Abstract
Purpose
Intestinal calcium absorption is thought to play a critical role in nephrolithiasis; however, no study has directly assessed this association. The purpose of this study was to explore the relationship between intestinal fractional calcium absorption, calcium intake, and nephrolithiasis.
Materials and Methods
The Study of Osteoporotic Fractures is a prospective cohort of 9704 post-menopausal women recruited from population-based listings in 1986 and followed for more than 20 years. Secondary analyses were performed of 7982 women who reported their history of nephrolithiasis, of which 5452 (68%) underwent oral radioactive calcium assay (45Ca). The impact of dietary and supplemental calcium on intestinal fractional calcium absorption was evaluated and factors independently associated with nephrolithiasis were determined.
Results
Fractional calcium absorption decreased with increased calcium intake, with no difference between dietary and supplemental calcium. Fractional calcium absorption was higher in women with a nephrolithiasis history among all calcium intake groups. Increased dietary calcium intake reduced the likelihood of nephrolithiasis by 45–54% (p=0.03). Women with a history of nephrolithiasis were less likely to supplement calcium (p<0.001). In adjusted analyses, women who supplemented calcium were 21–38% less likely to have a nephrolithiasis history (p=0.007) and there was a 24% increased risk of kidney stones for each 10% increase in fractional calcium absorption (p=0.008).
Conclusions
Fractional calcium absorption is higher in women with a history of nephrolithiasis. Higher intestinal fractional calcium absorption is associated with a greater risk of historic nephrolithiasis. Dietary and supplemental calcium decrease fractional calcium absorption and may protect against nephrolithiasis.
Keywords: Calcium absorption, nephrolithiasis, kidney stones, radioactive calcium, dietary calcium intake, calcium supplementation
INTRODUCTION
Several mechanisms can be responsible for hypercalciuria and it may be classified by the responsible organ system as intestinal absorptive, renal leak, or parathyroid resorptive hypercalciuria.1, 2 However, this classification system has not dramatically changed the management or understanding of the mechanisms of nephrolithiasis formation. Regardless of the organ responsible for the hypercalciuria, the end result may be either a primary or secondary increase in intestinal fractional calcium absorption and this may predispose to the formation of kidney stones.
Fractional calcium absorption varies widely between individuals, ranging from 10–70%.3–5 Patients with hypercalciuria tend to have higher intestinal calcium absorption.6, 7 Small studies (n≤14 patients) have used radioactive calcium isotopes to evaluate the relationship between intestinal fractional calcium absorption and hypercalciuria.8–10 However, these studies have examined changes in urine calcium, but not the more relevant clinical endpoint of stone formation or stone episodes. Until now, no previous study has directly evaluated the relationship between radioactive intestinal fractional calcium absorption and kidney stones.
Using data from the prospective multicenter Study of Osteoporotic Fractures (SOF), we explored the relationship between calcium absorption, calcium intake, and nephrolithiasis. We hypothesized that (1) higher intestinal fractional calcium absorption would be associated with an increased risk of historic nephrolithiasis; (2) higher dietary and supplemental calcium intake would decrease intestinal fractional calcium absorption; and (3) greater calcium intake would be associated with a decreased risk of historic nephrolithiasis.
MATERIALS AND METHODS
Patients
The Study of Osteoporotic Fractures is a prospective cohort of 9704 primarily Caucasian women age 65 years and older recruited from population-based listings in 1986 and followed for more than 20 years. Study rationale, design, and eligibility of SOF have been reported previously and are repeated here in brief.11–14 Participating women underwent a baseline physical examination, then follow-up visits every 2 years. Nephrolithiasis history was included from visits 2, 3 or 4. Secondary analyses were performed of 7982 women who presented in 1992 (visit 4) and had previously responded to the nephrolithiasis question. At the fourth clinical visit, 5452 women (68%) underwent oral radioactive calcium assay to evaluate intestinal fractional calcium absorption as described below.
