Summary
Liver enzyme elevations among patients on antiretroviral therapy (ART) were determined by prospectively evaluating aspartate aminotransferase (AST) data in a cohort of patients in Kampala over 36 months. A proportion of patients had hepatitis B virus (HBV) status determined. Hepatotoxicity was graded I to IV according to the AIDS Clinical Trial Group criteria. Of 546 patients, 377 (69%) were women; overall median baseline CD4+ T-cell was 97/μL (interquartile range [IQR] 20–164). Hepatitis B surface antigen (HBsAg) was detected in 42 (9%) of 470 persons. ART included lamivudine, with either nevirapine and d4T (74%) or efavirenz and AZT (26%). Median (IQR) AST level at baseline was 35 (27, 53 IU/L). Over 36 months, only eight patients had grade III AST elevation. Neither HBsAg nor ART regimen influenced AST levels. Male gender and CD4+ change from baseline were correlated with AST elevation. Patients with HIV/HBV co-infection were not at an increased risk of AST elevation, which occurred uncommonly in this setting.
Keywords: liver toxicity, AST, antiretroviral therapy, nevirapine
INTRODUCTION
Aspartate aminotranferase (AST) elevations are common in patients infected with the human immunodeficiency virus (HIV) and are associated with antiretroviral therapy (ART), especially nevirapine and high doses of ritonavir, and with chronic viral hepatitis.1,2 Explanations for AST elevations associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) are multifactorial, including immune reconstitution, emergence of HBV resistance while on HBV-active ART, HBV reactivation after withdrawal of HBV-active ART and direct hepatotoxicity.3–5
Early nevirapine hepatotoxicity has been described, especially in female patients who were initiated on ART with low body mass index, and the authors of the study specifically recommended discouraging the use of nevirapine in such patients.6 However, nevirapine hepatotoxicity occurs at higher HIV CD4+ counts than those at which patients in sub-Saharan Africa begin ART, and safe use of nevirapine-based regimens has been suggested in patients where CD4 count is low irrespective of HBV and HCV co-infections.7
In resource-rich countries, studies have demonstrated the rapid progression of liver disease in HIV-infected patients co-infected with HBV or HCV.3,8 In many sub-Saharan African countries both HIV and HBV are highly endemic. However, the natural history of chronic viral hepatitis and the association of ART with AST elevations have not been carefully examined in this setting. Therefore, we determined the frequency of AST elevation over 36 months following initiation of ART among HIV-infected patients attending our clinic in urban Kampala, Uganda.
PATIENTS AND METHODS
We prospectively studied AST elevations in a cohort of patients starting ART for the first time at the Infectious Diseases Clinic (IDC) in Kampala, Uganda. The IDC is a Center of Excellence for HIV Care and Research. From 2002, when the Infectious Diseases Institute started providing expanded HIV care, more than 23,000 adults have been registered in the clinic, of whom more than 6000 are on ART. In the period from April 2004 to April 2005, 559 clinic patients starting ART were consecutively recruited in an observational cohort study involving standardized follow-up during treatment including measurement of AST, which was selected as the proxy for liver enzymes due to available resources. Details of the cohort have been described elsewhere.9 Recruitment in the cohort was dependent on the need to initiate ART following the WHO guideline of CD4+ T-cells of 200/μL or less.
Basic demographic information (gender, age), as well as specific ART regimens, were recorded at enrollment into the cohort with updates of ART regimen changes noted during follow-up. Liver enzyme testing was performed at baseline and repeated every six months. However, in addition to these cohort-specific protocols, patients were examined monthly, asked about medication adherence and potential side-effects, and if indicated, clinicians could request additional liver enzyme testing in case of suspected hepatotoxicity.
The cohort protocol included measurement of aspartate aminotransferase (AST) and the levels were determined by Cobas Integra 400 using Roche reagents on fresh serum samples in a College of American Pathologists certified laboratory. In this assay, the upper limit of normal was 40 IU/L and values above were considered `elevated' and graded I to IV according to the AIDS Clinical Trial Group guidelines.1 Liver enzyme elevation was defined as any level of AST above 40 IU/L. Patients with grade III or IV AST elevation were not started on ART until AST levels declined.
