SUMMARY
To investigate the mechanism of fidelity in pre-mRNA splicing, we developed an in vitro assay sensitive to proofreading of 5′ splice site cleavage. We inactivated spliceosomes by disrupting a critical metal-ligand interaction at the catalytic center and discovered that when the DEAH-box ATPase Prp16 was disabled these spliceosomes successfully catalyzed 5′ splice site cleavage but at a reduced rate. Although Prp16 does not promote splicing of a genuine substrate until after 5′ splice site cleavage, we found that Prp16 can associate with spliceosomes before 5′ splice site cleavage, consistent with a role for Prp16 in proofreading 5′ splice site cleavage. We established that Prp16-mediated rejection is reversible, necessitating a downstream discard pathway that we found requires the DEAH-box ATPase Prp43, a spliceosome disassembly factor. These data provide evidence that splicing fidelity mechanisms discriminate against slow substrates and that the mechanisms for establishing the fidelity of 5′ splice site cleavage and exon ligation share a common ATP-dependent framework.
INTRODUCTION
Pre-mRNA splicing is catalyzed by the spliceosome, a dynamic ribonucleoprotein (RNP) machine, composed of five small nuclear RNAs (snRNA) and eighty conserved proteins (Wahl et al., 2009 and references therein). The snRNAs U1 and U4 do not participate in catalysis but do aid in the assembly of the spliceosome onto a pre-mRNA. U2, U5 and U6 snRNAs constitute the structural framework of the catalytically active spliceosome and form well-conserved interactions among themselves and with intronic and exonic sequences to juxtapose the reactants for splicing catalysis. Further, base paired U2/U6 appears to participate in splicing catalysis (Huppler et al., 2002; Madhani and Guthrie, 1992; Mefford and Staley, 2009; Valadkhan and Manley, 2001; Valadkhan et al., 2009; Yean et al., 2000). The protein components consist of both snRNP and non-snRNP protein factors, which together mediate a number of functions, including promoting RNP rearrangements and stabilizing RNP conformations during spliceosome assembly and catalysis (Wahl et al., 2009 and references therein).
To ensure fidelity in gene expression, the spliceosome must excise introns accurately and with single nucleotide precision. The spliceosome recognizes introns through consensus sequences at the 5′ splice site, the branch point and the 3′ splice site (Wahl et al., 2009 and references therein). These sequences also participate directly in splicing catalysis, which involves two sequential, phosphoryl transfer reactions. In the first step of this reaction, 5′ splice site cleavage, the 2′ hydroxyl of a conserved intronic adenosine attacks the 5′ splice site, forming a lariat intermediate and a free 5′ exon. The spliceosome then repositions the intermediates for the second step, exon ligation. In this step, the 3′ hydroxyl of the 5′ exon attacks the 3′ splice site, excising the intron and ligating the two exons to form the mRNA. Identifying the optimal splice site among a large number of nearly optimal splice sites is a daunting challenge for the spliceosome.
To promote fidelity, the spliceosome employs constitutive components to discriminate against suboptimal substrates through either an equilibrium or kinetic mechanism (Burgess and Guthrie, 1993b; Mayas et al., 2006; Query and Konarska, 2004; Xu and Query, 2007). Equilibration between the two catalytic states of the spliceosome contributes to fidelity by sequestering aberrant substrates in a spliceosomal conformation that is catalytic but inappropriate given the connectivity of the substrate (Query and Konarska, 2004). In kinetic proofreading, the spliceosome preferentially rejects suboptimal substrates through a branched pathway. This rejection is mediated by enzymes belonging to the DExD/H-box family of ATPases (Burgess and Guthrie, 1993b; Mayas et al., 2006; Xu and Query, 2007). DExD/H-box ATPases are a ubiquitous class of nucleic acid remodelling factors, which use the energy derived from ATP binding and/or hydrolysis to disrupt RNA-RNA or RNA-protein interactions (Rocak and Linder, 2004). In splicing, at least eight conserved members of this family mediate specific RNP rearrangements to facilitate splicing of an optimal substrate. In addition, at least three of these ATPases promote fidelity by antagonizing splicing of suboptimal substrates (Burgess and Guthrie, 1993b; Mayas et al., 2006; Xu and Query, 2007).
In a pioneering genetic study, Burgess and Guthrie discovered that the DEAH-box ATPase Prp16p not only promotes rearrangement of the spliceosome but also the fidelity of branch point recognition (Burgess and Guthrie, 1993b). Specifically, while prp16 mutants accumulate splicing intermediates and compromise mRNA formation with a wild-type substrate, with a mutated branch site substrate prp16 mutants increased the levels of both mutated lariat intermediates and mRNA products (Burgess and Guthrie, 1993b). Because these Prp16p mutants hydrolyze ATP inefficiently in vitro, Prp16p was proposed to enhance the fidelity of branch point recognition by a kinetic proofreading mechanism in which Prp16p- and ATP-dependent rejection competes with a step in splicing that requires the genuine branch site sequence (Burgess and Guthrie, 1993b). Prp16p was originally proposed to compete with a step at the lariat intermediate stage, because with a wild-type substrate Prp16p does not appear to either bind to the substrate or promote splicing until after 5′ splice site cleavage (Schwer and Guthrie, 1991) and because prp16 mutants did not appear to alter the levels of pre-mRNA having a mutated branch site (Burgess and Guthrie, 1993b). However, recent genetic studies (Query and Konarska, 2004; Villa and Guthrie, 2005) are also consistent with an alternative model in which Prp16p promotes the fidelity of branch point recognition by antagonizing mutated substrates prior to 5′ splice site cleavage. The lack of an in vitro assay for the fidelity of 5′ splice site cleavage has precluded a biochemical test of this model and a deeper investigation of the role of Prp16p in fidelity. Additionally, while Prp16 is required to reject a suboptimal substrate, the requirements for subsequently discarding a rejected substrate down a pathway that leads to turnover have not been defined.
Two additional DExD/H-box ATPases promote fidelity in pre-mRNA splicing – Prp5p, during early spliceosome assembly, and Prp22p, during exon ligation (Mayas et al., 2006; Xu and Query, 2007). While Prp5p promotes the association of U2 snRNP with a wild-type substrate (Perriman and Ares, 2000; Ruby et al., 1993; Xu et al., 2004), Prp5p also discriminates against substrates having branch site mutations (Xu and Query, 2007). Similarly, while Prp22p promotes the release of a wild-type mRNA product after exon ligation (Company et al., 1991; Schwer and Gross, 1998), Prp22p also generally discriminates against mutated substrates before exon ligation (Mayas et al., 2006). Together with the studies of Prp16p, these data suggest a general model in which DExD/H-box ATPases establish fidelity in pre-mRNA splicing both by promoting an optimal substrate along the productive splicing pathway and by rejecting a suboptimal substrate down a competitive, unproductive pathway (Burgess and Guthrie, 1993a; Query and Konarska, 2006). However, it has remained unclear how the DExD/H-box ATPases discriminate between an optimal and a suboptimal substrate.
To define further the mechanisms for establishing fidelity in splicing, we developed an in vitro assay sensitive to proofreading of 5′ splice site cleavage. We found that the DEAH box ATPase Prp16p rejects spliceosomes prior to 5′ splice site cleavage, when the rate of 5′ splice site cleavage is slow. Further, we found that pre-mRNA rejected by Prp16p is discarded by a pathway that requires another DEAH box ATPase, the spliceosome disassembly factor Prp43p (Arenas and Abelson, 1997; Martin et al., 2002). Our analysis establishes a pathway for proofreading and discard at the stage of 5′ splice site cleavage and provides evidence that DExD/H-box ATPases promote the fidelity of RNA-dependent processes by antagonizing slow substrates.