Fractional calcium absorption was evaluated as a continuous variable, and by quintiles. The fractional calcium absorption for the quintiles were: lowest quintile (0–26.2% absorption); second quintile (26.2–30.5% absorption); third quintile (30.5–34.3% absorption); fourth quintile (34.3–39.2% absorption); highest quintile (>39.2% absorption). Body mass index (BMI) was evaluated categorically (<25, 25–29.9, 30–34.9, ≥35 kg/m2). Self-reported health status was dichotomized into “excellent” or “good” and compared to women that reported “fair”, “poor”, or “very poor”. Average dose of current calcium supplementation (visit 4) at the time of the fractional calcium absorption assay was determined and women were categorized into five categories (“none” and then quartiles of dosage of calcium supplementation dose). To evaluate calcium supplementation history, women were divided into four categories: never supplemented; only prior to study participation; only since study participation; and both prior to and since study participation. Weekly dietary calcium intake was calculated at visit 4 using a detailed 21-item nutritional questionnaire specifically designed by Block Dietary Systems (Berkeley, CA), administered when women underwent the fractional calcium absorption assay.
Fractional calcium absorption assay
Calcium absorption testing was completed in the morning after a 5-hour fast. Participants were instructed to abstain from calcium supplements for 12 hours and alcoholic beverages for 24 hours prior to testing. Fractional 45Ca absorption was estimated from the appearance of 45Ca in blood after ingestion of 50 g of labeled apple juice (63 mg of calcium) and 120 g of unlabeled Speas Farm apple juice (Sundor Brands, Mt. Dora, Florida). The total calcium load was 215 mg. Labeled 45Ca was prepared by the Osteoporosis Research Center at Creighton University in Omaha, Nebraska, and was shipped to the four clinic sites, where it was mixed with the unlabeled juice. Actual dosing occurred midway through consumption of a standardized light test meal. Blood was drawn into a serum separator tube exactly 3 hours after ingestion of the tracer and was allowed to clot at room temperature. Serum was separated within 2 hours of collection and was frozen at −70 °C until analysis. Frozen serum samples were later shipped on dry ice by overnight delivery to Creighton University, where fractional calcium absorption was estimated by using the single isotope method.15, 16
Statistical analysis
Chi squared analyses were used to compare categorical variables between patients with and without a history of nephrolithiasis. Student’s t-test was used to compare continuous variables. The relationship between calcium intake and fractional calcium absorption was evaluated in several analyses. Differences in mean fractional calcium absorption between women with and without a history of kidney stones were evaluated stratified by dietary calcium intake quintile at the time of the calcium absorption assay (visit 4). These analyses were then repeated by stratifying current calcium supplement dosage and again by calcium supplementation history. Multivariate logistic regression analyses were used to evaluate variables independently associated with nephrolithiasis with a priori adjustment for known nephrolithiasis risk factors including age, hypertension, diabetes mellitus, self-reported health status, vitamin D supplementation, estrogen use, and thiazide diuretic use. An interaction term was added to the model to evaluate calcium supplementation and fractional calcium absorption, and their association with nephrolithiasis. Odds ratio (OR), adjusted odds ratio (aOR), and 95% confidence intervals (CI) are presented. All p-values were two tailed and statistical significance was set at p<0.05. Analyses were performed using SAS 9.2 (SAS Institute, Cary, NC).
Our study received University of California Institutional Review Board Exemption (10-03262). For the original SOF study, institutional review board approval was obtained at all participating institutions with written informed consent from all participants.
RESULTS
Of the 7982 women in this analysis, 490 (6.1%) reported a history of nephrolithiasis at visits 2, 3 or 4. Women with a history of kidney stones had a higher BMI, were more likely to have hypertension and diabetes mellitus, and were less likely to report their health status as “excellent” or “good”.
