Abstract
Cow milk curd was prepared using 2% v/v of Streptococcus thermophilus DG1 and a mixed culture (0.5:1.5 v/v) of S. thermophilus DG1 and Lactobacillus plantarum and incubating at 37 °C for 16 h. Soy milk curd was prepared using different ratios of lactic cultures as stated earlier and also a mixed culture containing S. thermophilus DG1, L. plantarum and Leuconostoc mesenteroides sub spp. mesenteroides in the ratio 1:1:1 v/v along with beet pulp (2% w/w) and incubating at 37 °C for 18 h. This improved functional and probiotic properties of curd. Structural changes in curd samples during fermentation were observed by Scanning Electron Microscope (SEM). Soy milk curd showed loosened structure. The degradation of proteins into peptides and amino acids were evaluated by SDS PAGE and amino acid analysis. Maximum production of amino acids i.e. cystine, histidine and asparagine were observed in both the cow and soymilk after fermentation.
Keywords: Probiotic lactic cultures, Microstructure of curd, SEM. SDS PAGE, Amino acid analysis
Introduction
Soybean is believed to be a functional food. The composition of soybean is now well described and it is cheap food stuff rich in valuable proteins, unsaturated fatty acids, soluble and insoluble fibres and isoflavones which are very important for human nutrition (Erdman 2000). Soybean proteins help in reduction of cholesterol level, alleviate menopause symptoms, reduce the risk of cancer, osteoporosis and gall stones (Wagner et al. 1997). Difficulties in soybean utilisation are due to the presence of indigestible oligosaccharides and beanie taste of raw soybean. These problems with soybean can be solved by fermentation.
Soy milk is consumed by people who can not consume cow milk due to lactose intolerance or allergy to milk proteins. Soymilk in association with probiotic bacteria can produce beverage having multiple functional properties. By the fermentation of soy milk with probiotic bacteria, the nutritional value of the product is improved and can be consumed by the people who do not consume milk (Beasley et al. 2000; Ghosh et al. 2011; Ghosh and Chattopadhyay 2011).
However, the growth of the probiotic bacteria such as Lacobacillus was found to be affected in the soy milk medium (Božanić et al. 2008). This is due to the complex growth requirement of Lactobacillus spp. They require low oxygen tension, fermentable carbohydrates, proteins and their breakdown products, B complex vitamin, unsaturated fatty acids, minerals such as Mg, Mn and Fe for their growth (Muller et al. 2009). Soy milk contains all the above nutrients except iron which may be the reason of poor growth (Sandberg 2002). As soy milk does not contain lactose (milk sugar) therefore, for the formation of soy milk curd during fermentation addition of skim milk powder was required as a source of lactose. Sugar beet pulp is a cheap source of iron and was used in this study to the extent of 2% (w/w) without imparting any colour or flavour to the soy milk. It is known that the probiotic food with health claim should contain at least 106 cfu ml−1 of the bacteria because 108–109 of the viable cells is the minimum therapeutic dose per day. In order to improve the functional properties of soy and cow milk, fermentation by pure and mixed culture of different lactic acid bacteria have been investigated. This also requires consumer acceptance of the product which is directly related to the textural characteristic of this type of fermented food.
Textural characteristics of cow and soy milk curd can be correlated well with their microstructures. Scanning electron microscopy (SEM) was used successfully by many investigators to reveal the microstructures of various semisolid gel like products such as yoghurt, soy curd, gelatin gel, bean curd and cheese (Kalab and Harwalkar 1974; Pino et al. 2009a, b; Quintana et al. 2009). Total solid content of cow milk was found to affect the textural characteristics of curd and so the consumer appeal. Lee and Rha (1978) found that the physical and textural characteristics of the soy protein curds are related to their microstructure. So the studies were essential to develop a milk based fermented food from cow and soy milk using pure or mixed culture of lactic acid bacteria (LAB) to improve its functional characteristics and bioavailability of protein.
Earlier scientists have also studied primary proteolysis, secondary proteolysis and changes in hydrophilic and hydrophobic peptides during ripening of a goats’ milk cheese (Murcia al Vino) manufactured with plant coagulant and calf rennet (Tejada et al. 2008). According to Jovanovic et al. (2007) during SDS-PAGE analysis of soluble proteins in reconstituted milk, changes occurred during treatments at different temperatures, proteins of heat-treated samples containing added demineralized whey have had significantly different solubility. Scientists also reported the molecular weights of soluble proteins after performing SDS-PAGE (Shapiro et al. 1967; Weber and Osborn 1969 and Schägger and Von Jagow 1987).