RESULTS
An ATP-dependent activity that competes with 5′ splice site cleavage
To test for an ATP-dependent activity that competes with 5′ splice site cleavage and thereby serves to reject aberrant substrates, we sought to investigate the basis of a defect in which the spliceosome stalls just before 5′ splice site cleavage. No substrate mutations are currently known to stall splicing in vitro at the stage of 5′ splice site cleavage. However, an atomic substitution of sulfur for the pro-Sp non-bridging oxygen at position U80 of U6 snRNA (U6/sU80 (Sp)) does stall splicing just before 5′ splice site cleavage (Yean et al., 2000). U6-U80 resides in a bulge of the U6 intramolecular stem loop (ISL), which is analogous to the catalytic domain V of group II introns (Huppler et al., 2002; Villa et al., 2002), a class of introns that self-splice by a pathway indistinguishable from pre-mRNA splicing. A recent crystal structure of a group II intron (Toor et al., 2008) has revealed that five ligands in domain V bind a pair of metals separated by a distance previously predicted to promote the catalysis of the two transesterification reactions in splicing (Steitz and Steitz, 1993). Importantly, the spliceosomal analog of one ligand, the pro-Sp non-bridging oxygen of U6-U80, was proposed to play a catalytic role in binding a Mg2+ ion during 5′ splice site cleavage, because the sulfur-substituted U6/sU80 (Sp) spliceosomes fail to splice in Mg2+, which coordinates sulfur poorly, but successfully splice with more thiophilic metals, such as Mn2+ or Cd2+ (Yean et al., 2000), although a structural role for Mg2+ binding has not yet been ruled out. Because U6/sU80 (Sp) spliceosomes in Mg2+ undergo assembly and activation and stall just before 5′ splice site cleavage (Yean et al., 2000), we hypothesized that the sulfur substitution sensitizes spliceosomes to ATP-dependent rejection.
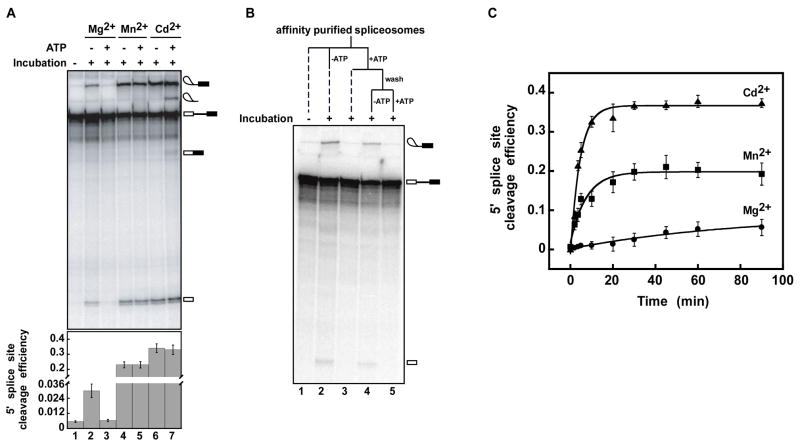
We indeed found evidence for an ATP-dependent rejection step that competes with 5′ splice site cleavage. To test our hypothesis, we assembled spliceosomes in the presence of Mg2+ on labeled ACT1 pre-mRNA in budding yeast extract that was depleted of endogenous U6 and reconstituted with U6/sU80 (Sp). After the spliceosomes stalled just prior to 5′ splice site cleavage, we affinity purified the spliceosomes using TAP-tagged Prp19p. The spliceosomes were then incubated in the presence of Mg2+, Mn2+ or Cd2+ to test for metal rescue and in the absence or presence of ATP to test for rejection. As expected for U6/sU80 (Sp) spliceosomes in the presence of ATP, 5′ splice site cleavage was prohibited in the presence of Mg2+ but was permitted in the presence of the more thiophilic divalent metal ions Mn2+ and Cd2+ (Figure 1A, lanes 3, 5 and 7). Remarkably, when U6/sU80 (Sp) spliceosomes were incubated in the absence of ATP, the spliceosome catalyzed 5′ splice site cleavage not only with Mn2+ and Cd2+ but also with Mg2+, 5-fold above background, suggesting that an ATP-dependent rejection activity was disabled (Figure 1A, lanes 2, 4 and 6). Splicing by U6/sU80 (Sp) spliceosomes in Mg2+ was also permitted when ATP was removed by gel filtration rather than by affinity purification or when affinity purified spliceosomes were supplemented with extract and incubated in the absence of ATP (Figure S1A, B), indicating that affinity purification did not remove a factor essential for repressing splicing by U6/sU80 (Sp) spliceosomes. To test the generality of the ATP-dependent rejection activity, we assembled U6/sU80 (Sp) spliceosomes on another, unrelated pre-mRNA substrate, UBC4; 5′ splice site cleavage of this substrate in Mg2+ was also permitted in the absence of ATP and repressed in the presence of ATP (Figure S1C). Importantly, although 5′ splice site cleavage is reversible (Tseng and Cheng, 2008), the formation of intermediates in the absence of ATP could not be reversed by the addition of ATP, implying that ATP can only reject U6/sU80 (Sp) spliceosomes before 5′ splice site cleavage (data not shown). Thus, the requirement of U6/sU80 (Sp) spliceosomes for more thiophilic, divalent metals in cleaving the 5′ splice site is established in part by an ATP-dependent rejection activity that competes with 5′ splice site cleavage.
Figure 1. An ATP-dependent activity rejects pre-mRNA reversibly prior to 5′ splice site cleavage, if the rate of 5′ splice site cleavage is slow.
(A) An ATP-dependent activity competes with 5′ splice site cleavage. U6/sU80 (Sp) spliceosomes stalled in Mg2+ on an ACT1 pre-mRNA substrate were affinity-purified and then either frozen (no incubation) or incubated with the indicated metals and with or without ATP-Mg2+. The migration of the splicing species is indicated on the right. The bottom panel shows the efficiency of 5′ splice site cleavage. Values are represented as means ± standard error of mean (SEM) from three experiments. (See also Figure S1).
(B) ATP-dependent rejection is reversible. Spliceosomes were stalled and purified as in A and then either frozen (no incubation) or incubated −/+ ATP. Spliceosomes incubated with ATP were then washed to remove ATP and incubated further −/+ ATP. Dashed lines indicate that samples were not incubated further.
(C) In the absence of ATP, U6/sU80 (Sp) spliceosomes catalyzed 5′ splice site cleavage in Mg2+ but with an apparent rate 10-fold lower than that in Mn2+ and 20 fold lower than that in Cd2+ (kMg = 0.010 ± 0.006 min−1; kMn = 0.13 ± 0.02 min−1; kCd = 0.22 ± 0.02 min−1 – errors reflect the error in the fit of the data points (see Experimental Procedures)). Reactions were as in A but without ATP. Error bars represent ± SEM of each data point for three experiments.
The catalysis of 5′ splice site cleavage by U6/sU80 (Sp) spliceosomes after removal of ATP (Figure 1A) suggested that the ATP-dependent activity may reject spliceosomes reversibly. To test explicitly for the reversibility of the ATP-dependent rejection step, we first incubated affinity-purified U6/sU80 (Sp) spliceosomes in Mg2+ in the presence of ATP to allow rejection and then, after washing away ATP, incubated the spliceosomes further in Mg2+ and in the presence or absence of ATP. During this second incubation in the presence of ATP, the spliceosome continued to repress 5′ splice site cleavage (Figure 1B, lane 5). However, in the absence of ATP, repression was relieved and the spliceosomes catalyzed 5′ splice site cleavage (Figure 1B, lane 4). Thus, the ATP-dependent activity rejects spliceosomes reversibly.
The role of the U80 pro-Sp non-bridging oxygen in exon ligation has been unclear – while more thiophilic metals rescue a block to 5′ splice site cleavage conferred by the U6/sU80 (Sp) sulfur substitution, a subsequent block to exon ligation could not be rescued (Yean et al., 2000). However, when we affinity-purified U6/sU80 (Sp) spliceosomes stalled on ACT1 pre-mRNA at the 5′ splice site cleavage stage in Mg2+ and then incubated in the presence of ATP and the thiophilic metal Cd2+, these spliceosomes not only catalyzed 5′ splice site cleavage but also exon ligation (Figures 1A, lane 7 and S1D, lane 3; see also Figure 3A, lane 3). In contrast, with the less thiophilic metal Mn2+, these spliceosomes only catalyzed 5′ splice site cleavage. These data provide evidence that the U6-U80 pro-Sp non-bridging oxygen binds a metal that is essential for both 5′ splice site cleavage and exon ligation, although the roles of this metal in each step remain to be determined.