Calcium intake and fractional calcium absorption
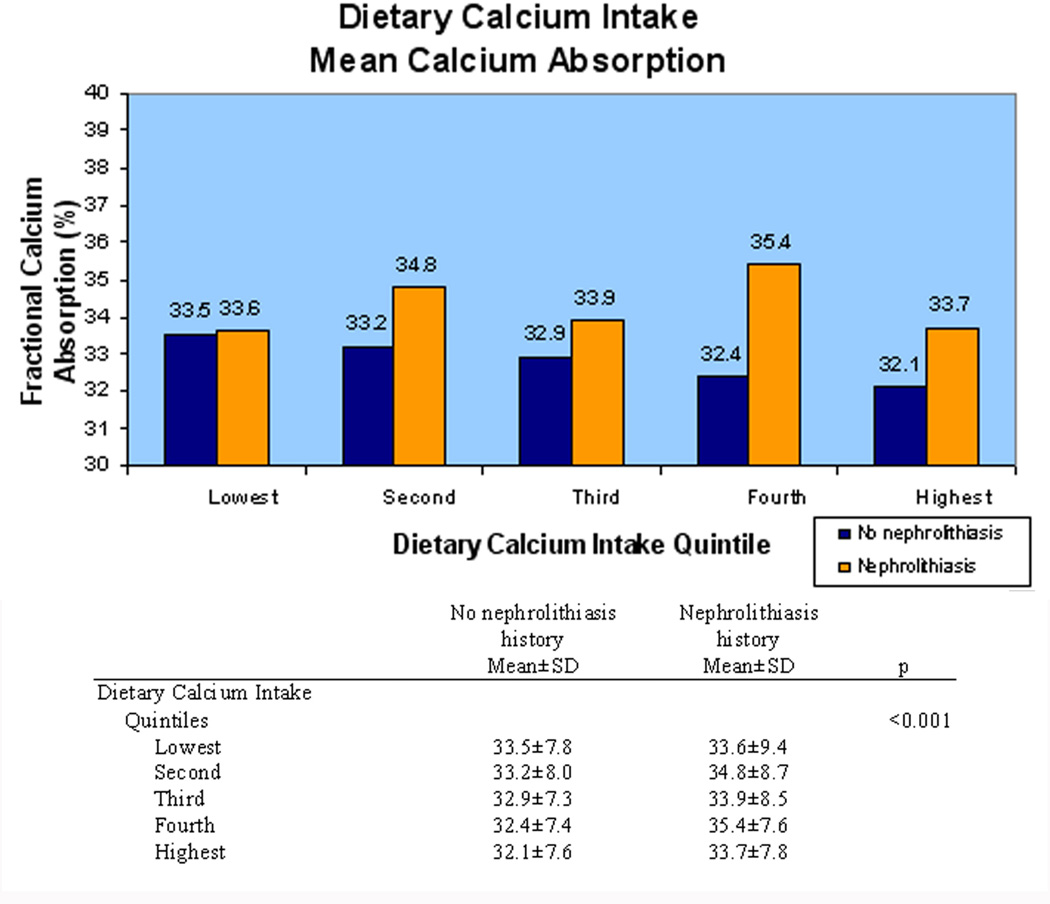
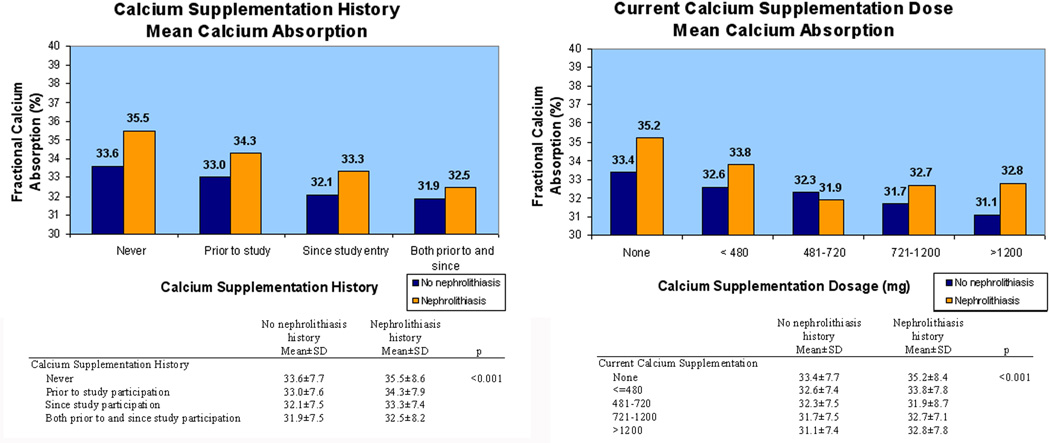
Greater current intake of calcium was associated with lower fractional calcium absorption with a similar association for dietary and supplemental calcium intake. For every dietary calcium intake quintile, fractional calcium absorption was higher in women with a history of nephrolithiasis (p<0.001) (Figure 1). For each calcium supplementation history category, fractional calcium absorption was higher in women with a history of kidney stones (p<0.001) (Figure 2). Similarly, when analyzed by current daily calcium supplementation dosage, fractional calcium absorption tended to be higher in women with a history of nephrolithiasis. Women without a history of nephrolithiasis had lower mean fractional calcium absorption as dietary and supplemental calcium increased, but this finding was less consistent in women with a history of nephrolithiasis.
Figure 1.
Average fractional calcium absorption decreased as dietary calcium intake quintile increased. Women with a history of nephrolithiasis (orange) had higher fractional calcium absorption than women with no history of nephrolithiasis (blue).
Figure 2.
Average fractional calcium absorption decreased with (A) greater calcium supplementation history and (B) increasing calcium supplementation dosage. Women with a history of nephrolithiasis (orange) had higher fractional calcium absorption than women with no history of nephrolithiasis (blue).
Dietary and supplemental calcium and nephrolithiasis