The aim of the present study was to observe the modification in microstructure of fermented milk samples inoculated with different ratios of lactic cultures by Scanning Electron Microscopy (SEM), and the proteolysis by Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) to find out the structural changes and protein degradation in both the cow and soy milk curd after fermentation. The bands obtained on gel electrophoresis were compared with the bands of known molecular weights of standard proteins.
Material and methods
Microbial cultures
A strain of LAB was isolated from home made curd using plate and dilution technique and later was identified as S. thermophilus DG1 using DNA base composition by Bangalore Genei (India) (GenBank accession number BIS-08-072). Other lactic cultures i.e. L. plantarum (MTCC 1325) and L. mesenteroides sub spp. mesenteroides (MTCC 107) were collected from the Institute of Microbial Technology (IMTECH), Chandigarh, India.
Composition of medium
Culture of S. thermophilus DG1 was developed in MRS broth with the following composition (g l−1): tryptone 10.0, yeast extract 5.0, D-glucose 20.0, tween - 80 1.0, potassium dihydrogen phosphate 6.0, ammonium citrate 2.0, sodium acetate, hydrated 25.0, glacial acetic acid 1.32, magnesium sulphate hydrated (MgSO4, 7H2O) 0.575, manganese sulfate, hydrated (MnSO4, 4H2O) 0.14, ferrous sulphate, hydrated (FeSO4, 7H2O) 0.034. The pH was adjusted to 5.4 (de Man et al. 1960). Medium composition for L. plantarum MTCC No: 1325 was: skim milk 100 g, tomato juice 100 ml, yeast extract (MERCK) 5 g, distilled water 1000 ml. pH of the medium was adjusted to 7.0 and the medium composition for L. mesenteroides sub spp. mesenteroides MTCC No:107 was: peptone 10 g, beef extract 10 g, yeast extract 5 g, glocose 20 g, twwwn 80: 1 ml, sodium hydrogen phosphate: 2 g, sodium acetate 5 g, tri ammonium citrate 2 g, magnesium sulfate, hydrated (MgSO4. 7H2O): 0.2 g, manganese sulfate (MnSO4. 4 H2O): 0.2 g, agar 15 g, distilled water 1000 ml. The pH of the medium was adjusted to 6.4 ± 0.2. All the chemicals were obtained from MERCK Pvt. Ltd.
Preparation of curd from cow milk
Cow milk (Amul Dairy; Dankuni, West Bengal) having the following composition: total fat - 3 g, cholesterol - 0.01 mg, total carbohydrate - 4.7 g, protein - 3.1 g 100% per 100 ml of Amul fresh milk, and skim milk powder (SAGAR) having the following composition: milk fat (max) 1%, milk protein 35%, carbohydrates 51%, minerals 7%, moisture 3.5% per 100 g of skim milk powder were purchased from the local market at Jadavpur. 60 g of skim milk powder was mixed with 240 ml of cow milk, stirred well and heated to 40 °C in a water bath. After cooling to about 37 °C each of two 100 ml of this milk sample were inoculated aseptically with 2% v/v S. thermophilus DG1 and 2% v/v of a mixed culture (0.5:1.5 v/v) of S. thermophilus DG1 and L. plantarum and 2% v/v of a mixed culture (0.5:1.5 v/v) of S. thermophilus DG1 and L. mesenteroides sub spp. mesenteroides.
The inoculated milk samples were incubated at 37 °C for 16 h to form curd. In the present study, 2% (v/v) S. thermophilus DG1 was added into cow milk and was allowed to incubate at 37 °C for 16 h to form cow milk curd. This curd was used as control and was considered as Home made milk curd.
Preparation of curd from soybean
Preparation of soy milk
Soybeans were purchased from the local market and carefully selected to remove infected ones. The beans were then soaked in water for about 8 h, dehulled by rubbing in hand and again soaked in water overnight to absorb sufficient water (water/bean ratio 6:1). The soaked beans were grounded in a blender for 15 min and then boiled and cooled to destroy trypsin inhibitor and improve flavour, and then filtered through a fine cloth (Steinkraus and Dekker 1996). The produced soymilk was then heated to boiling and cooled to 37 °C and was used for the preparation of curd. The composition of soy milk/100 ml is as follows: fat - 1.6 g, protein - 4 g, carbohydrate - 5.6 g, minerals - 0.089 g.