A subset of patients who had available samples were evaluated for hepatitis B surface antigen (HBsAg) status using commercially available Abbott Murex EIA kits (HBsAg version 3, Murex Biotech Ltd, Darford, UK) according to the manufacturer's manual of operation. This HBsAg test was performed more than a year after cohort enrolment.
The study was approved by the Scientific Review Committee of the Infectious Disease Institute, Makerere University Faculty of Medicine Research and Ethics committee, and Uganda National Council for Science and Technology.
STATISTICAL METHODS
The comparison of baseline characteristics between the patients with and without AST elevations was performed using the t-test for continuous variables.
To determine the difference in severity of AST elevation, we used the amount by which AST exceeded 40 IU/L as a response variable (`AST40'). To analyse factors associated with elevated AST values from ART start to 36 months, we utilized multilevel mixed effects linear regression modeling with AST40 as the dependent variable and CD4, age (in 5-year increments), ART regimen, gender, HBsAg, HIV RNA log copies viral loads, WHO staging and previous AST as independent variables. This multivariate analysis was performed on all patients who had an HBsAg test result. Previous AST was noted to mean AST level at the previous six-month visit. We included all factors with P values ≤0.2 in the model. A P value <0.05 was considered significant. All statistical analyses were performed using STATA 9.2 (College Station, TX, USA).
RESULTS
Patient characteristics
Of the 559 patients, 546 (97.7%) had complete baseline data available, 377 (69%) were women, the median age was 35 years (interquartile range [IQR], 30–41) and the median CD4+ T-cell count at ART initiation was 97 cells/μL (IQR 20–164). Most of the patients (88.7%) had WHO stages 3 and 4 disease. All patients were started on lamivudine-containing ART with either nevirapine (74%) or efavirenz (26%) in addition to d4T or AZT.
Liver enzymes
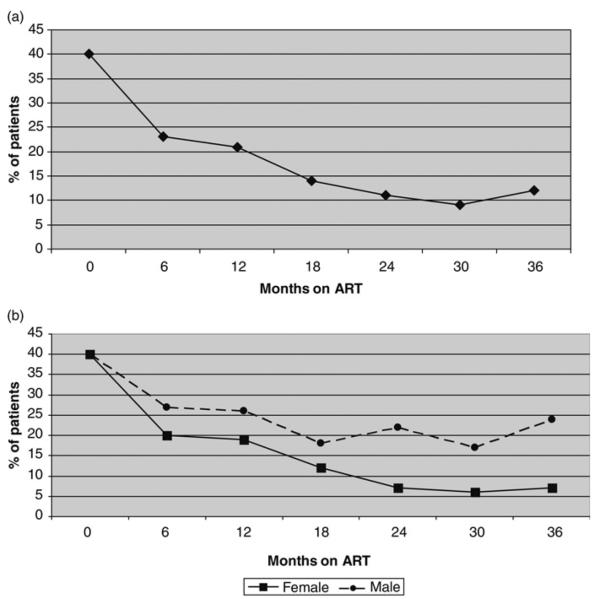
At baseline, 217/546 (40%) of the patients had an elevated AST level. However, only eight (1.0%) of these patients had grade III (6 patients) or IV elevations (2 patients) and these two patients reported symptoms of liver disease (both with jaundice) at the baseline visit. All these patients started ART after AST normalization within three months of the initial enrolment.
As shown in Figure 1a the proportion of patients with abnormal AST progressively declined with only 12% of the active patients with elevated AST at month 30. By 36 months on ART, there were eight new instances of grade III AST elevation including two that occurred in patients who were on antituberculosis treatment. Although three of these patients were symptomatic, they improved with ART discontinuation.
Figure 1.
(a) Proportion of patients with AST elevation in the first 36 months of ART. (b) Proportion of patients with AST elevation in the first 36 months of ART by gender. AST = aspartate aminotransferase; ART= antiretroviral therapy
At the time of ART initiation the same proportion of men and women had elevated AST levels (40%) and declines were noted in both men and women on ART. However, the AST decline on ART was attenuated in men, and 24 months after starting ART, the proportion of men with elevated AST was greater than in women (at month 24, men versus women: 22% versus 6%, P< 0.0001; at month 30: 24% versus 7%, P< 0.0001) (Figure 1b).
