Abstract
Background
Rhythm control for atrial fibrillation (AF) is cumbersome because of its progressive nature caused by structural remodelling. Upstream therapy refers to therapeutic interventions aiming to modify the atrial substrate, leading to prevention of AF.
Objective
The Routine versus Aggressive upstream rhythm Control for prevention of Early AF in heart failure (RACE 3) study hypothesises that aggressive upstream rhythm control increases persistence of sinus rhythm compared with conventional rhythm control in patients with early AF and mild-to-moderate early systolic or diastolic heart failure undergoing electrical cardioversion.
Design
RACE 3 is a prospective, randomised, open, multinational, multicenter trial. Upstream rhythm control consists of angiotensin converting enzyme inhibitors and/or angiotensin receptor blockers, mineralocorticoid receptor antagonists, statins, cardiac rehabilitation therapy, and intensive counselling on dietary restrictions, exercise maintenance, and drug adherence. Conventional rhythm control consists of routine rhythm control therapy without cardiac rehabilitation therapy and intensive counselling. In both arms, every effort is made to keep patients in the rhythm control strategy, and ion channel antiarrhythmic drugs or pulmonary vein ablation may be instituted if AF relapses. Total inclusion will be 250 patients. If upstream therapy proves to be effective in improving maintenance of sinus rhythm, it could become a new approach to rhythm control supporting conventional pharmacological and non-pharmacological rhythm control.
Keywords: Atrial fibrillation, Secondary prevention, Upstream therapy, Heart failure
Background and rationale
Atrial fibrillation (AF) is the most common cardiac arrhythmia, currently affecting more than six million people in Europe. The prevalence of AF is expected to increase twofold during the next 30–50 years, partly due to the ageing population and lifestyle changes [1]. AF is responsible for an increased risk of death, stroke, heart failure, hospitalisation, and an impaired quality of life. In order to improve outcome and to promote healthy ageing, it is important to develop safe and effective treatment strategies. Rhythm control is the therapeutic strategy of choice in patients who have symptomatic AF [2]. However, AF is difficult to treat because of the progressive nature of the arrhythmia.
Structural remodelling seems to play an important role in the initiation and maintenance of AF. It can be caused by risk factors for AF including increasing age, underlying diseases and other factors such as altered metabolism, autonomic changes, and genetic and environmental influences [3]. These factors induce atrial structural changes through various pathways including the renin-angiotensin-aldosterone system (RAAS) and inflammation, leading to enlarged atria, hypertrophy, fibrosis, and myolysis [4–6]. Structural remodelling creates a substrate for AF as a result of electrical dissociation between muscle bundles and local conduction heterogeneities facilitating the initiation and perpetuation of AF [5, 6]. Indeed, the first manifestation of AF usually occurs after years of atrial and ventricular remodelling [7, 8]. Once AF develops, it causes marked changes in atrial electrophysiology (‘electrical remodelling’) and the structural remodelling process further deteriorates, constituting a vicious cycle in which ‘AF begets AF’ [5, 6, 9], making it challenging to restore and maintain sinus rhythm (Fig. 1) [7].
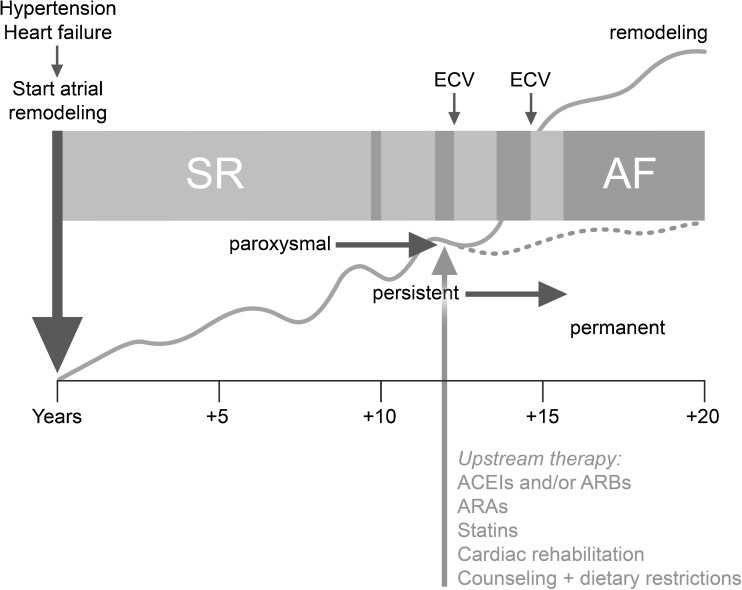
Fig. 1.
Time course of atrial substrate remodelling starting long before the first episode of atrial fibrillation (AF): hypothetical representation of how underlying disease such as hypertension or heart failure induces atrial remodelling long before the onset of AF and of how atrial remodelling progresses in relation to the clinical appearance of AF. The light grey arrow denotes early institution of upstream therapy in persistent AF, which may reduce or reverse atrial remodelling (dotted line) and could improve maintenance of sinus rhythm. ACEI angiotensin converting enzyme inhibitor, MRA mineralocorticoid receptor antagonist, ARB angiotensin-receptor blocker, ECV electrical cardioversion, SR sinus rhythm. Adapted with permission from Cosio et al. [7]
Outcome of current rhythm-control therapies is often poor because of progressive structural remodelling and limitations in efficacy and adverse events of the available ion-channel antiarrhythmic drugs and ablation techniques. Ablation and ion-channel antiarrhythmic drugs counteract electrical remodelling processes, but do not alter the underlying structural remodelling.