Preparation of soy milk curd
Skim milk powder (SAGAR- 60 g) was added to 240 ml of soymilk and mixed well and was homogenized. Each of 100 ml of soymilk and skim milk mix was inoculated with different ratios of lactic cultures (stated earlier) and also a mixed culture containing 2% v/v S. thermophilus DG1, (0.5:1.5 v/v) of S. thermophilus DG1, L. plantarum and a 2% v/v mixed culture of S. thermophilus DG1, L. plantarum and L. mesenteroides sub spp. mesenteroides in the ratio of 1:1:1 (v/v) containing 2% (w/w) beet pulp and samples were incubated at 37 °C for 18 h to form soy curd.
After preparation of soymilk curd a portion of the samples were dried in a desiccator. The method described under preparation of samples for SEM analysis.
Survivality of probiotic cultures after curd formation
Cow milk curd and soy curd samples (1 g each) were serially diluted with normal saline and plated in MRS agar medium. The plates were incubated at 37 °C for 24 h and the number of bacterial colonies was counted.
Textural analysis
The curd samples stored at 4 °C were cut into cubes before compression experiment and were kept at 4 °C until tested. From each sample of curd, 5 to 8 cubes were taken and tested. The compression force versus peak height curve for each cube was obtained from Instron (model no 4301). The cross head speed was 15 mm/min, and 10 Newton load was applied in case of curd samples; chart speed was 10 mm/min and the depth applied was 15 mm. The static load cell weight for curd samples was 2 kg. From the characteristic of the curve, hardness was determined as described by Bourne (Bourne 1976) from the maximal peak height in the first compression. Three replicates of each sample were tested and the data obtained were averaged with an error of ±1%.
Preparation of samples for SEM
After the formation of curd, the curd samples were cut into pieces and stirred for about 10 min to drain off the whey portion through a fine cheese cloth and stored in a covered beaker at 4 °C. The aluminium foil was cut into pieces and small amount of stored curd sample from different preparation was transferred into the petridish containing foil. Samples were then heated to 60 °C in a hot air oven for 2.5 h until the moisture in the semisolid curd gel was evaporated to dryness. Different samples prepared in this way covered in the petridish were kept in a desiccator (containing fused CaCl2) to prevent moisture absorption.
Microstructure examination by Scanning Electron Microscopy (SEM)
The dried samples in aluminium foil were fixed in 7% glutaraldehyde for 4 h at 22 °C. Specimens were washed twice with deionised water, dehydrated successively in 20, 40, 60, 80, 95% absolute alcohol, defatted twice in chloroform and kept in absolute alcohol. At regular intervals fresh alcohol was added to the sample. The aluminium foil containing samples in the petridish was kept in hot air oven at 60° -70 °C for 1–2 h and then placed in the desiccators. Each dried specimen was fractured to expose the internal structure, mounted in an aluminium stub coated with mica plate of 100°A thickness. Microscopy was carried out on a JSM 6360, Tokyo, Japan; Scanning Electron Microscope at an accelerating voltage of 17 KV and pictures were taken on polarized film.
Sodium dodecyl sulphate-poly acrylamide gel electrophoresis (SDS-PAGE)
The prepared curd samples were treated with NaOH solution of pH 12.0 for its complete dissolution. Aliquots (2 ml) were taken and mixed well with an equal volume of Tris buffer (pH 8–9) containing ß-mercaptoethanol for stabilizing the protein. After centrifuging at 5000 rpm for 10 min, the supernatants were separated. SDS-PAGE analysis method as described by Creighton (1997) was used for the evaluation of protein degradation using gradient gel (10% w/v). The gel was 1.5 mm thick and consisted of a 2 cm stacking gel and a 10 cm running gel. 20 μg of protein was applied to sample slots. The period of electrophoresis was 3.5 h at 115 volt. After the end of electrophoresis, the matter from gel was eluted. Protein bands were stained with Coomassie Brilliant Blue in methanol/water/acetic acid (5:5:1, v/v), and then distained in the same solvent (Moore and Stein 1963). Standard protein markers were used. The molecular weights of different proteins (in k Da) were as follow: ovalbumin, 43.0; glutamic dehydrogenase, 55.0; bovine serum albumin, 66.2; phosphorylase b, 97.4; ß-galactosidase, 116.0; myosin, 200.0. The gel images were observed and photographed using Photoshop version 7.0.