There was a trend towards higher mean daily dietary calcium intake in women without a history of nephrolithiasis (p=0.07). When evaluated by quintile, women in the highest four quintiles were 45–54% less likely to report a history of nephrolithiasis (p=0.03 for trend) (Table 1).
Table 1.
Reduced risk of nephrolithiasis from dietary calcium intake and supplemental calcium in unadjusted analyses.
| Unadjusted Dietary and Supplemental Calcium Intake and the Risk of Nephrolithiasis | ||||
|---|---|---|---|---|
| OR | 95% CI | p | ||
| Dietary calcium intake | ||||
| Quintile | mg/day | |||
| Lowest | <216 | Ref | Ref | 0.03 |
| Second | 216–387 | 0.49 | 0.37–0.65 | |
| Third | 388–564 | 0.48 | 0.37–0.64 | |
| Fourth | 565–808 | 0.46 | 0.35–0.62 | |
| Highest | >808 | 0.55 | 0.42–0.72 | |
| Calcium supplement history | <0.001 | |||
| Never | Ref | Ref | ||
| Prior to study participation | 0.71 | 0.55–0.91 | ||
| Since study participation | 0.79 | 0.59–1.05 | ||
| Both prior to and since study participation | 0.56 | 0.43–0.74 | ||
| Current calcium supplementation dose (mg/day) | ||||
| None | Ref | Ref | 0.02 | |
| ≤ 480 | 0.66 | 0.47–0.91 | ||
| 481–720 | 0.59 | 0.41–0.84 | ||
| 721–1200 | 0.52 | 0.36–0.75 | ||
| ≥ 1200 | 0.76 | 0.54–1.07 | ||
Women with a history of nephrolithiasis were less likely to supplement calcium (p<0.001) and supplemented calcium at lower doses (p=0.02). Women that supplemented calcium were 21–44% less likely to report a history of nephrolithiasis compared to women that never supplemented calcium (p<0.001 for trend) (Table 1). Furthermore, each calcium supplementation dose category was associated with a 24–48% decreased likelihood of reporting a history of kidney stones (p=0.02) compared to women who were not currently supplementing calcium.