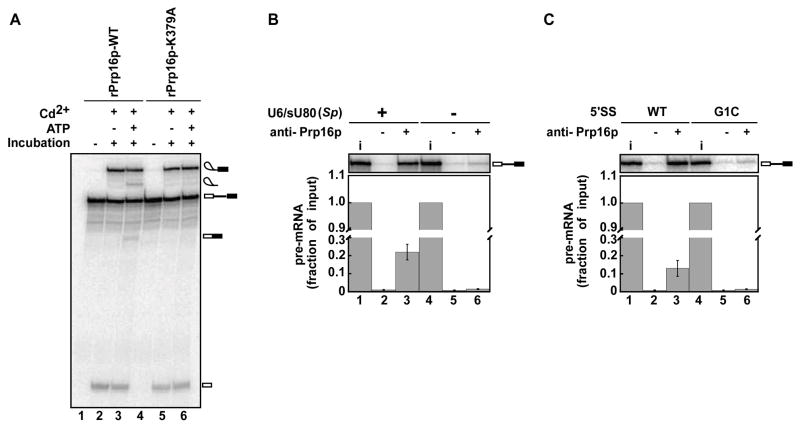
Figure 3. Prp16p can bind the spliceosome prior to 5′ splice site cleavage.
(A) In Cd2+, stalled and affinity purified U6/sU80 (Sp) spliceosomes undergo not only 5′ splice site cleavage but also exon ligation in an ATP- and Prp16p-dependent manner. Reactions were as in Figure 2B but included Cd2+ in the final incubation.
(B, C) Prp16p antibodies immunoprecipitate pre-mRNA bound to spliceosomes stalled prior to 5′ splice site cleavage but only if U6 is present (B) and the 5′ splice site conforms to the consensus sequence (C). Spliceosomes were stalled and purified as in Figure 1A, except in B, lanes 4–6, where U6/sU80 (Sp) was omitted and in C, lanes 4–6, where the 5′ splice site (5′SS) was mutated (G1C). Top panels show inputs (i) and immunoprecipitates, which were 10% and 40% of a splicing reaction, respectively; bottom panels show quantitation. Values and errors were as in Figure 1A.
Defective spliceosomes are rejected before 5′ splice site cleavage by the DEAH- box ATPase Prp16p
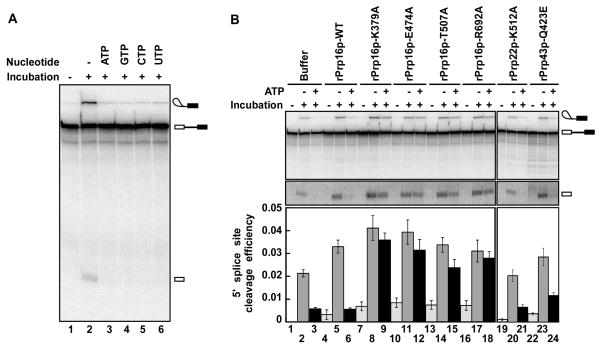
We next tested the prediction that this ATP-dependent activity that competes with 5′ splice site cleavage corresponds to the DEAH-box ATPase Prp16p, just as the ATP-dependent activity that competes with exon ligation corresponds to the DEAH-box ATPase Prp22p (Mayas et al., 2006). We first observed that 5′ splice site cleavage of U6/sU80 (Sp) spliceosomes in Mg2+ was repressed by GTP, CTP and UTP as efficiently as by ATP (Figure 2A), thereby implicating a nonspecific NTPase of the DEAH-box ATPase subfamily, such as Prp16p (Rocak and Linder, 2004; Schwer and Guthrie, 1992). To directly test for a role for Prp16p, we asked whether recombinant, mutated Prp16p compromised the ATP-dependent rejection of U6/sU80 (Sp) spliceosomes. We purified three dominant negative variants of Prp16p that permit 5′ splice site cleavage but block exon ligation when added to splicing reactions containing a wild-type substrate (Schneider et al., 2002). Additionally, we purified one hypomorphic variant (Prp16p-T507A) that is cold-sensitive but permits both 5′ splice site cleavage and exon ligation in vitro at the intermediate temperature of 23 °C (Hotz and Schwer, 1998; Schneider et al., 2002). We then added the mutated or wild-type rPrp16p to splicing extracts, reconstituted with U6/sU80 (Sp), affinity purified the stalled spliceosomes and then incubated in Mg2+ either in the absence or presence of ATP. As expected, in the case of spliceosomes purified from extracts supplemented with wild-type rPrp16p, 5′ splice site cleavage was repressed in the presence of ATP (Figure 2B, lane 6). Importantly, when spliceosomes purified from extracts supplemented with mutated rPrp16p were incubated in the presence of ATP, 5′ splice site cleavage was no longer repressed; rather, the spliceosomes permitted formation of lariat intermediate and 5′ exon, ~10-fold above the level permitted by wild-type rPrp16p for both the dominant negative mutants and the cold-sensitive mutant (Figure 2B, cf. lane 6 with 9, 12, 15 and 18). In contrast to the Prp16p mutations, mutations in the DEAH-box ATPases Prp22p and Prp43p did not compromise the ability of the spliceosome to repress 5′ splice site cleavage in the presence of ATP (Figure 2B, lanes 19–24). Verifying these observations, when U6/sU80 (Sp) spliceosomes were stalled in extract of the cold-sensitive mutant prp16-302, affinity-purified, and then incubated at low temperature, 5′ splice site cleavage was allowed even in the presence of ATP, ~8 fold above the level permitted in wild-type PRP16 extract (Figure S2). Thus, Prp16p is required for the ATP-dependent rejection of spliceosomes lacking an important interaction between Mg2+ and the pro-Sp non-bridging oxygen of U6-U80. These observations imply that Prp16p competes with 5′ splice site cleavage to promote the fidelity of this reaction.
Figure 2. The DEAH-box ATPase Prp16p rejects defective spliceosomes prior to 5′ splice site cleavage.
(A) 5′ splice site cleavage in U6/sU80 (Sp) spliceosomes is repressed by four different nucleoside triphosphates (NTPs). Reactions were as in Figure 1A, but with Mg2+ only.
(B) rPrp16p mutants but not wild type (WT) permit 5′ splice site cleavage by U6/sU80 (Sp) spliceosomes even in the presence of ATP. Reactions were as in Figure 1A, except spliceosomes were stalled in yeast extract supplemented with recombinant variants of Prp16p, Prp22p or Prp43p and then incubated only with Mg2+. The intensity of the middle panel is increased. Values and errors were as in Figure 1A. (See also Figure S2).
Prp16p can bind spliceosomes before 5′ splice site cleavage
Although Prp16p does not appear to bind to or function on a wild-type substrate until after 5′ site cleavage (Schwer and Guthrie, 1991; Schwer and Guthrie, 1992; Villa and Guthrie, 2005; Wang and Guthrie, 1998), our data showing a role for Prp16p in rejecting defective spliceosomes before 5′ splice site cleavage (Figure 2B) imply that Prp16p can bind to the spliceosome before 5′ splice site cleavage. Further, when U6/sU80 (Sp) spliceosomes are stalled just before 5′ splice site cleavage in Mg2+, affinity-purified and then rescued with Cd2+ in the presence of ATP, these spliceosomes not only catalyzed 5′ splice site cleavage but also exon ligation, again implying binding of Prp16p to the spliceosome before 5′ splice site cleavage (Figures 1A, lane 7; 3A, lane 3; S1D, lane 3 and S1E). Note that in these experiments exon ligation required Prp16p, because in parallel experiments utilizing extract supplemented with the dominant negative mutant rPrp16p-K379A rather than wild-type rPrp16p, the affinity-purified spliceosomes failed to catalyze exon ligation (Figure 3A, cf. lanes 3 and 6). Although previous studies have only found evidence for binding of Prp16p to splicing intermediates (Schwer and Guthrie, 1991; Schwer and Guthrie, 1992; Villa and Guthrie, 2005; Wang and Guthrie, 1998), these previous studies would have been unable to detect binding to pre-mRNA if Prp16p binds the substrate just before 5′ splice site cleavage in the context of a short-lived spliceosomal intermediate.
