Abstract
Ribonucleotides have shown many promising applications in food and pharmaceutical industries. The aim of the present study was to produce ribonucleotides (RNA) by Kluyveromyces marxianus ATCC 8,554 utilizing cheese whey, a dairy industry waste, as a main substrate under batch fermentation conditions. The effects of temperature, pH, aeration rate, agitation and initial cellular concentration were studied simultaneously through factorial design for RNA, biomass production and lactose consumption. The maximum RNA production (28.66 mg/g of dry biomass) was observed at temperature 30°C, pH 5.0 and 1 g/l of initial cellular concentration after 2 h of fermentation. Agitation and aeration rate did not influence on RNA concentration (p > 0.05). Maximum lactose consumption (98.7%) and biomass production (6.0 g/l) was observed after 12 h of incubation. This study proves that cheese whey can be used as an adequate medium for RNA production by K. marxianus under the optimized conditions at industrial scale.
Keywords: Cheese whey, Kluyveromyces marxianus, Ribonucleotides, Fermentation
Introduction
Cheese whey is an important residue generated from dairy industries is recognized as a source of many bioactive compounds of commercial significance (Guimarães et al. 2010; Koushki et al. 2011). It is produced in large amounts from dairy industries causing significant environmental problem (Ozmihci and Kargi 2007; Sansonetti et al. 2010). The world production of cheese whey is estimated at over 108 tones per year could be an attractive raw material for the production of value-added compounds such as ethanol, single cell protein, organic acids, biodegradable plastics and oligonucleotides (Koushki et al. 2011; Kolaei et al. 2007; Li and Shahbazi 2006; Nath et al. 2008; Belem and Lee 1999). However, only 15% of the available whey is used by industries (Calil et al. 2009), being the great remaining part discarded as residual water in the environment (Saraiva et al. 2009). Whey has high biological (40,000 ppm) and chemical oxygen demand (60,000 ppm) causing serious environmental problems for dairy industries (Ghaly et al. 2007).
Cheese whey is separated during the conventional cheese preparations and was found to contain 7% solids comprising of about 10–12% proteins, 74% lactose, 8% minerals and 3% fat (Morr 1989; Florentino et al. 2005). The compositional analysis, available volume and disposal problems attribute its capacity to serve as a cheap and sustainable feedstock for the production of several value-added products. In the present study, we aimed to evaluate its potential for the production of ribonucleotides by Kluyveromyces marxianus ATCC 8774. Ribonucleotides have been successfully used in pharmaceutical industry as an immunological modulator (Schaller et al. 2004) and in food industry as a flavour enhances (Ventanas et al. 2010). Ribonucleotides produced by K. fragilis also have been reported to have anti-tumour, anti-viral and probiotic effects (Belem and Lee 1997). Thus, these applications justify our interest in its production using a low-cost fermentation.
Kluyveromyces yeast has been used in the elaboration of dairy products because of its ability in lactose assimilation (Koutinas et al. 2009). They are classified as Generally Regarded as Safe (GRAS) microorganisms according to the Food and Drug Administration (FDA) guidelines, which is an important security aspect since its fermentation products have applicability in pharmaceutical and food industries (Lane and Morrissey 2010). In past, several microorganisms such as Candida utilis, Bacillus subtilis, Saccharomyces cerevisiae, Micrococcus glutamicus and Penicillium citrinum have been employed for the production of ribonucleotides (Belem and Lee 1997). However, K. marxianus has shown high growth and product titers due to its β-galactosidase production when lactose from whey utilized as carbon source (Revillion et al. 2003; Gupte and Nair 2010). The pH of the culture medium and the maintenance of appropriate temperature during fermentation process are very critical parameters to attain the desired yields of metabolites (Belem and Lee 1997; Longhi et al. 2004). Till now, however, nothing is reported concerning the optimization studies for ribonucleotides production through statistically designed approaches. In the present study, we aimed to find the influence between five factors and the possible interactions which contribute to ribonucleotides production by K. marxianus ATCC 8774 from whey by using a fractional factorial design. Selected parameters were temperature, pH, aeration rate, agitation and initial cellular concentration. Measured responses were biomass production, lactose consumption and ribonucleotides production.
