Abstract
Storage of tomato (Solanum lycopersicum) as originally tropical fruit is limited by the risk of chilling injury (CI). To develop an effective technique to reduce CI, the effects of treatment with 0, 50 and 100 μM prohexadione-calcium (Pro-Ca) on CI, electrolyte leakage (EL), malondialdehyde (MDA) and proline contents, and activities of phospholipase D (PLD) and lipoxygenase (LOX), were investigated in tomato fruit stored at 1°C for 21 days. Treatment with Pro-Ca, without significant difference between two applied concentrations, significantly mitigated chilling injury. Also, Pro-Ca treatment maintained lower levels of EL and MDA content, higher level of proline content and inhibited the increases in PLD and LOX activities compared with the control fruit. These results suggest that Pro-Ca might mitigate CI by inhibiting PLD and LOX activities and by enhancing membrane integrity.
Keywords: Prohexadione-calcium, Tomato, Chilling Injury, Postharvest
Introduction
Storage of tomatoes (Solanum lycopersicum) as originally tropical fruit is limited by the risk of chilling injury (CI) (Bourne 2006). Chilled tomato fruits develop several symptoms, such as sunken areas on the fruits (blemishes), diseases caused by pathogen, and losing their ability to develop full color, which lead to substantial degradation of produce quality (Zhao et al. 2009). Maintenance of membrane integrity at low temperature has been reported to be important for the resistance to CI (Wonsheree et al. 2009). Lipolytic cascade in membrane lipids deterioration during CI was achieved by the concerted activities of membranous lipolytic enzymes such as phospholipase D (PLD) and lipoxygenase (LOX) (Pinhero et al. 1998).
Prohexadione-calcium (Pro-Ca) mimics the structure of 2-oxoglutaric acid, and inhibits 2-oxoglutarate-dependent dioxygenases activity, which are pivotal for formation of growth-active gibberellins and flavonoids metabolism (Rademacher 2000). Pro-Ca as a bioregulator affects plant metabolism such as hormonal balance. In apple, the alteration of flavonoid biosynthesis leads to the accumulation of luteoforol, a novel molecule with phytoalexin activity responsible for increased resistance against pathogens (Halbwirth et al. 2002; Spinelli et al. 2005). In addition, Pro-Ca blocks 1-aminocyclopropane-1-carboxylate-oxidase (ACC-oxidase) activity, which is a key enzyme in the ethylene biosynthesis (Rademacher 2000), and it affects fruit quality via improving sugar accumulation in fruits (Rademacher et al. 2004; Costa et al. 2004, 2005). Furthermore, Pro-Ca has very favorable toxicological and eco-toxicological features, a low propensity for crop residues and no health risk for user or consumer is indicated (Winkler 1997; Evans et al. 1999). However, no information is available on the effects of Pro-Ca applied after harvest on chilling injury in fruits and vegetables. The objective of this study was to determine the effects of Pro-Ca as an environmentally friendly technology on MDA and proline contents, electrolyte leakage and PLD and LOX enzyme activities and their relation to CI in tomato fruit.
Materials and methods
Fruit and treatment
Tomato fruit (Solanum lycopersicum cv. Newton) were harvested at mature green stage in July 2011 from a greenhouse in Ahar, Iran. About 900 fruit were manually picked and immediately transferred to the laboratory. Those with defects were discarded. 810 fruit were selected and divided into 3 lots of 270 for the following treatment in triplicate (90 fruit per replicate): control (0) and Pro-Ca at 50 or 100 μM. Prohexadione-calcium (Pro-Ca) was purchased from Sigma–Aldrich. For each treatment and replicate, fruit were immersed in a fresh 10 L solution for 5 min. Following treatment, fruit were allowed to completely dry at room temperature before storage at 1 °C and 85–90% RH for 3 weeks. 15 fruit per replicate of each treatment were removed immediately from cold storage after 7, 14 or 21 days for analyses of electrolyte leakage, PLD and LOX enzyme activity, MDA and proline content. These samples were mixed and frozen immediately in liquid nitrogen, then stored at −80 °C. For CI evaluation, 15 fruit per replicate of each treatment were sampled weekly from cold storage and held at 25 °C for 3 days. Each treatment was replicated three times.