Fractional calcium absorption and nephrolithiasis
Women were 30% more likely to report a history of nephrolithiasis for every 10% increase in fractional calcium absorption (OR 1.30, 95% CI 1.12–1.52, p<0.001) in unadjusted analyses. Similarly, when compared to those in the lowest quintile, women with higher fractional calcium absorption had significantly increased likelihood of historic nephrolithiasis (p<0.001 for trend), particularly in the highest quintile (OR 1.66, 95% CI 1.14–2.41).
Fractional calcium absorption, calcium supplementation and nephrolithiasis
In multivariate analyses, fractional calcium absorption, BMI, and calcium supplementation were independently associated with nephrolithiasis after adjustment for age, health status, hypertension, diabetes mellitus, vitamin D supplementation, and thiazide diuretic use (Table 2). There was a 24% increased likelihood of having a history of nephrolithiasis for each 10% increase in fractional calcium absorption (aOR 1.24, 95% CI 1.05–1.45 fold increase, p=0.009). These trends were similar when fractional calcium absorption was analyzed by quintile (p=0.008 for trend), where women in the highest quintile of fractional calcium absorption had the greatest risk of having a history of nephrolithiasis (aOR 1.48, 95% CI 1.00–2.19). The likelihood of reporting a history of kidney stones was significantly reduced in women who supplemented calcium prior to study participation (21%), since study participation (23%), and both prior to and since study participation (38%) (p=0.007) compared to women who never supplemented calcium. Higher BMI category was associated with a 70–82% increased likelihood of nephrolithiasis (p=0.001) compared to patients with BMI <25 kg/m2. Participant’s quintiles of dietary calcium intake was not independently associated with nephrolithiasis (p=0.61) and inclusion in the multivariate models did not change the point estimates, confidence intervals or significance of any of the independent predictors.
Table 2.
Factors independently associated with nephrolithiasis in multivariate model adjusted for age, health status, hypertension, diabetes mellitus, vitamin D supplementation, and thiazide diuretic use. Adjusted odds ratio (aOR), 95% CI and p-values for BMI, and calcium supplementation are from a final multivariate model using continuous fractional calcium absorption.
| Factors Independently Associated with Nephrolithiasis | ||||
|---|---|---|---|---|
| aOR* | 95% CI | p | ||
| Fractional calcium absorption | ||||
| Risk per 10% increase | 1.24 | 1.05–1.45 | 0.009 | |
| Quintile | Raw fractional absorption | |||
| Lowest | <26.2% | Ref | Ref | 0.008 |
| Second | 26.2–30.5 | 0.85 | 0.55–1.30 | |
| Third | 30.5–34.3 | 1.14 | 0.76–1.70 | |
| Fourth | 34.3–39.2 | 1.18 | 0.79–1.77 | |
| Highest | >39.2 | 1.48 | 1.00–2.19 | |
| Calcium supplement history | 0.007 | |||
| Never | Ref | Ref | ||
| Prior to study participation | 0.79 | 0.57–1.10 | ||
| Since study participation | 0.77 | 0.53–1.11 | ||
| Both prior to and since study participation | 0.62 | 0.44–0.87 | ||
| BMI (kg/m2) | 0.001 | |||
| <25 | Ref | Ref | ||
| 25–30 | 1.74 | 1.29–2.33 | ||
| 30–35 | 1.70 | 1.18–2.45 | ||
| >35 | 1.82 | 1.08–3.08 | ||
adjusted for age, hypertension, health status, diabetes mellitus, vitamin D supplementation, estrogen and thiazide diuretic use.
