Abstract
In vitro antioxidant activities and protective effects of sesame cake extract in stabilising sunflower and soybean oils were tested. Total phenolic, flavonoid and flavonol contents in the extract of sesame cake were 1.94 ± 0.02 (mg gallic acid equivalent (GAE) g−1 dry weight (DW)), 0.88 ± 0.02 (mg quercetin equivalent (QE) g−1 DW), and 0.40 ± 0.02 (mg QE g−1 DW), respectively. Protective effects of sesame cake extract in stabilizing sunflower and soybean oils were tested, compared to synthetic antioxidants, by measuring their peroxide values (PV), conjugated dienes (CD), conjugated trienes (CT), and p-anisidine value during accelerated storage. Results indicated that sesame cake extract exhibited stronger antioxidant activity than BHT and BHA. However, its antioxidant activity was less than that of TBHQ.
Keywords: Sesame, Vegetable oils, Accelerated storage, Antioxidant activity, Oxidative stability
Introduction
Edible oils with higher levels of unsaturation, especially polyunsaturated fatty acids (PUFA), are more susceptible to oxidation. Oxidation of oils modifies organoleptic properties and affecting the shelf life of the product (Lercker and Rodriguez-Estrada 2002). Lipid oxidation of oils not only produces rancid odours, unpleasant flavours and discoloration, but also decreases the nutritional quality. Many methods have been reported to measure the oxidative stability (OS) of edible oils. The oxidative stability of oils and fats with added antioxidants can be determined during storage under normal ambient conditions and packing. However, in general, oxidation take a long time to occur which is impractical for routine analysis. For this reason, accelerated oxidation tests or aging tests are conducted. Tests like Schaal oven test, Sylvester test and Swift test are used for testing accelerated oxidation at elevated temperatures (Mahuya et al. 2008; Ramadan 2008). Storage of oils at high temperatures has been employed for monitoring OS of oils. The extent of oxidation in oils has been frequently evaluated by measuring the peroxide value (PV). This index is related to the hydroperoxides, the primary oxidation products, which are unstable and readily decompose to form mainly mixtures of volatile aldehyde compounds. The oxidative degradation compounds that derived from degradation of hydroperoxides are generally termed as secondary oxidative products which are determined in oils and fats by methods such as p-anisidine (AV) (Ramadan and Mörsel 2004).
To overcome the stability problems of oils and fats, synthetic antioxidants, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butyl hydroquinone (TBHQ) have widespread use as food additives in many countries. Recent reports reveal that these compounds may be implicated in many health risks, including cancer and carcinogenesis (Prior 2004). Hence, there is a tendency towards the use of natural antioxidants of plant origin to replace these synthetic antioxidants. Natural antioxidants such as flavonoids, tannins, coumarins, curcumanoids, xanthones, phenolics, lignans and terpenoids are found in various plant products (such as fruits, leaves, seeds and oils) (Jeong et al. 2004; Yang et al. 2010) and they are known to protect easily-oxidizable constituents of food from oxidation.
The number of studies on residual sources of antioxidants has increased considerably in recent years (Ramadan and Mörsel 2002; Moure et al. 2001; Sachindra et al. 2010). Shui and Leong (2006) found that antioxidants obtained from star fruit residues slowed the rancidity process of oil to a greater extent than did BHT. They stressed the high potential of this residue for preventing oil rancidity. The antioxidant compounds from agri-waste may not only increase the stability of foods, by preventing lipid peroxidation, but may also protect biomolecules and supramolecular structures e.g. membranes, ribosomes from oxidative damage in humans or animals.
Sesame (Sesamum indicum L., Pedaliaceae) is one of the most important oilseed crops, because of its high content of lipid, in the world (Shyu and Hwang 2002). It is not only a source of edible oil, but also widely used in baked goods and confectionery products (Namiki 1995). In Egypt, the major part of the imported sesame is essentially transformed to Halaweh. This food product is obtained after mixing the white tehineh (white sesame seed dehulled, roasted and ground), saponin (Saponaria officinalis) and nougat (heat-treated sucrose) (Abu-Jdayil et al. 2002). The sesame cake is a by-product of the oil industry which could be recovered and used as a value added product. However, in some sesame processing countries this by-product is generally discarded or used in animal feeding. Preliminary studies showed that an appreciable amount of antioxidants was still present in sesame cake. In this study, the sesame cake has been evaluated as a source of natural antioxidants. The aim was to compare in vitro antioxidant activities and stabilizing effects of sesame cake extract in sunflower and soybean oils against oxidation during accelerated storage in comparision with synthetic antioxidants BHA, BHT and TBHQ. The content of total phenolics, flavonoids and flavonols were also determined.
Materials and methods
Materials
Sesame cake (Sesamum indicum cv Shandweel -3) was obtained from local market (Giza, Egypt) and stored in at −20 °C until use. BHA, BHT, chlorogenic acid, and 2,2′-Azinobis (3-ethylbenzothiazoline-6 sulfonic acid) (ABTS) were purchased from Sigma (St Louis,MO, USA). 1,1-diphenyl-2-picrylhydrazyl (DPPH), caffeic acid, TBHQ, p-Anisidine (4-Amino-anisol; 4-Methoxy-anilin), and β-carotene were obtained from Fluka (Buchs, Switzerland). All other chemicals used were analytical grade. Sunflower oil produced by Brökelmann-Oelmühle GmbH (Hamm, Germany) was obtained from Aldi supermarket (Berlin, Germany), while soybean oil which was obtained from Edeka aktivmarkt (Berlin, Germany) was produced by Kunella Feinkost GmbH (Cottbus, Germany) and the oils were free of any synthetic antioxidant.
Sample preparation
Sesame cake was washed, dried in hot air at 40 ºC and then ground into a fine powder in a mill. One hundred grams of the sample was initially extracted with hexane (three times with a total of 1.5 L of hexane) at room temperature. The defatted residue was washed with distilled water (three times with a total of 1.5 L of distilled water) to remove soluble sugars and proteins and dried at 40 °C. Ten grams of the above purified residue was extracted with 150 mL methanol for 16 h in a soxhlet extractor. The extract was filtered, solvent removed in a rotary evaporator below 40 °C, weighed and the residue was redissolved in 100 mL of methanol to give an antioxidant extract of known concentration and stored at −20 °C for further use.
Proximate composition of material
The major chemical constituents, moisture, ash, crude fat, crude fiber, and crude protein were determined in triplicate according to AOAC standard methods (1990). Carbohydrate content was calculated by difference.
Determination of total phenolics
Total phenolic content of the extract was determined by the Folin–Ciocalteu method (Saeedeh and Asna 2007; Ramadan et al. 2010). A 20 μL aliquot of extract solution was mixed with 1.16 mL distilled water and 100 μL of Folin–Ciocalteu reagent, followed by addition of 300 μL of Na2CO3 solution (20%). Subsequently, the mixture was incubated in a shaking incubator at 40 °C for 30 min and its absorbance at 760 nm was measured. Gallic acid was used as a standard for calibration curve. Total phenolic content expressed as gallic acid equivalent (GAE) was calculated using the following linear equation based on the calibration curve:
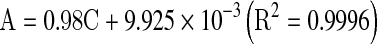 |
where A is the absorbance and C is the concentration (mg GAE g−1 dry weight (DW)).