Material and methods
Microorganism and inoculum preparation
The strain used was Kluyveromyces marxianus var. marxianus ATCC 8554, obtained from the Culture Collection of voor Centraalbureau Schimmelcultures (Utrecht, Holland). The microorganism was rehydrated and conserved in assay tubes with skim-milk (medium base of rehydrated bovine milk and glycerol) at −20°C. Yeast cells were replicated in Sabouraud agar slants, and stored at a temperature of 4°C. The yeast was replicated every 7 days in order to keep the culture with its initial characteristics.
K. marxianus cells were inoculated in 250 mL Erlenmeyer flasks containing 100 ml of treated whey (pH: 6.0). Two mililiters of cellular suspension with 4% of transmittance (600 nm) were added to the medium. Inoculum preparation was carried out at 100 rpm, 30°C for 18 h.
Whey treatment
The substrate was fresh whey obtained from enzymatic coagulation of pasteurized milk from manufacture of Minas Frescal cheese, Minas Geiras, Brazil. It was treated with chloridric acid (32% hydrochloric acid) until pH reached 4.3 for de-proteinization to avoid Maillard reaction during sterilization by autoclaving. Thereafter, the material was cooled at room temperature and filtered with cotton sheets, to remove fines and cream. It was finally added to a 500 mL flask for posterior sterilization (121°C, 1 atm, 20 min). Temperature, pH and aeration rate were also taken into the consideration according to the factorial design. All the fermentation runs were continued up to 12 h. The samples were taken aseptically after every 2 h.
Fermentation
Batch fermentations were conducted in a 3 L bench stirred tank bioreactor (Braun Biostast®, USA), supplied by 1 L of treated whey. The inoculum was added to obtain the concentration determined according to the factorial design. A fractioned factorial 25-1 with three repetitions at central point was designed. Studied parameters were initial cellular concentration, process temperature, aeration rate, pH and agitation. Levels and values of each independent variable are presented in Table 1. According to the designed matrix, 19 fermentation runs were carried out with the changed parameters including three repetitions at central point.
Table 1.
Fractioned factorial design investigating the effects of temperature, pH, aeration rate, agitation and initial cellular concentration to ribonucleotides production by Kluyveromyces marxianus ATCC 8554
| Run | Variables in coded levels | Measured response | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | RNA (mg/g cell) | Biomass (g/l) | Lactose Consumption (% | |||||||
| T2 | T6 | T12 | T2 | T6 | T12 | T2 | T6 | T12 | ||||||
| 1 | +1 | +1 | +1 | +1 | +1 | 12.7 | 8.9 | 6.9 | 0.7 | 2.6 | 3.7 | 19.8 | 71.3 | 97.0 |
| 2 | +1 | +1 | +1 | −1 | −1 | 18.5 | 14.1 | 12.4 | 0.4 | 1.5 | 2.5 | 6.3 | 30.5 | 66.4 |
| 3 | +1 | +1 | −1 | +1 | −1 | 25.1 | 15.6 | 13.1 | 0.3 | 2.2 | 2.8 | 12.1 | 58.4 | 94.4 |
| 4 | +1 | +1 | −1 | −1 | +1 | 24.4 | 14.5 | 10.2 | 0.3 | 1.6 | 2.4 | 23,9 | 65.5 | 96.0 |
| 5 | +1 | −1 | +1 | +1 | −1 | 32.1 | 22.1 | 25.1 | 0.7 | 1.8 | 1.8 | 14.1 | 76.4 | 97.0 |
| 6 | +1 | −1 | +1 | −1 | +1 | 10.5 | 8.5 | 9.6 | 1.1 | 2.1 | 2.2 | 30.3 | 95.2 | 98.0 |