Upstream therapy refers to the use of non-ion-channel antiarrhythmic drugs that modify the atrial substrate to prevent the occurrence of new-onset AF (i.e., primary prevention) or recurrence of AF (i.e., secondary prevention). It includes treatment with RAAS blockers (angiotensin-converting-enzyme inhibitors [ACEIs], angiotensin-receptor blockers [ARBs], and mineralocorticoid receptor antagonists [MRAs]), and statins (Fig. 1) [10]. RAAS blockers may prevent or reduce atrial structural remodelling by decreasing fibrosis and by improving haemodynamics through lowering of blood pressure and reduction of ventricular and atrial wall stress [11–13]. RAAS blockers may also prevent AF by counteracting the effects of aldosterone on potassium channels leading to shortening of the action potential [14]. Statins, known for their lipid-lowering capacities, have pleiotropic properties such as reduction of inflammation and oxidative stress. Through these properties, statins may play a protective role against the development of AF [11, 15–17]. Moderate exercise may also reduce AF by targeting structural remodelling. Moderate physical activity is associated with a significantly lower incidence of AF [18–20]. In addition, peak oxygen consumption increases by 20 %, left ventricular remodelling may reverse, and diastolic function may improve [21, 22].
Upstream therapy may improve the outcome of rhythm-control therapy and could prevent or postpone the need for ion-channel antiarrhythmic drugs and/or ablation, while having fewer adverse events. Results of upstream therapy for the prevention of AF in animal experiments, hypothesis-generating small clinical studies, and retrospective analyses in selected patient categories have been encouraging [23–26]. ACEIs and ARBs seem to be beneficial in secondary AF prevention especially when atrial remodelling is less advanced, and when added to amiodarone [27]. On the other hand, other trials did not show any beneficial effect for secondary prevention [28, 29]. MRAs may be even more effective in preventing AF recurrences but only few data are available [30]. Results regarding effectiveness of statins in preventing AF recurrences have been inconclusive [31–33].
Overall, upstream therapy seems to be most effective in primary prevention. The disappointing results regarding secondary AF prevention may have been caused by inclusion of patients in whom the extent of remodelling was too severe and irreversible due to a long history of AF and underlying heart disease [34, 35]. Inclusion of patients in whom remodelling processes are less advanced, i.e. patients with a short history of both AF and underlying heart disease, may improve outcome. These patients have not been studied before. In addition, the institution of several types of upstream therapies instead of just one type of upstream therapy may improve success. In heart failure, upstream therapy can further reduce AF through reduction or reversion of ventricular remodelling. Reversal of heart failure has been shown to be followed by normalised atrial function and decreased duration of AF, though not all substrate for AF [36]. Therefore, upstream therapies may be most effective in AF that occurs in patients with heart failure [37].
The Routine versus Aggressive upstream rhythm Control for prevention of Early atrial fibrillation in heart failure (RACE 3) study tests the hypothesis that aggressive upstream rhythm control increases persistence of sinus rhythm in patients with short-lasting (i.e., early) AF and mild-to-moderate early systolic or diastolic heart failure. Aggressive upstream rhythm control consists of a combination of non-ion-channel antiarrhythmic drugs (ACEIs and/or ARBs, MRAs, and statins), cardiac rehabilitation therapy, counselling and dietary restrictions in addition to conventional heart failure drugs. We postulate that the institution of a combination of different classes of upstream therapy may have synergistic effects on the atrial substrate by decreasing AF directly through reduction of atrial remodelling and indirectly through reduction of ventricular remodelling (Fig. 1). This may ultimately enhance persistence of sinus rhythm and possibly also improve prognosis [35].
Study design and methods
Study oversight
The RACE 3 study (ClinicalTrials.gov Identifier NCT00877643) is a prospective, randomised, open-label, multinational, multicenter, superiority trial designed to compare two rhythm-control strategies in patients with short-lasting (i.e. early) persistent AF. The study is initiated and coordinated by the Interuniversity Cardiology Institute of the Netherlands, the University Medical Center Groningen, and the Working Group on Cardiovascular Research the Netherlands. The trial is funded by major grants from the Netherlands Heart Foundation (NHS B 2008 035), the Interuniversity Cardiology Institute Netherlands and the Working Group on Cardiovascular Research the Netherlands and by unrestricted educational grants from pharmaceutical and device companies. None of the sponsors are involved in the study design and data collection, data analysis, or manuscript preparation. The steering committee (see Appendix) designed the study and is responsible for the conduct of the study, data analyses and reporting, and manuscript preparation. Study monitoring, data management and validation are independently performed at the Trial Coordination Center (University Medical Center Groningen, the Netherlands). The study was approved by the institutional review boards of all participating centers. All patients are asked to give written informed consent.
Study recruitment started in 2009, and randomisation is expected to be concluded in the summer of 2014. Follow-up for the primary endpoint will be 1 year. In addition, an exploratory randomised long-term extension of the RACE 3 study will be performed to study the long-term effects of the two treatment strategies. Total follow-up will be 5 years. The study is being conducted in 15 centers in the Netherlands and five centers in the United Kingdom. At present, 168 patients have been included.
Objectives
The primary objective is to investigate whether in patients with early AF and mild-to-moderate early systolic or diastolic heart failure an aggressive upstream rhythm-control approach, including ACEIs and/or ARBs, MRAs, statins, cardiac rehabilitation therapy, counselling, and dietary restrictions, increases persistence of sinus rhythm compared with conventional rhythm control.
Secondary objectives will investigate whether aggressive upstream rhythm control improves persistence of sinus rhythm without the need for class III ion-channel antiarrhythmic drugs or pulmonary vein ablation, quality of life, left ventricular function, and exercise capacity, and decreases the number of patients with permanent AF, number of electrical cardioversions, numbers of pulmonary vein and atrioventricular node ablations, hospitalisations for heart failure or other cardiovascular reasons, and all-cause mortality. Cost-effectiveness will also be studied. In addition, the association of biomarkers of processes related to remodelling and thrombosis, and the role of genetic factors in AF recurrences, the development of heart failure hospitalisations and other cardiovascular morbidity and mortality will be investigated.
