Abstract
Stachys floridana schuttl. E x benth, also named yinmiao, is a special and traditional Chinese vegetable that is usually used by some diabetes patients as a pharmaceutical plant for its high content of stachyose. Due to the lower cost and higher economic reward, Stachys floridana schuttl. Ex benth is a potentially new source to extract stachyose in the medicine and food industry. Here we optimized the extraction of stachyose from Stachys floridana schuttl. Ex benth by response surface methodology, the yeild can reach as high as up to 47.0% of stachyose at temperature of 60 °C, extraction time of 40 min, ethanol volume of 60% and ratio of solid–liquid at 1:10. Our primary result holds great promising for Stachys floridana schuttl. Ex bent extracting industry as well as diabetic medicine.
Keywords: Stachys floridana Schuttl. ex Benth, Stachyose, Extraction, Response surface methodology
Introduction
Stachyose is a non-reducing tetrasaccharide consisting of one glucose, one fructose and two galactoses molecules (Pukacka et al. 2009; Yin et al. 2006). It is commonly highly reserved in the vegetative storaged organs and seeds of plants (Muzquiz et al. 1999). It can be acted as important freezing protectant in the roots in winter (Regier et al. 2010). Stachyose belongs to a classic member of raffinose family oligosaccharides (RFOs; raffinose, stachyose, and verbascose) (Dierking and Bilyeu 2009). Because humans and monogastric animals do not possess the α-galactosidase enzyme to break down these oligosaccharides (Csáky and Fekete 2004), it is not digested by monogastric animals in the small intestine. Instead, it enters the lower gut and be fermented by gas-producing bacteria. A considerable amount of gas (mainly containing carbon dioxide, hydrogen and methane) is produced from the formation of flatulence, which makes α-galactosides considered as arduous factors (Muzquiz et al. 1999).
However, stachyose is proved to be a prebiotics (Gibson and Roberfroid 1995; Seo et al. 2007; Rabelo et al. 2009), which can stimulate the growth of bifidobacteria in the human and animal intestine and protect the function of organs by eliminating harmful substances (Grmanová et al. 2010; Rada et al. 2002 and 2008; Liying et al. 2003). It is also regarded as a potential substitute for sugar in diabetes patients (Yin et al. 2006; Zhang et al. 2004). Therefore, stachyose has been approved as the special health care foods by many countries (Yin et al. 2006).
Stachys floridana Schuttl. ex Benth (SFSEB), a kind of Stachys sieboldii Miq, is a special and traditional Chinese vegetable usually used as food and pharmaceutical plant due to the high stachyose content. It mainly originates from Yanshi city, located in North China, which occupies over 95% of the output of Stachys floridana schuttl in China. It is a potentially new raw material which can be used to extract stachyose in industry due to its better economic output.
The objective of this study was to investigate the effect of extraction time, extraction temperature, ethanol volume, and the ratio of solid–liquid on percentage of stachyose extraction, as well as to optimize the condition of the stachyose extraction using response surface methodology (RSM). An empirical statistical technique would be employed for multiple regression analysis by using quantitative data obtained from properly designed experiments to solve multivariate equations simultaneously (Li et al. 2009; Shafiq et al. 2010; Jain et al. 2011).
Material and methods
Samples and sample preparation
The sample SFSEB was purchased from the supplier in Yanshi city in Henan province, China. The fresh SFSEB were selected, washed, and dried at 60 °C for 18 h, then smashed into dry superfine powder.
Reagent and working standard curve
Analytical grade stachyose, sucrose, and raffinose (all contents >99%) were obtained from Sigma-Aldrich (Missouri, USA). Stock standard solutions of stachyose, sucrose, and raffinose were prepared at a concentration of 2.0 mg/ml and stored at 4 °C in the refrigerator. The standard solutions were prepared as needed in distilled water. The standard curve of stachyose was shown as a straight line by HPLC analysis, with the regressive equation of
 |
1 |
x was peak area of stachyose, y was concentration of stachyose, and coefficient of (R2) =0.9995.
All of the sample solutions were filtered through a 0.45 μm Millipore membrane filter before the HPLC analysis.