| 7 | +1 | −1 | −1 | +1 | +1 | 9.7 | 16.7 | 11.9 | 1.2 | 2.3 | 2.5 | 42.2 | 97.2 | 98.0 |
| 8 | +1 | −1 | −1 | −1 | −1 | 25.8 | 23.1 | 23.6 | 0.5 | 2.2 | 2.3 | 27.1 | 77.1 | 97.9 |
| 9 | −1 | +1 | +1 | +1 | −1 | 24.2 | 26.5 | 15.2 | 0.2 | 1.4 | 3.6 | 3.1 | 14.7 | 89.0 |
| 10 | −1 | +1 | +1 | −1 | +1 | 18.6 | 15.7 | 11.7 | 0.4 | 1.9 | 3.1 | 9.3 | 57.0 | 86.4 |
| 11 | −1 | +1 | −1 | +1 | +1 | 21.8 | 18.5 | 13.6 | 0.3 | 1.5 | 3.1 | 11.0 | 58.0 | 96.9 |
| 12 | −1 | +1 | −1 | −1 | −1 | 31.0 | 17.0 | 15.4 | 0.2 | 1.4 | 2.0 | 5.8 | 22.2 | 64.1 |
| 13 | −1 | −1 | +1 | +1 | +1 | 24.7 | 17.5 | 17.3 | 0.1 | 1.8 | 2.7 | 14.3 | 73.2 | 97.6 |
| 14 | −1 | −1 | +1 | −1 | −1 | 30.2 | 26.6 | 17.4 | 0.3 | 1.5 | 3.0 | 1.6 | 36.6 | 94.9 |
| 15 | −1 | −1 | −1 | +1 | −1 | 26.5 | 19.3 | 12.9 | 0.5 | 2.3 | 3.5 | 16.9 | 64.5 | 97.2 |
| 16 | −1 | −1 | −1 | −1 | +1 | 17.3 | 14.9 | 14.5 | 0.1 | 0.9 | 1.7 | 19.1 | 68.9 | 97.4 |
| 17 | 0 | 0 | 0 | 0 | 0 | 10.9 | 16.6 | 15.6 | 0.5 | 2.2 | 2.8 | 28.3 | 94.8 | 98.8 |
| 18 | 0 | 0 | 0 | 0 | 0 | 28.9 | 20.4 | 21.0 | 0.4 | 2.1 | 2.7 | 17.8 | 79.9 | 97.4 |
| 19 | 0 | 0 | 0 | 0 | 0 | 7.5 | 24.7 | 18.4 | 0.6 | 2.4 | 2.9 | 19.5 | 94.0 | 97.4 |
| Factors | Real levels | |||||||||||||
| −1 | +1 | |||||||||||||
| X1 | Temperature (°C) | 30 | 40 | |||||||||||
| X2 | pH | 5.0 | 7.0 | |||||||||||
| X3 | Aeration rate (vvm) | 0.5 | 1.5 | |||||||||||
| X4 | Agitation (rpm) | 100 | 300 | |||||||||||
| X5 | Initial cellular concentration (g/l) | 1.0 | 3.0 | |||||||||||
T2 – Analysis after 2 h of fermentation
T6 – Analysis after 6 h of fermentation
T12 – Analysis after 12 h of fermentation
Samples were collected at baseline and at every two hour period until 12 h. Concentrations of biomass, lactose, and ribonucleotide were evaluated for each sample.
Analytical methods
The cellular concentration was determined by measuring the turbidity of a diluted sample at 600 nm using the previously obtained standard curve of absorbance against dry biomass (Silva et al. 2004). This procedure was carried out in triplicate.
Lactose concentration was determined using the DNSA (3, 5 dinitrosalicylic acid) method of Miller (1959). The first step in ribonucleotide determination is its extraction from the yeast cells. Cells were centrifuged at 2,000 rpm for 15 min at 4°C. Afterwards, the supernatant was reserved for lactose quantification, while the biomass was washed three times with distilled water and then centrifuged again for the removal of the lactose and proteins residuals. Centrifuged cells were suspended in 10 ml of phosphate buffer solution (0.1 M, pH: 7.5). The autolysis was carried out in a water batch at 60°C, 80 rpm for 24 h. After the autolysis the solution was centrifuged at 2,000 rpm for 15 min at 4°C and the supernatant was separated for ribonucleotides quantification. Then, 1 ml of distilled water and 3 ml of orcinol solution (0.1% orcinol and 0.1% of hexa-hydrated ferric chloride in concentrated hydrochloric acid) were added to 2 mL of the supernatant rich in RNA (resulted from cell autolysis). This mixture was boiled in a water bath for 30 min to promote the reaction. After this period the sample was cooled and its absorbance was measured at 660 nm. Adenosine monophosphate (10–80 mg/ml) (Sigma-Aldrich, USA) was used as the standard.