To test explicitly for the association of Prp16p with spliceosomes before 5′ splice site cleavage, we accumulated a spliceosomal intermediate stalled just before 5′ splice site cleavage using the U6/sU80 (Sp) modification and then assayed for immunoprecipitation of pre-mRNA using an anti-Prp16p antibody. Consistent with the association of Prp16p with spliceosomes prior to 5′ splice site cleavage, anti-Prp16p antibodies immunoprecipitated pre-mRNA 10-fold above background (Figure 3B, cf. lanes 2, 3). This association of Prp16p with the pre-mRNA depended on spliceosome assembly, because in the absence of U6 reconstitution, the levels of immunoprecipitated pre-mRNA decreased 8-fold (Figure 3B, cf. lanes 3, 6) and because a substrate defective for spliceosome assembly due to a G1C mutation at the 5′ splice site (Cheng and Abelson, 1987) decreased the levels of immunoprecipitated pre-mRNA 10-fold (Figure 3C, cf. 3 and 6). Although these experiments do not test whether Prp16p binds to the spliceosomes before 5′ splice site cleavage when cleavage is fast, these results indicate that Prp16p can bind to the spliceosome prior to 5′ splice site cleavage when cleavage is slow, consistent with a role in proofreading at this stage.
Evidence that Prp16p proofreads the rate of 5′ splice site cleavage
By competing with 5′ splice site cleavage, Prp16p could establish specificity and promote fidelity by rejecting a spliceosome faster than 5′ splice site cleavage in the case of a defective spliceosome and slower than 5′ splice site cleavage in the case of an intact spliceosome. This dependence of the relative rates on the integrity of the spliceosome could be established by one of two non-mutually exclusive mechanisms. First, the integrity of the spliceosome could alter the rate of Prp16p-dependent rejection. Second, the integrity of the spliceosome could alter the rate of 5′ splice site cleavage, a possibility that we tested. Specifically, after affinity purification of U6/sU80 (Sp) spliceosomes stalled in Mg2+, we measured the rate of 5′ splice site cleavage in Mg2+, Mn2+ or Cd2+ and in the absence of ATP, to eliminate Prp16p-mediated competition. The apparent rate of 5′ splice site cleavage in Mg2+ is ten fold slower than that in Mn2+ and twenty fold slower than that in Cd2+, correlating with the relative thiophilicity of each metal ion (Figure 1C; Pecoraro et al., 1984; Sigel, 1997). Thus, the spliceosomes that were rejected also spliced the slowest. These observations provide evidence that Prp16p proofreads splicing by distinguishing rates of 5′ splice site cleavage.
Discard of stalled pre-mRNA is mediated by the DEAH-box ATPase Prp43p
Because Prp16p rejects U6/sU80 (Sp) spliceosomes reversibly (Figures 1B), to promote fidelity, the spliceosome must also initiate a discard pathway to favor turnover of rejected pre-mRNA. Discard could be established by degrading the rejected pre-mRNA while still bound to the spliceosome (Bousquet-Antonelli et al., 2000) or by releasing the pre-mRNA from the spliceosome and then exporting and degrading the pre-mRNA, just as lariat intermediates rejected by Prp22p are released from the spliceosome and then exported and degraded (Hilleren and Parker, 2003; Mayas et al., 2010).
To test for discard of pre-mRNA in terms of release from the spliceosome, we assayed for release by glycerol gradient centrifugation. While we were unable to utilize an ACT1 transcript to assay for release of pre-mRNA from stalled spliceosomes, due to significant levels of unassembled pre-mRNA that would obscure discarded pre-mRNA (data not shown), we were able to use a UBC4 transcript for the discard experiments, because this substrate efficiently incorporates pre-mRNA into spliceosomes (Figure 4A, B). UBC4, similar to ACT1, suffers rejection when reconstituted with U6/sU80 (Sp) (Figure S1C). While a discarded population of UBC4 pre-mRNA was not evident at steady state, we considered the possibility that the pre-mRNA cycled between spliceosome-bound and discarded populations. To assay for discard of pre-mRNA by release from U6/sU80 (Sp)-stalled spliceosomes, we tested whether the spliceosome-bound pre-mRNA could be chased into a released population. Specifically, after stalling U6/sU80 (Sp) spliceosomes on labeled UBC4 in the presence of Mg2+, we added 100-fold excess of unlabeled UBC4 transcript to prevent reassembly of the spliceosome on discarded pre-mRNA. Before the chase, the majority of the pre-mRNA migrated rapidly peaking in fractions 16–20 where spliceosomes migrate (Figures 4A, B and S3; see below). After the chase, the faster migrating population of spliceosome-bound pre-mRNA decreased over time with a concomitant appearance of a new, slower migrating population of pre-mRNA peaking in fractions 11–14 (Figures 4A, B and S3A, B). To test whether this slower migrating pre-mRNA reflects free pre-mRNA released from the spliceosome, we assessed the migration of pre-mRNA that cannot assemble a spliceosome either due to a G1C/U2G double mutation of the 5′ splice site consensus sequence or due to an absence of ATP in the splicing reaction. While pre-mRNA in the absence of extract migrated near the top of the gradient, the unassembled pre-mRNA in the presence of extract peaked in fractions 11–14, coincident with the discarded population (cf. Figures 4A, B and S3A, B with 4E, F). These results suggest that the spliceosome discards stalled pre-mRNA through dissociation.
Figure 4. The DEAH-box ATPase Prp43p mediates discard of pre-mRNA stalled prior to 5′ splice site cleavage.
(A, B, C, D) The spliceosome discards stalled pre-mRNA and this discard requires Prp43p. U6/sU80 (Sp) spliceosomes were assembled on labeled UBC4 pre-mRNA in Mg2+ and in extracts supplemented with wild-type (WT) (A, B) or mutated (Q423E) (C, D) rPrp43p. The stalled spliceosomes were then chased by adding excess unlabeled UBC4 pre-mRNA. The reactions were analyzed by glycerol gradient before (top) and after (bottom) the chase (A, C). Inputs (i) represent 10% of the reaction. The pre-mRNA in each fraction was quantitated (B, D). (See also Figure S3).
(E, F) Discarded and unassembled pre-mRNA migrate similarly. (E) Glycerol gradient analysis is shown for pre-mRNA in the absence of extract (top) or in the presence of extract but in the absence of ATP (middle) or with a G1C/U2G double mutation of the 5′ splice site consensus sequence (bottom). (F) Quantitation of E.
(G, H) Efficient discard of stalled pre-mRNA is prevented by mutated rPrp16p. (G) Reactions were as in Figure 4A and C, except extracts were supplemented with wild-type (WT) or mutated (K379A) rPrp16p, and reactions were analyzed only after the chase.
Recently, we identified a role for the DEAH-box ATPase Prp43p in discarding splicing intermediates (Mayas et al., 2010), in addition to its role in releasing the excised intron and disassembling the spliceosome once splicing of a genuine substrate is complete (Arenas and Abelson, 1997; Martin et al., 2002; Small et al., 2006; Tsai et al., 2005). Further, mutations in either prp43 or its cofactors suppress splicing factor mutations in vivo that compromise spliceosome activation in vitro, suggesting a role for Prp43p in disassembling defective spliceosomes (Pandit et al., 2006). To test whether Prp43p discards stalled pre-mRNA, we supplemented extract with either wild-type rPrp43p or mutated rPrp43p having the dominant negative mutation Q423E. As expected, when spliceosomes were assembled on labeled UBC4 pre-mRNA in extracts supplemented with wild-type rPrp43p and then chased with unlabeled UBC4 pre-mRNA, the faster migrating population of spliceosome-bound pre-mRNA decreased with a concomitant appearance of the slower migrating population of discarded pre-mRNA (Figure 4A, B); discard of the pre-mRNA was also observed in the absence of supplemental rPrp43p (Figure S3A, B). However, when spliceosomes were assembled on labeled UBC4 pre-mRNA in extracts supplemented with mutated rPrp43p and then chased with unlabeled UBC4 pre-mRNA, the spliceosome bound fraction of pre-mRNA remained intact and no discarded pre-mRNA appeared (Figure 4C, D); in contrast, mutated rPrp22p did not compromise the discard of pre-mRNA (Figure S3E, F). Validating this observation, when U6/sU80 (Sp) spliceosomes were stalled in extract from the cold-sensitive mutant prp43-Q423N or the isogenic wild-type strain and then chased with unlabeled UBC4, in the mutant extract the pre-mRNA remained bound to the spliceosome (data not shown). Together, these results provide evidence that Prp43p mediates the discard of stalled pre-mRNA.