Analysis of amino acids of curd samples by amino acid analyser
Waters Pico·Tag amino acid analyser were used to determine the amino acids present in the curd samples. For this, 0.2 g of prepared curd samples was taken in Eppendrof tubes and freeze-dried for 8 h to evaporate into dryness. Then the samples were allowed for acid hydrolysis at high pressure (10 atm) at 105 °C for 20 h with 6 (N) HCl. Distilled phenol of 10 μl was used to prevent oxidation during hydrolysis, and the samples was allowed for rehydration at normal temperature (37 °C). The samples were derivatised using PITC (phenyl isothiocyanate) at 37 °C for 20 min so that the free amino groups present in the protein sample can react with PITC to form PTH (phenylthiohydantoin); and the free amino acids were detected by Waters 2487 Dual λ absorbance Detector at 253 nm. The samples were then ready for HPLC (containing C18 hydrophobic column) sample of 100 μl volume was injected for 35 min runtime. The amino acids based on their hydrophobic nature bind to the column at the optimum temperature of 38 °C. Standards provided by the Waters Pico·Tag PVT LTD. were used for calculation of amino acid concentrations in the curd samples. The essential amino acid contents were also analysed by earlier authors (Swendseid et al. 1958; Ray Sarkar et al. 1953).
Calculation of amino acid concentrations from standards
From the chromatogram, peak area and peak heights were obtained. For each of the amino acids the obtained area was divided by the standard peak height and then was multiplied by a standard factor of 0.625. Thus for all amino acids, concentrations in picomol amount were obtained. Then all the values were added and equal to 100% and thus actual percent concentration of amino acid was obtained.
Statistical analysis
The experiments were conducted for three replications. The experimental data were statistically analyzed using SPSS 16.0 for Windows (general) linear model, univariate analysis and Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA) (Ghosh and Chattopadhyay 2010).
Results and discussion
The viable count of S. thermophilus DG1, L. plantarum and L. mesenteroides sub spp. mesenteroides was found to be increased from 3.70 to 6.50, 6.68 to 7.09 and 6.34 to 6.78 log cfu/g, respectively, in cow milk curd, whereas, it increased from 6.50 to 7.12, 6.57 to 8.11 and 6.48 to 6.72 log cfu/g, respectively, in soy curd.
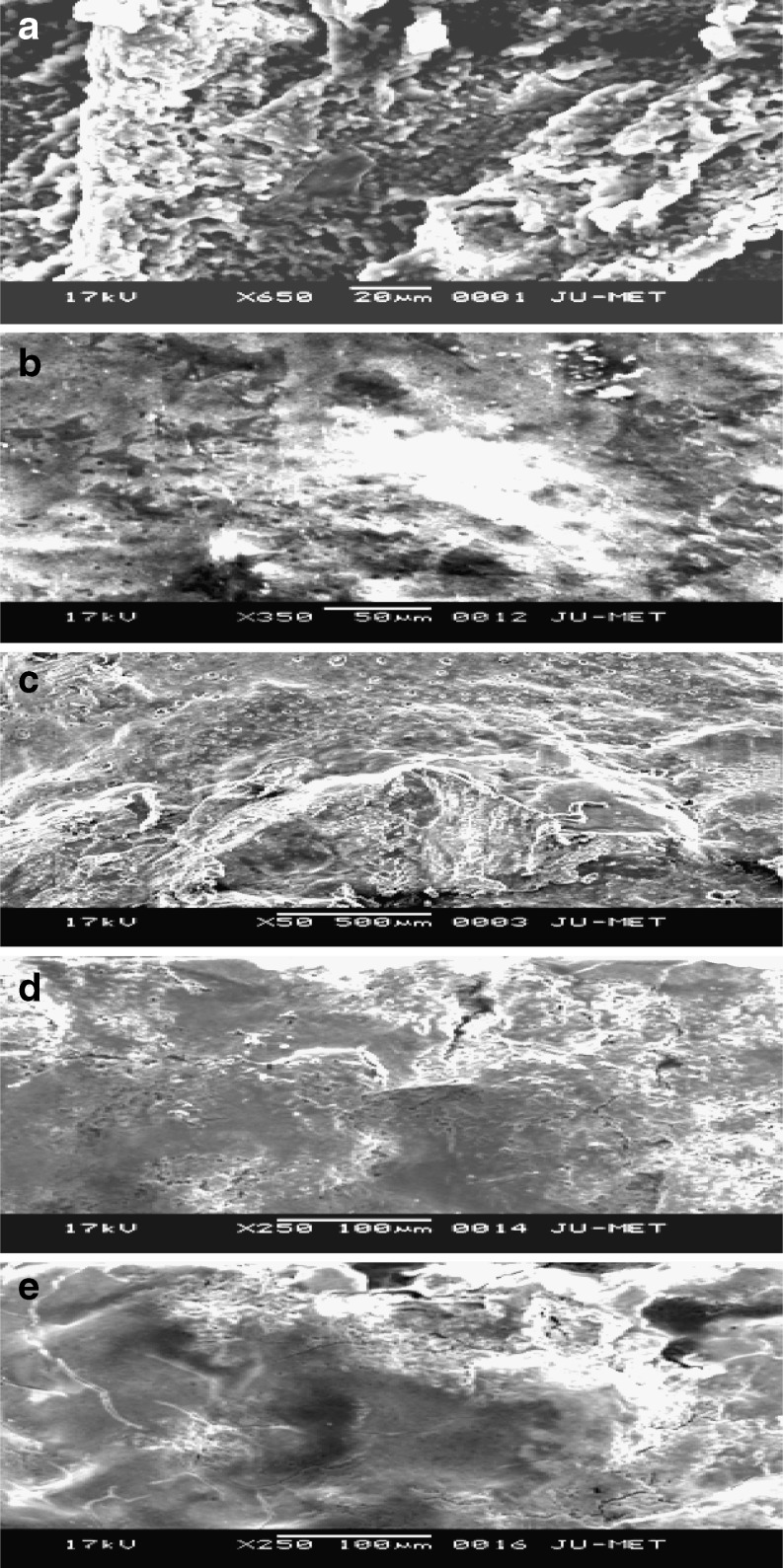