Determination of total flavonoids
Total flavonoid content was determined by the method of Ordon et al. (2006) of sample solution. A 0.5 mL aliquot of ethanolic 2% AlCl3 was added to 0.5 mL of extract solution. After 1 h incubation at room temperature the absorbance at 420 nm was measured. Extract samples were evaluated at a final concentration of 0.1 mg mL−1. Total flavonoid content expressed as quercetin equivalent (QE) was calculated using the following equation based on the calibration curve:
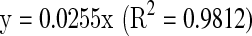 |
where x is the absorbance and y is the concentration (mg QE g−1 DW).
Determination of total flavonols
Total flavonol content was determined by the method of Kumaran and Joel (2007). To 2.0 mL of extract solution, 2.0 mL of ethanolic 2% AlCl3 and 3.0 mL (50 g/L) sodium acetate solutions were added. The absorption at 440 nm was read after 2.5 h incubation at 20 °C. Extract samples were evaluated at a final concentration of 0.1 mg/mL. Total flavonol content expressed as QE was calculated using the following equation based on the calibration curve:
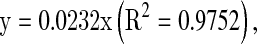 |
where x is the absorbance and y is the concentration (mg QE g−1 DW).
Antioxidant activity of the extract
According to the differences among the wide number of the systems available, the results of a single method can give only a reductive suggestion of the antioxidant properties of the extracts (Gianni et al. 2005). Thus, the antioxidant capacity of each extract was determined through three procedures
DPPH· radical scavenging capacity
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay (Lee et al. 2003; Ramadan et al. 2007) was utilised with some modifications. The stock reagent solution (1 x 10−3 M) was prepared by dissolving 22 mg of DPPH in 50 mL methanol and stored at −20 °C until use. The working solution (6 x 10−5 M) was obtained by mixing 6 mL of the stock solution with 100 mL methanol to obtain an absorbance value of 0.8 ± 0.02 at 515 nm, using a spectrophotometer. The extract of sesame cake and the synthetic antioxidant (TBHQ, BHA, and BHT prepared in ethanol) with different concentrations (0.1 mL of each) were allowed to react with 3.9 mL of the DPPH solution and vortexed for 30 s and then the absorbance was measured at 515 nm, at the end of a 30 min incubation. A control sample with no added extract was also analysed and the scavenging percentage was calculated according to the following equation:
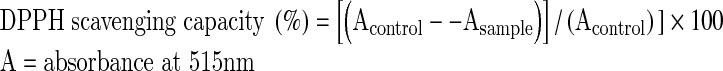 |
ABTS radical scavenging assay
For ABTS assay, the method of Re et al. (1999) was adopted. The stock solutions included 7 mM ABTS solution and 2.4 mM potassium persulfate solution. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12–16 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS•+ solution with 60 mL methanol to obtain an absorbance of 0.706 ± 0.001 units at 734 nm using the spectrophotometer. ABTS•+ solution was freshly prepared for each assay. Plant extract with different concentrations (1 mL of each) were allowed to react with 1 mL of the ABTS•+ solution and the absorbance was recorded at 734 nm after 7 min using the spectrophotometer. The ABTS•+ scavenging capacity of the extract was compared with different concentrations of BHT, BHA, and TBHQ (1 mL of each) and percentage inhibition calculated as ABTS radical scavenging activity
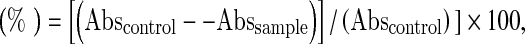 |
where Abscontrol is the absorbance of ABTS radical + methanol; Abssample is the absorbance of ABTS radical + sample extract/standard.
β-Carotene-linoleic acid bleaching test
The ability of the extract to prevent the bleaching of β-carotene was assessed as described by Keyvan et al. (2007). In brief, 0.2 mg β-carotene in 1 ml chloroform, 20 mg of linoleic acid and 200 mg of Tween 20 were transferred into a round-bottom flask. Once the chloroform had been removed in a rotary evaporator, 50 mL distilled H2O was added and the resulting mixture was stirred vigorously. Six mL aliquots of the emulsion were transferred to tubes containing different concentrations of extract/standard. After mixing, an aliquot was transferred into a cuvette and the absorbance (Abs0) at 470 nm was recorded. The remaining samples were placed in a water bath at 50 °C for a period of 2 h. Thereafter, the absorbance of each sample was measured at 470 nm (Abs120). The data are presented as antioxidant activity% (AA%) values, calculated using the equation:
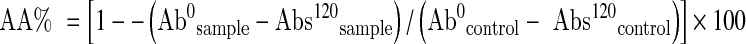 |
Determination of oxidative stability
Selection of test oils was based on the presence of varying PUFA levels. Sunflower oil (SFO) which has a high level of linoleic acid (18:2n-6) and soybean oil (SBO) which is rich in α-linolenic acid (18:3n-3) and linoleic acid was used. The schaal oven test (Fennema 1976) was conducted to evaluate the effect of antioxidants against oxidation during the accelerated oxidative storage of oils. Sesame cake extract was added to commercial edible sunflower and soybean oils obtained from local market at different concentrations (5, 10, 50, 100 and 200 ppm, based on extract weight), in a series of transparent glass bottles having a volume of 20 mL each. TBHQ, BHA and BHT at a level of 0.02% were also applied for comparison. The bottles were completely filled with oil and sealed. A control sample was prepared by using the same amount of methanol used to dissolve the antioxidant and the extracts. The antioxidant-enriched oil samples were evaporated in a vacuum evaporator below 40 °C to evaporate the solvent and subjected to accelerated oxidation in the dark in an oven at 70 °C for 72 h. Samples (20 g) were removed periodically every 4, 8, 24, 32, 48, 56, and 72 h for analysis. Immediately after storage period, oil samples were withdrawn for triplicate analyses. The oils were sampled for each measurement from separate bottles.
Analytical procedures
Peroxide value (PV)
Peroxide value of samples was measured according to the standard method of IUPAC (1987). The method is based on iodometric titration, which measures the iodine produced from potassium iodide by the peroxides present in the oil. In a conical flask (250 mL) vegetable oils sample (4 ± 0.5 g) was taken along with chloroform (10 mL), glacial acetic acid (15 mL) and fresh saturated aqueous potassium iodide solution (1 mL). The flask was stoppered and shaken vigorously for 1 min and then kept in the dark for 5 min more. In the next step, double distilled water (10 mL) was mixed thoroughly with the solution and titrated against 0.002 N sodium thiosulphate solution until the yellow colour almost disappeared. Then about 0.5 mL of soluble starch indicator (1%) solution was added. Titration was continued until the blue colour just disappeared. One blank reagent (without sample) was prepared.