To determine whether discard of pre-mRNA from the spliceosome requires Prp16p-mediated rejection, we supplemented extract with either wild-type rPrp16p or mutated rPrp16p having the dominant negative mutation K379A. When spliceosomes were assembled in extracts supplemented with wild-type rPrp16p and then chased, the faster migrating population of spliceosome-bound pre-mRNA decreased with a concomitant appearance of the slower migrating population of discarded pre-mRNA (Figure 4G, H). However, when spliceosomes were assembled in extracts supplemented with mutated rPrp16p and then chased, the spliceosome bound fraction of pre-mRNA remained intact and no discarded pre-mRNA appeared. Consistent with an upstream requirement for Prp16p, mutated rPrp43p does not detectably compromise the fidelity of 5′ splice site cleavage (Figure 2B and data not shown). Although we cannot exclude the possibility that mutated rPrp16p prevents the binding of Prp43p and subsequent discard of pre-mRNA in these experiments, these results provide evidence that Prp43p-mediated discard of stalled pre-mRNA requires Prp16p-mediated rejection.
DISCUSSION
DExD/H-box ATPases have been implicated in proofreading multiple steps in pre-mRNA splicing (Burgess and Guthrie, 1993b; Mayas et al., 2006; Mayas et al., 2010; Xu and Query, 2007), and may proofread other RNA processes as well. To investigate the mechanism of fidelity during 5′ splice site cleavage in pre-mRNA splicing, we developed an in vitro assay sensitive to proofreading at this critical step. Proofreading required ATP and the DEAH-box ATPase Prp16p (Figures 1 and 2). Although Prp16p is not required until after 5′ splice site cleavage of a wild-type substrate (Schwer and Guthrie, 1991), Prp16p could bind to the spliceosome before 5′ splice site cleavage, consistent with a role for Prp16p in proofreading 5′ splice site cleavage (Figure 3). Significantly, Prp16p proofreads a putative catalytic metal-ligand interaction important for 5′ splice site cleavage, and we discovered evidence that Prp16p does so by distinguishing between slow and fast rates of 5′ splice site cleavage (Figure 1C). Finally, Prp16p-mediated rejection was reversible and the spliceosome discarded pre-mRNA through dissociation by the DEAH-box ATPase Prp43p (Figures 1B, 4). Our data establish a pathway for proofreading and discard at the stage of 5′ splice site cleavage (Figure 5), and provide evidence that DExD/H-box ATPases establish specificity in splicing by discriminating against substrates that proceed slowly.
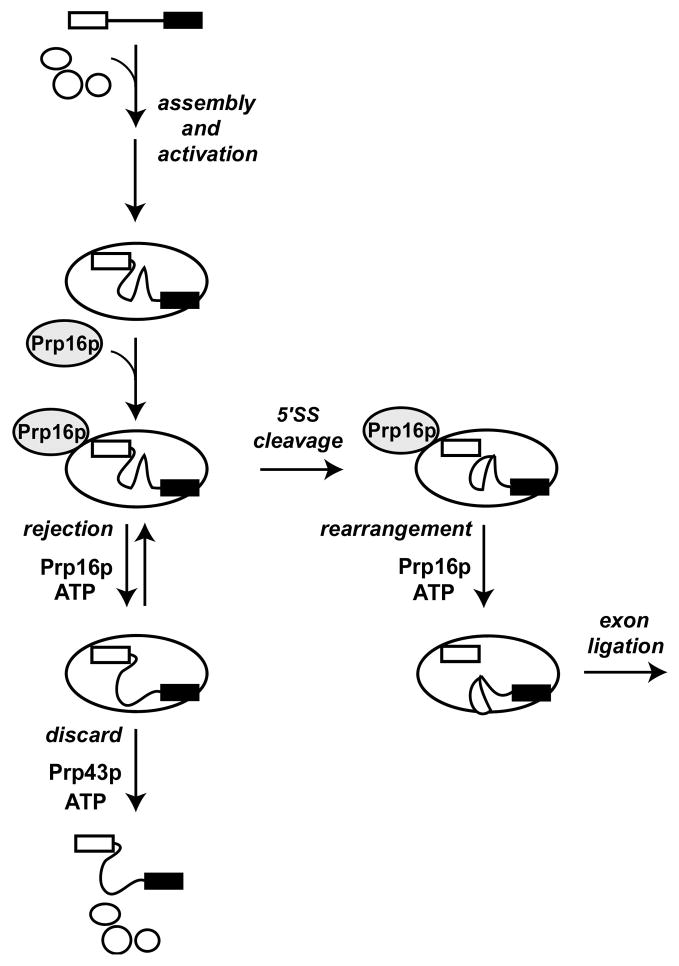
Figure 5. A collaborative role for Prp16p and Prp43p in the fidelity of 5′ splice site cleavage.
A simplified pathway for splicing is shown, emphasizing the two roles for Prp16p and the role for Prp43p at the stage of 5′ splice site cleavage. Before 5′ splice site cleavage, Prp16p antagonizes splicing by competing with 5′ splice site cleavage and thereby permits rejection of suboptimal pre-mRNA through a kinetic proofreading mechanism. After rejection, a pre-mRNA may return to the 5′ splice site cleavage conformation or discard in a Prp43p-dependent manner. After 5′ splice site cleavage, Prp16p promotes splicing by allowing exon ligation. In a model for kinetic proofreading, the fidelity of 5′ splice site cleavage is enhanced by a competition between 5′ splice site cleavage and the ATP-dependent activity of Prp16p. Specificity for a wild-type substrate would be established if k5′ splice site cleavage (wild-type) > k5′ splice site cleavge (aberrant) and/or if krejection(aberrant) > krejection(wild-type). Our data provide evidence for the former case.
Prp16p promotes the fidelity of branch point recognition (Burgess and Guthrie, 1993b). Genetic studies suggested that Prp16p proofreads the branch site either before or after 5′ splice site cleavage (Burgess and Guthrie, 1993b; Query and Konarska, 2004; also see Introduction). By demonstrating that Prp16p can bind spliceosome-bound pre-mRNA and repress 5′ splice site cleavage, our data provide biochemical evidence that Prp16p antagonizes mutated substrates before 5′ splice site cleavage.
Still, Prp16p promotes exon ligation of a wild-type substrate by rearranging the spliceosome immediately after 5′ splice site cleavage (Query and Konarska, 2004; Schwer and Guthrie, 1991; Schwer and Guthrie, 1992; Villa and Guthrie, 2005). This dual role for Prp16p at 5′ splice site cleavage parallels the dual role for Prp22p at exon ligation (Mayas et al., 2006). In the case of a mutated substrate, Prp22p rejects intermediates by rearranging the spliceosome before exon ligation, while in the case of a wild-type substrate, Prp22p promotes release of mRNA by rearranging the spliceosome immediately after exon ligation (Company et al., 1991; Schwer and Gross, 1998). Together, these data indicate that the chemical steps of splicing are proofread by a common ATP-dependent framework mediated by DExD/H-box ATPases.