Extraction of Stachyose
The reflux defatting technique was considered in the laboratory in order to improve the defatting effects and decrease the effect of fat on the stachyose yield. And previous studies showed that chloroform may be used as defating agent in reflux defatting technique (Cohen et al. 2009; Schober et al. 2010). Here, dried powder of SFSEB (5.0 g) was placed into 50 mL of chloroform and refluxed for 2 h at 60 °C for defatting. The solvent was then removed by filtration and the defatted residue was extracted by different concentrations of ethanol and refluxed at a designed time, temperature and ratio of solid–liquid. The mixture was filtered and the supernatant, as the crude extraction solution of stachyose, was analyzed by the HPLC system.
HPLC analysis
Stachyose was analyzed by using an HPLC system equipped with RI detector. 20 μL sample was loaded on to a Hypersil NH2 column (250 mm × 4.6 mm i.d.,Thermo Fisher Scientific, USA). A mixture of acetonitrile and water (70:30 v/v) was used as a mobile phrase with a flow rate of 1.0 mL/min. The temperature of the column was set at 25 °C. The yield of stachyose was calculated from the peak area and its regressive Eq. 1.
Experimental design for single factor and response surface methodology
The aim of single factor tests was to optimize the main effective factors and the better ranges of parameters for RSM (Table 1). Here, the single factor were extraction temperature, extraction time, ethanol volume and the ratio of solid–liquid, respectively. And their ranges for RSM were as follows: extraction temperature (X1) ranged from 50 °C to 70 °C; time (X2) ranged from 30 to 50 min; ethanol volume (X3) ranged from 50 to 70%; the ratio of solid–liquid ranged from 1:8 to 1:12. The coded and corresponding uncoded independent variables used in the RSM design were listed in Table 2.
Table 1.
The results of single factors on the yield of stachyose
| Temperature (°C) | Y (%) | Time (min) | Y (%) | The Ethanol Volume (% v/v) | Y (%) | The Ratio of Solid–liquid (°C) | Y (%) |
|---|---|---|---|---|---|---|---|
| 30 | 39.3 ± 1.23 | 20 | 37.7 ± 0.93 | 30 | 34.4 ± 1.33 | 1:8 | 35.1 ± 1.40 |
| 40 | 41.5 ± 1.20 | 30 | 40.0 ± 1.14 | 40 | 38.8 ± 1.11 | 1:10 | 45.4 ± 1.51 |
| 50 | 42.6 ± 1.03 | 40 | 45.6 ± 1.53 | 50 | 42.1 ± 0.93 | 1:12 | 41.5 ± 1.36 |
| 60 | 45.2 ± 1.29 | 50 | 39.2 ± 1.09 | 60 | 45.1 ± 1.18 | 1:14 | 41.2 ± 1.09 |
| 70 | 41.1 ± 1.32 | 60 | 37.6 ± 0.79 | 70 | 40.2 ± 1.14 | 1:16 | 39.8 ± 1.17 |
Table 2.
The levels of different extraction variables in coded and uncoded form for the extraction of stachyose
| Independent variables | Symbol | Levels | |||
|---|---|---|---|---|---|
| uncoded | coded | -1 | 0 | 1 | |
| Extraction Temperature (°C) | X1 | x1 | 50 | 60 | 70 |
| Extraction Time (min) | X2 | x2 | 30 | 40 | 50 |
| Ethanol Volume (%) | X3 | x3 | 50 | 60 | 70 |
| Ratio of Solid–Liquid | X4 | x4 | 1:8 | 1:10 | 1:12 |
A central composite design of Box-Behneken was used to investigate the effects of the four independent variables on the yield of stachyose (Y). The independent variables were coded at three levels (−1, 0, 1). The whole design consisted of 29 experimental points, including five replications of the center points (all variables were coded as zero) and 24 factorial points (Table 3). The 29 sets of experiments were performed in a random order. The purpose of the center points was to estimate the pure error and curvature.
Table 3.