Statistical analysis
A fractionated factorial design 25−1 with three replicates in the central point was performed, totalizing 19 assays. The influence of the independent variables on lactose consumption, RNA and biomass production was determined by response surface methodology. Pearson correlations (r) were performed to evaluate possible correlations between the response variables. The level of significance was 5%. The statistical analysis was performed in the version 14 of the MINITAB® Statistical Software program.
Results and discussion
Cheese whey is a major pollutant of the dairy industry characterized by high BOD (30–50 g/l) and COD (60–80 g/l). Bioconversion of cheese whey into commercial product such as ribonucleotides by fermentative process provides an environmental benign and near term solution to fulfill the demand of ribonucleotides for various applications (Belem and Lee 1997; Christensen et al. 2011). Generally, cheese whey has been found to retain 55% of milk nutrients and represents about 85–95% of the milk volume (Guimarães et al. 2010). However, the selection of process parameters and their application at optimized levels is significant to attain the satisfactory yield of ribonucletodes making an overall impact on process economization. We studied the process variables (effect of temperature, pH, aeration rate, agitation and initial cellular concentration) on ribonucleotides production, through a factorial experimental design, by K. marxianus from cheese whey. Biomass production and lactose consumption were also evaluated as responses.
Treated whey showed 48.6 ± 3.2 g/L of lactose after deproteinization and sterilization and was used as a direct carbon source by K. marxianus for ribonucleotides production. Lactose consumption by K. marxianus showed an inverse relation with pH and aeration rate, and a direct relation with time of cultivation and initial cellular concentration. However, production of ribonucleotides was indirectly dependent upon temperature, pH, time and initial cellular concentration. The regression presented the best fitness (pFaj = 0.318) with 88.6% of lactose consumption. Lactose consumption can be demonstrated by the linear regression lactose consumption (g/l):
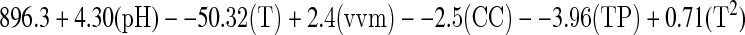 |
where T = temperature, vvm = aeration rate, CC = initial cellular concentration, TP = time, T2 = squared temperature.
Lactose consumption was also compared with the reduction of BOD. Experimental run (12) showed 64% reduction in BOD. A remarkable reduction was noticed in BOD levels (98%) at the central points.
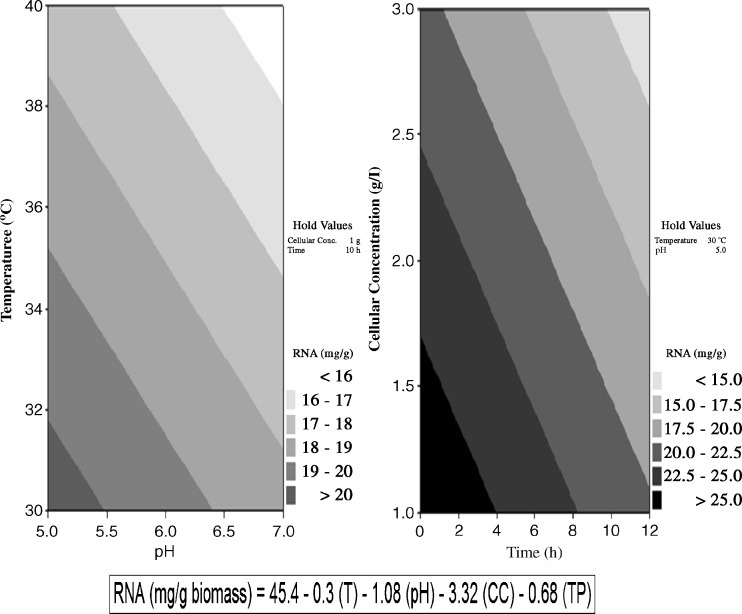
The highest ribonucleotides concentration, about 28.66 mg/g of biomass, was obtained after 2 h of fermentation at 30°C, pH 5.0 and 1 g/l of initial cellular concentration (Fig. 1). The independent variables such as agitation and aeration rate did not influence on RNA concentration (p > 0.05). The regression model presented the best fitness (pFaj = 0.084) and showed 57.8% of RNA concentration. Production of ribonucleotides can be stated by the linear model RNA (mg g−1 biomass):
 |
where T = temperature, CC = initial cellular concentration, TP = time.