Over 30 years ago, Hopfield and Ninio independently proposed a general kinetic proofreading model for fidelity in which the specificity of an enzyme-substrate interaction is increased by expending energy to establish additional inspections of the substrate after initial binding (Hopfield, 1974; Ninio, 1975). In this model the specificity of an interaction is enhanced by a branch in the reaction pathway through which mutated substrates are preferentially rejected (Burgess and Guthrie, 1993a; Hopfield, 1974; Ninio, 1975; Query and Konarska, 2006; Yarus, 1992a; Yarus, 1992b). In support of the importance of kinetic proofreading in splicing, at least three of the eight spliceosomal DExD/H-box ATPases establish branched pathways that compete with splicing to promote fidelity (Burgess and Guthrie, 1993b; Mayas et al., 2006; Xu and Query, 2007). However, it has remained unclear how such competitions establish specificity – how optimal substrates are favored on the productive splicing pathway and suboptimal substrates are favored on a rejection pathway.
In a kinetic proofreading model, specificity can be established by one of two non-mutually exclusive mechanisms. In the case of 5′ splice site cleavage, specificity could be established because either the rate of Prp16p-mediated rearrangement or the rate of 5′ splice site cleavage varies with the identity of the substrate or the integrity of the spliceosome (Figure 5). In the first case, Prp16p would act as a sensor of the authenticity of a substrate. In this case, a suboptimal substrate would trigger Prp16p activity before 5′ splice site cleavage, perhaps by directly activating Prp16p or by destabilizing or loosening the 5′ splice site cleavage conformation of the spliceosome. In contrast, an optimal substrate would resist Prp16p activity by repressing Prp16p or by stabilizing or compacting the spliceosome until after 5′ splice site cleavage when the altered connectivity of the substrate would activate Prp16p. Supporting this mechanistic possibility for specificity, cofactors both repress and activate DExD/H-box ATPases (Alcazar-Roman et al., 2006; Ballut et al., 2005; Bellare et al., 2008; Maeder et al., 2009; Small et al., 2006; Tanaka et al., 2007; Weirich et al., 2006). Additionally, the activity of a DExD/H box-ATPase can depend on the stability of its substrate (Bhaskaran and Russell, 2007). Whether Prp16p or any other spliceosomal DExD/H box ATPase functions as a sensor of the authenticity of a substrate remains to be determined.
In the second case, Prp16p would act as a sensor of time, effectively limiting the window for catalysis. In this case, with an optimal substrate that splices fast, Prp16p would act after 5′ splice site cleavage, resulting in advancement of the substrate along the splicing pathway. With a mutated substrate that splices slowly, Prp16p would act before 5′ splice site cleavage, resulting in rejection of the substrate. In support of this timer model, in the absence of ATP the apparent rate of 5′ splice site cleavage with U6/sU80 (Sp) spliceosomes was significantly slower in Mg2+ than in Mn2+ or Cd2+, establishing that a slower rate in Mg2+ correlates with the ATP- and Prp16-dependent rejection of U6/sU80 (Sp) spliceosomes in Mg2+ (Figures 1 and 2B). These observations provide evidence that DExD/H-box ATPases enhance the fidelity of pre-mRNA splicing by discriminating between different rates for genuine and aberrant substrates during proofreading. For example, these data predict that Prp22p discriminates against suboptimal 3′ splice sites because such splice sites undergo exon ligation slowly, although this prediction remains to be tested.
In the simplest model Prp16p antagonizes suboptimal substrates and promotes optimal substrates before and after 5′ splice site cleavage by the same mechanism. Evidence suggests that the spliceosome toggles between closed catalytic and open substrate-binding conformations and that Prp16p mediates opening of the closed catalytic conformation after 5′ splice site cleavage (Hilliker et al., 2007; Liu et al., 2007; Perriman and Ares, 2007; Query and Konarska, 2004; Villa and Guthrie, 2005). Because Prp16p unwinds RNA duplexes in vitro (Wang et al., 1998), Prp16p likely promotes this closed-to-open transition through disruption of RNA and/or RNP structures that stabilize the 5′ splice site cleavage conformation. Indeed, prp16 mutants are suppressed by disrupting base pairing interactions in two key snRNA structures that stabilize the 5′ splice site cleavage conformation - U2 stem IIc and U2/U6 helix I (Hilliker et al., 2007; Madhani and Guthrie, 1994; Mefford and Staley, 2009; Perriman and Ares, 2007). Further, prp16 mutants are suppressed by disrupting undefined interactions involving U6, Prp8p and Isy1p that also stabilize the 5′ splice site cleavage conformation (Madhani and Guthrie, 1994; Query and Konarska, 2004; Villa and Guthrie, 2005). Moreover, disruption of U2 stem IIc and U2/U6 helix I as well as mutation of PRP8 and deletion of ISY1 enhance discrimination against a mutated branch site substrate and thereby increase fidelity mediated by Prp16p (Hilliker et al., 2007; Madhani and Guthrie, 1994; Perriman and Ares, 2007; Query and Konarska, 2004; Villa and Guthrie, 2005). Thus, Prp16p appears to increase the specificity of 5′ splice site cleavage by simply disrupting equivalent interactions before or after 5′ splice site cleavage, depending on the identity of the substrate.
Importantly, Prp16p-mediated rejection is reversible (Figures 1B). We propose that this reversibility delays discard of a substrate to allow sampling of alternative splice sites and potentially regulation of splice site choice. This reversibility also necessitates a downstream discard pathway that provides an opportunity to abort splicing. Spliceosome-bound pre-mRNA can be degraded by a nuclear pathway mediated by Rat1p or the nuclear exosome (Bousquet-Antonelli et al., 2000). However, lariat intermediates stalled by a mutation at the 3′ splice site are degraded by a cytoplasmic pathway mediated by Xrn1p or the cytoplasmic exosome (Hilleren and Parker, 2003). These mutated lariat intermediates are not only rejected by Prp22p (Mayas et al., 2006) but also exported after dissociation from the spliceosome by the DEAH-box ATPase Prp43p (Mayas et al., 2010), a factor also required to dissociate a genuine excised intron (Arenas and Abelson, 1997; Martin et al., 2002).
In vitro the spliceosome similarly dissociates stalled pre-mRNA by a pathway that requires Prp43p (Figure 4), suggesting that in vivo rejected pre-mRNA may be discarded by dissociation, export and degradation. Consistent with this model, mutations in prp43 and its cofactors suppress splicing factor mutations defective in spliceosome activation (Pandit et al., 2006). In addition, mutated pre-mRNA can be exported and degraded in the cytoplasm (Hilleren and Parker, 2003; Legrain and Rosbash, 1989; Rain and Legrain, 1997; Rutz and Seraphin, 2000). In such cases, it has been assumed that the pre-mRNA is exported due to a failure to bind the spliceosome. However, our data suggests that some of the mutated pre-mRNA degraded in the cytoplasm may have successfully engaged the spliceosome but subsequently suffered rejection and then discard through dissociation, export and degradation. In support of this hypothesis, a mutated ACT1 pre-mRNA substrate having the U257A branch site mutation is not only degraded in the cytoplasm but also proofread by Prp16p (Burgess and Guthrie, 1993b; Hilleren and Parker, 2003). Thus, our data suggest that mutated precursors and lariat intermediates stalled at the chemical steps are not only rejected by parallel mechanisms but also discarded by parallel mechanisms.
The role for Prp43p at the precursor, intermediate and product stages of splicing suggests a target for this molecular motor that is common to all three stages of splicing. Such a target remains to be defined but formally could comprise U2, U5, U6 and/or the intron itself. Notably, Prp43p is also associated with pre-rRNA and histone pre-mRNA processing (Combs et al., 2006; Friend et al., 2007; Lebaron et al., 2005; Leeds et al., 2006); suggesting that Prp43p may play an analogous discard role in these reactions. Intriguingly, histone pre-mRNA processing also requires U2 (Friend et al., 2007), and human Prp43p has been identified as a component of the U2 snRNP (Behzadnia et al., 2007), raising the possibility that Prp43p may target a U2-substrate interaction.