Study population
The study population consists of patients with early symptomatic persistent AF and mild-to-moderate early heart failure who are to undergo an electrical cardioversion and who are otherwise stable. A total of 250 patients are to be included. Early symptomatic persistent AF is defined as a total history of AF <5 years before randomisation and a total persistent AF duration of >7 days but <6 months (more than one episode is allowed), with a maximum of one previous electrical cardioversion during the last 2 years; neither electrical nor chemical cardioversion ≥2 years ago are allowed. Mild-to-moderate early heart failure is defined as a total heart failure history <1 year and either diastolic heart failure (left ventricular ejection fraction [LVEF] ≥45 %, New York Heart Association [NYHA] functional class II-III, and additional criteria consisting of echo parameters and/or elevated N-terminal pro-brain natriuretic peptide [NTproBNP]), or systolic heart failure (LVEF 25–45 % and NYHA class I–III). The most important exclusion criteria include severe heart failure, i.e. NYHA class IV or LVEF <25 %, left atrial size >50 mm, severe valvular disease, and present MRA use. A list of inclusion and exclusion criteria is provided in Table 1.
Table 1.
Inclusion and exclusion criteria for the RACE 3 study
| Inclusion criteria | Exclusion criteria |
|---|---|
| - Early symptomatic persistent AF defined as: | - Symptoms not allowing electrical cardioversion to be delayed for 3 weeks |
| 1. Total AF history <5 years, and | - Heart failure NYHA functional class IV |
| 2. Total persistent AF duration >7 days and <6 months, and | - LVEF <25 % |
| 3. ≤ 1 previous electrical cardioversion during the last 2 years; neither electrical nor chemical cardioversion ≥2 years ago are allowed. | - Left atrial size >50 mm (parasternal axis) |
| - Mild-to-moderate early heart failure, defined as: | - Severe valvular disease (previous valve repair/replacement is permitted) |
| 1. Total heart failure history <1 year, and | - Present MRA use |
| 2. One of the following: | - Patients with cardiac resynchronisation therapy |
| - LVEF ≥45 % and NYHA II-III, and normal pulmonary function and body mass index <40 kg/m2, and | - Previous use of continuous prophylactic class I or class III antiarrhythmic drugs |
| - Previously documented heart failure-related NT-proBNP elevation (> 400 ng/l (= 48 pmol/l)), or | - Postoperative AF |
| - Evidence of structural heart disease including left ventricular hypertrophy (posterior wall and/or septum diameter ≥11 mm or ≥10 mm using Penn’s method) or regional left ventricular dysfunction (akinesia, hypokinesia), or | - Myocardial infarction within last 3 months |
| - Previous admission for heart failure, or | - Hypersensitivity against MRAs |
| - Evidence of diastolic dysfunction on echocardiography (average annular e’ <8 cm/s, and deceleration time >220 ms, and average E/e’ >8 or in case of colour coded TDI E/e’ >11) | - Uncontrolled hypertension (systolic blood pressure >160 mmHg and/or a diastolic blood pressure >95 mmHg |
| - LVEF 25–45 % and NYHA class I–III | - Unstable angina pectoris |
| - Optimal documentation and treatment of underlying heart disease | - Open heart surgery within the last 3 months |
| - Otherwise stable patients | - Serum potassium >5 mmol/l |
| - No contraindication for oral anticoagulation | - Acute and reversible illnesses |
| - Eligible for cardiovascular rehabilitation program | - Alcohol or drug abuse or a severe progressive extracardiac disease |
| - Age ≥40 years | - Untreated manifest and latent hyper- or hypothyroidism or <3 months euthyroidism |
| - Moderate to severe renal insufficiency (creatinine clearance less than 50 ml/min) | |
| - Patients with liver cirrhosis (Child-Pugh class C) or repeated ALAT/ASAT 1.5 times the upper limit or other significant liver disease | |
| - Co-administration of strong CYP3A4 inhibitors (i.e. grapefruit juice, ketoconazole, itraconazole, posaconazole, voriconazole, clarithromycin, erythromycin, telithromycin, indinavir, nelfinavir, ritonavir, saquinavir, HIV-protease inhibitors) or strong CYP3A4 inductors (i.e. carbamazepine, rifampicin, St John’s Wort) | |
| - Pregnancy | |
| - Complex congenital heart diseases. The only congenital heart diseases that can be included are atrial septal defect, ventricular septal defect, bicuspid aortic stenosis | |
| - Patients unlikely to comply with the protocol |
AF atrial fibrillation, MRA mineralocorticoid receptor antagonist, LVEF left ventricular ejection fraction, NT-proBNP N-terminal related pro-B-type natriuretic peptide, NYHA New York Heart Association
Randomisation and treatment
Patients are randomised to either aggressive upstream rhythm control or conventional rhythm control. Randomisation is performed 3 weeks prior to electrical cardioversion in order to start upstream therapy 3 weeks before cardioversion (Fig. 2).