In exploratory stratified analyses, we evaluated whether the relationship between fractional calcium absorption and the history of nephrolithiasis was affected by the use of calcium supplements. In women who never supplemented calcium, there was a 38% increased likelihood of nephrolithiasis for each 10% increase in fractional calcium absorption (OR 1.38, 95% CI 1.10–1.73, p=0.006). Among women with any history of calcium supplementation the increased likelihood of nephrolithiasis was slightly lower and no longer statistically significant (OR 1.20, 95% CI 0.97–1.47, p=0.09). However, the interaction between calcium supplementation (any supplementation versus never supplemented) and fractional calcium absorption was not significant (p=0.45).
DISCUSSION
This is the first large study to evaluate the relationship between intestinal fractional calcium absorption and kidney stones, in contrast to prior studies which have only evaluated urine calcium levels. This is important because stone occurrences represent true clinical events, whereas increased urinary calcium is a physiologic finding that may or may not lead to subsequent nephrolithiasis.
In this study, increasing calcium intake was associated with lower fractional calcium absorption, with no difference in the source of calcium (diet or supplemental). Furthermore, women with a history of nephrolithisis tended to have higher fractional calcium absorption when calcium intake was analyzed by dietary calcium intake quintile, calcium supplementation history, and current calcium supplementation dosage. Fractional calcium absorption was independently associated with nephrolithiasis. We hypothesize that stone formers have higher baseline fractional calcium absorption contributing to their risk of kidney stones and that this is a marker of women decreasing their calcium intake due to a prior stone event. Greater calcium intake, which decreases fractional calcium absorption,17 may offset some of this risk for stone formers.
Women that currently or previously supplemented calcium were 21–38% less likely to have a history of kidney stones, after adjustment for other nephrolithiasis risk factors. Though it is possible that calcium supplementation may protect against the formation of kidney stones, most of the nephrolithiasis events in this study occurred prior to study participation, and thus we suspect that women with a history of kidney stones were reluctant to supplement calcium or supplemented at lower doses. In fact, previous studies have reported that supplemental calcium may increase the risk of nephrolithiasis by 17–20%.13, 18–20
There was a borderline difference in average dietary calcium intake between women with and without a history of kidney stones (p=0.07). However, women in the highest four quintiles of dietary calcium intake were significantly less likely to have had kidney stones. Thus, similar to previous studies, limited dietary calcium intake may predispose women to nephrolithiasis.19, 20 This may be due to a lower concentration of calcium in the intestinal tract to bind oxalate, increasing intestinal oxalate absorption and subsequent excretion in the urine. Previous studies have reported more than a 50% decrease in the development of nephrolithiasis for women consuming the highest amounts of dietary calcium, after adjustment for known nephrolithiasis risk factors.18–20 It is likely that increased dietary calcium intake is associated with greater intake of unrecognized factors which protect against kidney stone formation.21
This study has several limitations. This is a retrospective study using previously collected data. Participants were older, post-menopausal white women, and our results may not be generalizable to other populations. Nephrolithiasis was self-reported, but other large cohort studies have demonstrated this to be a reliable measure of the event.22–24 The timing and frequency of kidney stone formation were not assessed. While this study gives evidence that increased fractional calcium absorption is associated with nephrolithiasis, it was unfortunately not possible to evaluate the temporal relationship between nephrolithiasis and intestinal calcium absorption. Based on previous epidemiologic studies, it was assumed that 75–85% of the stones in this cohort were calcium oxalate as stone analyses were not available.25 Dietary questionnaires did not assess fluid intake, dietary oxalate or alkali intake and these could represent potentially uncontrolled confounders.26 It is possible that enrollees may have changed their behavior or activity level due to their participation in the study. Analyses were adjusted for thiazide diuretic, estrogen and vitamin D use, but the doses, duration and timing of therapy may have varied over the patient’s lifetime. Serum calcium, phosphorous, other electrolytes and parathyroid hormone levels as well as urinary calcium levels were not assessed in this study. Fortunately, calcium absorption was evaluated prior to the widespread use of bisphosphonates for bone disease with <1% of women in this study taking bisphosphonates.