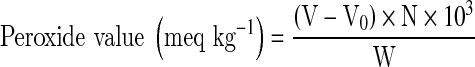 |
where V is the titre value (mL) of sodium thiosulphate solution for sample, V0 the titre value (mL) of sodium thiosulphate solution for blank, N the normality of sodium thiosulphate solution and W the weight of sample in gram.
p-Anisidine value (AV)
p-Anisidine value (AV) was determined according to AOCS method (1995). The method is based on the spectrophotometric determination of products formed in the reaction between aldehydic compounds in the oil and p-anisidine. Oil samples (0.5–2.0 g) were dissolved in 25 mL isooctane and absorbance of this fat solution was measured at 350 nm using a spectrophotometer (Hitachi U-3000, Tokyo, Japan). Five millilitres of the above mixture was mixed with 1 mL 0.25% p-anisidine in glacial acetic acid (w/v) and after standing for 10 min, absorbance was read at 350 nm using a spectrophotometer. The p-anisidine value (AV) was calculated according to the equation:
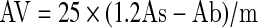 |
where As is the absorbance of the fat solution after reaction with the p-anisidine reagent; Ab is the absorbance of the fat solution and m is the mass of oil sample (g).
Conjugated dienes (CD) and conjugated trienes (CT)
Specific extinctions at 232 and 270 nm (i.e., CD and CT) were determined using a spectrophotometer. Oil samples were diluted with isooctane to bring the absorbance within limits following the standard method of IUPAC method II. D. 23 (1979).
Statistical analysis
Statistical analyses were conducted using SPSS (Statistical Program for Social Sciences, SPSS Corporation, Chicago, IL, USA) version 16.0 for Windows. All analyses were performed in triplicate and data reported as means ± standard deviation (SD). Data were subjected to analysis of variance (ANOVA). The confidence limits used in this study were based on 95% (P < 0.05).
Results and discussion
Analysis of sesame cake showed that the extract had 5.71% moisture, 30.39% crude fat, 23.76% crude protein, 6.12% ash, 10.86% crude fibre, and 28.87% carbohydrates, indicating that this by-product could be an alternative source of fat, carbohydrate, and protein. The lipid content in sesame cake used in the current work was lower than that reported by Suja et al. (2004).
Total phenolics
Phenolic compounds serve as important antioxidants because of their ability to donate a hydrogen atom or an electron in order to form stable radical intermediates. Hence, they prevent the oxidation of various biological molecules (Cuvelier et al. 1992). In fact, several oilseeds and their byproducts have been investigated for phenolic compounds in search for safe sources of natural antioxidants (Wettasinghe et al. 2002). In the case of cereal grains it has been shown that their outer layers such as husk, pericarp, testa and aleurone cells contain the highest concentration of total phenolics (Kähkönen et al. 1999). Naczk and Shahidi (1998) have shown that canola hulls also contain a high proportion of phenolic compounds. Since the antioxidant activity is not always in direct correlation with the presence of large quantities of polyphenolic compounds, both data need to be examined together. Flavonoids possess a broad spectrum of chemical and biological activities including radical scavenging properties. Such properties are especially distinct for flavonols. Therefore, all extracts were analyzed for total phenolic compounds, flavonoid and flavonol contents. The Folin–Ciocalteu method measures the reduction of the reagent by phenolic compounds with the formation of a blue complex that can be measured at 760 nm against gallic acid as a standard (Imeh and Khokhar 2002). Total phenolic, flavonoid and flavonol contents in the extract of sesame cake were 1.94 ± 0.02 (mg GAE g−1 dry weight (DW)), 0.88 ± 0.02 (mg QE g−1 DW), and 0.40 ± 0.03 (mg QE g−1 DW), respectively. The total phenolic content of sesame cake extract was found to be lower than potato peels (2.91 mg GAE g−1 DW) (Mohdaly et al. 2010) and banana (2.32 mg GAE g−1 DW) (Nagendran et al. 2006), but greater than carrot (1.52 mg GAE g−1 DW) (Kequan and Liangli 2006) and wheat bran (1.0 mg GAE g−1 DW) (Kähkönen et al. 1999).
Antioxidant activity of the extract
As stressed by Huang et al. (2005) no single method is adequate for evaluating the antioxidant capacity of foods, since different methods can yield widely diverging results. Various methods, based on different mechanisms, must be used. Here, we have applied the ABTS, DPPH, and β-carotene/linoleic acid bleaching assays to the extracts.
DPPH radical scavenging activity
Using DPPH• radical, the free radical scavenging ability of the sesame cake extracts was evaluated considering that DPPH• radical is commonly used for the assessment of antioxidant activity in vitro and is foreign to biological systems. DPPH• is a very stable organic free radical with deep violet color which gives absorption maxima within 515–528 nm range. Upon receiving proton from any hydrogen donor, mainly from phenolics, it loses it chromophore and becomes yellow. As the concentration of phenolic compounds or degree of hydroxylation of the phenolic compounds increases their DPPH radical scavenging activity also increases, and can be defined as antioxidant activity (Zhou and Yu 2004).
DPPH radical-scavenging abilities of sesame cake extracts along with the reference standards BHA, BHT and TBHQ are shown in Fig. 1a. The extract demonstrated a concentration-dependent scavenging activity against DPPH radicals. Scavenging activities of sesame cake extract were higher (p < 0.05) than that of BHT and BHA but less than that of TBHQ. It has been proven that antioxidant activity of plant extracts is mainly ascribed to the concentration of the phenolic compounds present in the plants (Heim et al. 2002). The results of the DPPH• free radical scavenging assay suggest that components within the extract are capable of scavenging free radicals via electron- or hydrogen-donating mechanisms and thus could be able to prevent the initiation of deleterious free radical mediated chain reactions in susceptible matrices, e.g. biological membranes. This further showed the capability of the extracts to scavenge different free radicals in different systems, indicating that they may be useful therapeutic agents for treating radical-related pathological damage.
Fig. 1.