Chilling injury (CI) index
CI of fruits was evaluated at 25 °C for 3 days after 7, 14 or 21 days in cold storage period. The fruits were returned to ambient temperature (25 °C) for development of CI symptoms. Symptoms were manifested as surface pitting according to the method of Ding et al. (2002), where 0 = no pitting; 1 = pitting covering <25% of the fruit surface; 2 = pitting covering 25% to 50% of the surface; 3 = pitting covering >50% to 75% of the surface, and 4 = pitting covering >75% of the surface. The average extent of cold damage was expressed as the CI index, which was calculated using the following formula:
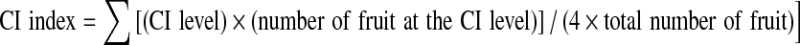 |
Electrolyte leakage (EL), malondialdehyde (MDA) and proline content
EL was measured using the method of Jiang et al. (2001). 3 mm thick of mesocarp tissue were excised from equator part of 5 fruits. Disks were put into aqueous 0.1Mmannitol under constant shaking. The conductivity of the solution (L1) was measured with a conductivity meter. Solutions were boiled for 10 min and then cooled to 20 °C. The conductivity of tissues (L2) was measured. The percentage of electrolyte leakage was calculated using the following formula: % Electrolyte leakage = (L1 = L2) × 100. MDA content was measured by the thiobarbituric acid method described by Ding et al. (2007). Absorbance at 532 nm was recorded and corrected for nonspecific absorbance at 600 nm. MDA content was expressed as μmol g−1 fresh weight (FW). Proline content was measured using the acid ninhydrin method described by Shan et al. (2007). Proline in tissues was extracted with 30 mL L − 1 sulfosalicylic acid at 100 °C for 10 min with shaking. The extract was mixed with an equal volume of glacial acetic acid and acid ninhydrin reagent and boiled for 30 min. After cooling, the reaction mix was partitioned against toluene and the absorbance of the organic phase was recorded at 520 nm. Proline content expressed as μg proline g−1 fresh weight (FW).
Enzyme assays
For PLD and LOX, 5 g of tissue was ground with 5 mL of 50 mmol L−1 Tris–HCl (pH 8), containing 10 mmol L−1 KCl, 500 mmol L−1 sucrose and 0.5 mmol L−1 phenylmethylsulfonylfluoride. The extracts were then homogenized and centrifuged at 12 000×g for 10 min at 4 °C. The supernatants were used for the enzyme assays.
PLD assay was determined according to Karakurt and Huber (2003). One unit of PLD was defined as the amount of enzyme that catalyzed the formation of 1 nmol D-nitrophenol h−1. LOX activity was assayed using the method of Todd et al. (1990). One unit of LOX is defined as the amount of enzyme which causes an increase in absorption of 0.01 min−1 at 234 nm and 25 °C when linoleic acid is used as the substrate. Protein content in the enzyme extracts was estimated according to Bradford (1976), using bovine serum albumin as a standard. All the activity of the enzymes was expressed as units (U) mg−1 protein.
Statistical analysis
The experiment was arranged as split plots in time on the basis of completely randomized design with three replications. Analysis of variance (ANOVA) was carried out with SPSS software. Differences between means were assessed by Duncan’s multiple range tests with differences being considered significant at P < 0.05.