These findings have important clinical implications. This study helps to clarify some of our understanding of the relationship between calcium metabolism and kidney stones. Though this is a cross-sectional analysis of fractional calcium absorption, these findings are consistent with previous studies of calcium absorption demonstrating the highest net and fractional calcium absorption in patients with low calcium intake and decreasing fractional calcium absorption in patients with higher calcium intakes.17 This is attributed to active absorption of intestinal calcium at low calcium intakes and a transition to a more linear absorption of calcium due to passive paracellular diffusion at higher calcium intakes.17, 27 Though an increase in absolute calcium absorption likely remains important as it could contribute to elevated urinary calcium excretion, calcium is always in relative excess in the urine. Thus it is likely that an elevated fractional calcium absorption would leave less intestinal calcium available to bind oxalate, thus increasing oxalate absorption and urinary oxalate excretion. Dietary and supplemental calcium decrease fractional calcium absorption, and this may partially protect women from the subsequent risk of kidney stones. Future studies examining specific patient groups that would not benefit from increased calcium intake would be valuable.
CONCLUSIONS
Results of this large study suggest that intestinal fractional absorption of calcium may play an important role in the development of nephrolithiasis. Women with a history of kidney stones had higher fractional calcium absorption and higher intestinal fractional calcium absorption is an independent risk factor for nephrolithiasis. Greater calcium intake is associated with lower fractional calcium absorption and this may protect against nephrolithiasis.
ACKNOWLEDGEMENTS
The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1.
Abbreviations
- 45Ca
radioactive calcium45
- SOF
Study of Osteoporotic Fractures
- BMI
body mass index
- OR
odds ratio
- aOR
adjusted odds ratio
- CI
95% confidence intervals
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the AUA Annual Meeting, Washington DC, May 18, 2011
Winner 2011 Excellence in Research Award, American College of Surgeons Clinical Congress, San Francisco CA, October 24th, 2011
Contributor Information
Mathew D. Sorensen, Department of Urology, University of Washington School of Medicine, Seattle.
Brian H. Eisner, Department of Urology, Massachusetts General Hospital, University of California, San Francisco.
Katie L. Stone, Research Institute, California Pacific Medical Center, University of California, San Francisco.
Arnold J. Kahn, Research Institute, California Pacific Medical Center, University of California, San Francisco.
Li-Yung Lui, Research Institute, California Pacific Medical Center, University of California, San Francisco.
Natalia Sadetsky, Department of Urology, University of California, San Francisco.
Marshall L. Stoller, Department of Urology, University of California, San Francisco.
References
- 1.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995;98:50. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 2.Pak CY, Britton F, Peterson R, et al. Ambulatory evaluation of nephrolithiasis. Classification, clinical presentation and diagnostic criteria. Am J Med. 1980;69:19. doi: 10.1016/0002-9343(80)90495-7. [DOI] [PubMed] [Google Scholar]
- 3.Alevizaki CC, Ikkos DG, Singhelakis P. Progressive decrease of true intestinal calcium absorption with age in normal man. J Nucl Med. 1973;14:760. [PubMed] [Google Scholar]
- 4.Bullamore JR, Wilkinson R, Gallagher JC, et al. Effect of age on calcium absorption. Lancet. 1970;2:535. doi: 10.1016/s0140-6736(70)91344-9. [DOI] [PubMed] [Google Scholar]
- 5.Heaney RP, Recker RR. Distribution of calcium absorption in middle-aged women. Am J Clin Nutr. 1986;43:299. doi: 10.1093/ajcn/43.2.299. [DOI] [PubMed] [Google Scholar]