Experimental design and results of Response Surface Analysis for the extraction rate of stachyose
| Run | X1 | X2 | X3 | X4 | Y(%) | |
|---|---|---|---|---|---|---|
| Actual value | Predicted value | |||||
| 1 | −1 | −1 | 0 | 0 | 32.5 | 33.4 |
| 1 | −1 | 0 | 0 | 32.3 | 32.4 | |
| 3 | −1 | 1 | 0 | 0 | 33.4 | 32.9 |
| 4 | 1 | 1 | 0 | 0 | 32.8 | 31.7 |
| 5 | 0 | 0 | −1 | −1 | 43.9 | 42.3 |
| 6 | 0 | 0 | 1 | −1 | 25.6 | 26.6 |
| 7 | 0 | 0 | −1 | 1 | 37.1 | 37.1 |
| 8 | 0 | 0 | 1 | 1 | 41.8 | 41.8 |
| 9 | −1 | 0 | 0 | −1 | 34.1 | 34.1 |
| 10 | 1 | 0 | 0 | −1 | 30.9 | 30.3 |
| 11 | −1 | 0 | 0 | 1 | 39.0 | 39.0 |
| 12 | 1 | 0 | 0 | 1 | 36.6 | 36.6 |
| 13 | 0 | −1 | −1 | 0 | 32.9 | 32.9 |
| 14 | 0 | 1 | −1 | 0 | 31.7 | 31.7 |
| 15 | 0 | −1 | 1 | 0 | 35.0 | 35.0 |
| 16 | 0 | 1 | 1 | 0 | 30.2 | 30.0 |
| 17 | −1 | 0 | −1 | 0 | 40.4 | 40.4 |
| 18 | 1 | 0 | −1 | 0 | 36.3 | 36.2 |
| 19 | −1 | 0 | 1 | 0 | 30.0 | 30.0 |
| 20 | 1 | 0 | 1 | 0 | 33.8 | 33.8 |
| 21 | 0 | −1 | 0 | −1 | 31.6 | 31.6 |
| 22 | 0 | 1 | 0 | −1 | 35.0 | 35.0 |
| 23 | 0 | −1 | 0 | 1 | 40.1 | 39.6 |
| 24 | 0 | 1 | 0 | 1 | 37.8 | 36.2 |
| 25 | 0 | 0 | 0 | 0 | 46.7 | 47.4 |
| 26 | 0 | 0 | 0 | 0 | 45.2 | 47.4 |
| 27 | 0 | 0 | 0 | 0 | 45.4 | 47.4 |
| 28 | 0 | 0 | 0 | 0 | 48.6 | 47.4 |
| 29 | 0 | 0 | 0 | 0 | 49.1 | 47.4 |
Statistical analysis
All analyses were performed in triplicate. Mean values being compared were considered to be significantly different if the p-value was less than 0.05. All statistical evaluations of response surface methodology were done with the statistical software Design-Expert 6.0.5.
Results and discussion
HPLC analysis of stachyose
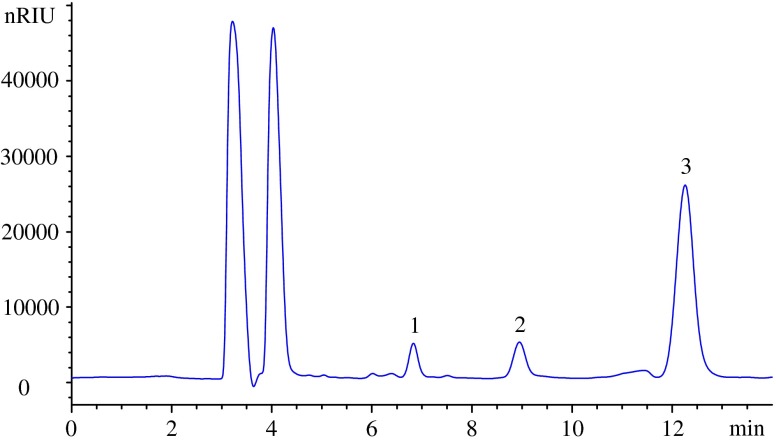
The crude extraction solution of stachyose was analyzed with HPLC after chloroform defatting under different extraction conditions. The content of water in mobile phase showed a great effect on the retention time of the carbohydrates. Lower content of water in mobile phase results in a longer retention times for the carbohydrates. However, the peak in the chromatogram became broad and asymmetric when the content of water was below 10% (v/v) (Yin et al., 2006). After testing several solvent systems, a solvent mixture of acetonitrile–water (70: 30 v/v) with a flow rate of 1.0 mL/min was chosen, and the retention times were 6.8 min for sucrose, 8.9 min for raffinose and 12.3 min for stachyose, respectively (Fig. 1).