Fig. 1.
Effect of pH, temperature, initial cellular concentration and time of cultivation on ribonucleotides production by Kluyveromyces marxianus ATCC 8554 using cheese whey. Maximum ribonucleotides production (28.66 mg/g) was observed at the equation of optimized set of conditions. 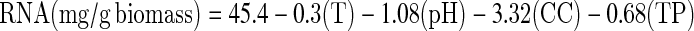 where T = temperature (°C), CC = initial cellular concentration (g/l), TP = time (h)
where T = temperature (°C), CC = initial cellular concentration (g/l), TP = time (h)
Ribonucleotides content produced by fermentation can be influenced by the type of microorganism, cultivation conditions, the extraction method and the analytical method applied for the quantification (Calil et al. 2009). For instance, Belem and Lee (1999) reported RNA production (6.3 mg/g biomass) by K. marxianus var marxianus ATCC 28244 when grown on cheese whey. Revillion et al. (2003), at similar conditions, obtained 17.2 mg of RNA/g dry biomass using K. marxianus CBS 6556 in whey fermentation. These reports differ from our findings in the present work. However, Belem and Lee (1999) clearly observed the maximum ribonucleotides production at the beginning of the log phase of the batch fermentation. The results obtained in our study were similar to those found by Kim et al. (2002) who reported the production of ribonucleotides AMP (adenosine mono phospahate), 28.6 mg/g dry biomass, GMP (guanidine mono phosphate) 12.6 mg/g dry biomass (both after 12 h of autolysis) and IMP (Inosine mono phospahate) 20.4 mg/g dry biomass (after 24 h of autolysis), using the residue of ginseng (Panax ginseng) process (rich in ginenoids, sugars and proteins) in Hansenula anomala KCM 11473 fermentation.
Maximum biomass concentration was obtained after 12 h of fermentation and ranged from 2.8 to 6.4 g/l. The variability in biomass concentration depended on the independent variables. The biomass production was influenced by temperature, cellular initial concentration and time of fermentation. Aeration rate, agitation and pH did not influence biomass production at the evaluated levels. The regression model explained 89.7% of the variability of biomass production and presented a good fitness (pFaj = 0.148) and can be stated by the linear regression (g L−1):
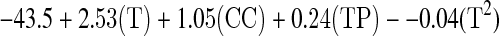 |
where T = temperature, CC = initial cellular concentration, TP = time and T2 = squared temperature
We found maximum ribonucleotide after 2 h of incubation. Probably, because of the K. marxianus previous growth during 18 h of inoculum preparation, the highest RNA production could have happened in this phase. Production of oligonucleotides does not depend upon the biomass concentration. Belem and Lee (1999) also reported that RNA production was not dependent upon biomass production. Ribonucleotides concentration can decrease if a long fermentation time is used (Belem and Lee 1999; Kim et al. 2002). These studies revealed the maximum ribonucleotides production by K. marxianus in whey fermentation under fed-batch or continuous cultivations after maintaining a logarithmic growth phase of K. marxianus. The cellular growth rate of any microorganism is controlled by a variety of factors involved in the metabolic processes (Groeneveld et al. 2009). It is therefore difficult to explain that which metabolic process or combined processes are responsible for the hikes in growth. K. marxianus is known as fast growing eukaryote showing its potential for the application in industrial biotechnology processes. Groeneveld et al. (2009) demonstrated a “cell selection method” to reveal the effect of metabolic process or pool of metabolic processes on growth control of K. marxianus by applying pH-auxostat cultivation. They were able to select a strain with a 30% increased growth rate by the cell selection method reporting decreased cell-cycle time to 52 min, much below that reported to date for any eukaryote.
In the present study, after 12 h of fermentation, substrate concentration varied between 63.7 to 98.7% and the lowest lactose concentration was observed at 35°C, pH 5.0, 0.5 vvm and 3 g/l of initial cellular concentration. Agitation did not influence on lactose consumption. Similar results were observed by Santiago et al. (2004), who demonstrated a consumption of 50 g/l of lactose after 12 h of fermentation in a whey based medium fermented by K. marxianus to produce β-galactosidase. Ghaly and Kamal (2004) observed a reduction of 99% of lactose concentration after 28 h of whey fermentation using K. fragilis to produce single-cell protein. These authors also demonstrated the reduction of 98% of the original whey biochemical oxygen demand (BOD) after biomass removal by ultrafiltration. We also observed a reduction of BOD (64%) in experimental run. However, the maximum reduction in BOD (98%) was noticed at the central points. Fermentation process for the production of various bioactive compounds utilizing whey seems to be a promising alternative to reduce whey pollutant potential, as observed in the present study and by Christensen et al. (2011).