Results and discussion
Effects of Pro-Ca treatment on CI index
Treatment with the Pro-Ca, without significant difference between two applied concentrations, resulted in a lower CI index (P < 0.01) (Table. 1). Slight CI symptoms appeared after 7 days at 1 °C plus 25 °C for 3 days in fruit from all treatment, and continued to progress over time. Chilling injury is a major factor that reduces postharvest quality and limits storage life of tomato fruit. In this study, Pro-Ca was applied and could significantly reduce postharvest CI in tomato fruit (Table. 1). Albrecht et al. (2004) reported that the application of Pro-Ca mitigated the frost injury in apple flowers and leaves. The application of Pro-Ca induces alteration of the flavonoid metabolism in the plant tissue compared to the control (Halbwirth et al. 2002; Roemmelt et al. 2002). Flavonoids can scavenge reactive oxygen species (ROS). Flavonoids are hydrophilic and are primarily located in the cytosol and vacuole. Albrecht et al. (2004) suggested that the mitigation of frost injury in apple flowers and leaves treated with Pro-Ca can possibly be explained by an alteration of flavonoids, which may reduce oxidative stress, decrease the freezing point or scavenge ROS production under frost stress.
Table 1.
Effects of prohexadione-calcium (Pro-Ca) on chilling injury (CI) index, electrolyte leakage, malondialdehyde (MDA) and proline contents and phospholipase D (PLD) and lipoxygenase (LOX) activities in tomato fruit during storage at 1 °C
| Storage time (days) | Treatment | CI index | Electrolyte leakage % | MDA content (μmol g−1 FW) | Proline content (μg g−1 FW) | PLD activity (U mg−1 protein) | LOX activity (U mg−1 protein) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 8.2 ± 2.24c | 45.7 ± 36.21c | 10.2 ± 1.22c | 31.2 ± 2.25c | 0.92 ± 0.23h | |
| 7 | Control | 0.26 ± .11cde | 24.1 ± 5.34b | 64.8 ± 22.69b | 11.5 ± 3.03c | 36.4 ± 1.37bc | 1.6 ± 0.41fg |
| Pro-Ca 50 μM | 0.06 ± .11de | 19.3 ± 5.58b | 58.8 ± 15.54b | 11.7 ± 1.08c | 35.2 ± 1.51c | 1.6 ± 0.40gh | |
| Pro-Ca 100 μM | 0.03 ± .14e | 8.9 ± 6.65c | 35.4 ± 5.96c | 12.8 ± 2.38c | 34.2 ± 0.94c | 1.5 ± 0.44h | |
| 14 | Control | 1.5 ± .25b | 63.3 ± 9.86a | 71.2 ± 16.78ab | 17.7 ± 1.52b | 42.8 ± 0.65b | 2.2 ± 0.11b |
| Pro-Ca 50 μM | 0.26 ± .11cde | 42.5 ± 7.85b | 60.5 ± 11.82b | 24.7 ± 1.31a | 38.5 ± 1.30bc | 1.7 ± 0.25de | |
| Pro-Ca 100 μM | 0.13 ± 0.11cde | 39.9 ± 3.74b | 30.5 ± 7.34c | 26.8 ± 0.81a | 36.8 ± 0.69bc | 1.7 ± 0.36ef | |
| 21 | Control | 2.6 ± 0.34a | 84.5 ± 11.17a | 94.8 ± 12.93a | 17.6 ± 1.26b | 45.9 ± 1.55a | 2.5 ± 0.12a |
| Pro-Ca 50 μM | 0.46 ± 0.11c | 53.1 ± 10.40b | 65.2 ± 6.87b | 25.2 ± 0.84a | 41.9 ± 1.41b | 2.1 ± 0.26c | |
| Pro-Ca 100 μM | 0.41 ± 0.11cd | 47.6 ± 6.65bc | 56.4 ± 10.61bc | 27.5 ± 0.44a | 38.4 ± 0.47bc | 1.8 ± 0.32d | |
| Significance | |||||||
| Treatment (T) | df2 | * * | * * | * | * * | * * | * * |
| During (D) | df2 | * | ** | ns | ** | * * | * |
| T × D | df4 | ** | * | * | * | * | * |
Mean values ± SD (n = 3). Different letters indicate significant differences at significance level P = 0.05
** and * represent significance at the 0.01, and 0.05 levels, respectively, and ns represents non-significance at P < 0.05
Effects of Pro-Ca treatment on electrolyte leakage, MDA and proline content
Membrane leakage in fruit, as evaluated by relative EL measurements, was significantly reduced in Pro-Ca treated fruit (Table. 1; P < 0.05). As shown in Table 1, Pro-Ca treatments significantly reduce MDA content of tomato fruit (P < 0.05). Also, during storage times, proline content was higher in Pro-Ca treated fruit than in control fruit (P < 0.05; Table. 1). CI involves membrane damage, which can be measured indirectly by electrolyte leakage. Zhao et al. (2009) have found that the correlation coefficient between CI index and electrolyte leakage was high irrespective of differences in chilling susceptibility between tomato cultivars. Accordingly, both the CI index and electrolyte leakage (Table. 1) were significantly lower in Pro-Ca treated fruit than in control fruit. Treatment with Pro-Ca decreased the accumulation of the lipid peroxidation product MDA (Table. 1), which is regarded as an indicator of the loss of structural integrity in membranes, being associated with cold stress. ROS accumulation may cause oxidative damage to plant cell membranes, forming toxic products such as MDA, a secondary end product of polyunsaturated fatty acid oxidation. Thus, MDA is usually considered to be an indicator of the degree of plant oxidative stress and of the structural integrity of the membranes of plants subjected to low temperatures (Hodges et al. 1999). Treatment with Pro-Ca resulted in a decrease in MDA content (Table. 1), i.e. inhibited lipid peroxidation under chilling stress, which clearly indicated that Pro-Ca could strongly protect plants from oxidative damage and thus enhance chilling tolerance.