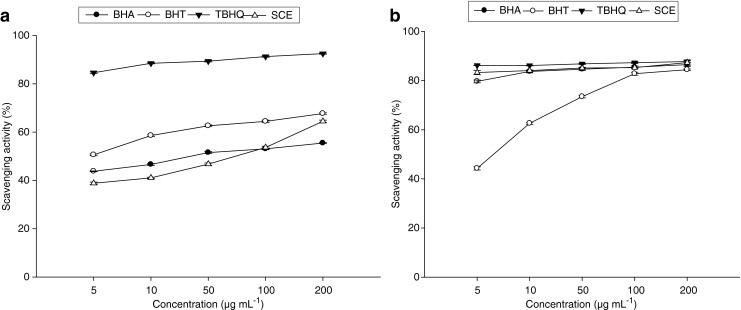
Scavenging activity of sesame cake extract against a DPPH and b ABTS radicals compared with that of BHA, BHT, and TBHQ at different concentrations. Data are mean ± SD of three determinations
ABTS radical scavenging activity
Although the DPPH• free radical is ubiquitously used to estimate the potential free radical scavenging activity of natural products, the ABTS•+ free radical is commonly used when issues of solubility or interference arise and the use of DPPH•-based assays becomes inappropriate. Having considered the solubility of the test sample and the advantages and disadvantages of the use of the DPPH• free radical, it was considered necessary to further assess the extracts against the ABTS•+ free radical. ABTS, a protonated radical, has characteristic absorbance maxima at 734 nm which decrease with the scavenging of the proton radicals. This method measures the antioxidant activity of both water-soluble and lipid-soluble antioxidants, as well as extracts from natural sources (Mathew and Abraham 2006). In our study, the extract exhibited antioxidant activity and showed comparable activity to the synthetic antioxidants BHA, BHT and TBHQ (Fig. 1b). The scavenging of the ABTS+ radical by the extract was found to be higher than that of DPPH radical. Factors like stereoselectivity of the radicals or the solubility of the extract in different testing systems have been reported to affect the capacity of extracts to react and quench different radicals (Yu et al. 2002). Wang et al. (1998) found that some compounds which have ABTS+ scavenging activity did not show DPPH scavenging activity. However, this was not the case in the current study.
β-carotene-linoleic acid bleaching
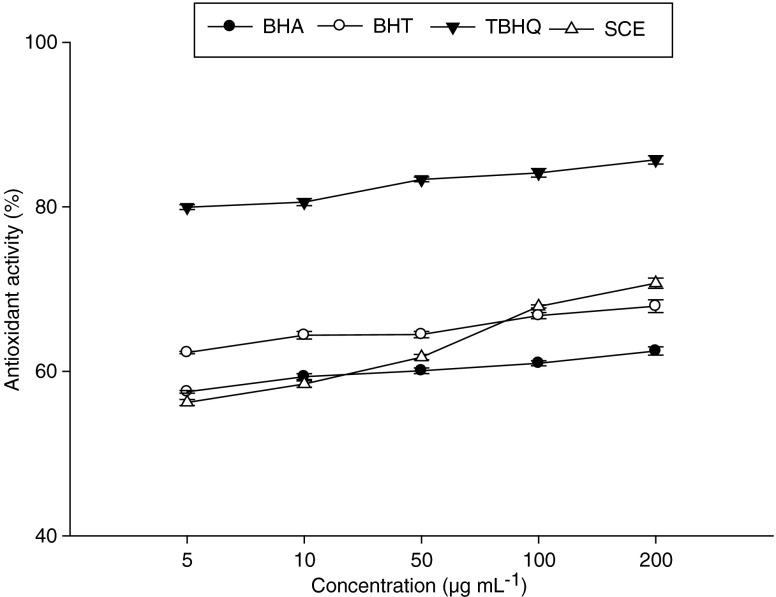
Synthetic free radical scavenging (ABTS and DPPH) models are valuable tools to indicate the potential antioxidant activity of plant extracts. However, these systems do not use a food or biologically relevant oxidizable substrate so no direct information on an extract’s protective action can be determined (Dorman et al. 2003; Ramadan and Mörsel 2003). Therefore, it was considered important to assess the extracts in a β-carotene–linoleic acid lipid: water emulsion assay despite its reported limitations. In this assay, the oxidation of linoleic acid generates peroxyl free radicals due to the abstraction of hydrogen atom from diallylic methylene groups of linoleic acid. The free radical then will oxidize the highly unsaturated β-carotene. The presence of antioxidants in the extract will minimize the oxidation of β-carotene by hydroperoxides. An extract capable of retarding/inhibiting the oxidation of β-carotene may be described as a free radical scavenger and primary antioxidant. Effect of sesame cake extracts on oxidation of β-carotene/linoleic acid is shown in Fig. 2. It was clear that the presence of antioxidants in sesame cake extract reduced the oxidation of β-carotene by hydroperoxides from these extracts. There were differences (P < 0.05) between the extract, control and reference standards effect. Sesame cake extracts were better in their effect on reducing the oxidation of β-carotene than BHA and BHT but lower than that of TBHQ. According to the β-carotene-linoleic acid bleaching data, the extracts were capable of scavenging free radicals in a complex heterogenous medium. This may suggest that the extract may have potential use as antioxidative preservatives in emulsion-type systems.
Fig. 2.
Antioxidant activity of sesame cake extract in β-carotene/linoleic acid system compared with that of BHA, BHT, and TBHQ at different concentrations. Data are mean ± SD of three determinations
Phenolic compounds could probably be responsible for the high antioxidant capacity because some authors have reported that there is no correlation between the content of these main antioxidant compounds and the radical scavenging capacity (Yu et al. 2002; Ramadan and Mörsel 2003). The results obtained by us do not support these claims. The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in neutralizing free radicals, quenching singlet and triplet oxygen, or decomposing peroxides (Osawa 1994).
Oil stability is usually determined under accelerated oxidation conditions (60 °C or more) because ambient conditions demand an excessively long period. To evaluate the antioxidant efficacies of the extracts in soybean and sunflower oils, PV, AV and UV absorptivity were determined as indices of lipid oxidation. The oxidative stability studies were carried out at 70 °C in an oven. This temperature was ideal, because at higher temperatures the peroxides will decompose very fast (Mariod et al. 2010).
Effect of the extract on sunflower oil oxidation
PV is one of the most widely used tests for the measurement of oxidative rancidity in oils and fats. In this paper, degree of oxidation of sunflower oil samples was determined by measuring PV in the absence and presence of antioxidants at 70 °C for 72 h. The influence of antioxidants during storage on PV in the sunflower oil samples is shown in Fig. 3a. Results showed that PV increased linearly with storage time and increased in acceleration after 32 h. Sunflower oil samples without the antioxidant (control) reached a maximum PV of 25.82 ± 0.24 meq kg−1 after 72 h of storage. A significant difference (p < 0.05) in PV was observed between the control and sunflower oil samples containing sesame cake extract and synthetic antioxidants, all of which slowed the rate of peroxide formation. The PV of sunflower oil samples with 200 ppm extract, BHA, BHT and TBHQ were 13.68 ± 0.26, 13.86 ± 0.24, 13.44 ± 0.26, and 11.04 ± 0.26 meq kg−1, respectively. The p-anisidine value (AV) is one of the best methods for evaluating secondary lipid oxidation products produced during the oxidative degradation of oil. It is based on the reactiveness of the aldehyde carbonyl bond on the p-anisidine amine group, leading to the formation of a Schiff base that absorbs at 350 nm (Ying et al. 2010):
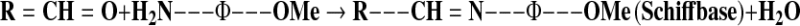 |
Fig. 3.