Changes in microstructure of curds prepared from cow and soy milk during fermentation by different strains of LAB were studied. Lee and Marshall (1981) reported the relation between microstructure and texture of processed cheese, milk curds. The microstructure of both the milk curd and soy curd during fermentation stages were examined by SEM technique. Scanning electron micrographs obtained are shown in the figures (Figs. 1, 2). In Fig. 1 (a), the texture was uniform. There was a decrease in porosity along with an increase in size of casein aggregates throughout the production process. Fig. 1 (c) showed non uniform structure of the milk curd because of the addition of mixed lactic cultures. A very interesting observation was obtained from Fig. 1 (d) of soymilk curd where uniform pore size was appeared. Cracks were appeared in few places on the curd surface. Addition of Beet pulp (2% w/w) into the mixture of soymilk- skim milk inoculated with the lactic cultures showed an interesting change in Fig. 1 (e). The pore size became bigger, indicating the significant interaction of iron of beet pulp with the soy curd. The looseness of the structure of soy curd was consistent with its significant lower hardness compared to other curd samples. Textural characteristics of curd samples with respect to hardness are shown in Table 1.
Fig. 1.
a Cow milk curd prepared with 2% (v/v) S. thermophilus DG1. Photograph shows uniform texture of the curd sample. b Soy curd prepared with 2% (v/v) S. thermophilus DG1. Photograph shows non uniform texture of the curd sample. c Cow milk curd prepared with 0.5:1.5 (v/v) of S. thermophilus DG1 & L. plantarum. Photograph shows non uniform texture of the curd sample. d Soy curd prepared with 0.5:1.5 (v/v) of S. thermophilus DG1 & L. plantarum showing uniform pore size. e Soy curd prepared with 1:1:1 (v/v) of S. thermophilus DG1, L. plantarum & L. mesenteroides in addition to 2% beet pulp showing appearance of few cracks in the texture
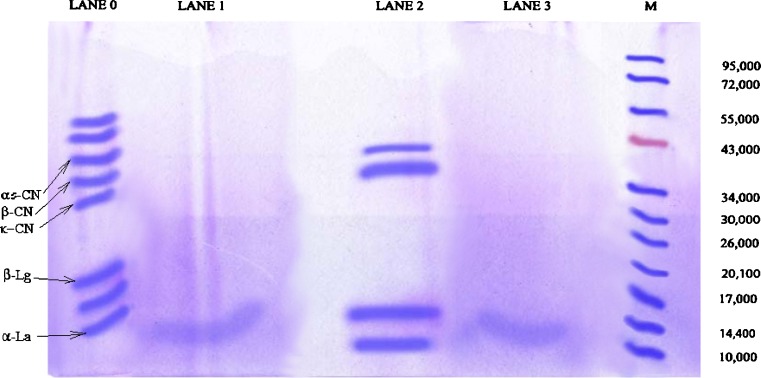
Fig. 2.
SDS-PAGE analysis of curd proteins. Lane M, protein markers with standard molecular weight ranged from 10 to 95 kDa; Lane 0, soy (whey) protein, Lane 1 soy curd protein, Lane 2, cow milk (whey) protein, and Lane 3, cow milk curd protein. The SDS-electrophoretic profile of the milk proteins (lane 0) contains five major bands identified as α s–casein, ß-casein, α –La, 71 kDa-band, and three minor bands identified as β-Lg, κ-casein
Table 1.