Fig. 1.
HPLC chromatogram of the sample 1.sucrose, 2.raffinrose, 3.stchayose
It should be mentioned when the column temperature or mobile phase changed within a small range, the baseline still remained flat and stable, and the responses of the oligosaccharides were not obviously influenced. Under the selected conditions of 20 μL of the standard mixed solution injected into the HPLC system at 25 °C, the Hypersil NH2 column could provide good separation for stachyose, sucrose and raffinose from the extracts within 15 min, with no other interfering peak presented near the peak of the analyte. According to the relationship in double logarithmic of peak areas versus sample concentrations, the contents of the stachyose in dry tubers of yinmiao could be calculated and the results were shown in Table 3.
The results and analysis of single factors
The effects of each extraction factor on the yield of stachyose were shown in Table 1, where Y (%) was the yield of stachyose. When one factor was investigated, other independent variables were kept constant. At extraction temperature of 60 °C, extraction time of 40 min, ethanol volume at 60% and the ratio of solid–liquid of 1:10, respectively, the extraction yield arrived at maximum value. Thus, the parameters of the central points of RSM were determined as the above.
Model fitting
Experimental set and corresponding experimental data obtained were shown in Table 3. The analysis of variance (ANOVA) was also performed and the results were given in Table 3. In Table 4, a summary of the ANOVA (F-test) and p-value, was used as a means to check the significance of each coefficient and indicated the interaction strength between each parameter.
Table 4.
ANOVA for response surface quadratic model analysis of variance
| Source | DF | Sum of square | Mean square | F-value | Probability (p) |
|---|---|---|---|---|---|
| Model | 14 | 973.3 | 69.5 | 13.5 | <0.0001* |
| Residual | 14 | 72.2 | 5.2 | ||
| Lack of fit | 10 | 59.5 | 6.0 | 1.9 | 0.29 |
| Pure error | 4 | 12.8 | 3.2 | ||
| Cor total | 28 | 1045.5 | |||
| R2 = 0.931 | Adj.R2 = 0.862 |
In the present experiment, the model F-value of 13.5 implied that the model was significant. While, the “Lack of fit F-value” of 1.9 implied that the “Lack of fit” was less obvious than the pure error. There was a 28.72% chance that a "Lack of fit F-value" could occur. Non-significant lack of fit was good. The coefficient of determination (R2) and adjusted coefficient of determination (Adj.R2) were 0.931 and 0.862, respectively. High values of R2 (93.1%) and adj R2 (86.2%) indicated a high dependence and correlation between the observed and the predicted values of response. In addition, the value of R2 = 93.1% also indicated that the total variation can be explained by this model.
Multivariable linear regression was used to estimate the coefficients on the second-order polynomial equation and the obtained regression coefficients, where the significance was determined using the Student t-test and p-value, and summarized in Table 5. The corresponding variable would be more significant if the absolute value of t increase and the p-value decreases (Wei et al. 2009).
Table 5.