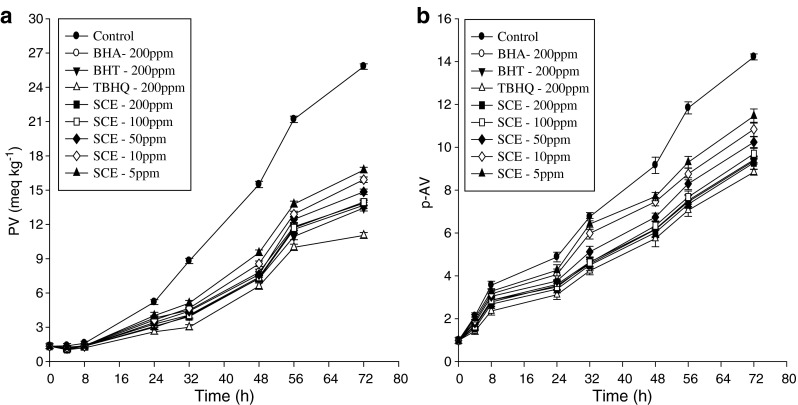
Relative increase in peroxide value (a) and p-anisidine value (b) of treated sunflower oil samples with sesame cake extract under accelerated storage. Error bars show the variations of three determinations in terms of standard deviation
Figure 3b depicts the AV for sunflower oil samples stabilized with the extract, TBHQ, BHT, BHA, and control. AV increased throughout the storage time, which increased in acceleration after 32 h. The p-anisidine value of control reached a maximum of 14.22 ± 0.14 from an initial value of 0.98 ± 0.13 after 72 h of storage. The difference in antioxidant activity may be accounted for on the basis of chemical structures. The stability of phenoxy radicals reduces the rate of propagation and further reactions and thus increases the oxidative stability of lipids (Ying et al. 2010). The results demonstrated that sesame cake extract had higher antioxidant activity than BHA and BHT but lower activity than that of TBHQ for all concentrations. In addition, in most cases, the synthetic antioxidant TBHQ which has two para-hydroxyl groups can donate hydrogen atoms to active free radicals to interrupt the chain reaction of oxidation (Jiang and Wang 2006) and this is responsible for superior antioxidant activity in various edible oils (Ying et al. 2010). In this study, in comparison with synthetic antioxidants, effects of sesame cake extract may also play an important role in the observed trends.
Conjugated dienes (CD) and triene (CT) contents of sunflower oil samples stabilized with extract, BHT, BHA, TBHQ, and control are shown in Table 1. The CD and CT contents increased with increase in storage time with greater rate for control. The oil samples stabilized with sesame cake extract showed lower levels of CD and CT compared to control, indicating antioxidant potential of the components of sesame cake extract. The results of the CD and CT in present study revealed that the antioxidant activity of sesame cake extract applied at 200 was stronger than those of BHA and BHT but weaker than that of TBHQ, when used in their legal amounts. Measurement of CD and CT is a good parameter for the determination of oxidative stability of the oils. Lipids containing methylene-intrupted dienes or polyenes show a shift in their double bond position during oxidation. The resulting conjugated dienes exhibit intense absorption at 232 nm, similarly conjugated trienes absorb at 270 nm. The increase in CD and CT contents is proportional to the uptake of oxygen. Greater the levels of CD and CT lower will be the oxidative stability of the oils (Bushra et al. 2007).
Table 1.
Conjugated dienes (ג232 nm) and conjugated trienes (ג270 nm) of treated sunflower oil samples with sesame cake extract during oven test
| Time (h) | Control | BHA- 200 ppm | BHT- 200 ppm | TBHQ- 200 ppm | Sesame cake extract | ||||
|---|---|---|---|---|---|---|---|---|---|
| 200 ppm | 100 ppm | 50 ppm | 10 ppm | 5 ppm | |||||
| Conjugated dienes (ג232 nm) | |||||||||
| 0 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 | 1.22 ± 0.13 |
| 4 | 2.80 ± 0.21 | 2.12 ± 0.21 | 2.10 ± 0.21 | 1.82 ± 0.21 | 2.04 ± 0.17 | 2.14 ± 0.17 | 2.30 ± 0.17 | 2.34 ± 0.17 | 2.48 ± 0.17 |
| 8 | 4.60 ± 0.23 | 3.26 ± 0.23 | 3.22 ± 0.23 | 2.90 ± 0.23 | 3.12 ± 0.23 | 3.26 ± 0.33 | 3.42 ± 0.33 | 3.48 ± 0.33 | 3.66 ± 0.33 |
| 24 | 6.32 ± 0.33 | 4.24 ± 0.33 | 4.20 ± 0.33 | 3.92 ± 0.33 | 4.10 ± 0.33 | 4.30 ± 0.11 | 4.44 ± 0.11 | 4.52 ± 0.11 | 4.76 ± 0.11 |
| 32 | 7.78 ± 0.31 | 5.42 ± 0.31 | 5.36 ± 0.31 | 5.12 ± 0.31 | 5.24 ± 0.20 | 5.42 ± 0.31 | 5.68 ± 0.33 | 5.84 ± 0.31 | 6.08 ± 0.33 |
| 48 | 9.84 ± 0.24 | 6.80 ± 0.21 | 6.78 ± 0.23 | 6.54 ± 0.23 | 6.72 ± 0.33 | 6.86 ± 0.43 | 7.06 ± 0.43 | 7.14 ± 0.43 | 7.36 ± 0.43 |
| 56 | 13.12 ± 0.31 | 8.08 ± 0.31 | 8.00 ± 0.31 | 7.78 ± 0.33 | 7.96 ± 0.33 | 8.18 ± 0.33 | 8.36 ± 0.33 | 8.52 ± 0.31 | 8.84 ± 0.33 |
| 72 | 15.16 ± 0.24 | 9.14 ± 0.21 | 9.14 ± 0.21 | 8.82 ± 0.23 | 9.06 ± 0.23 | 9.30 ± 0.23 | 9.48 ± 0.23 | 9.80 ± 0.31 | 10.20 ± 0.31 |
| Conjugated trienes (ג270 nm) | |||||||||
| 0 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 | 0.54 ± 0.13 |
| 4 | 1.06 ± 0.13 | 0.78 ± 0.13 | 0.78 ± 0.11 | 0.66 ± 0.11 | 0.72 ± 0.11 | 0.78 ± 0.11 | 0.84 ± 0.11 | 0.88 ± 0.13 | 0.92 ± 0.13 |
| 8 | 3.04 ± 0.12 | 1.50 ± 0.12 | 1.48 ± 0.11 | 1.22 ± 0.13 | 1.38 ± 0.12 | 1.58 ± 0.21 | 1.74 ± 0.21 | 1.88 ± 0.21 | 2.10 ± 0.21 |
| 24 | 4.86 ± 0.23 | 2.56 ± 0.24 | 2.50 ± 0.23 | 2.30 ± 0.23 | 2.40 ± 0.27 | 2.60 ± 0.27 | 2.78 ± 0.31 | 2.98 ± 0.31 | 3.10 ± 0.33 |
| 32 | 5.92 ± 0.22 | 3.58 ± 0.22 | 3.54 ± 0.22 | 3.32 ± 0.23 | 3.46 ± 0.24 | 3.68 ± 0.32 | 3.68 ± 0.32 | 3.96 ± 0.33 | 4.28 ± 0.32 |
| 48 | 7.38 ± 0.21 | 4.72 ± 0.21 | 4.70 ± 0.21 | 4.52 ± 0.22 | 4.64 ± 0.22 | 4.70 ± 0.22 | 4.94 ± 0.31 | 5.16 ± 0.31 | 5.40 ± 0.31 |
| 56 | 8.82 ± 0.31 | 5.96 ± 0.32 | 5.92 ± 0.32 | 5.68 ± 0.32 | 5.88 ± 0.22 | 6.10 ± 0.22 | 6.26 ± 0.33 | 6.48 ± 0.33 | 6.74 ± 0.33 |
| 72 | 9.98 ± 0.23 | 6.80 ± 0.23 | 6.76 ± 0.23 | 6.60 ± 0.23 | 6.72 ± 0.23 | 6.84 ± 0.33 | 6.98 ± 0.33 | 7.08 ± 0.32 | 7.30 ± 0.21 |
Effect of the extract on soybean oil oxidation