A positive linear relationship was observed between the time of cultivation and the initial cellular concentration at the biomass production. The maximum value of biomass (6.0 g/l) was obtained after 12 h of fermentation with 3 g/l of initial cellular concentration at 35°C. This temperature is known as the ideal one for K. marxianus growth. Earlier, Barba et al. (2001) reported biomass production (10 g/l) after 12 h of whey batch fermentation using K. lactis at 30°C, pO2 20% and pH of 4.0. Higher biomass concentration (30 g/l) was obtained by Revillion et al. (2003) in K. marxianus CBS 6,556 whey fermentations after 20 h due to the high aeration rate, 3.5 vvm, which was superior to the levels studied in the present work (0.5, 1 and 1.5 vvm). In another study, Lukondeh et al. (2005) achieved biomass concentrations between 4.7 and 23 g/l after 12 to 16 h of K. marxianus batch fermentation at 30°C and pH at 5.0, and demonstrated a positive correlation between lactose concentration and biomass production.
Although a higher lactose concentration was used in the present study, lower biomass concentration was observed when compared to other published reports discussed earlier. It may be probably due to the low aeration rate used in the present study. Studies conducted by Ghaly and El-Taweel (1995) and Pinheiro et al. (2000) indicated that the elevation of the aeration rate allowed an improvement in the biomass production due to the inhibition of ethanol production.
Higher biomass production can be obtained if fed-batch process is applied. Belem and Lee (1999) cultivated K. marxianus var marxianus ATCC 28244 using whey based medium as the substrate in fed-batch fermentation with aeration rate, 2 vvm and reported 28.13 g/l of biomass concentration.
All response variables presented correlation when analyzed in pairs, as shown in Table 2. Although Belem and Lee (1999) did not correlate ribonucleotides and biomass production, but the variables such as RNA and biomass presented a moderate negative correlation, which indicated that at high biomass production, lower ribonucleotides concentration was obtained. Moreover, ribonucleotides production presented a low positive correlation with lactose, which demonstrates that higher RNA production is associated with the beginning of the fermentative process. The higher correlation observed, the negative one, for lactose consumption and biomass production illustrated the substrate conversion on biomass in the assayed conditions.
Table 2.
Pearson correlation (r) between the response variables ribonucleotides and biomass production and lactose consumption obtained in Kluyveromyces marxianus ATCC 8554 whey fermentation process
| RNA (mg/g) | Biomass (g/l) | Lactose (g/l) | |
|---|---|---|---|
| RNA (mg/g) | 1 | −0.69 (p < 0.001) | 0.40 (p < 0.001) |
| Biomass (g/l) | 1 | −0.75 (p < 0.001) | |
| Lactose (g/l) | 1 |
RNA – ribonucleotides concentration (mg/g dry biomass).
Conclusions
This study has demonstrated the feasibility of ribonucleotides production from cheese whey by K. marxianus var. marxianus ATCC 8554. In the present study, we found maximum ribonucleotides (28.66 mg/g of dry biomass) at the initial phase of incubation. Temperature (30°C), pH (5.0) and initial cell concentration (1 g/l) were the main influencing factors on RNA production under the employed fermentation conditions. Whey utilization for the production of ribonucleotides by fermentative process is an ideal strategy to overcome its pollutant effect by reducing BOD (98%) after the incubation. Looking at the today’s environmental concerns, production of value-added products such as ribonucleotides from cheese whey can be a cost effective, appropriate and safe-environment method.
Acknowledgement
The authors would like to acknowledge FAPEMIG (EDT 2700/06) for the financial support to carry out this work.
Contributor Information
Anuj Kumar Chandel, Email: anuj.kumar.chandel@gmail.com.
Silvio Silvério da Silva, Phone: +55-12-31595146, Email: silvio@debiq.eel.usp.br, Email: silviosilverio@gmail.com.