Regression coefficient of polynomial function of response surface of stachyose yield
| Term | Coefficient Estimate | Standard Error | t | p |
|---|---|---|---|---|
| X1 | −0.56 | 0.66 | 0.73 | 0.406 |
| X2 | −0.30 | 0.66 | 0.20 | 0.658 |
| X3 | −2.15 | 0.66 | 10.7 | 0.006 |
| X4 | 2.6 | 0.66 | 15.7 | 0.001 |
| X21 | −6.75 | 0.89 | 57.4 | < 0.0001 |
| X22 | −7.64 | 0.89 | 73.4 | < 0.0001 |
| X23 | −5.95 | 0.89 | 44.5 | < 0.0001 |
| X24 | −4.09 | 0.89 | 21.1 | 0.0004 |
| X1X2 | −0.058 | 1.1 | 2.618E-003 | 0.960 |
| X1 X3 | 2.0 | 1.1 | 3.1 | 0.101 |
| X1 X4 | 0.21 | 1.1 | 0.034 | 0.856 |
| X2 X3 | −0.92 | 1.1 | 0.65 | 0.433 |
| X2 X4 | −1.42 | 1.1 | 1.6 | 0.231 |
| X3 X4 | 5.6 | 1.1 | 25.7 | 0.0002 |
For the present experiments, the model terms are significant when values of p was less than 0.050, therefore x3, x4, x21, x22, x23, x24, x3x4 were observed as significant model terms. The results showed that x3, x4 were the key impact factors on the yield of stachyose (p = 0.001, p = 0.006, respectively). The effective order of test variables on the yield of stachyose was as follows: x4 > x3 > x1 > x2.
The second-order polynomial model was used to express the stachyose yield (Y) as a function of independent variables (Modha and Pal 2011). Equation 2 was shown as follows (in terms of coded levels):
 |
2 |
From Table 4 and Eq. 2, it can be seen that the factors with the largest effect on the yield were the quadratic term of time, temperature, ethanol volume, and ratio of solid–liquid (p < 0.001). All the second-order terms had positive effects on yield. From all the results, it had been proved that the model was fully applicable.
Analysis of response surfaces
Response surfaces were drawn to determine the individual and interactive effect of test variable on rate of extraction (Chakraborty et al. 2011). Response surface graphs were plotted between two independent variables while the remaining independent variables were kept at the zero coded level. The relationship between variables was illustrated by these response surface plots (Lu et al. 2008). The contour map could indicate the interaction strength in which the ellipse represented significance and the rotundity in verse.
The effects of the four factors as well as their interactive effect on the extraction rate were shown in Fig. 2. Figure 2a denoted the three dimensional surfaces plots of effect of extraction temperature (x1) and extraction time (x2) on response: when the extraction temperature (from 50 to 70 °C) and the extraction time (from 30 to 50 min) increased, Y gradually mounted up to the highest value (59.6 °C and 39.6 min, respectively), and decreased gradually. Its contour map was close to rotundity, indicating no significant interaction.
Fig. 2.
Response surface and contour plot showing the effect of different extraction variables
Previous studies showed that the heat treatment could increase the swelling degree of materials and the movement speed of molecules. But by using this method, some unwanted components might diffuse into the solution (Ruiz-Matute et al. 2007; Sanz et al. 2001). When the extraction temperature is too low (under 50 °C), the materials do not dissolve fully. As a result, stachyose would not obtain enough energy to free themselves from the chemical bonds such as hydrogen bonding and Van Der Waals force. On the other hand, when the extraction temperature is too high (above 70 °C), the molecular movement of ethanol becomes too fast, shortening the contact time between stachyose and ethanol which leads to the stachyose solubility decreased. Here, Y is the highest at the extraction temperature of 60 °C.
When the extraction time increased, Y gradually mounted up to the highest value (40 min), and then decreased gradually. One possible reason is that the materials are not dissolved and the cell membrane is not disrupted fully in shorter time (Sogi et al. 2010). So, the intracellular components are not released totally, which causes a lower yield of stachyose. With the extension of time, increased ethanol concentration would help more stachyose molecules diffuse into the solvent. However, the yield of stachyose would decrease at higher temperature and longer extraction time due to slight increase in hydrolysis of the stachyose,increasing the yield of monomer sugars after heat treatment. Palm and Zacchi (Palm and Zacchi 2003) studied the effect of extraction performed at different extraction temperature and extraction time on hemicellulosic oligosaccharides from spruce. They also observed that the higher temperature and longer treating time are related to the hydrolysis of the oligosaccharides. In addition, when the extraction time is longer, the energy consumption and the investment cost of the actual production would also increase, which is detrimental to the actual production.