It has been suggested that proline protects plants by functioning as a cellular osmotic regulator between cytoplasm and vacuole, and by detoxifying of ROS, thus protecting membrane integrity and stabilizing antioxidant enzymes (Bohnert and Jensen 1996). Positive correlations between the accumulation of endogenous proline and improved cold tolerance have been found mostly in chilling-sensitive plants (Zhao et al. 2009). As shown in Table 1, treatment with Pro-Ca increased proline content in tomato fruit. This increase in proline content of Pro-Ca treated fruit along with the mitigation of CI in this study confirms this finding. Bekheta et al. (2009) reported that the application of Pro-Ca enhanced the contents of proline, photosynthetic pigments (chlorophyll a, chlorophyll b and carotenoids), total carbohydrates and total soluble sugars in faba bean seedlings grown under salt stress. Bekheta et al. (2009) suggested that the growth retardant Pro-Ca may mitigate the harmful effects of salinity on the growth and development of Vicia faba seedlings.
Effects of treatment with Pro-Ca on PLD and LOX activities
Activities of PLD and LOX increased during storage, but Pro-Ca treatment inhibited the increases in activities of both enzymes and maintained lower enzyme activities at 21 days of storage (P < 0.05; Table. 1). Consequently, at the end of storage the activities of PLD and LOX were lower in fruit treated with Pro-Ca than in control fruit. To our knowledge, there is no report on the response of PLD and LOX activities to Pro-Ca treatment in tomato fruit. Therefore, in this study we examined the possible role that PLD and LOX may play in response to chilling stress in tomato fruit and investigated the response of PLD and LOX enzymes activity to treatment with Pro-Ca. In control fruit, we observed a significant increase in PLD and LOX activities accompanying the development of CI in tomato fruit (Table. 1). From this we suggest that activation of membranous lipolytic enzymes such as PLD and LOX under chilling temperature might cause irreversible membrane damage and finally the occurrence of CI (Mao et al. 2007). From our observations that treatment with Pro-Ca significantly reduced the PLD and LOX activities and mitigated CI in tomato fruit, we conclude that the reduction of CI by Pro-Ca may be related to the reduction of the PLD and LOX activities. PLD and LOX catalyse peroxidation of polyunsaturated fatty acids and are believed to be major contributors to chilling-induced membrane damage and thus CI in plant tissue (Pinhero et al. 1998; Wang 2001). Accordingly, Mao et al. (2007) showed that the development of CI in cucumber fruit was accompanied by increases in PLD and LOX activities when exposed to chilling stress, and that the enhanced tolerance to CI by heat treatment was related to the reduction in activities of both enzymes. Rui et al. (2010) reported that in loquat fruit heat treatment was associated with reduced internal browning (IB). They suggested that the reduction of IB in loquat fruit under chilling stress by heat treatment might be due to maintenance of membrane integrity, higher unsaturated/saturated fatty acid ratio, and reduced PLD and LOX activities. Consequently, increases in PLD and LOX activities might promote the development of IB and CI in loquat fruit due to a loss of membrane integrity; i.e. these two enzymes might be associated with the initiation of CI by being involved in membrane deterioration and signaling pathway in response to chilling stress. Lyons (1973) considered changes in membrane structure and composition as the primary event of CI by leading to a loss of permeability control and metabolic dysfunctioning.