Hepatitis B surface antigen
Four hundred and seventy patients (84.1%) were evaluated retrospectively for HBsAg, of whom 42 (9%) tested positive. Figure 2 shows the proportion of patients with elevated AST at baseline and during the follow-up visits by HBsAg baseline result. No significant differences were detected in the proportion of persons with elevated AST according to HBsAg status at baseline or while on ART.
Figure 2.
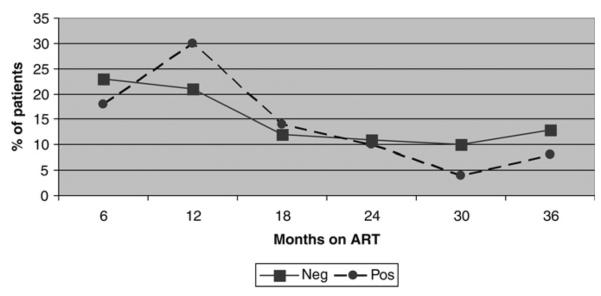
Graph comparing liver enzymes among 470 patients who were tested for hepatitis B virus. The figure shows no significant difference in the liver enzymes among HIV/HBV co-infected compared with those with HIV mono-infection over the 36 months of antiretroviral therapy. ART= antiretroviral therapy
Correlates of increasing AST level
At univariate analysis, factors that were associated with mean AST level at baseline were male gender (P = 0.001), HBsAg positive (P = 0.018), a CD4+ T-cell count <100 cells/μL (P = 0.005) and WHO stage 3–4 (P = 0.001). However, in a multivariable model, only gender remained statistically correlated with AST level (Table 1). While on ART, current CD4 count (P< 0.001), current WHO stages 3 or 4 (P = 0.03) and HIV viral load log10 copies ≥5 (P< 0.0001) were all associated with AST level.
Table 1.
Correlates of elevated AST levels at baseline and while on antiretroviral therapy by multivariate analysis
| Variable | β (95% CI) | P value |
|---|---|---|
| Baseline | ||
| Age (5-year increase) | 0.07(−0.33–0.47) | |
| Baseline AST | 0.02 (−0.00–0.04) | |
| Male sex | 1.63 (0.16–3.11) | 0.030 |
| Hepatitis B status | 0.26 (−2.20–2.73) | |
| Baseline CD4 <100 cell/μL | 0.53 (−0.86,1.92) | |
| Baseline WHO | ||
| 1 and 2 | 0 | |
| 3 and 4 | 0.93 (−0.38–2.24) | |
| Baseline ART regimen | ||
| NVP 1st line | 0 | |
| EFV | −1.76 (−3.99–0.46) | |
| Baseline HIV RNA log copies/mL | ||
| <5 | 0 | |
| ≥5 | −0.04 (−1.75–1.68) | |
| CD4 change from baseline | 0 | |
| − 0.005 (−0.009–0.001) | 0.005 | |
| Current ART regimen | ||
| NVP – first line | 0 | |
| EFV – first line | 1.62 (1.34–2.57) | |
| Second line* | −1.81 (−10.45–6.82) | |
| Others† | ||
| Current WHO stage | ||
| 1–2 | Ref | |
| 3–4 | −0.60 (−1.87–0.66) | |
| Current HIV RNA log copies/mL | ||
| <5 | 0 | |
| ≥5 | 0.52 (−2.57–3.62) |
AST = aspartate aminotransferase; NVP = nevirapine; EFV = efavirenz; ART = antiretroviral therapy; CI = confidence interval
Second-line regimens consisted of lopinavir/ritonavir (Kaletra) plus either didanosine/zidovudine, zidovudine/lamivudine, tenofovir/emtricitabine or lamivudine/efavirenz
Other regimens included two who were off therapy for sometime, one on abacavir lamivudine and efavirenz, and one on zidovudine, didanosine and Alluvia (lopinavir/ritonavir)
In a multivariate model that adjusted for age, baseline AST, previous visit AST, HBsAg status, baseline and current CD4+ T-cell count, baseline and current WHO stage, baseline and current HIV viral loads as well as baseline and current regimens, factors that remained significantly associated with AST elevations included the male gender, β = 1.63 (95% confidence interval [CI] 0.16–3.33, P = 0.03), CD4 change from baseline, β −0.005 (95% CI 0.009 to −0.001, P = 0.005). Gender difference in AST elevation was not accounted for by loss to follow-up since this was equal across both genders.