Figure 4a shows the PV developments during the storage of soybean oil at 70 °C for 72 h with various concentrations of sesame cake extract. A continuous increase in PV with increase in storage period was observed for all the samples. Initially, increase in PV was very slow, but it started increasing approaching 8 h of storage and went on increasing further with increase in storage period. A significant difference (P < 0.05) in PV was observed between the control and soybean oil containing extracts, BHT, BHA, and TBHQ. These results indicated that sesame cake extract inhibited soybean oil oxidation. Further, the antioxidant effect of sesame cake extract at 200 ppm was better than those of BHT and BHA. During incubation, TBHQ maintained the lowest PV. The effect of sesame cake extract on soybean oil oxidation (measured by AV) is shown in Fig. 4b. In control sample formation of carbonyls was higher than in samples with added extracts (P < 0.05). When compared with the control, sesame cake extract at 200 ppm was found to be even more effective in retarding the formation of carbonyl components than BHT and BHA after 72 h of storage, which predicted their high antioxidant potential. However, at all the stages of storage period this extract was less effective than TBHQ. This is in accordance with results of Aruoma et al. (1992) who reported that in soybean oil carnosic acid was more active than BHT and BHA, but less active than TBHQ.
Fig. 4.
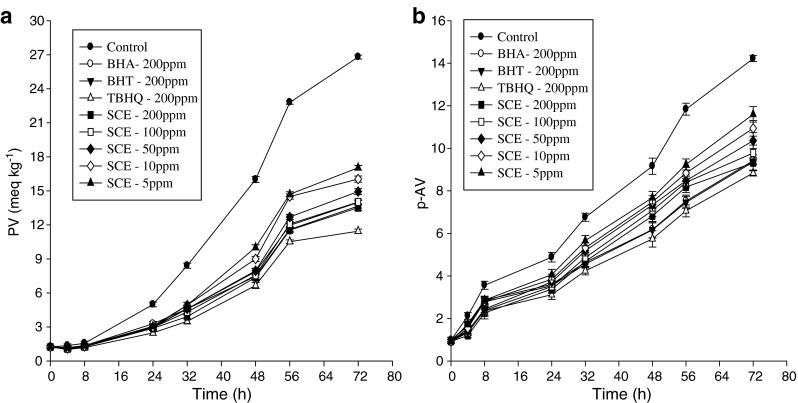
Relative increase in peroxide value (a) and p-anisidine value (b) of treated soybean oil samples with sesame cake extract under accelerated storage. Error bars show the variations of three determinations in terms of standard deviation
Absorption at 232 nm and 270 nm, due to the formation of primary and secondary compounds of oxidation (Table 2), showed a pattern in good agreement with that of the PV and AV. CD and CT contents went on increasing with the increase in storage time. Initially, rate of formation of CD was higher, and went on decreasing with increase in storage time, while the reverse behavior was observed for CT content, i.e. initial rate was lower, and went on increasing with the storage time. Formation of high contents of CD may be related to the presence of higher contents of polyunsaturated fatty acids (Liu and White 1992) in soybean oil. Conjugated trienes may be produced by dehydration of conjugated diene hydroperoxides. Highest contents were observed for control, indicating greater intensity of oxidation, followed by BHA, BHT, sesame cake extract, and TBHQ.
Table 2.
Conjugated dienes (ג232 nm) and conjugated trienes (ג270 nm) of treated soybean oil samples with sesame cake extract during oven test
| Time (h) | Control | BHA- 200 ppm | BHT- 200 ppm | TBHQ- 200 ppm | Sesame cake extract | ||||
|---|---|---|---|---|---|---|---|---|---|
| 200 ppm | 100 ppm | 50 ppm | 10 ppm | 5 ppm | |||||
| Conjugated dienes (ג232 nm) | |||||||||
| 0 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 | 1.08 ± 0.13 |
| 4 | 2.24 ± 0.23 | 1.82 ± 0.23 | 1.82 ± 0.23 | 1.66 ± 0.23 | 1.74 ± 0.21 | 1.84 ± 0.21 | 1.88 ± 0.21 | 1.96 ± 0.21 | 2.02 ± 0.21 |
| 8 | 4.18 ± 0.17 | 3.00 ± 0.17 | 2.98 ± 0.17 | 2.70 ± 0.21 | 2.88 ± 0.31 | 3.00 ± 0.31 | 3.16 ± 0.31 | 3.30 ± 0.31 | 3.46 ± 0.31 |
| 24 | 6.54 ± 0.21 | 4.36 ± 0.21 | 4.36 ± 0.21 | 4.12 ± 0.21 | 4.32 ± 0.33 | 4.50 ± 0.43 | 4.62 ± 0.43 | 4.76 ± 0.43 | 5.28 ± 0.43 |
| 32 | 7.98 ± 0.27 | 6.18 ± 0.27 | 6.14 ± 0.27 | 5.88 ± 0.31 | 6.08 ± 0.31 | 6.22 ± 0.31 | 6.50 ± 0.31 | 6.68 ± 0.32 | 6.84 ± 0.32 |
| 48 | 10.08 ± 0.33 | 7.50 ± 0.33 | 7.46 ± 0.33 | 7.22 ± 0.33 | 7.40 ± 0.41 | 7.58 ± 0.41 | 7.76 ± 0.41 | 7.98 ± 0.41 | 8.42 ± 0.41 |
| 56 | 13.52 ± 0.32 | 9.16 ± 0.32 | 9.14 ± 0.32 | 8.88 ± 0.32 | 9.04 ± 0.41 | 9.24 ± 0.41 | 9.36 ± 0.43 | 9.54 ± 0.43 | 9.92 ± 0.43 |
| 72 | 15.84 ± 0.23 | 10.42 ± 0.23 | 10.38 ± 0.23 | 10.10 ± 0.31 | 10.30 ± 0.31 | 10.52 ± 0.31 | 10.78 ± 0.31 | 10.98 ± 0.31 | 11.24 ± 0.31 |
| Conjugated trienes (ג270 nm) | |||||||||
| 0 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 | 0.48 ± 0.13 |
| 4 | 0.98 ± 0.13 | 0.64 ± 0.13 | 0.64 ± 0.13 | 0.58 ± 0.11 | 0.62 ± 0.11 | 0.68 ± 0.11 | 0.74 ± 0.11 | 0.74 ± 0.11 | 0.80 ± 0.11 |
| 8 | 2.84 ± 0.12 | 1.40 ± 0.12 | 1.38 ± 0.12 | 1.20 ± 0.12 | 1.30 ± 0.13 | 1.48 ± 0.13 | 1.48 ± 0.13 | 1.62 ± 0.13 | 1.82 ± 0.13 |
| 24 | 4.66 ± 0.21 | 2.32 ± 0.21 | 2.26 ± 0.21 | 2.00 ± 0.21 | 2.18 ± 0.11 | 2.30 ± 0.11 | 2.52 ± 0.11 | 2.72 ± 0.11 | 2.88 ± 0.11 |
| 32 | 6.14 ± 0.24 | 3.28 ± 0.24 | 3.28 ± 0.24 | 3.08 ± 0.24 | 3.22 ± 0.22 | 3.26 ± 0.32 | 3.50 ± 0.32 | 3.66 ± 0.32 | 3.80 ± 0.32 |
| 48 | 7.78 ± 0.22 | 4.66 ± 0.22 | 4.62 ± 0.22 | 4.42 ± 0.21 | 4.56 ± 0.31 | 4.70 ± 0.31 | 4.82 ± 0.31 | 5.12 ± 0.31 | 5.42 ± 0.31 |
| 56 | 9.10 ± 0.21 | 5.76 ± 0.21 | 5.70 ± 0.21 | 5.50 ± 0.21 | 5.64 ± 0.31 | 5.86 ± 0.31 | 6.02 ± 0.31 | 6.22 ± 0.31 | 6.38 ± 0.33 |
| 72 | 10.34 ± 0.13 | 6.94 ± 0.13 | 6.90 ± 0.11 | 6.70 ± 0.21 | 6.86 ± 0.21 | 7.10 ± 0.21 | 7.24 ± 0.21 | 7.36 ± 0.23 | 7.64 ± 0.23 |
Conclusion