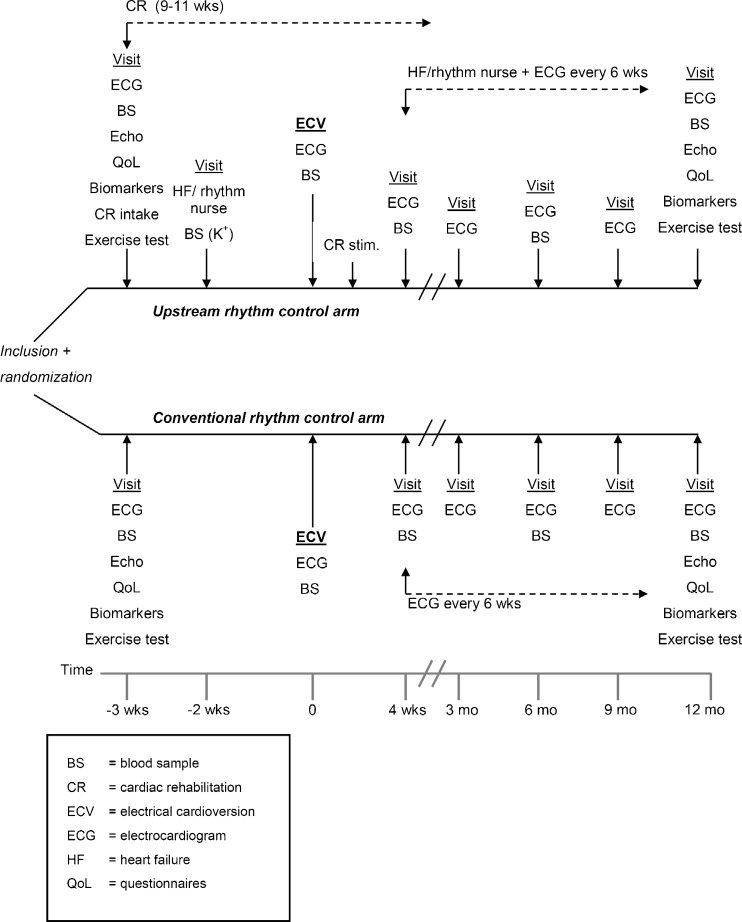
Fig. 2.
Flowchart of the RACE 3 study schedule
Aggressive upstream rhythm control consists of: 1) Rhythm control for AF including electrical cardioversion and antiarrhythmic treatment as in routine clinical practice, according to the guidelines [2, 38]. The first ECV will be performed without institution of class III ion-channel antiarrhythmic drugs but with a beta-blocker. In addition, patients are started on upstream medication to prevent AF (i.e. ACEIs and/or ARBs, and MRAs, and statins); 2) Optimal medication for heart failure according to the guidelines; 3) Dietary (salt and fluid) restrictions; 4) Counselling by heart failure/rhythm nurse (visits every 6 weeks); and 5) Cardiac rehabilitation. Treatment with upstream therapy is started at randomisation and continued during follow-up. Every effort is made to titrate each patient to the highest dose free from unacceptable side effects for all upstream drugs. Counselling by the heart failure/rhythm nurse is performed every 6 weeks to check and stimulate physical activity and drug adherence and to make patients aware of dietary factors that can reduce disease burden. Heart rhythm is assessed on ECG. Furthermore, patients will be stimulated to quit smoking. Cardiac rehabilitation aims to stimulate patients to be physically active. Cardiac rehabilitation starts immediately after inclusion and lasts 9–11 weeks (at least 6–8 weeks after electrical cardioversion, even if cardioversion needs to be postponed). Supervised physical training takes place at least two, but preferably three times a week. Training is performed according to perceived exertion, in which patients are stimulated to exercise at the highest pace or intensity that still allows for comfortable conversation.
Conventional rhythm control consists of: 1) Rhythm control for AF including electrical cardioversion and antiarrhythmic treatment as in routine clinical practice, according to the guidelines [2, 38]. The first ECV will be performed without institution of class III ion-channel antiarrhythmic drugs but with a beta-blocker; 2) Optimal medication for heart failure according to the guidelines; 3) Follow-up visits (without counselling) every 6 weeks for documentation of heart rhythm with ECG only.
The difference between upstream rhythm control and conventional rhythm control consists of the aggressive institution of upstream drugs (ACEIs and/or ARBs, and MRAs, and statins), dietary restrictions, counselling visits, and cardiac rehabilitation.
Background therapy in both treatment arms: In both treatment arms re-electrical cardioversion is performed after a recurrence with institution of ion-channel antiarrhythmic drugs and/or pulmonary vein ablation according to the guidelines [2].
Follow-up
Follow-up visits take place at one, three, six, nine, and 12 months after the first study electrical cardioversion. Thereafter in the long-term extension, patient visits are twice a year in the aggressive group, alternated with telephone counselling by the research nurse every 6 months between the visits, and once a year in the conventional group. Total follow-up will be 5 years. A blood sample for biomarker and genetic analyses, 24-h urine to assess sodium secretion, echocardiogram, bicycle exercise testing and questionnaires testing quality of life and costs are collected at baseline and at 1 year of follow-up. During follow-up in both treatment arms every effort is made to keep the patient in a rhythm-control strategy. An electrocardiogram is conducted every 6 weeks to aid documentation of recurrent AF. Recurrence of persistent AF is defined as documented, symptomatic AF episodes lasting longer than 7 days or that do not terminate spontaneously. The persistence of AF is preferably verified by 24-h Holter monitoring to show continuous (and not paroxysmal) AF. Cardiovascular hospitalisations and mortality are carefully documented and monitored. A committee of experts who are unaware of the treatment assignments will adjudicate cardiovascular morbidity and mortality.