DISCUSSION
This study shows that in HIV-infected patients in Uganda there is a low frequency of clinically significant AST elevation during the first three years of ART. Symptomatic monitoring for ART-related liver toxicity during this period may therefore provide a more cost-effective approach than routine laboratory testing of AST levels in this setting.
The finding of a low frequency of AST elevations in persons on ART is consistent with some but not all prior reports. Weidle et al.10 in 2008 reported an overall fall in liver enzymes in patients in rural Uganda, and hepatitis B status did not influence the liver enzyme pattern even though the HBsAg prevalence was 23%. Likewise, a study comparing laboratory and clinical monitoring and clinically driven monitoring of patients on ART in Uganda and Zimbabwe followed for a median of five years showed no difference in grade IV or ART-modifying toxicity.11Also a South African study in which there was a high HBsAg prevalence, found a low proportion of liver enzyme elevations attributable to ART.10 Interestingly, in studies from resource-rich countries the risk of grade III–IV liver enzyme elevation appears higher.1,12 For example, a study from Spain by Núnñez et al. demonstrated that close to 10% of patients who started ART developed significant hepatotoxicity, with risk factors being hepatitis C infection, older age group and alcohol.12
There are a number of factors that must be considered when comparing studies from sub-Saharan Africa and those from resource-rich countries. HIV-infected persons in the resource-rich countries are more likely to have chronic HCV infection, which itself is associated with grade III–IV liver enzyme elevation.1 In contrast, co-infection with HBV occurs in 9–17% of HIV-infected persons in sub-Saharan Africa.13 The types of antiretroviral drugs also differ, with sub-Saharan Africa having a greater prevalence of nevirapine-based ART and concurrent treatment for tuberculosis than in the West.14–16
In our study, two of the eight patients who developed grade III AST elevation were on treatment for tuberculosis as well. Resource-rich countries may also differ from sub-Saharan African settings in the CD4+ lymphocyte counts at which ART is started and increased frequency of laboratory monitoring, and the latter would be expected to increase detection of liver enzyme elevations.
In resource-rich countries, the development of resistance to lamivudine in chronic HBV/HIV co-infection occurs predictably in up to 90% of patients by approximately four years of therapy.17,18 In addition, withdrawal of lamivudine has been shown to lead to HBV flares.19 Nonetheless, despite the fact that all the patients in our study were initiated on lamivudine-containing ART along with nevirapine (74%) and efavirenz (26%) and 9% had chronic hepatitis B, we found few instances of hepatotoxicity, as was also seen in other studies.10,20 In our study, it is possible that these patients started ART at CD4+ cell counts that were too low to induce nevirapine-hypersensitivity reaction, which has been reported to occur at higher CD4+ cell count. Also, the monitoring of AST in this study protocol was less frequent (6-monthly) and might have missed some subclinical transient elevations that could have occurred during the early ART initiation period. Another possible explanation for low AST elevations overall, and no increased risk associated with HBsAg status, is that the stage of hepatitis B might have been inactive in most patients. Recently, there are data that suggest marked differences in the proportion of persons with inactive chronic hepatitis B worldwide, explained largely by HBV genotype.21 Indeed, there was little evidence that AST levels were more frequently elevated at baseline in the HBsAg-positive patients. Since persons with inactive chronic hepatitis B typically have HBV DNA levels <2000 IU/mL, we would also not anticipate seeing lamivudine resistance/breakthrough. Further studies including evaluation of HBV genotyping, hepatitis B antigen serologies and DNA quantification, which are not currently available in Uganda, are necessary to confirm this hypothesis.