The trend of the results with the three dimensional surface plots by interaction of extraction temperature (x1) and ethanol volume (x3) (Fig. 2b), extraction temperature (x1) and ratio of solid–liquid (x4) (Fig. 2c), extraction time (x2) and ethanol volume (x3) (Fig. 2d), extraction time (x2), and ratio of solid–liquid (x4) (Fig. 2e) were the same, which indicated that there was no significant interaction. While the interaction of ethanol volume (x3) and ratio of solid–liquid (x4) (Fig. 2f) was significant for the contour map was close to ellipse, the p-value was only 0.0002. When ethanol volume was 59.6%, and the ratio of solid–liquid was 1:10.6, the stachyose yield was the highest.
When the ratio of solid–liquid was at 1:10, Y was the highest in the study. The effect of the ratio of solid–liquid on the yield was very significant (p = 0.001, p < 0.05). When the ratio was lower (1:8), the contact opportunity of the superfine powder and solvent became less and the stachyose residues were much higher in the solid phase, Y was lower. The higher concentration solvent (1:10) was useful in the diffusion of the stachyose. But when the ratio of solid–liquid increased sequentially (1:12), it would decrease the yield of stachyose due to larger amount of solvent.
Solvent extraction is a classical method for the extraction of oligosaccharides. In this study, when the ethanol volume was 30–50%, Y was found to be lower. It is probably due to some more contaminated components in water-extractable fractions than the alcohol extracted ones. The water-extractable oligosaccharides are also easier to undergo hydrolysis to form monosaccharide (Hromádková et al. 1999). In addition, Y mounted up to the highest value at 60% of the ethanol volume here. It was possible that some precipitates (such as protein and polysaccharides) that are formed by the higher ethanol concentration might hinder the stachyose from diffusing into the alcohol solution. The result obtained from the experiment is the same as the study of Ekvall et al. (Ekvall et al. 2007). It was noticed that the volume of ethanol is closely related to the yield of stachyose, which in turn proves that ethanol concentration is another key impact factor on the stachyose extraction (p = 0.006, p < 0.05).
Optimization and verification
Based on the above, the predicted optimal conditions for stachyose yield were as follow: extraction temperature of 59.6 °C, extraction time of 39.6 min, ethanol volume of 59.6%, and the ratio of solid–liquid at 1:10.6. The predicted stachyose yield was 47.4%.
But in terms of the actual production, it was difficult to control these optimum conditions. Fortunately, these factors were close to the central point. In order to operate conveniently, the optimum conditions for extracting stachyose from yinmiao were regulated as follows: extraction temperature of 60 °C, extraction time of 40 min, ethanol volume of 60%, and the ratio of solid–liquid at 1:10. Therefore the mean experimental values of the five replications on the optimum were 47.0%, where the error was at 1.2%.
Previous studies show that soybean holds high content of the stachyose, and it was identified as the exclusive source of stachyose (Grmanová et al. 2010). However, through our designed protocal, stachyose could be extracted from yinmiao, and the yield of stachyose is 46.99% by RSM. So yinmiao can be new potentical source of stachyose in industry due to its lower cost and higher economic efficiency.
Conclusion
The factor of extraction conditions including extraction temperature, extraction time, the ethanol volume, and the ratio of liquid–solid on the yield of stachyose was investigated here. The results of RSM were used to identify a mathematical statistical relationship between explanatory variable levels and the response and to optimize the system response. The model of the multivariable linear regression is more significant in the study as compared to the “Lack of fit F-value” of the analysis of variance. All of these results indicat that RSM was successfully applied to determine the optimum factors for stachyose extraction. The experimental values match well with the predicted data. The ethanol volume and the ratio of liquid–solid are the key impact factors of the yield of stachyose. The effective order of test variables on the yield of stachyose was determined as follows: the ratio of liquid–solid > ethanol volume > the extraction temperature > the extraction time. The requirements and conditions of extracting stachyose from yinmiao were as follows: extraction temperature of 60 °C, extraction time of 40 min, ethanol volume of 60%, and ratio of solid–liquid at 1:10. The extraction yield of stachyose was measured at as high as 47.0% under these optimized conditions.