It can be concluded that sesame cake extract can stabilize both sunflower and soybean oils very effectively at all concentrations. Sesame cake extract showed varying degrees of antioxidant activity in different test systems in a dose-dependent manner. The pattern of activity of the extract within the assays also differed. Sesame cake extract at concentration of 200 ppm has stabilization efficiency comparable to commonly-employed synthetic antioxidants BHT and BHA at their legal limit, but has lower efficiency than that of the synthetic antioxidant TBHQ. Sesame cake extract has a strong antioxidative effect during initial and final steps of oxidation in the dark in an oven at 70 °C for 72 h. Therefore, sesame cake extract can be recommended as a potent source of antioxidants for the stabilization of food systems, especially vegetable oils with high levels of PUFA. Phenolic compounds might be responsible for the antioxidant activity of sesame cake, although further studies are required to reveal whether they contain other antioxidative constituents.
References
- Abu-Jdayil B, Al-Malah K, Asoud H. Rheological characterization of milled sesame (tehineh) Food Hydrocoll. 2002;16:55–61. doi: 10.1016/S0268-005X(01)00040-6. [DOI] [Google Scholar]
- Official methods of analysis. 15. Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- AOCS (1995) Official Methods and Recommended practices of the American Oil Chemists’ Society, 4th ed. AOCS, Champaign, IL. (USA)
- Aruoma OI, Halliwell B, Aeschbach R, Loliger J. Antioxidant and prooxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- Bushra S, Farooq A, Roman P. Antioxidant potential of corncob extracts for stabilization of corn oil subjected to microwave heating. Food Chem. 2007;104:997–1005. doi: 10.1016/j.foodchem.2006.12.061. [DOI] [Google Scholar]
- Cuvelier ME, Richard H, Berst C. Comparison of the antioxidative activity of some acid-phenols: structure–activity relationship. Biosci Biotechnol Biochem. 1992;56:324–325. doi: 10.1271/bbb.56.324. [DOI] [Google Scholar]
- Dorman HJD, Kosar M, Kahlos K, Holm Y, Hiltunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J Agric Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- Fennema OR (1976) Principles of food science, Part 1, Food chem, Marcel Dekker Inc.
- Gianni S, Silvia M, Mariavittoria M, Martina S, Stefano M, Matteo R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005;91:621–632. doi: 10.1016/j.foodchem.2004.06.031. [DOI] [Google Scholar]
- Heim KE, Taigliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/S0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Huang D, Band O, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- Imeh U, Khokhar S. Distribution of conjugated and free phenols in fruits: antioxidant activity and cultivar variations. J Agric Food Chem. 2002;50:6301–6306. doi: 10.1021/jf020342j. [DOI] [PubMed] [Google Scholar]
- Standard methods for the analysis of oils and fats and derivatives. Toronto: Pergamon; 1979. [Google Scholar]
- Standard methods for the analysis of oils, fats, and derivatives. 7. Oxford: Blackwell Scientific Publication; 1987. [Google Scholar]
- Jeong SM, Kim SY, Kim DR, Nam KC, Ahn DU, Lee SC. Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extracts. Food Chem Toxicol. 2004;69:377–381. [Google Scholar]
- Jiang AL, Wang CH. Antioxidant properties of natural components from Salvia plebeia on oxidative stability of ascidian oil. Process Biochem. 2006;41:1111–1116. doi: 10.1016/j.procbio.2005.12.001. [DOI] [Google Scholar]
- Kähkönen MP, Hopia AI, Vuorela HJ, Raucha JP, Pihlaja K, Kujala TS. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kequan Z, Liangli Y. Total phenolic contents and antioxidant properties of commonly consumed vegetables grown in Colorado. LWT – Food Sci Technol. 2006;39:1155–1162. doi: 10.1016/j.lwt.2005.07.015. [DOI] [Google Scholar]
- Keyvan DHJ, Damien D, Into L, Raimo H. Chemical composition and antioxidative activity of Moldavian balm (Dracocephalum moldavica L.) extracts. LWT – Food Sci Technol. 2007;40:1655–1663. doi: 10.1016/j.lwt.2006.11.013. [DOI] [Google Scholar]
- Kumaran A, Joel KR. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT – Food Sci Technol. 2007;40:344–352. doi: 10.1016/j.lwt.2005.09.011. [DOI] [Google Scholar]
- Lee SC, Kim JH, Jeong SM, Kim DR, Ha JU, Nam KC, et al. Effect of far infrared radiation on the antioxidant activity of rice hulls. J Agric Food Chem. 2003;51:4400–4403. doi: 10.1021/jf0300285. [DOI] [PubMed] [Google Scholar]
- Lercker G, Rodriguez-Estrada MT. Cholesterol oxidation mechanism. In: Guardiola F, Dutta PC, Codony R, Savage GP, editors. Cholesterol and phytosterol oxidation products: Analysis, occurrence and biological effects Champaign. IL: AOCS; 2002. pp. 1–26. [Google Scholar]
- Liu H, White PJ. Oxidative stability of soybean oils with altered fatty acid compositions. J Am Oil Chem Soc. 1992;69:528–532. doi: 10.1007/BF02636103. [DOI] [Google Scholar]
- Mahuya B, Runu C, Utpal R. Antioxidant activity of natural plant sources in dairy dessert (Sandesh) under thermal treatment. LWT– Food. Sci Technol. 2008;41:816–825. [Google Scholar]