References
- Barba D, Beolchini F, Del Re G, Di Giacomo G, Veglio F. Kinetic analysis of Kluyveromyces lactis fermentation on whey: batch and fed-batch operations. Process Biochem. 2001;36:531–536. doi: 10.1016/S0032-9592(00)00242-9. [DOI] [Google Scholar]
- Belem MAF, Lee BH. Production of RNA derivatives by Kluyveromyces fragilis grown on whey. Food Sci Technol Int. 1997;3:437–444. doi: 10.1177/108201329700300605. [DOI] [Google Scholar]
- Belem MAF, Lee BH. Fed-batch fermentation to produce oligonucleotides from Kluyveromyces marxianus grown on whey. Process Biochem. 1999;34:501–509. doi: 10.1016/S0032-9592(98)00118-6. [DOI] [Google Scholar]
- Calil NO, Húngaro HM, Ferraz FO, Ferreira AS, Silva SS. Growth of Kluyveromyces marxianus yeasts strains in deproteined whey obtained from dairy industry. In: Mendez-Vilas A, editor. Current research topics in applied microbiology and microbial biotechnology. USA: Seville; 2009. pp. 442–444. [Google Scholar]
- Christensen AD, Kadar Z, Oleskowicz-Popiel P, Thomsen MH. Production of bioethanol from organic whey using Kluyveromyces Marxianus. J Ind Microbiol Biotechnol. 2011;38:283–289. doi: 10.1007/s10295-010-0771-0. [DOI] [PubMed] [Google Scholar]
- Florentino ER, Macedo GR, Santos ES, Pereira FM, Santos FN, Silva SF. Caracterização do soro de queijo visando processo de aproveitamento. Hig Aliment. 2005;19:30–32. [Google Scholar]
- Ghaly AE, El-Taweel AA. Effect of micro-aeration on the growth of Candida tropicalis and production of ethanol during batch fermentation of cheese whey. Bioresour Technol. 1995;52:203–217. doi: 10.1016/0960-8524(95)00026-B. [DOI] [Google Scholar]
- Ghaly AE, Kamal MA. Submerged yeast fermentation of acid cheese whey for protein production and pollution potential reduction. Water Res. 2004;38:631–644. doi: 10.1016/j.watres.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Ghaly AE, Rushton DG, Mahmoud NS. Potential air and groundwater pollution from continuous high land application of cheese whey. Am J Appl Sci. 2007;4:619–627. doi: 10.3844/ajassp.2007.619.627. [DOI] [Google Scholar]
- Groeneveld P, Stouthamer AH, Westerhoff HV (2009) Super life – how and why ‘cell selection’ leads to the fastest-growing eukaryote. FEBS J 276:254–270 [DOI] [PubMed]
- Guimãraes PMR, Teixeira JA, Domingues L. Fermenation of lactose to bio-ethanol by yeasts as part of integrated solutions for the valorization of cheese whey. Biotech Adv. 2010;28:375–384. doi: 10.1016/j.biotechadv.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Gupte AM, Nair JS. β –galactosidase production and ethanol fermentation from whey using Kluyveromyces marxianus NCIM 3551. J Sci Ind Res. 2010;69:855–859. [Google Scholar]
- Kim JH, Lee BH, Lee JS. Production of ribonucleotides by autolysis of Hansenula anomala grown on Korean Ginseng steaming effluent. J Biosci Bioeng. 2002;93:318–321. doi: 10.1263/jbb.93.318. [DOI] [PubMed] [Google Scholar]
- Kolaei VSM, Karimzadeh R, Alsadati SAS, Toufighi J. Modeling of single cell protein production from cheese whey using tanks-in-series model. Iran J Biotechnol. 2007;5:87–92. [Google Scholar]
- Koushki M, Jafari M, Azizi M. Comparison of ethanol production from cheese whey permeate by two yeast strains. J Food Sci Technol. 2011 doi: 10.1007/s13197-011-0309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutinas AA, Papapostolou H, Dimitrellou D, Kopsahelis N, Katechaki E, Bekatorou A, Bosnea LA. Whey valorisation: A complete and novel technology development for dairy industry starter culture production. Bioresour Technol. 2009;100:3734–3739. doi: 10.1016/j.biortech.2009.01.058. [DOI] [PubMed] [Google Scholar]