Texture characteristic of curd samples
| Curd samples | Hardness (in Newton) |
|---|---|
| Home made curd (control) | 4.2 ± 0.4 |
| Cow milk curd (inoculated with 2% v/v S. thermophilus DG1) | 3.6 ± 0.8 |
| Cow milk curd (inoculated with 0.5: 1.5 v/v of S. thermophilus DG1, L. plantarum | 3.2 ± 0.2 |
| Soy milk curd (inoculated with 2% v/v S. thermophilus DG1) | 3.5 ± 0.6 |
| Soy milk curd (inoculated with 1:1:1 v/v S. thermophilus DG1, L. plantarum and L. mesenteroides) in addition to 2% beet pulp | 3.2 ± 0.2 |
Hardness data presented are average values. ± indicates standard deviation (SD) of n = 3 experiments
The distribution of protein bands for curd samples are shown in Fig. 2. It can be found that proteolysis of curd protein occurred during fermentation. Molecular weights of protein subunits ranged from 10 to 72 k Da in the ascending direction from bottom to top as characterized by band lane 0 and lane 3. With the progress of fermentation cow and soy milk proteins in the curd samples were degraded by proteases. The best resolution of band in cow milk curd prepared from 2% v/v S, thermophilus DG1 was obtained after proteolysis, whereas, cow milk curd prepared with the mixed lactic culture of 0.5:1.5 v/v S. thermophilus DG1 and L. plantarum gave bands beyond 10 k Da that could not be visible. It was obtained from SDS-PAGE analysis that soy milk curd prepared with the addition of 2% v/v S. thermophilus DG1 gave band of α lactoglobulin at the molecular wt of 14 k Da and soy milk curd prepared from mixed culture of 0.5:1.5 v/v S. thermophilus DG1 and L. plantarum and 1:1:1 v/v S. thermophilus DG1, L. plantarum and L. mesenteroides sub spp. mesenteroides addition of 2% w/w beet pulp provided bands beyond the band size of 10 k Da, and those could not be visualized with naked eye. Most of the peptides in curd proteins had molecular weight less than 20 k Da, indicating that those proteins in soy curd and cow milk curd were degraded into small peptides. Casein components of curd samples were obtained as a broad band (Lanes 1 and 3) indicating that small peptides were produced during separation which have been eluted from the protein gel and most of them have molecular weights <20 k Da, whereas whey proteins, serum albumins and other proteins were clearly visualized in the protein gel which indicated that their molecular weights were >20 k Da and they were not degraded before loading to the gel (Lane 0 and 2). At pH 12.0, casein protein forming the curd gel was degraded extensively to form the polypeptides of smaller molecular weights. When this solution was put into the SDS gel at pH 7.0, the degraded proteins were separated in the gel on application of electrical field as shown in Fig. 2. Detected polypeptides were identified using the standards of α-La, β-Lg, κ-casein, α-casein, β-casein (Sigma, USA) and the low molecular weight kit (LKB-Pharmacia, Sweden).
Amino acid analysis
The percentage of amino acids (in terms of picomol) were calculated with respect to the reference values (Table 2) and it was shown that the amino acids such as cystine, histidine, aspartic acid and asparagine were present in both types of curd samples and found to be increased significantly (Table 2). The concentrations of amino acids (Table 2) vary with the different ratios of inoculum added to the curd. The soy milk curd (inoculated with 1:1:1 v/v of S. thermophilus DG1, L. plantarum and L. mesenteroides and addition of 2% w/w beet pulp) had maximum amount of amino acid compared to other probiotic curd samples. This is due to the iron fortification with addition of beet pulp in the soy milk. It was observed that the amino acids namely histidine, cystine, asparagine were present in considerable amounts in the curd samples after hydrolysis (Table 2).
Table 2.