- Mariod AA, Ibrahim RM, Ismail M, Norsharina I. Antioxidant activities of phenolic rich fractions (PRFs) obtained from black mahlab (Monechma ciliatum) and white mahlab (Prunus mahaleb) seedcakes. Food Chem. 2010;118:120–127. doi: 10.1016/j.foodchem.2009.04.085. [DOI] [Google Scholar]
- Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Mohdaly AA, Sarhan MA, Smetanska I, Mahmoud A. Antioxidant properties of various solvent extracts of potato peels, sugar beet pulp, and sesame cake. J Sci Food Agric. 2010;90(2):218–226. doi: 10.1002/jsfa.3796. [DOI] [PubMed] [Google Scholar]
- Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H. Natural antioxidants from residual sources. Food Chem. 2001;72:145–171. doi: 10.1016/S0308-8146(00)00223-5. [DOI] [Google Scholar]
- Naczk M, Shahidi F (1998) Insoluble condensed tannins canola. Book of abstracts of the 58th annual IFT meeting, Atlanta, GA, June 25–29
- Nagendran B, Kalyana S, Samir S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- Namiki M. The chemistry and physiological functions of sesame. Food Rev Int. 1995;11:281–329. doi: 10.1080/87559129509541043. [DOI] [Google Scholar]
- Ordon JD, Gomez MA, Vattuone MI. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97:452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- Osawa T. Novel natural antioxidants for utilization in food and biological systems. In: Uritani I, Garcia VV, Mendoza EM, editors. Postharvest biochemistry of plant food materials in the tropics. Tokyo: Japan Scientific Societies Press; 1994. pp. 241–251. [Google Scholar]
- Prior RL. Absorption and metabolism of anthocyanins: Potential health effects. In: Meskin WR M, Bidlack AJ, Davies DS, Lewis RKR, editors. Phytochemicals: mechanisms of action. Boca Raton: CRC; 2004. p. 1. [Google Scholar]
- Ramadan MF. Quercetin increases antioxidant activity of soy lecithin in a triolein model system. LWT-Food Sci Technol. 2008;41:581–587. doi: 10.1016/j.lwt.2007.05.008. [DOI] [Google Scholar]
- Ramadan MF, Mörsel J-T. Characterization of phospholipid composition of black cumin (Nigella sative L.) seed oil. Nahrung/Food. 2002;46:240–244. doi: 10.1002/1521-3803(20020701)46:4<240::AID-FOOD240>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ramadan MF, Mörsel J-T. Recovered lipids from prickly pear [(Opuntia ficus-indica (L.) Mill)] peel: a good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem. 2003;83:447–456. doi: 10.1016/S0308-8146(03)00128-6. [DOI] [Google Scholar]
- Ramadan MF, Mörsel JT. Oxidative stability of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) crude seed oils upon stripping. Euro J Lipid Sci Tech. 2004;106:1–9. [Google Scholar]
- Ramadan MF, Zayed R, El-Shamy H. Screening of bioactive lipids and radical scavenging potential of some solanaceae plants. Food Chem. 2007;103:885–890. doi: 10.1016/j.foodchem.2006.09.040. [DOI] [Google Scholar]
- Ramadan MF, Kinni SG, Seshagiri M, Mörsel J-T. Fat-soluble bioactives, fatty acid profile and radical scavenging activity of Semecarpus anacardium seed oil. J Am Oil Chem Soc. 2010;87:885–894. doi: 10.1007/s11746-010-1567-0. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sachindra NM, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47:94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedeh A, Asna U. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007;102:1233–1240. doi: 10.1016/j.foodchem.2006.07.013. [DOI] [Google Scholar]
- Shui G, Leong LP. Residue from star fruit as valuable source for functional food ingredients and antioxidant nutraceuticals. Food Chem. 2006;97:277–284. doi: 10.1016/j.foodchem.2005.03.048. [DOI] [Google Scholar]
- Shyu YS, Hwang LS. Antioxidative activity of the crude extract of lignan glycosides from unroasted Burma black sesame meal. Food Res Int. 2002;35:357–365. doi: 10.1016/S0963-9969(01)00130-2. [DOI] [Google Scholar]
- Suja KP, John TA, Selvam NT, Jayalekshmy A, Armugham C. Antioxidant efficacy of sesame cake extract in vegetable oil protection. Food Chem. 2004;84:393–400. doi: 10.1016/S0308-8146(03)00248-6. [DOI] [Google Scholar]
- Wang M, Li J, Rangarajan M, Shao Y, La Voie EJ, Huang T. Antioxidative phenolic compounds from sage (Salvia officinalis) J Agric Food Chem. 1998;46:4869–4873. doi: 10.1021/jf980614b. [DOI] [Google Scholar]
- Wettasinghe M, Shahidi F, Amarowicz R. Identification and quantification of low molecular weight phenolic antioxidants in seeds of evening primrose (Oenothera biennis L.) J Agric Food Chem. 2002;50:1267–1271. doi: 10.1021/jf010526i. [DOI] [PubMed] [Google Scholar]
- Yang CY, Mandal PK, Han K-H, Fukushima M, Choi K, Kim C-J, Lee C-H. Capsaicin and tocopherol in red pepper seed oil enhances the thermal oxidative stability during frying. J Food Sci Technol. 2010;47:162–165. doi: 10.1007/s13197-010-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Lei Y, Yuangang Z, Xiaoqiang C, Fuji W, Fang L. Oxidative stability of sunflower oil supplemented with carnosic acid compared with synthetic antioxidants during accelerated storage. Food Chem. 2010;118:656–662. doi: 10.1016/j.foodchem.2009.05.038. [DOI] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yu L. Effects of extraction solvent on the wheat bran antioxidant activity estimation. LWT– Food. Sci Technol. 2004;37:717–721. [Google Scholar]