- 6.Pak CY, East DA, Sanzenbacher LJ, et al. Gastrointestinal calcium absorption in nephrolithiasis. J Clin Endocrinol Metab. 1972;35:261. doi: 10.1210/jcem-35-2-261. [DOI] [PubMed] [Google Scholar]
- 7.Worcester EM, Coe FL. New insights into the pathogenesis of idiopathic hypercalciuria. Semin Nephrol. 2008;28:120. doi: 10.1016/j.semnephrol.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brannan PG, Morawski S, Pak CY, et al. Selective jejunal hyperabsorption of calcium in absorptive hypercalciuria. Am J Med. 1979;66:425. doi: 10.1016/0002-9343(79)91063-5. [DOI] [PubMed] [Google Scholar]
- 9.Zerwekh JE, Sakhaee K, Pak CY. Utility and limitation of calciuric response to oral calcium load as a measure of intestinal calcium absorption: comparison with isotopic fractional calcium absorption. Invest Urol. 1981;19:161. [PubMed] [Google Scholar]
- 10.Liberman UA, Sperling O, Atsmon A, et al. Metabolic and calcium kinetic studies in idiopathic hypercalciuria. J Clin Invest. 1968;47:2580. doi: 10.1172/JCI105940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud KE, Duong T, Cauley JA, et al. Low fractional calcium absorption increases the risk for hip fracture in women with low calcium intake. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 2000;132:345. doi: 10.7326/0003-4819-132-5-200003070-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ettinger B, Black DM, Nevitt MC, et al. Contribution of vertebral deformities to chronic back pain and disability. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1992;7:449. doi: 10.1002/jbmr.5650070413. [DOI] [PubMed] [Google Scholar]
- 13.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 14.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003;18:1947. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 15.Heaney RP, Recker RR. Estimation of true calcium absorption. Ann Intern Med. 1985;103:516. doi: 10.7326/0003-4819-103-4-516. [DOI] [PubMed] [Google Scholar]
- 16.Heaney RP, Recker RR. Estimating true fractional calcium absorption. Ann Intern Med. 1988;108:905. doi: 10.7326/0003-4819-108-6-905_2. [DOI] [PubMed] [Google Scholar]
- 17.Heaney RP, Saville PD, Recker RR. Calcium absorption as a function of calcium intake. J Lab Clin Med. 1975;85:881. [PubMed] [Google Scholar]
- 18.Bataille P, Achard JM, Fournier A, et al. Diet, vitamin D and vertebral mineral density in hypercalciuric calcium stone formers. Kidney Int. 1991;39:1193. doi: 10.1038/ki.1991.151. [DOI] [PubMed] [Google Scholar]
- 19.Curhan GC, Willett WC, Rimm EB, et al. A prospective study of dietary calcium and other nutrients and the risk of symptomatic kidney stones. N Engl J Med. 1993;328:833. doi: 10.1056/NEJM199303253281203. [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Willett WC, Speizer FE, et al. Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med. 1997;126:497. doi: 10.7326/0003-4819-126-7-199704010-00001. [DOI] [PubMed] [Google Scholar]
- 21.Heller HJ. The role of calcium in the prevention of kidney stones. J Am Coll Nutr. 1999;18:373S. doi: 10.1080/07315724.1999.10718901. [DOI] [PubMed] [Google Scholar]
- 22.Ensrud KE, Ewing SK, Taylor BC, et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;168:382. doi: 10.1001/archinternmed.2007.113. [DOI] [PubMed] [Google Scholar]
- 23.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988–1994. Am J Kidney Dis. 2008;51:741. doi: 10.1053/j.ajkd.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848. doi: 10.1097/01.ju.0000152082.14384.d7. [DOI] [PubMed] [Google Scholar]
- 26.Taylor EN, Curhan GC. Diet and fluid prescription in stone disease. Kidney Int. 2006;70:835. doi: 10.1038/sj.ki.5001656. [DOI] [PubMed] [Google Scholar]
- 27.Ireland P, Fordtran JS. Effect of dietary calcium and age on jejunal calcium absorption in humans studied by intestinal perfusion. J Clin Invest. 1973;52:2672. doi: 10.1172/JCI107461. [DOI] [PMC free article] [PubMed] [Google Scholar]