Concentration of amino acids in curd samples
| Amino acids | Concentration of amino acids present in curd samples (% picomol) | |||
|---|---|---|---|---|
| Cow milk curd1 | Cow milk curd 2 | Soy milk curd3 | Soy milk curd4 | |
| Asparagine | 20.03 ± 1.18 a | 17.91 ± 1.89 a | 22.59 ± 2.55 b | 24.29 ± 2.56 b |
| Serine | 3.80 ± 0.16 c | 5.11 ± 1.02 a | 6.23 ± 1.44 a | 4.75 ± 1.47 a |
| Glycine | 0.14 ± 0.03 d | 1.52 ± 0.16 c | 1.19 ± 0.25 c | 1.78 ± 1.36 a |
| Histidine | 15.04 ± 1.54 a | 17.44 ± 2.54 b | 20.04 ± 2.69 b | 14.50 ± 2.85 b |
| Arginine | 1.33 ± 0.25 c | 5.35 ± 1.22 a | 4.03 ± 0.25c | 4.13 ± 2.11 b |
| Proline | 4.71 ± 0.55 c | 0.16 ± 0.01 d | 2.25 ± 0.48 c | 0.001 ± 0.00 e |
| Tyrosine | 2.04 ± 0.14 c | 2.27 ± 1.13 a | 2.02 ± 0.36 c | 2.88 ± 0.49 c |
| Valine | 4.36 ± 0.12 c | 6.78 ± 1.25 a | 6.76 ± 1.55 a | 6.29 ± 1.17 a |
| Methionine | 1.05 ± 0.02 d | 0.92 ± 0.04 d | 0.53 ± 0.02 d | 1.20 ± 0.15 c |
| Cystine | 32.06 ± 2.56 b | 20.11 ± 2.36 b | 14.65 ± 2.95 b | 21.79 ± 2.55 b |
| Isoleucine | 3.16 ± 0.45 c | 13.53 ± 1.55 a | 1.78 ± 0.25 c | 1.82 ± 0.54 c |
| Leucine | 5.34 ± 0.12 c | 1.72 ± 0.23 c | 9.19 ± 1.33 a | 7.73 ± 1.69 a |
| Phenylalanine | 2.74 ± 0.06 d | 3.39 ± 0.58 c | 3.56 ± 0.58 c | 3.98 ± 1.03 a |
| Lysine | 4.15 ± 0.11c | 3.87 ± 1.08 a | 5.22 ± 1.42 a | 4.90 ± 1.27 a |
Means ± SD with the same superscript within each column are not significantly different (P < 0.05) (n = 3)
Cow milk curd1 (prepared with 2% v/v S. thermophilus), Cow milk curd 2(prepared with 0.5:1.5 v/v of S. thermophilus DG1 and L. plantarum), Soy milk curd3 (prepared with 0.5:1.5 v/v of S. thermophilus DG1 and L. plantarum) and Soy milk curd4 (prepared with 1:1:1 v/v of S. thermophilus DG1, L. plantarum and L. mesenteroides along with 2% beet pulp)
Besides 12 amino acids obtained from different curd samples, the peaks for remaining eight amino acids present in protein were missing from all curd samples studied by us. The presence of insignificant peaks was observed in few portions of chromatogram and this indicated the presence of remaining four amino acids in trace amounts.
Conclusion
The present study demonstrated that during fermentation of curd (cow and soymilk curd) with the mixture of lactic cultures, the texture was modified. There was significant difference in hardness in terms of consistency. Photographs of SEM showed that protein network was destroyed during fermentation with homogeneous and dense microstructure formed in curd samples. Results from SDS-PAGE analysis indicated that casein was degraded into small biogenic peptides (molecular weight <20 k Da) during fermentation. Amino acid analysis also showed the production of increased amounts of amino acids in the curd samples leading to the improvement in nutritional quality (bioavailability) of cow milk and soy milk by mixed culture fermentation.
Acknowledgements
This work is a part of Major Research Project of the last author financially supported by the University Grants Commission (UGC), New Delhi, India. We are thankful to the Director and scientists of Indian Institute of Chemical Biology (IICB), Jadavpur; Kolkata-700032, for their kind cooperation in experimental works.
References
- Beasley S, Tuorila H, Saris PEJ. Fermented soymilk with a monoculture of Lactococcus lactis. Int J Food Microbiol. 2000;81:159–162. doi: 10.1016/S0168-1605(02)00196-4. [DOI] [PubMed] [Google Scholar]
- Bourne MC. Interpretation of force curves from instrumental texture measurements, rheology and texture in food quality. Westport: AVI Publishing Co; 1976. pp. 244–274. [Google Scholar]
- Božanić R, Brletić S, Lovković S. Influence of temperature and sugar addition on soymilk fermentation by probiotic bacteria. Mljekarstvo. 2008;58:61–68. [Google Scholar]
- Creighton TE (1997) Protein structure: a practical approach, 2nd edn, Oxford University Press
- de Man JC, Rogosa M, Sharpe ME. 69964 MRS Agar (Lactobacillus Agar) Appl Bact. 1960;23:130–135. doi: 10.1111/j.1365-2672.1960.tb00188.x. [DOI] [Google Scholar]