Conclusion
The results suggest that the development of CI in tomato fruit includes increases in PLD and LOX activities and thus a loss of membrane integrity. Consequently, applications of Pro-Ca might be used in order to reduce CI in tomato fruit under low temperature-conditions by reducing PLD and LOX activities, increasing proline content, and thus maintaining membrane integrity. We suggest that the Pro-Ca as a new, promising and safe technology can be used for mitigation of chilling injury in tomato fruit.
References
- Albrecht E, Schmitz-Eiberger M, Barauckmann M, Rademacher W, Noga G. Use of prohexadion-calcium, vitamin E and glycerin for the reduction of frost injury in apple (Malus domestica) flowers and leaves. Eur J Hortic Sci. 2004;69:59–65. [Google Scholar]
- Bekheta MA, Abdelhamid MT, El-Morsi AA. Physiological response of Vicia faba to prohexadione–calcium under saline conditions. Planta Daninha. 2009;27:769–779. doi: 10.1590/S0100-83582009000400015. [DOI] [Google Scholar]
- Bohnert HJ, Jensen RG. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996;14:89–97. doi: 10.1016/0167-7799(96)80929-2. [DOI] [Google Scholar]
- Bourne MC. Selection and use of postharvest technologies as a component of the food chain. J Food Sci. 2006;69:43–46. doi: 10.1111/j.1365-2621.2004.tb15491.x. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of micro-gram quantities of protein utilizing the principle of protein-dye binding. Analy Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Costa G, Sabatini E, Spinelli F, Andreotti C, Bomben C, Vizzotto G. Two years of application of prohexadione-ca on apple: effect on vegetative and cropping performance, fruit quality, return bloom and residual effect. Acta Hortic. 2004;653:35–40. [Google Scholar]
- Costa G, Andreotti C, Spinelli F, Rademacher W. Prohexadione-Ca: more than a growth regulator for pome fruit trees. Acta Hortic. 2005;727:107–116. [Google Scholar]
- Ding CK, Wang CY, Gross KC, Smith DL. Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta. 2002;214:895–901. doi: 10.1007/s00425-001-0698-9. [DOI] [PubMed] [Google Scholar]
- Ding ZS, Tian SP, Zheng XL, Zhou ZW, Xu Y. Responses of reactive oxygen metabolism and quality in mango fruit to exogenous oxalic acid or salicylic acid under chilling temperature stress. Physiol Plantarum. 2007;130:112–121. doi: 10.1111/j.1399-3054.2007.00893.x. [DOI] [Google Scholar]
- Evans JR, Evans RR, Regusci CL, Rademacher W. Mode of action, metabolism, and uptake of BAS 125W, prohexadione-calcium. HortSci. 1999;34:1200–1201. [Google Scholar]
- Halbwirth H, Kampan W, Stich K, Fischer TC, Meisel B, Forkmann GW, Rademacher W. Biochemical and molecular biological investigations with respect to induction of fire blight resistance in apple and pear by transiently altering the flavonoid metabolism with specific enzyme inhibitors. Acta Hortic. 2002;590:485–492. [Google Scholar]
- Hodges DM, Delong JM, Forney CF, Prange RK. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Shiina T, Nakamura N, Nakahara A. Electrical conductivity evaluation of postharvest strawberry damate. J Food Sci. 2001;66:1392–1395. doi: 10.1111/j.1365-2621.2001.tb15220.x. [DOI] [Google Scholar]