Definitions
Permanent AF is defined as accepted AF [2]. Hospitalisation for heart failure is defined as a hospitalisation (≥1 overnight stay) for heart failure requiring intravenous diuretics. Stroke is defined as a disabling haemorrhagic, ischaemic or undetermined stroke confirmed by a neurologist on the basis of computerised tomography or magnetic resonance imaging and necessitating hospitalisation. Systemic emboli must be confirmed by a physician and necessitate hospitalisation. Bleeding is defined as bleeding with a decrease in the haemoglobin value of >20 g/l or requiring blood transfusion, or symptomatic bleeding in a critical organ or area (intra-cranial, retroperitoneal, spinal, ocular, pericardial, atraumatic articular), or as a fatal bleeding. Syncope is defined as sudden temporary loss of consciousness with spontaneous recovery accompanied by trauma necessitating hospital admission (≥1 overnight stay). Life-threatening adverse effects of antiarrhythmic drugs include drug-induced heart failure, conduction disturbances and ventricular arrhythmias necessitating hospital admission (≥1 overnight stay). Myocardial infarction requires at least two of the following: 1) typical chest pain for at least 20 min; 2) electrocardiogram showing signs of acute myocardial infarction; 3) cardiac enzyme elevation more than twice the upper limit of normal. Life-threatening arrhythmias are defined as those arrhythmias requiring hospitalisation. All-cause mortality is categorised by means of blinded adjudication into four categories: death from cardiac arrhythmia, death from non-arrhythmic cardiac causes, death from non-cardiac vascular causes, and death from non-cardiovascular causes.
Endpoints
The presence of the primary endpoint requires that: 1) the patient is still in a rhythm-control strategy according to the attending physician, and 2) that sinus rhythm is maintained after 1 year of follow-up, defined as sinus rhythm during ≥6/7th of the assessable time of continuous 7-day Holter monitoring during the last week of the study. Failure to fulfil these requirements is considered therapy failure. The primary endpoint therefore requires that a 7-day Holter is performed during the last 7 days of follow-up. At least 5/7th of the continuous 7-day Holter monitoring should be assessable; otherwise, the 7-day Holter monitoring is to be repeated.
Secondary endpoints include: 1) The presence of sinus rhythm during 7-day Holter monitoring after 1 year of follow-up with or without antiarrhythmic drugs; 2) Sinus rhythm after 1 year of follow-up during 7-day Holter monitoring without the need for pulmonary vein isolation; 3) Permanent AF; 4) Number of electrical cardioversions; 5) Pulmonary vein ablations; 6) Atrioventricular node ablation; 7) Left ventricular systolic and diastolic function; 8) Exercise capacity; 9) Hospitalisations for heart failure; 10) Hospitalisations for other cardiovascular reasons and 11) All-cause mortality. Additional analyses include quality of life, biomarker and genetic analyses and costs and cost-effectiveness assessment. Quality of life assessment includes the Short Form Health Survey (SF)-36, Multidimensional Fatigue Index (MVI)-20, Eysenck Personality Questionnaire (EPQ), Toronto AF Questionnaire, Center for Epidemiologic Studies Questionnaire (CES-D), Minnesota Questionnaire, European Heart Failure Self-Care Behaviour Scale, Hospital Anxiety and Depression Scale (HADS), and the Ladder of Life. Biomarker analyses will include markers associated with collagen metabolism, inflammation, dedifferentiation, thrombosis, neurohumoral factors and proteomic profiles in order to study the association between severity of remodelling, heart failure and activation of thrombosis and outcome of AF. In addition, genetic analyses will be performed. Costs will be calculated from a societal perspective. All relevant costs inside and outside the health care system are taken into account. Direct medical costs, direct non-medical costs, and indirect non-medical costs will be calculated. The time horizon of the economic evaluation will be equal to that of the clinical study. For the exploratory randomised long-term extension all the above-mentioned endpoints will be assessed as secondary endpoints after 5 years of follow-up.
Electronic data collection
All data are recorded electronically and are transferred to the server holding the central database at the Trial Coordination Center, which is regularly backed up and password protected. The electronic case record forms are monitored at regular times by the study monitor.
Data safety monitoring board
A data safety monitoring board (DSMB) has been established to perform on-going safety surveillance and to perform interim analyses on the safety data, in addition to assessing scientific content. The DSMB is an independent committee that meets on a regular basis to evaluate (serious) adverse events and clinical endpoints.
Endpoint adjudication committee
A committee of experts, masked to the treatment assignments, will adjudicate all possible (clinical) endpoints.
Statistical considerations
The RACE 3 study is a superiority trial; the null hypothesis (H0) is that the number of patients in sinus rhythm in the upstream rhythm-control arm will be equal to the number of patients in sinus rhythm in the conventional rhythm-control arm. The alternative hypothesis (Ha) is that there will be more patients in sinus rhythm in the upstream rhythm-control arm than in the conventional rhythm-control arm. To test these hypotheses, chi-square analysis and logistic regression (univariate and multivariate) will be conducted.
The primary efficacy population (intent-to-treat) consists of all randomised patients. Patients will be analysed according to treatment received. The number of subjects who enrol and are randomised will be summarised by treatment group. The number of subjects withdrawing from the study will be tabulated by reason for withdrawal and by treatment group. The time of withdrawal and reason for withdrawal will be listed by treatment group. Potential biases due to withdrawal of subjects will be investigated. Patients’ demographics and disease characteristics at baseline will be tabulated and evaluated for treatment group differences. If differences are found, caution will be taken when interpreting the results of the analyses between groups and the methods may be modified to adjust for these differences.
For quantitative parameters, descriptive statistics will be mean, standard deviation, median, inter-quartile range and range of minimum and maximum, and number of valid observations by treatment group. Mean, median and inter-quartile range will be reported to a precision of one or two decimal places more than the individual measurements; standard deviation will be reported to a precision of two decimal places more than the individual measurements; range will have the same precision as the individual measurements. Appropriate statistics will be used to compare treatment groups. In all statistical analyses, a two-sided p < 0.05 is considered statistically significant.