In this study, AST levels decreased with initiation of ART. In most studies of liver enzymes following ART, the focus is on the risk of hepatotoxicity and thus on the individuals whose liver enzymes increase. However, it is also interesting to consider why liver enzymes decline with initiation of ART. Our data do not provide a conclusive answer. Nonetheless, it is possible that HIV replication in Kupffer cells and possibly other liver cells might stimulate local inflammation. The SMART study has suggested that indeed HIV itself may also be harmful for the liver.22 Reductions in liver enzyme elevations following ART would then be expected as would the correlation of these reductions with HIV RNA suppression (and the inverse correlation with CD4+ T-cell count restoration).
Our study has several limitations. First AST levels were measured only six-monthly and therefore we may have missed transient AST elevations especially in the first 12 weeks of ART. It is likely, however, that these AST elevations were not clinically relevant because patients were seen monthly by dedicated cohort physicians who asked subjects about symptoms and ordered additional liver tests if clinically indicated. In fact, in resource-limited settings where regular testing is costly, our study, as is the recently published DART study, is reassuring that frequent testing for liver enzymes is not needed.11
Hepatitis B viral loads were not performed in our study. Therefore, we do not know whether the low levels of increased AST in HBV-infected patients were related to low HBV viral load levels. A recent study performed in Uganda, however, indeed suggests that this could be a possible explanation.23 Finally, 23 patients were lost to follow-up: 13 in the first, eight in the second and two in the third year. We do not know how many of them died. A previous publication from the same cohort reported high mortality especially within the first 12 months of follow-up. Tuberculosis and cryptococcal meningitis were the main causes of death; no patient died because of clinically evident liver disease.24
Our study also found that male gender was associated with AST elevations. It is possible that the male predominance could be due to other factors that may include alcohol consumption. In addition, in this study, only AST was used to measure liver enzymes, and we cannot exclude the possibility that additional cases of ALT-related grade III–IV toxicity would have been detected by inclusion of ALT testing. However, although the choice of AST was purely practical, it is interesting that in following HIV/HCV co-infected subjects, co-author DT found that AST is more strongly correlated than ALT with liver fibrosis25 and liver fibrosis progression.26 In addition, there is very tight correlation between the two markers and liver enzyme elevations after ART.1 We therefore do not expect there would have been many with grade III–IV ALT elevation and not AST elevation.27 In addition, the overall AST levels declined and there were no other symptomatic cases aside from the three detected by AST testing.
In conclusion, in this setting, the risk of clinically significant ART-related hepatotoxicity was low, even among HIV/HBV co-infected persons.
ACKNOWLEDGEMENTS
We would like to acknowledge Kenneth Opio and Joseph Sempa for performing some of the statistical analysis, Yuka Manabe who supported the program as Head of Research at Infectious Diseases Institute and Gilead for sponsoring the fellowship. This study was funded in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. DT was supported for this work by R01DA16078.