- Karakurt Y, Huber DJ. Activities of several membrane and cell wall hydrolases, ethylene biosynthetic enzymes, and cell wall polyuronide degradation during low-temperature storage of intact and fresh-cut papaya (Carica papaya) fruit. Postharvest Biol Technol. 2003;28:219–229. doi: 10.1016/S0925-5214(02)00177-1. [DOI] [Google Scholar]
- Lyons JM. Chilling injury in plants. Ann Rev Plant Physiol. 1973;24:445–466. doi: 10.1146/annurev.pp.24.060173.002305. [DOI] [Google Scholar]
- Mao LC, Pang HG, Wang GZ, Zhu CG. Phospholipase D and lipoxygenase activity of cucumber fruit in response to chilling stress. Postharvest Biol Techno. 2007;44:42–47. doi: 10.1016/j.postharvbio.2006.11.009. [DOI] [Google Scholar]
- Pinhero RG, Paliyath G, Yada RY, Murr DP. Modulation of phospholipase D and lipoxygenase activities during chilling Relation to chilling tolerance of maize seedlings. Plant Physiol Biochem. 1998;36:213–224. doi: 10.1016/S0981-9428(97)86878-7. [DOI] [Google Scholar]
- Rademacher W. Growth retardants: effects on gibberellins biosynthesis and other metabolic pathways. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:501–531. doi: 10.1146/annurev.arplant.51.1.501. [DOI] [PubMed] [Google Scholar]
- Rademacher W, Saarloos K, Garuz-Porte JA, Riera Forcades F, Senechal Y, Andreotti C, Sinelli F, Sabatini E, Costa G. Impact of Prohexadione–Ca on the vegetative and reproductive performance of apple and pear trees. Eur J Hortic Sci. 2004;69:221–228. [Google Scholar]
- Roemmelt S, Peterek S, Treutter D, Rademacher W, Speakman JB, Andreotti C, Costa G, Sponza G, Tortoreto L, Bazzi C, Halbwirth N, Stich K, Forkmann G. Alteration of phenylpropanoid biosynthesis of fruit trees as a tool for enhancement of fire blight resistance. Acta Hortic. 2002;590:477–484. [Google Scholar]
- Rui H, Cao S, Shang H, Jin P, Wang K, Zheng Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J Sci Food Agric. 2010;90:1557–1561. doi: 10.1002/jsfa.3993. [DOI] [PubMed] [Google Scholar]
- Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao ZG, Zheng CC. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- Spinelli F, Speakman JB, Rademacher W, Halbwirth H, Stich K, Costa G. Luteoforol, a flavan 4-ol, is induced in pome fruits by prohexadione-Ca and shows phytoalexin-like properties against Erwinia amylovora and other plant pathogens. Eur J Plant Pathol. 2005;111:1–10. doi: 10.1007/s10658-004-1602-9. [DOI] [Google Scholar]
- Todd JF, Paliyath G, Thompson JE. Characteristics of a membrane associated lipoxygenase in tomato fruit. Plant Physiol. 1990;94:1225–1232. doi: 10.1104/pp.94.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Plant phospholipases. Ann Rev Plant Physiol Plant Mol Biol. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- Winkler V. Reduced risk concept for prohexadione-calcium, a vegetative growth control plant growth regulator in apples. Acta Hortic. 1997;451:667–671. [Google Scholar]
- Wonsheree T, Kesta S, van Doorn WG. The relationship between chilling injury and membrane damage in lemon basil (Ocimum citriodourum) leaves. Postharvest Biol Tech. 2009;51:91–96. doi: 10.1016/j.postharvbio.2008.05.015. [DOI] [Google Scholar]
- Zhao DY, Shen L, Fan B, Liu KL, Yu MM, Zheng Y, Dign Y, Sheng JP. Physiological and genetic properties of tomato fruits from 2 cultivars differing in chilling tolerance at cold storage. J Food Sci. 2009;74:348–352. doi: 10.1111/j.1750-3841.2009.01156.x. [DOI] [PubMed] [Google Scholar]