While at least three of the eight spliceosomal DExD/H box ATPases function at specific steps to promote proofreading, our data suggest that a single DExD/H box ATPase Prp43p functions to discard suboptimal substrates at two stages, minimally, in addition to discarding the excised intron. This implication raises the questions of how Prp43p is regulated. Although discard of the excised intron requires the prior activity of Prp22p, discard of the pre-mRNA and the lariat intermediate does not (Figure S3E, F; Mayas et al., 2010), implying that discard does not simply follow the pathway for splicing an optimal substrate. Given the roles of the DExD/H box ATPase Brr2p, the GTPase Snu114p and the Prp43p cofactors Ntr1 and Ntr2 in discard of the excised intron (Boon et al., 2006; Small et al., 2006; Tanaka et al., 2007; Tsai et al., 2005), these factors represent potential collaborators in the discard of suboptimal substrates. Finally, given the general role of Prp43p in terminating splicing, this ATPase represents an intriguing potential target in the regulation of splicing.
EXPERIMENTAL PROCEDURES
Plasmids, Strains and Synthetic U6 snRNA
See Supplemental Experimental Procedures for details.
In vitro splicing
Pre-mRNA substrates and S. cerevisiae splicing extracts were prepared as described (Mayas et al., 2006). U6 was depleted and reconstituted from extract, as described with modifications (see Supplemental Experimental Procedures; Yean et al., 2000). Splicing reactions and immunoprecipitations were performed as described with modifications (see Supplemental Experimental Procedures; Schwer and Guthrie, 1992; Stevens and Abelson, 2002).
Recombinant proteins
His6-tagged variants of Prp16p, Prp22p and Prp43p were purified as described (Martin et al., 2002; Schneider et al., 2002).
Glycerol gradient centrifugation
Glycerol gradients were performed as described (Mayas et al., 2010). In the discard assays, U6/sU80 (Sp)-stalled spliceosomes were chased with a 100-fold excess of unlabeled transcript for 30 min.
Supplementary Material
Acknowledgments
We thank the Staley lab for discussions and critically reading the manuscript; J. Abelson for the UBC4 transcription template; C. Guthrie for anti-Prp16 antibodies; S.-C. Cheng for anti-Ntc20 antibodies; R. Mayas, D. Semlow and S. Fica for reagents; and C. Jordan, and M. Norman for experimental support. This work was supported by an American Cancer Society Illinois Division Stephen F. Sener, M.D. - Research Scholar Award to J.P.S. (06-099-01-GMC) and a grant to J.P.S. from the U.S. National Institutes of Health (GM62264).
Footnotes
The Supplemental Data includes three Figures, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at _.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- Arenas JE, Abelson JN. Prp43: An RNA helicase-like factor involved in spliceosome disassembly. Proc Natl Acad Sci USA. 1997;94:11798–11802. doi: 10.1073/pnas.94.22.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballut L, Marchadier B, Baguet A, Tomasetto C, Seraphin B, Le Hir H. The exon junction core complex is locked onto RNA by inhibition of eIF4AIII ATPase activity. Nat Struct Mol Biol. 2005;12:861–869. doi: 10.1038/nsmb990. [DOI] [PubMed] [Google Scholar]
- Behzadnia N, Golas MM, Hartmuth K, Sander B, Kastner B, Deckert J, Dube P, Will CL, Urlaub H, Stark H, Luhrmann R. Composition and three-dimensional EM structure of double affinity-purified, human prespliceosomal A complexes. Embo J. 2007;26:1737–1748. doi: 10.1038/sj.emboj.7601631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellare P, Small EC, Huang X, Wohlschlegel JA, Staley JP, Sontheimer EJ. A role for ubiquitin in the spliceosome assembly pathway. Nat Struct Mol Biol. 2008;15:444–451. doi: 10.1038/nsmb.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran H, Russell R. Kinetic redistribution of native and misfolded RNAs by a DEAD-box chaperone. Nature. 2007;449:1014–1018. doi: 10.1038/nature06235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon KL, Auchynnikava T, Edwalds-Gilbert G, Barrass JD, Droop AP, Dez C, Beggs JD. Yeast ntr1/spp382 mediates prp43 function in postspliceosomes. Mol Cell Biol. 2006;26:6016–6023. doi: 10.1128/MCB.02347-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Antonelli C, Presutti C, Tollervey D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell. 2000;102:765–775. doi: 10.1016/s0092-8674(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Burgess SM, Guthrie C. Beat the clock: paradigms for NTPases in the maintenance of biological fidelity. Trends Biochem Sci. 1993a;18:381–384. doi: 10.1016/0968-0004(93)90094-4. [DOI] [PubMed] [Google Scholar]
- Burgess SM, Guthrie C. A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell. 1993b;73:1377–1391. doi: 10.1016/0092-8674(93)90363-u. [DOI] [PubMed] [Google Scholar]
- Cheng SC, Abelson J. Spliceosome assembly in yeast. Genes Dev. 1987;1:1014–1027. doi: 10.1101/gad.1.9.1014. [DOI] [PubMed] [Google Scholar]
- Combs DJ, Nagel RJ, Ares M, Jr, Stevens SW. Prp43p is a DEAH-box spliceosome disassembly factor essential for ribosome biogenesis. Mol Cell Biol. 2006;26:523–534. doi: 10.1128/MCB.26.2.523-534.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Company M, Arenas J, Abelson J. Requirement of the RNA helicase-like protein Prp22 for release of messenger RNA from spliceosomes. Nature. 1991;349:487–493. doi: 10.1038/349487a0. [DOI] [PubMed] [Google Scholar]
- Friend K, Lovejoy AF, Steitz JA. U2 snRNP binds intronless histone pre-mRNAs to facilitate U7-snRNP-dependent 3′ end formation. Mol Cell. 2007;28:240–252. doi: 10.1016/j.molcel.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren PJ, Parker R. Cytoplasmic degradation of splice-defective pre-mRNAs and intermediates. Mol Cell. 2003;12:1453–1465. doi: 10.1016/s1097-2765(03)00488-x. [DOI] [PubMed] [Google Scholar]
- Hilliker AK, Mefford MA, Staley JP. U2 toggles iteratively between the stem IIa and stem IIc conformations to promote pre-mRNA splicing. Genes Dev. 2007;21:821–834. doi: 10.1101/gad.1536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfield JJ. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz HR, Schwer B. Mutational analysis of the yeast DEAH-box splicing factor Prp16. Genetics. 1998;149:807–815. doi: 10.1093/genetics/149.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler A, Nikstad LJ, Allmann AM, Brow DA, Butcher SE. Metal binding and base ionization in the U6 RNA intramolecular stem-loop structure. Nat Struct Biol. 2002;9:431–435. doi: 10.1038/nsb800. [DOI] [PubMed] [Google Scholar]
- Lebaron S, Froment C, Fromont-Racine M, Rain JC, Monsarrat B, Caizergues-Ferrer M, Henry Y. The splicing ATPase prp43p is a component of multiple preribosomal particles. Mol Cell Biol. 2005;25:9269–9282. doi: 10.1128/MCB.25.21.9269-9282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds NB, Small EC, Hiley SL, Hughes TR, Staley JP. The splicing factor Prp43p, a DEAH box ATPase, functions in ribosome biogenesis. Mol Cell Biol. 2006;26:513–522. doi: 10.1128/MCB.26.2.513-522.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain P, Rosbash M. Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell. 1989;57:573–583. doi: 10.1016/0092-8674(89)90127-x. [DOI] [PubMed] [Google Scholar]
- Liu L, Query CC, Konarska MM. Opposing classes of prp8 alleles modulate the transition between the catalytic steps of pre-mRNA splicing. Nat Struct Mol Biol. 2007;14:519–526. doi: 10.1038/nsmb1240. [DOI] [PubMed] [Google Scholar]
- Madhani HD, Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992;71:803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- Madhani HD, Guthrie C. Genetic interactions between the yeast RNA helicase homolog Prp16 and spliceosomal snRNAs identify candidate ligands for the Prp16 RNA-dependent ATPase. Genetics. 1994;137:677–687. doi: 10.1093/genetics/137.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder C, Kutach AK, Guthrie C. ATP-dependent unwinding of U4/U6 snRNAs by the Brr2 helicase requires the C terminus of Prp8. Nat Struct Mol Biol. 2009;16:42–48. doi: 10.1038/nsmb.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Schneider S, Schwer B. Prp43 is an essential RNA-dependent ATPase required for release of lariat-intron from the spliceosome. J Biol Chem. 2002;277:17743–17750. doi: 10.1074/jbc.M200762200. [DOI] [PubMed] [Google Scholar]