Acknowledgements
The authors would like to express their gratitude to Professor Yusheng Cao for his technical support and also acknowledge supports to the Opening Foundation of the State Key Laboratory of Food Science and Technology in Nanchang University, China (No. NCU200508)
References
- Chakraborty SK, Kumbhar BK, Chakraborty S, Yadav P. Influence of processing parameters on textural characteristics and overall acceptability of millet enriched biscuits using response surface methodology. J Food Sci Technol. 2011;48:167–174. doi: 10.1007/s13197-010-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Deeds JR, Wong ES, Hanner RH, Yancy HF, White KD, Thompson TM, Wahl M, Pham TD, Frances G, Huh I, Austin C, Dizikes G, Gerber S. Public Health Response to Puffer Fish (Tetrodotoxin) Poisoning from Mislabeled Product. Journal of Food Protection. 2009;72:810–817. doi: 10.4315/0362-028x-72.4.810. [DOI] [PubMed] [Google Scholar]
- Csáky I, Fekete S. Soybean: feed quality and safety. Part 1: biologically active components. A review. Acta Vet Hung. 2004;52:299–313. doi: 10.1556/AVet.52.2004.3.6. [DOI] [PubMed] [Google Scholar]
- Dierking EC, Bilyeu KD. Raffinose and Stachyose metabolism are not required for efficient soybean seed germination. J Plant Physiol. 2009;166:1329–1335. doi: 10.1016/j.jplph.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Ekvall J, Stegmark R, Nyman M. Optimization of extraction methods for determination of the raffinose family oligosaccharides in leguminous vine peas (Pisum sativum L.) and effects of blanching. J Food Compos Anal. 2007;20:13–18. doi: 10.1016/j.jfca.2006.06.010. [DOI] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Grmanová M, Rada V, Sirotek K, Vlková E. Naturally occurring prebiotic oligosaccharides in poultry feed mixtures. Folia Microbiol (Praha) 2010;55:326–328. doi: 10.1007/s12223-010-0050-5. [DOI] [PubMed] [Google Scholar]
- Hromádková Z, Ebringerová A, Valachovic P. Comparison of classical and ultrasound-assisted extraction of polysaccharides from Salvia officinalis L. Ultrason Sonochem. 1999;5:163–168. doi: 10.1016/S1350-4177(98)00046-7. [DOI] [PubMed] [Google Scholar]
- Jain SK, Verma RC, Murdia LK, Jain HK, Sharma GP. Optimization of process parameters for osmotic dehydration of papaya cubes. J Food Sci Technol. 2011;48:211–217. doi: 10.1007/s13197-010-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wang ZC, Dai HJ, Cui L, Xu QG, Li J. Response surface optimization of polysaccharides extraction from liriope roots and its modulatory effect on sjogren syndrome. Int J Biol Macromol. 2009;45:284–288. doi: 10.1016/j.ijbiomac.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Liying Z, Li D, Qiao S, Johnson EW, Li B, Thacker PA, Han IK. Effects of Stachyose on performance, diarrhoea incidence and intestinal bacteria in weanling pigs. Arch Tierernahr. 2003;57:1–10. [PubMed] [Google Scholar]
- Lu CH, Engelmann NJ, Lila MA, Erdman JW., Jr Optimization of lycopene extraction from tomato cell suspension culture by response surface methodology. J Agric Food Chem. 2008;56:7710–7714. doi: 10.1021/jf801029k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modha H, Pal D. Optimization of Rabadi-like fermented milk beverage using pearl millet. J Food Sci Technol. 2011;48:190–196. doi: 10.1007/s13197-010-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzquiz M, Burbano C, Pedrosa MM, Folkman W, Gulewicz K. Lupins as a potential source of raffinose family oligosaccharides preparative method for their isolation and purification. Ind Crops Prod. 1999;9:183–188. doi: 10.1016/S0926-6690(98)00030-2. [DOI] [Google Scholar]