- Lane MM, Morrissey JP. Kluyveromyces marxianus: a yeast emerging from its sister’s shadow. Fungal Biol Rev. 2010;30:1–10. [Google Scholar]
- Li Y, Shahbazi A. Lactic acid recovery from cheese whey fermetation broth using combined ultrafiltration and nanofiltration membranes. Appl Biochem Biotechnol. 2006;132:985–996. doi: 10.1385/ABAB:132:1:985. [DOI] [PubMed] [Google Scholar]
- Longhi LGS, Luvizetto DJ, Ferreira LS, Rech R, Ayub MAZ, Secchi AR. A growth kinetic model of Kluyveromyces marxianus cultures on cheese whey as substrate. J Ind Microbiol Biotechnol. 2004;31:35–40. doi: 10.1007/s10295-004-0110-4. [DOI] [PubMed] [Google Scholar]
- Lukondeh T, Ashbolt NJ, Rogers PL. Fed-batch fermentation for production of Kluyveromyces marxianus FII 510700 cultivated on lactose based medium. J Ind Microbiol Biotechnol. 2005;32:284–288. doi: 10.1007/s10295-005-0245-y. [DOI] [PubMed] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Morr CV. Whey proteins: Manufacture. In: Fox PF, editor. developments in Dairy Chemistry-4: Functional Milk Proteins. New York: Elsevier Appl Sci; 1989. pp. 245–284. [Google Scholar]
- Nath A, Dixit M, Bandiya A, Chavda S, Desai AJ. Enhanced PHB production and scale up studies using cheese whey in fed batch culture of Methylobacterium sp. ZP24. Bioresour Technol. 2008;99:5749–5755. doi: 10.1016/j.biortech.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Ozmihci S, Kargi F. Kinetics of batch ethanol fermentation of cheese-whey powder (CWP) solution as function of substrate and yeast concentrations. Bioresour Technol. 2007;98:2978–2984. doi: 10.1016/j.biortech.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Pinheiro R, Belo I, Mota M. Air pressure effects on biomass yield of two different Kluyveromyces strains. Enzyme Microb Technol. 2000;26:756–762. doi: 10.1016/S0141-0229(00)00168-X. [DOI] [PubMed] [Google Scholar]
- Revillion JP, Brandelli A, Ayub M. Production of yeast extract from whey using Kluyveromyces marxianus. Braz Arch Biol Technol. 2003;49:121–127. doi: 10.1590/S1516-89132003000100017. [DOI] [Google Scholar]
- Sansonetti S, Curcio S, Calabrò V, Dorio G. Optimization of ricotta cheese whey (RCW fermentation by response surface methodology. Bioresour Technol. 2010;101:9156–9162. doi: 10.1016/j.biortech.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Santiago PA, Marquez LDS, Cardoso VL, Ribeiro EJ. Estudos da produção de beta-galactosidase por fermentação de soro de queijo com Kluyveromyces marxianus. Cienc Tecnol Aliment. 2004;24:567–572. doi: 10.1590/S0101-20612004000400015. [DOI] [Google Scholar]
- Saraiva CB, Mendonça RCS, Pereira DA. Diagnóstico ambiental de um laticínio de pequeno porte. Ver Bras Agroecologia. 2009;4:2051–2054. [Google Scholar]
- Schaller JP, Kuchan MJ, Thomas DL, Cordle CT, Winship TR, Buck RH, Baggs GE, Wheeler JG. Effect of dietary ribonucleotides on infant immune status. Part 1: humoral responses. Pediatr Res. 2004;56:883–890. doi: 10.1203/01.PDR.0000145576.42115.5C. [DOI] [PubMed] [Google Scholar]
- Silva DDV, Felipe MGA, Mancilha IM, Paula FP. Biotechnological production of xylitol from lignocellulosic materials. Biof Eur. 2004;8:56–57. [Google Scholar]
- Ventanas S, Mustonen S, Puolanne E, Tuorila H. Odour and flavour perception in flavoured model systems: Influence of sodium chloride, umami compounds and serving temperature. Food Qual Prefer. 2010;21:453–462. doi: 10.1016/j.foodqual.2009.11.003. [DOI] [Google Scholar]