- Mayas RM, Maita H, Semlow DR, Staley JP. Spliceosome discards intermediates via the DEAH box ATPase Prp43p. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0906022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayas RM, Maita H, Staley JP. Exon ligation is proofread by Prp22p, a DExD/H-box ATPase. Nat Struct Mol Biol. 2006;13:482–490. doi: 10.1038/nsmb1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford MA, Staley JP. Evidence that U2/U6 helix I promotes both catalytic steps of pre-mRNA splicing and rearranges in between these steps. RNA. 2009;15:1386–1397. doi: 10.1261/rna.1582609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57:587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- Pandit S, Lynn B, Rymond BC. Inhibition of a spliceosome turnover pathway suppresses splicing defects. Proc Natl Acad Sci U S A. 2006;103:13700–13705. doi: 10.1073/pnas.0603188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecoraro VL, Hermes JD, Cleland WW. Stability constants of Mg2+ and Cd2+ complexes of adenine nucleotides and thionucleotides and rate constants for formation and dissociation of MgATP and MgADP. Biochemistry. 1984;23:5262–5271. doi: 10.1021/bi00317a026. [DOI] [PubMed] [Google Scholar]
- Perriman R, Ares M., Jr ATP can be dispensable for prespliceosome formation in yeast. Genes Dev. 2000;14:97–107. [PMC free article] [PubMed] [Google Scholar]
- Perriman RJ, Ares M., Jr Rearrangement of competing U2 RNA helices within the spliceosome promotes multiple steps in splicing. Genes Dev. 2007;21:811–820. doi: 10.1101/gad.1524307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Query CC, Konarska MM. Suppression of multiple substrate mutations by spliceosomal prp8 alleles suggests functional correlations with ribosomal ambiguity mutants. Mol Cell. 2004;14:343–354. doi: 10.1016/s1097-2765(04)00217-5. [DOI] [PubMed] [Google Scholar]
- Query CC, Konarska MM. Splicing fidelity revisited. Nat Struct Mol Biol. 2006;13:472–474. doi: 10.1038/nsmb0606-472. [DOI] [PubMed] [Google Scholar]
- Rain JC, Legrain P. In vivo commitment to splicing in yeast involves the nucleotide upstream from the branch site conserved sequence and the Mud2 protein. EMBO J. 1997;16:1759–1771. doi: 10.1093/emboj/16.7.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocak S, Linder P. DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol. 2004;5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- Ruby SW, Chang TH, Abelson J. Four yeast spliceosomal proteins (PRP5, PRP9, PRP11, and PRP21) interact to promote U2 snRNP binding to pre-mRNA. Genes Dev. 1993;7:1909–1925. doi: 10.1101/gad.7.10.1909. [DOI] [PubMed] [Google Scholar]
- Rutz B, Seraphin B. A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. EMBO J. 2000;19:1873–1886. doi: 10.1093/emboj/19.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Hotz HR, Schwer B. Characterization of dominant-negative mutants of the DEAH-box splicing factors Prp22 and Prp16. J Biol Chem. 2002;277:15452–15458. doi: 10.1074/jbc.M112473200. [DOI] [PubMed] [Google Scholar]
- Schwer B, Gross CH. Prp22, a DExH-box RNA helicase, plays two distinct roles in yeast pre- mRNA splicing. EMBO J. 1998;17:2086–2094. doi: 10.1093/emboj/17.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwer B, Guthrie C. Prp16 is an RNA-dependent ATPase that interacts transiently with the spliceosome. Nature. 1991;349:494–499. doi: 10.1038/349494a0. [DOI] [PubMed] [Google Scholar]
- Schwer B, Guthrie C. A conformational rearrangement in the spliceosome is dependent on Prp16 and ATP hydrolysis. EMBO J. 1992;11:5033–5039. doi: 10.1002/j.1460-2075.1992.tb05610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel RKO, Song B, Sigel H. Stabilities and structures of metal ion complexes of Adenosine 5′-O-Thiomonophosphate (AMPS2−) in comparison with those of its parent nucleotide (AMP2−) in aqueous solution. J Am Chem Soc. 1997;119:744–755. [Google Scholar]
- Small EC, Leggett SR, Winans AA, Staley JP. The EF-G–like GTPase Snu114 regulates spliceosome dynamics mediated by Brr2p, a DExD/H-box ATPase. Mol Cell. 2006;23:389–399. doi: 10.1016/j.molcel.2006.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci USA. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SW, Abelson J. Yeast pre-mRNA splicing: methods, mechanisms, and machinery. Methods Enzymol. 2002;351:200–220. doi: 10.1016/s0076-6879(02)51849-8. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Aronova A, Schwer B. Ntr1 activates the Prp43 helicase to trigger release of lariat-intron from the spliceosome. Genes Dev. 2007;21:2312–2325. doi: 10.1101/gad.1580507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor N, Keating KS, Taylor SD, Pyle AM. Crystal structure of a self-spliced group II intron. Science. 2008;320:77–82. doi: 10.1126/science.1153803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RT, Fu RH, Yeh FL, Tseng CK, Lin YC, Huang YH, Cheng SC. Spliceosome disassembly catalyzed by Prp43 and its associated components Ntr1 and Ntr2. Genes Dev. 2005;19:2991–3003. doi: 10.1101/gad.1377405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CK, Cheng SC. Both catalytic steps of nuclear pre-mRNA splicing are reversible. Science. 2008;320:1782–1784. doi: 10.1126/science.1158993. [DOI] [PubMed] [Google Scholar]
- Valadkhan S, Manley JL. Splicing-related catalysis by protein-free snRNAs. Nature. 2001;413:701–707. doi: 10.1038/35099500. [DOI] [PubMed] [Google Scholar]
- Valadkhan S, Mohammadi A, Jaladat Y, Geisler S. Protein-free small nuclear RNAs catalyze a two-step splicing reaction. Proc Natl Acad Sci U S A. 2009;106:11901–11906. doi: 10.1073/pnas.0902020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T, Guthrie C. The Isy1p component of the NineTeen complex interacts with the ATPase Prp16p to regulate the fidelity of pre-mRNA splicing. Genes Dev. 2005;19:1894–1904. doi: 10.1101/gad.1336305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa T, Pleiss JA, Guthrie C. Spliceosomal snRNAs: Mg(2+)-dependent chemistry at the catalytic core? Cell. 2002;109:149–152. doi: 10.1016/s0092-8674(02)00726-2. [DOI] [PubMed] [Google Scholar]
- Wahl MC, Will CL, Luhrmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guthrie C. PRP16, a DEAH-box RNA helicase, is recruited to the spliceosome primarily via its nonconserved N-terminal domain. RNA. 1998;4:1216–1229. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wagner JD, Guthrie C. The DEAH-box splicing factor Prp16 unwinds RNA duplexes in vitro. Curr Biol. 1998;8:441–451. doi: 10.1016/s0960-9822(98)70178-2. [DOI] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- Xu YZ, Newnham CM, Kameoka S, Huang T, Konarska MM, Query CC. Prp5 bridges U1 and U2 snRNPs and enables stable U2 snRNP association with intron RNA. EMBO J. 2004;23:376–385. doi: 10.1038/sj.emboj.7600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YZ, Query CC. Competition between the ATPase Prp5 and branch region-U2 snRNA pairing modulates the fidelity of spliceosome assembly. Mol Cell. 2007;28:838–849. doi: 10.1016/j.molcel.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. Proofreading, NTPases and translation: constraints on accurate biochemistry. Trends Biochem Sci. 1992a;17:130–133. doi: 10.1016/0968-0004(92)90320-9. [DOI] [PubMed] [Google Scholar]
- Yarus M. Proofreading, NTPases and translation: successful increase in specificity. Trends Biochem Sci. 1992b;17:171–174. doi: 10.1016/0968-0004(92)90257-a. [DOI] [PubMed] [Google Scholar]
- Yean SL, Wuenschell G, Termini J, Lin RJ. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature. 2000;408:881–884. doi: 10.1038/35048617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.