the nigra as a whole or the most vulnerable ventrolateral tier. This suggests that the SNc, like the terminal field in the putamen, suffers a profound loss of dopaminergic function soon after diagnosis and that the entire neuron is apparently impaired. These findings suggest that clinical deterioration after this time likely represents a loss of a compensatory mechanism or degeneration of non-dopaminergic neurons. The pattern of degeneration seen in melanin-containing SNc cells seems somewhat slower. In this case, there were minimal reductions when the nigra was assessed as a whole, but extensive loss of melanin cells in the ventrolateral tier and the pars lateralis subregion. Interestingly, this same case showed a profound loss of tyrosine hydroxylase-positive neurons in both analyses. It is noted that there was considerable variability in the numbers of melanin-positive neurons in early-stage disease with several early patients exhibiting profound loss (Fig. 8A). From 3–7 years post-diagnosis, there was a highly variable 33–80% reduction in melanin-containing neurons, and thereafter, a substantial loss at all time points, albeit with some variability. After the first decade following diagnosis, there was a slower rate of degeneration, and further loss was subtle, gradual and with much less variability. Nonetheless, loss of melanized neurons was less than the loss of tyrosine hydroxylase neurons at all time points, suggesting that some cells may be dysfunctional but still potentially capable of being restored by trophic therapies or similar therapies.
Our findings, analysing both the cell bodies and terminal areas of the nigrostriatal system, suggest that at symptom onset, there is a moderate loss of at least 50% of fibres in the putamen that continue to express sufficient tyrosine hydroxylase to be detected and a comparable loss of tyrosine hydroxylase-positive stained cells in the SNc. Although the loss tyrosine hydroxylase-positive staining in the SNc neurons was profound at the earliest time points, the loss of melanin containing neurons occurred more gradually over the course of the first decade following diagnosis for most subregions (except the ventrolateral tier and pars lateralis subregion), and the difference in the number of tyrosine hydroxylase-positive and melanin-positive neurons continued at all post-diagnostic time points. As Parkinson’s disease progresses beyond the first decade, there are relatively stable numbers of residual tyrosine hydroxylase-positive and melanin containing neurons persisting, suggesting that there may be two subpopulations of substantia nigra neurons with substantially different vulnerabilities to Parkinson’s disease neurodegeneration and anatomical targets.
Collectively, these findings indicate that phenotypic changes in the nigrostriatal systems are greater than structural changes and suggest that clinical dysfunction in the early stages is likely due to a severe loss of function of nigrostriatal neurons. This may be related to degeneration of dopamine terminals, but it is also possible that SNc neurons may be dysfunctional but still potentially viable. The loss of a dopaminergic phenotype is well described in primates as the loss of phenotypic markers occurs as a function of normal ageing and Parkinson’s disease (Bartus et al., 2010). This has obvious implications for the timing of studies testing putative neuroprotective or trophic therapies where it might be preferable to study patients at a relatively early stage of the disease when there is a greater chance of surviving dopamine neurons that could benefit from therapy. Indeed, degeneration of dopamine terminals, or severe dysfunction of dopamine terminals that limit axonal transport to the SNc could explain why adeno-associated viral delivery of the trophic factor neurturin (CERE-120) into the putamen failed to improve Unified Parkinson’s Disease Rating Scale motor scores at 12 months in patients with moderately advanced Parkinson’s disease who were >5 years after diagnosis, but did provide Unified Parkinson’s Disease Rating Scale motor benefit for the subpopulation that was followed for 18 month (Marks et al., 2010). In support of the concept that axonal dysfunction may have contributed to a delayed trophic response, we observed minimal SNc neuronal staining for neurturin with only sparse tyrosine hydroxylase staining in the putamen in autopsies performed 2–3 months following gene delivery of CERE-120 (Bartus et al., 2010), but more robust SNc neurturin expression with dense tyrosine hydroxylase-immunoreactive fibre network at autopsy performed four years following treatment (Marks et al., 2010). These observations suggest that dopaminergic fibres that do not stain for tyrosine hydroxylase and dopamine transporter may be dysfunctional but not completely degenerated and potentially recoverable.
These findings have important implications for considering which patients should be included in studies of putative neuroprotective agents. Although our findings may reflect a phenotypic downregulation of dopaminergic markers, it remains equally plausible that absence of staining for dopamine terminals in the putamen at 4 years and beyond reflects complete degeneration of dopamine terminals and a time point at which no benefit could be expected, even from a potentially effective agent. These data suggest that there will be a better chance of detecting a beneficial effect of a putative neuroprotective agent if patients with Parkinson’s disease are enrolled at earlier time points in their illness, and further emphasize the importance of trying to define patients with pre-motor or prodromal Parkinson’s disease where it is likely that there is a greater number of preserved dopamine neurons and a greater chance of a neuroprotective or restorative agent providing a clinically meaningful benefit.
Several caveats must be considered when interpreting the data from our study. Pathological studies are potentially biased dependent upon the population of individuals studied. In the present report, Parkinson’s disease cases in the Arizona cohort were obtained from a retirement community with an environment that facilitates rapid autopsy. This cohort is comprised primarily of elderly individuals with a higher socio-economic status and greater education than a random Parkinson’s disease population. However, we found the same pattern of results with a dramatic and early loss of striatal dopaminergic terminals in the Australia cohort, which included younger patients with relatively short disease duration. Thus, it appears that neither socio-economic factors, nor an age-related inability to maintain compensatory mechanisms accounts for our data. We had limited numbers of brains, particularly in the early stages of the illness when it appears that degeneration occurs at the most rapid rate. Finally, we were not able to make firm conclusions regarding how our data are associated with clinical status.