Unless stated otherwise, all secondary analyses will be performed using the same patients included in the primary analysis. Secondary variables will be analysed using an appropriate statistical test, depending on the nature of the variable. Changes in parameters over time in the different treatment groups will be analysed using repeated measurement analysis or techniques that evaluate the timing of endpoints, when appropriate.
The sample size is based on data of other studies. Persistence of sinus rhythm has been observed in 20 % to 50 % of patients 1 year after electrical cardioversion. These numbers approach 20 % to 30 % when patients have a history of heart failure [39–42]. In a treatment strategy using electrical cardioversions and amiodarone, persistence of sinus rhythm was observed in 50 % after 1 year of follow-up [43]. Therefore, we expect that after 1 year of follow-up, persistence of sinus rhythm will be observed in 50 % of patients randomised to conventional rhythm control versus 70 % of patients randomised to upstream rhythm control. Based on a type 1 error of 0.05 and a power of 80 %, we calculated an estimated sample size of 100 patients in each study group. Taking into account a dropout rate of 20 %, the sample size should be 250 patients in total. Stratification is done for LVEF< and ≥45 %. An interim analysis consisting of a preliminary open assessment of the primary endpoint will be conducted by an independent data monitoring committee after 100 patients have completed the protocol. This will be done in order to have the option to adapt the sample size if the incidence rates at the interim analyses of the two treatment arms are different than originally expected to find the predefined treatment effect.
Conclusion
Increasing knowledge concerning the structural remodelling processes in the atria before and during AF at least in part clarifies the failure of current rhythm-control therapies in AF. Upstream therapy that prevents or attenuates the remodelling process may be effective or at least support conventional rhythm-control therapies in maintaining sinus rhythm, especially in patients in whom structural remodelling processes are less advanced. The RACE 3 study is a randomised trial investigating whether an aggressive upstream rhythm-control approach, including ACEIs and/or ARBs, MRAs, statins, cardiac rehabilitation therapy, counselling, and dietary restrictions, increases persistence of sinus rhythm compared with conventional rhythm control. The study is conducted in patients with early persistent AF, in whom structural remodelling processes are expected to be less advanced and in whom upstream rhythm control may therefore be beneficial. If such upstream therapy proves to be effective in improving maintenance of sinus rhythm, it could become a new approach to rhythm control, supporting conventional pharmacological and non-pharmacological rhythm control.
Acknowledgments
Disclosure statement
The trial is funded by major grants from the Netherlands Heart Foundation (NHS B 2008 035), the Interuniversity Cardiology Institute Netherlands and the Working Group on Cardiovascular Research the Netherlands and unrestricted educational grants from AstraZeneca, Bayer, Biotronik, Boehringer-Ingelheim, Boston Scientific, Medtronic, Sanofi Aventis, St Jude Medical paid to the Interuniversity Cardiology Institute Netherlands.
Dr. Van Gelder reports receiving consulting fees from MSD and Boehringer-Ingelheim. Dr. Crijns reports receiving consulting fees from Boehringer-Ingelheim, Sanofi-Aventis and AstraZeneca, grant support from St. Jude Medical, Boston Scientific, Boehringer-Ingelheim, Sanofi-Aventis, Medapharma and Merck, and honoraria from Medtronic, Sanofi-Aventis, Medapharma, Merck, Boehringer-Ingelheim and Biosense Webster, Dr. Alings reports receiving consulting fees from Bayer, Boehringer-Ingelheim, MSD, and Sanofi-Aventis, lecture fees from Bayer, Boehringer-Ingelheim, MSD, and AstraZeneca and travel support from Bristol-Myers Squibb and Boston Scientific. Dr. Lane reports receiving research fees from Bayer and Boehringer-Ingelheim, lecture fees from Bayer, Boehringer-Ingelheim, BMS/Pfizer, consulting fees from Sanofi-Aventis. Dr Lip has served as a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Portola and Boehringer-Ingelheim and has been on the speakers bureau for Bayer, BMS/Pfizer, Boehringer-Ingelheim, and Sanofi Aventis. Dr Smeets reports receiving lecture fees from Boston Scietific. Dr. Tukkie reports receiving consulting fees and grant support from Medtronic, Dr. Van Veldhuisen has received Board Membership fees from Pfizer, in relation to his Steering Committee membership of EMPHASIS-HF (Study of Eplerenone).
No other potential conflict of interest relevant to this article was reported.
Appendix
Participating centers
The Netherlands: G.C.M. Linssen, Hospital Group Twente, Almelo/Hengelo; G.S. De Ruiter, Onze Lieve Vrouwe Gasthuis, Amsterdam; H.A. Bosker, Hospital Rijnstate, Arnhem/Velp; R.H.J. Peters, Ter Gooi Hospital, Blaricum; M. Alings, Amphia Hospital, Breda; V. Hagens, Ommelander Hospital Group, location Delfzijl; Y.S. Tuininga, Deventer Hospital, Deventer; A.H. Liem, Admiraal de Ruyter Hospital, Goes; R.G. Tieleman, Martini Hospital, Groningen; I.C. Van Gelder, University Medical Center Groningen, Groningen; G.J.E. Verdel, Kennemer Gasthuis, Haarlem; H.J.G.M. Crijns, Maastricht University Medical Center, Maastricht; G.E. Cramer, University Medical Center Nijmegen, Nijmegen; A. Van der Galiën, Ommelander Hospital, Winschoten, J.G. Meeder, Viecuri Hospital, location Venlo.
United Kingdom: G.Y.H. Lip, Birmingham City Hospital, Birmingham; J.S. de Bono, University Hospitals Birmingham, Birmingham; S. Nadar, Good Hope Hospital, Sutton Coldfield; M.H. Tayebjee, Leeds Teaching Hospitals, Leeds; C.J. Boos, Poole Hospital, Poole.