- Erdman JW. Soy Protein and cardiovascular disease: a statement for healthcare professionals from the nutrition committee of the AHA. Circulation. 2000;102:2555–2559. doi: 10.1161/01.CIR.102.20.2555. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Chattopadhyay P (2010) Application of principal component analysis (PCA) as a sensory assessment tool for fermented food products. J Food Sci Technol. doi:10.1007/s13197-011-0280-9 [DOI] [PMC free article] [PubMed]
- Ghosh D, Chattopadhyay P (2011) Survivality of probiotic microorganisms for improvement of nutritional quality of ice cream. Ind Chem Engg 53(2) (Accepted)
- Ghosh D, Ray LG, Chattopadhyay P (2011) Nutraceutical potential of fermented dairy products. Ind Chem Engg 52(4) (Accepted)
- Jovanovic S, Barac M, Macej O, Vucic T, Lacnjevac C. SDS-PAGE analysis of soluble proteins in reconstituted milk exposed to different heat treatments. Sensors. 2007;7:371–383. doi: 10.3390/s7030371. [DOI] [Google Scholar]
- Kalab M, Harwalkar VR. Milk gel structure II. Relation between firmness and ultrastructure of heat induced skim milk gels containing 40–60% total solids. J Dairy Res. 1974;41:131–135. doi: 10.1017/S0022029900015004. [DOI] [Google Scholar]
- Lee YH, Marshall RT. Microstructure and Texture of Process Cheese, Milk curds, and caseinate curds containing native or boiled soy proteins. J Dairy Sci. 1981;64:2311–2317. doi: 10.3168/jds.S0022-0302(81)82852-4. [DOI] [Google Scholar]
- Lee CH, Rha C. Microstructure of soybean protein aggregates and its relation to the physical and textural properties of the curd. J Food Sci. 1978;43:79–84. doi: 10.1111/j.1365-2621.1978.tb09740.x. [DOI] [Google Scholar]
- Moore S, Stein WH. Chromatographic determination of amino acids by use of autonomic recording equipment. S. P. Colowick and N. O. Kalpan, ed. Methods Enzymol. 1963;6:819. doi: 10.1016/0076-6879(63)06257-1. [DOI] [Google Scholar]
- Muller JA, Ross RP, Fitzgerald GF, Stanton C (2009) Manufacture of Probiotic Bacteria. Prebiotics and Probiotics Sci Technol, Publisher Springer, New York 725–759
- Pino A, Prados F, Galán E, Paul LH, McSweeney Salguero JF. Proteolysis during the ripening of goats’ milk cheese made with plant coagulant or calf rennet. Food Research Int. 2009;42:324–330. doi: 10.1016/j.foodres.2008.12.009. [DOI] [Google Scholar]
- Pino A, Francisco P, Elena G, Rafael V, José FS. Amino acids evolution during ripening of goats’ milk cheese manufactured with different coagulants. Int J Food Sci Technol. 2009;44:2062–2069. doi: 10.1111/j.1365-2621.2009.02031.x. [DOI] [Google Scholar]
- Quintana AMV, López MAB, Revilla I, Martín IG, Hierro JMH, Perez CG. Seasonal evolution of hydrophilic and hydrophobic peptide contents in cheeses made from ewe’s goat’s or cow’s milk. Czech J Food Sci. 2009;27:S106–S108. [Google Scholar]
- Ray Sarkar BC, Rykala AJ, Duncan CW. The essential amino acid content of the proteins isolated from milk of the cow, ewe, sow, and mare. J Dairy Sci. 1953;36:859–864. doi: 10.3168/jds.S0022-0302(53)91573-2. [DOI] [Google Scholar]
- Sandberg AS. Bioavailability of minerals in legumes. Br J Nutr. 2002;88:S281–S285. doi: 10.1079/BJN/2002718. [DOI] [PubMed] [Google Scholar]
- Schägger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shapiro AL, Viñuela E, Maizel JV., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967;28:815–820. doi: 10.1016/0006-291X(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Steinkraus KH, Dekker M (1996) Handbook of indigenous fermented foods. 2nd edn, Inc, New York
- Swendseid ME, Feeley RJ, Harris CL, Tuttle SG. Egg protein as a source of the essential amino acids. J Nutr. 1958;17:203–211. doi: 10.1093/jn/68.2.203. [DOI] [PubMed] [Google Scholar]
- Tejada L, Abellán A, Cayuela JM, Cacha AM, Salguero JF. Proteolysis in goats’ milk cheese made with calf rennet and plant coagulant. Int Dairy J. 2008;18:139–146. doi: 10.1016/j.idairyj.2007.08.010. [DOI] [Google Scholar]
- Wagner JD, Cefalu WT, Anthony MS, Litwak KN, Zhang L, Clarkson TB. Dietary soy protein and estrogen replacement therapy improve cardiovascular risk factors and decrease aortic cholesteryl ester content in ovariectomized cynomolgus monkeys. Metabolism. 1997;46:698–705. doi: 10.1016/S0026-0495(97)90016-0. [DOI] [PubMed] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]