In summary we have performed one of the largest pathology studies to date, evaluating the integrity of the nigrostriatal dopamine system in patients with Parkinson’s disease at multiple time points following diagnosis. Using markers for dopamine function, we show that fibres in the dorsal striatum, which underlie motor function in Parkinson’s disease, are only mildly affected at 1 year, are variably affected at 3 years, and are virtually absent by Years 4–5 and thereafter. A similarly profound loss of the dopaminergic marker, tyrosine hydroxylase, was seen in the SNc neuronal cell bodies, demonstrating that the entire neuron is affected early after diagnosis. Importantly, the number of tyrosine hydroxylase-positive neurons is less than the number of melanized neurons at all time points, suggesting that some nerve cells are likely to be damaged but to have not yet degenerated, and thus might be capable of responding to a protective or trophic factor therapy. We also demonstrate a population of dopamine neurons that continue to express tyrosine hydroxylase and persist for decades after diagnosis. These neurons presumably innervate the ventral striatum and other non-striatal regions and are not vulnerable to the Parkinson’s disease degenerative process.
Our findings suggest that there may be significant challenges for ‘neuroprotective’ therapies that include patients with >4 years disease duration given the substantial loss of dopamine markers in the nigrostriatal terminals that has occurred by this time point and supports the use of patients in early stages of the disease for clinical trials. On the other hand, the persistence of populations of melanin containing neurons in comparison to the number of tyrosine hydroxylase-immunoreactive neurons for decades after diagnosis, suggests that trophic or regenerative therapies might still have value even in the later stages of the illness. A determination as to whether the loss of dopaminergic staining in fibres of the dorsal putamen is due to frank neurodegeneration or phenotypic downregulation will help guide the development of novel experimental therapies. Finally, the early changes in dopaminergic markers that we observe in both the SNc perikarya and in fibres projecting to the dorsal putamen emphasize the importance of efforts aimed at developing biomarkers that permit diagnosing Parkinson’s disease at early time points, perhaps even before the emergence of the classical motor features of the disease when there is greater preservation of dopaminergic markers and a greater potential for a beneficial effect with neuroprotective or trophic therapies.
Funding
This research was supported in part by a Centre Grant from the Parkinson’s Disease Foundation to Rush University Medical Centre. The Sun Health Research Institute Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Centre), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 PubMed and 1001 to the Arizona Parkinson's Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. The Human brain tissue samples received from the Sydney Brain Bank was supported by Neuroscience Research Australia, the University of New South Wales and the National Health and Medical Research Council of Australia (NHMRC). GMH has a NHMRC Senior Principal Research Fellowship 630434.
Glossary
Abbreviation
- SNc
substantia nigra pars compacta
References
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains. Mov Disord. 2010;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008a;115:445–51. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, et al. The Sun Health Research Institute Brain Donation Program: description and experience, 1987-2007. Cell Tissue Bank. 2008b;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, Kordower JH. Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol. 2002;450:203–14. doi: 10.1002/cne.10261. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease? Neurobiol Dis. 2007;25:134–49. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–48. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, et al. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–80. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–44. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system [in German] Klin Wochenschr. 1960;38:1236–9. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, et al. Motor score of the Unified Parkinson Disease Rating Scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63:584–8. doi: 10.1001/archneur.63.4.584. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Halliday GM, McRitchie DA, Cartwright H, Pamphlett R, Hely MA, Morris JG. Midbrain neuropathology in idiopathic Parkinson's disease and diffuse Lewy body disease. J Clin Neurosci. 1996;3:52–60. doi: 10.1016/s0967-5868(96)90083-1. [DOI] [PubMed] [Google Scholar]
- Herzog CB, Kordower JH, Chu Y, Wilson A, Brown L, Siffert J, et al. Long-term neurturin gene expression and upregulation of tyrosine hydroxylase in a PD patient with CERE-120. Soc Neurosci Abstr. in press. [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–8. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Neuropathology of sporadic Parkinson's disease: Evaluation and changes of concepts. Mov Disord. 2012;27:8–30. doi: 10.1002/mds.23795. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Ma SY, Roytta M, Rinne JO, Collan Y, Rinne UK. Correlation between neuromorphometry in the substantia nigra and clinical features in Parkinson's disease using disector counts. J Neurol Sci. 1997;151:83–7. doi: 10.1016/s0022-510x(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–72. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Marsden CD. Parkinson's disease. Lancet. 1990;335:948–52. doi: 10.1016/0140-6736(90)91006-v. [DOI] [PubMed] [Google Scholar]
- McRitchie DA, Cartwright HR, Halliday GM. Specific A10 dopaminergic nuclei in the midbrain degenerate in Parkinson's disease. Exp Neurol. 1997;144:202–13. doi: 10.1006/exnr.1997.6418. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Conner JM, Kordower JH. Nerve growth factor in Alzheimer's disease: defective retrograde transport to nucleus basalis. Neuroreport. 1995;6:1063–6. doi: 10.1097/00001756-199505090-00028. [DOI] [PubMed] [Google Scholar]
- Nandhagopal R, Kuramoto L, Schulzer M, Mak E, Cragg J, Lee CS, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain. 2009;132:2970–9. doi: 10.1093/brain/awp209. PubMed. [DOI] [PubMed] [Google Scholar]
- Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer's and Parkinson's diseases: rostrocaudal distribution. Brain. 1999;122(Pt 8):1449–68. doi: 10.1093/brain/122.8.1449. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O. Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology. 2008;70:1403–10. doi: 10.1212/01.wnl.0000285082.18969.3a. PubMed. [DOI] [PubMed] [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38:277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–85. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]