Steering committee
I.C. Van Gelder1,2 (chair), M. Alings3, H.J.G.M. Crijns4, M.D. Smit1, R. Tukkie5, J. Brügemann1,6, J.R.L.M. Smeets7, F.F. Willems8, H.L. Hillege1,9, J.G. Tijssen10, R.G. Tieleman11, D.J. Van Veldhuisen1, G.Y.H. Lip12.
1Department of Cardiology, Thoraxcenter, University of Groningen, University Medical Center Groningen, Groningen; 2Interuniversity Cardiology Institute Netherlands, Utrecht; 3Department of Cardiology, Amphia Hospital, Breda; 4Department of Cardiology, Maastricht University Medical Center, Maastricht; 5Department of Cardiology, Kennemer Gasthuis, Haarlem; 6Cardiac Rehabilitation Center, University Medical Center Groningen, Groningen; 7University Medical Center Nijmegen, Nijmegen; 8Hospital Rijnstate, Arnhem/Velp; 9Trial Coordination Center, Department of Epidemiology, University Medical Center Groningen, Groningen; 10Academic Medical Center, Amsterdam; 11Martini Hospital Groningen, Groningen; all the Netherlands; 12Department of Cardiology, City Hospital, Birmingham, United Kingdom.
Data safety monitoring board
H.J.J. Wellens1 (chair), A.M. Wilde2, Y.M. Pinto2, J.G. Tijssen2 (reporter of steering committee).
1Cardiovascular Research Institute, Maastricht; 2Academic Medical Centre, Amsterdam; all the Netherlands.
Endpoint adjudication committee
R.A. Tio (chair) 1, J. van Melle1, G.J. Luijckx2
1Department of Cardiology and 2Neurology, University Medical Center Groningen, Groningen, the Netherlands.
Trial management, statistics and data management
H.L. Hillege1, W.J.M. Mol1, J.G. Tijssen2.
1Trial Coordination Center, University Medical Center Groningen, Groningen; 2Academic Medical Center, Amsterdam; all the Netherlands.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12(10):1360–420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhof P, Lip GY, Van Gelder IC, et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options–a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace. 2012;14(1):8–27. doi: 10.1093/europace/eur241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jong AM, Maass AH, Oberdorf-Maass SU, et al. Mechanisms of atrial structural changes caused by stretch occurring before and during early atrial fibrillation. Cardiovasc Res. 2011;89(4):754–65. doi: 10.1093/cvr/cvq357. [DOI] [PubMed] [Google Scholar]
- 5.Schotten U, Verheule S, Kirchhof P, et al. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91(1):265–325. doi: 10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki YK, Nishida K, Kato T, et al. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124(20):2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 7.Cosio FG, Aliot E, Botto GL, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10(1):21–7. doi: 10.1093/europace/eum276. [DOI] [PubMed] [Google Scholar]
- 8.Lau DH, Mackenzie L, Kelly DJ, et al. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Hear Rhythm. 2010;7(3):396–404. doi: 10.1016/j.hrthm.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Wijffels MC, Kirchhof CJ, Dorland R, et al. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92(7):1954–68. doi: 10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 10.Smit MD, Van Gelder IC. Upstream therapy of atrial fibrillation. Expert Rev Cardiovasc Ther. 2009;7(7):763–78. doi: 10.1586/erc.09.59. [DOI] [PubMed] [Google Scholar]
- 11.Tsai CT, Lai LP, Kuo KT, et al. Angiotensin II activates signal transducer and activators of transcription 3 via Rac1 in atrial myocytes and fibroblasts: implication for the therapeutic effect of statin in atrial structural remodeling. Circulation. 2008;117(3):344–55. doi: 10.1161/CIRCULATIONAHA.107.695346. [DOI] [PubMed] [Google Scholar]
- 12.Milliez P, Deangelis N, Rucker-Martin C, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26(20):2193–9. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 13.Sicouri S, Cordeiro JM, Talarico M, et al. Antiarrhythmic effects of losartan and enalapril in canine pulmonary vein sleeve preparations. J Cardiovasc Electrophysiol. 2011;22(6):698–705. doi: 10.1111/j.1540-8167.2010.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammers C, Dartsch T, Brandt MC, et al. Spironolactone prevents aldosterone induced increased duration of atrial fibrillation in rat. Cell Physiol Biochem. 2012;29(5–6):833–40. doi: 10.1159/000178483. [DOI] [PubMed] [Google Scholar]
- 15.Sicouri S, Gianetti B, Zygmunt AC, et al. Antiarrhythmic effects of simvastatin in canine pulmonary vein sleeve preparations. J Am Coll Cardiol. 2011;57(8):986–93. doi: 10.1016/j.jacc.2010.08.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bang CN, Greve AM, Abdulla J, Kober L, Gislason GH, Wachtell K. The preventive effect of statin therapy on new-onset and recurrent atrial fibrillation in patients not undergoing invasive cardiac interventions: a systematic review and meta-analysis. Int J Cardiol. 2012. [DOI] [PubMed]
- 17.Yu T, Zhu W, Gu B, et al. Simvastatin attenuates sympathetic hyperinnervation to prevent atrial fibrillation during post-myocardial infarction remodeling process. J Appl Physiol. 2012. [DOI] [PubMed]