- Palm M, Zacchi G. Extraction of hemicellulosic oligosaccharides from spruce using microwave oven or steam treatment. Biomacromolecules. 2003;4:617–623. doi: 10.1021/bm020112d. [DOI] [PubMed] [Google Scholar]
- Pukacka S, Ratajczak E, Kalemba E. Non-reducing sugar levels in beech (Fagus sylvatica) seeds as related to withstanding desiccation and storage. J Plant Physiol. 2009;2:1–10. doi: 10.1016/j.jplph.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Rabelo MC, Fontes CP, Rodrigues S. Enzyme synthesis of oligosaccharides using cashew apple juice as substrate. Bioresour Technol. 2009;100:5574–5580. doi: 10.1016/j.biortech.2009.06.060. [DOI] [PubMed] [Google Scholar]
- Rada V, Bartonová J, Vlková E. Specific growth rate of bifidobacteria cultured on different sugars. Folia Microbiol (Praha) 2002;47:477–480. doi: 10.1007/BF02818784. [DOI] [PubMed] [Google Scholar]
- Rada V, Nevoral J, Trojanová I, Tománková E, Smehilová M, Killer J. Growth of infant faecal bifidobacteria and clostridia on prebiotic oligosaccharides in vitro conditions. Anaerobe. 2008;14:205–208. doi: 10.1016/j.anaerobe.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Regier N, Streb S, Zeeman SC, Frey B. Seasonal changes in starch and sugar content of poplar (Populus deltoides x nigra cv. Dorskamp) and the impact of stem girdling on carbohydrate allocation to roots. Tree Physiol. 2010;30:979–987. doi: 10.1093/treephys/tpq047. [DOI] [PubMed] [Google Scholar]
- Ruiz-Matute AI, Sanz ML, Corzo N, Martín-Alvarez PJ, Ibañez E, Martínez-Castro I, Olano A. Purification of lactulose from mixtures with lactose using pressurized liquid extraction with ethanol-water at different temperatures. J Agric Food Chem. 2007;55:3346–3350. doi: 10.1021/jf070018u. [DOI] [PubMed] [Google Scholar]
- Sanz MA, Blázquez I, Sierra I, Medrano MA, Frias J, Vidal-Valverde C, Hernández A. Nutritional evaluation of ethanol-extracted lentil flours. J Agric Food Chem. 2001;49:1854–1860. doi: 10.1021/jf001293i. [DOI] [PubMed] [Google Scholar]
- Schober TJ, Moreau RA, Bean SR, Boyle DL. Removal of surface lipids improves the functionality of commercial zein in viscoelastic zein-starch dough for gluten-free breadmaking. Journal of Cereal Science. 2010;52:417–425. doi: 10.1016/j.jcs.2010.07.004. [DOI] [Google Scholar]
- Seo DM, Kim SY, Eom HJ, Han NS. Synbiotic synthesis of oligosaccharides during milk fermentation by addition of leuconostoc starter and sugars. J Microbiol Biotechnol. 2007;17:1758–1764. [PubMed] [Google Scholar]
- Shafiq MA, Amarjit S, Sawhney BK. Response surface optimization of osmotic dehydration process for aonla slices. J Food Sci Technol. 2010;47:47–54. doi: 10.1007/s13197-010-0014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogi DS, Sharma S, Oberoi DPS, Wani IA. Effect of extraction parameters on curcumin yield from turmeric. J Food Sci Technol. 2010;47:300–304. doi: 10.1007/s13197-010-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei ZJ, Liao AM, Zhang HX, Liu J, Jiang ST. Optimization of supercritical carbon dioxide extraction of silkworm pupal oil applying the response surface methodology. Bioresour Technol. 2009;100:4214–4219. doi: 10.1016/j.biortech.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Yin J, Yang G, Wang S, Chen Y. Purification and determination of Stachyose in Chinese artichoke (Stachys Sieboldii Miq.) by high-performance liquid chromatography with evaporative light scattering detection. Talanta. 2006;70:208–212. doi: 10.1016/j.talanta.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Jia ZP, Kong LY, Ma HP, Ren J, Li MX, Ge X. Stachyose extract from Rehmannia glutinosa Libosch. to lower plasma glucose in normal and diabetic rats by oral administration. Pharmazie. 2004;59:552–556. [PubMed] [Google Scholar]