REFERENCES
- 1.Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Elevated liver enzymes following initiation of antiretroviral therapy. JAMA. 2000;19:2526–7. [PubMed] [Google Scholar]
- 2.Lana R, Nunez M, Mendoza JL, Soriano V. Rate and risk factors of liver toxicity in patients receiving antiretroviral therapy. Med Clin (Barc) 2001;117:607–10. doi: 10.1016/s0025-7753(01)72194-x. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski MS. Management of hepatic complications in HIV-infected persons. J Infect Dis. 2008;197(Suppl. 3):S279–93. doi: 10.1086/533414. [DOI] [PubMed] [Google Scholar]
- 5.Ofotokun I, Smithson SE, Lu C, Easley KA, Lennox JL. Liver enzymes elevation and immune reconstitution among treatment-naïve HIV-infected patients instituting antiretroviral therapy. Am J Med Sci. 2007;334:334–41. doi: 10.1097/MAJ.0b013e31811ec780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanne I, Mommeja-Martin H, Hinkle J, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J Infect Dis. 2005;191:825–9. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- 7.Mbougua JB, Laurent C, Kouanfack C, et al. Hepatotoxicity and effectiveness of a Nevirapine-based antiretroviral therapy in HIV-infected patients with or without viral hepatitis B or C infection in Cameroon. BMC Public Health. 2010;10:105–15. doi: 10.1186/1471-2458-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus and risk of liver related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 9.Kamya M, Mayanja-Kizza H, Kambugu A. Predictors of long-term viral failure among Ugandan children and adults treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;46:187–93. doi: 10.1097/QAI.0b013e31814278c0. [DOI] [PubMed] [Google Scholar]
- 10.Weidle PJ, Moore D, Mermin J, et al. Liver enzymes improve over twenty-four months of first line non-nucleoside reverse trancriptase inhibitor-based therapy in rural Uganda. AIDS Patient Care STDS. 2008;10:787–95. doi: 10.1089/apc.2008.0020. [DOI] [PubMed] [Google Scholar]
- 11.Mugyenyi P, Walker S, Hakim J, et al. Routine versus clinicaly driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomized non-inferiority trial. Lancet. 2010;375:123–31. doi: 10.1016/S0140-6736(09)62067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Núñez M, Lana R, Mendoza JL, Martín-Carbonero L, Soriano V. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:426–31. doi: 10.1097/00126334-200108150-00002. [DOI] [PubMed] [Google Scholar]
- 13.Burnett RJ, Francois G, Kew MC, et al. Hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver Int. 2005;25:201–13. doi: 10.1111/j.1478-3231.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Ocama P, Katwere M, Piloya T, et al. The spectrum of liver diseases in HIV infected individuals at an HIV treatment clinic in Kampala, Uganda. Afr Health Sci. 2008;8:8–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Iwalokun BA, Hodonu SO, Olaleye BM, Olabisi OA. Seroprevalence and biochemical features of hepatitis B surface antigenemia in patients with HIV-1 infection in Lagos, Nigeria. Afr J Med Sci. 2006;35:337–43. [PubMed] [Google Scholar]
- 16.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21:1301–8. doi: 10.1097/QAD.0b013e32814e6b08. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of Chronic Hepatitis B. J Hepatol. 2009;50:227–43. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Thio C, Locarnini S. Treatment of HIV-HBV co-infection: clinical and virological issues. AIDS Rev. 2007;9:40–53. [PubMed] [Google Scholar]
- 19.Bellini C, Keiser O, Chave JP, et al. Liver enzyme elevation after lamivudine withdrawal in HIV-hepatitis B virus co-infected patients: the Swiss HIV Cohort Study. HIV Med. 2009;10:12–18. doi: 10.1111/j.1468-1293.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya D, Katzenstein D, Wong A, et al. Alanine aminotransferase levels are not significantly elevated in patients with HIV/HBV co-infection and lamivudine resistance. Int J STD AIDS. 2008;19:780–1. doi: 10.1258/ijsa.2008.008020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingston SE, Simonetti JP, Bulkow LR, et al. Clearance of hepatitis B e antigen in patients with chronic hepatitis B and genotypes A, B, C, D, and F. Gastroenterology. 2007;133:1452–7. doi: 10.1053/j.gastro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Tedaldi E, Peters L, Neuhaus J, et al. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis. 2008;47:1468–75. doi: 10.1086/593102. [DOI] [PubMed] [Google Scholar]
- 23.Seremba E, Ocama P, Opio KC, et al. validity of Rapid Strip Assay test for detecting HBsAg in patients admitted to hospital in Uganda. J Med Virol. 2010;82:1334–40. doi: 10.1002/jmv.21813. [DOI] [PubMed] [Google Scholar]
- 24.Castelnuovo B, Yuka M, Kiragga A, Moses K, Philippa E, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first three years after antiretroviral therapy in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 25.Mehta SH, Thomas DL, Torbenson M, et al. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology. 2005;41:123–31. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 26.Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;16:2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 27.Sellier P, Schnepf N, Jarrin I, et al. Description of liver disease in a cohort of HIV/HBV coinfected patients. J Clin Virol. 2010;47:13–17. doi: 10.1016/j.jcv.2009.10.010. [DOI] [PubMed] [Google Scholar]