- 18.Mozaffarian D, Furberg CD, Psaty BM, et al. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2008;118(8):800–7. doi: 10.1161/CIRCULATIONAHA.108.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Everett BM, Conen D, Buring JE, et al. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes. 2011;4(3):321–7. doi: 10.1161/CIRCOUTCOMES.110.951442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grundvold I, Skretteberg PT, Liestol K, et al. Importance of physical fitness on predictive effect of body mass index and weight gain on incident atrial fibrillation in healthy middle-age men. Am J Cardiol. 2012;110(3):425–32. doi: 10.1016/j.amjcard.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 21.Erbs S, Linke A, Gielen S, et al. Exercise training in patients with severe chronic heart failure: impact on left ventricular performance and cardiac size. A retrospective analysis of the Leipzig Heart Failure Training Trial. Eur J Cardiovasc Prev Rehabil. 2003;10(5):336–44. doi: 10.1097/01.hjr.0000099031.38268.27. [DOI] [PubMed] [Google Scholar]
- 22.Edelmann F, Gelbrich G, Dungen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58(17):1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Shroff SC, Ryu K, Martovitz NL, et al. Selective aldosterone blockade suppresses atrial tachyarrhythmias in heart failure. J Cardiovasc Electrophysiol. 2006;17(5):534–41. doi: 10.1111/j.1540-8167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 24.Gillis AM, Morck M, Exner DV, et al. Beneficial effects of statin therapy for prevention of atrial fibrillation following DDDR pacemaker implantation. Eur Heart J. 2008;29(15):1873–80. doi: 10.1093/eurheartj/ehn192. [DOI] [PubMed] [Google Scholar]
- 25.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. 2002;106(3):331–6. doi: 10.1161/01.CIR.0000022665.18619.83. [DOI] [PubMed] [Google Scholar]
- 26.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):712–9. doi: 10.1016/j.jacc.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 27.Galzerano D, Di Michele S, Paolisso G, et al. A multicentre, randomized study of telmisartan versus carvedilol for prevention of atrial fibrillation recurrence in hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2012. [DOI] [PubMed]
- 28.Disertori M, Latini R, Barlera S, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009;360(16):1606–17. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 29.Goette A, Schon N, Kirchhof P, et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol. 2012;5(1):43–51. doi: 10.1161/CIRCEP.111.965178. [DOI] [PubMed] [Google Scholar]
- 30.Swedberg K, Zannad F, McMurray JJ, et al. Eplerenone and atrial fibrillation in mild systolic heart failure: results from the EMPHASIS-HF (Eplerenone in Mild Patients Hospitalization And SurvIval Study in Heart Failure) study. J Am Coll Cardiol. 2012;59(18):1598–603. doi: 10.1016/j.jacc.2011.11.063. [DOI] [PubMed] [Google Scholar]
- 31.Dabrowski R, Borowiec A, Smolis-Bak E, et al. Effect of combined spironolactone-beta-blocker +/− enalapril treatment on occurrence of symptomatic atrial fibrillation episodes in patients with a history of paroxysmal atrial fibrillation (SPIR-AF study) Am J Cardiol. 2010;106(11):1609–14. doi: 10.1016/j.amjcard.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Negi S, Shukrullah I, Veledar E, et al. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial) J Cardiovasc Electrophysiol. 2011;22(4):414–9. doi: 10.1111/j.1540-8167.2010.01925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koutbi L, Roy D, Talajic M, et al. Statins and atrial fibrillation in patients with left ventricular dysfunction: Insights from the AF-CHF trial. Int J Cardiol. 2012. [DOI] [PubMed]
- 34.Smit MD, Van Gelder IC. Valsartan and recurrent atrial fibrillation. N Engl J Med. 2009;361(5):532. doi: 10.1056/NEJMc091057. [DOI] [PubMed] [Google Scholar]
- 35.Van Gelder IC, Haegeli LM, Brandes A, et al. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace. 2011;13(11):1517–25. doi: 10.1093/europace/eur192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinagawa K, Shi YF, Tardif JC, et al. Dynamic nature of atrial fibrillation substrate during development and reversal of heart failure in dogs. Circulation. 2002;105(22):2672–8. doi: 10.1161/01.CIR.0000016826.62813.F5. [DOI] [PubMed] [Google Scholar]
- 37.van Veldhuisen DJ, Aass H, El Allaf D, et al. Presence and development of atrial fibrillation in chronic heart failure. Experiences from the MERIT-HF Study. Eur J Heart Fail. 2006;8(5):539–46. doi: 10.1016/j.ejheart.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 38.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Hear Rhythm. 2011;8(1):157–76. doi: 10.1016/j.hrthm.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 39.Hemels ME, Wiesfeld AC, Van Veldhuisen DJ, et al. Outcome of pharmacological rhythm control for new-onset persistent atrial fibrillation in patients with systolic heart failure: a comparison with patients with normal left ventricular function. Europace. 2007;9(4):239–45. doi: 10.1093/europace/eum011. [DOI] [PubMed] [Google Scholar]
- 40.van den Berg MP, Tuinenburg AE, van Veldhuisen DJ, et al. Cardioversion of atrial fibrillation in the setting of mild to moderate heart failure. Int J Cardiol. 1998;63(1):63–70. doi: 10.1016/S0167-5273(97)00273-8. [DOI] [PubMed] [Google Scholar]
- 41.Tuinenburg AE, Van Gelder IC, Van Den Berg MP, et al. Lack of prevention of heart failure by serial electrical cardioversion in patients with persistent atrial fibrillation. Heart. 1999;82(4):486–93. doi: 10.1136/hrt.82.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lehto M, Kala R. Persistent atrial fibrillation: a population based study of patients with their first cardioversion. Int J Cardiol. 2003;92(2–3):145–50. doi: 10.1016/S0167-5273(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed S, Rienstra M, Crijns HJ, et al. Continuous vs episodic prophylactic treatment with amiodarone for the prevention of atrial fibrillation: a randomized trial. JAMA. 2008;300(15):1784–92. doi: 10.1001/jama.300.15.1784. [DOI] [PubMed] [Google Scholar]