Abstract
Background
HIV prevalence is low in the Middle East and North Africa (MENA) region, though the risk or potential for further spread in the future is not well understood. Behavioral surveys are limited in this region and when available have serious limitations in assessing the risk of HIV acquisition. We demonstrate the potential use of herpes simplex virus-2 (HSV-2) seroprevalence as a marker for HIV risk within MENA.
Methods
We designed a mathematical model to assess whether HSV-2 prevalence can be predictive of future HIV spread. We also conducted a systematic literature review of HSV-2 seroprevalence studies within MENA.
Results
We found that HSV-2 prevalence data are rather limited in this region. Prevalence is typically low among the general population but high in established core groups prone to sexually transmitted infections such as men who have sex with men and female sex workers. Our model predicts that if HSV-2 prevalence is low and stable, then the risk of future HIV epidemics is low. However, expanding or high HSV-2 prevalence (greater than about 20%), implies a risk for a considerable HIV epidemic. Based on available HSV-2 prevalence data, it is not likely that the general population in MENA is experiencing or will experience such a considerable HIV epidemic. Nevertheless, the risk for concentrated HIV epidemics among several high-risk core groups is high.
Conclusions
HSV-2 prevalence surveys provide a useful mechanism for identifying and corroborating populations at risk for HIV within MENA. HSV-2 serology offers an effective tool for probing hidden risk behaviors in a region where quality behavioral data are limited.
Keywords: Sexually transmitted infections, genital herpes, epidemiology, mathematical modeling, review, Middle East and North Africa
Introduction
The Middle East and North Africa (MENA) region is perceived as “a real hole in terms of HIV/AIDS epidemiological data” (Mark Kline) (Bohannon 2005). Despite emerging data revealing low HIV prevalence, surveys suggest pockets of high-risk (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b; Egypt Ministry of Health and Population National AIDS Program 2006; Elrashied 2006; Jahani et al. 2009; Khattabi et al. 2005; Kriitmaa et al. 2010; Mahfoud et al. 2010; Marcelin et al. 2002; Pakistan National AIDS Control Program 2008). There is a notion that risky sexual practices do occur in MENA but that these practices are hidden due to the conservative nature of MENA societies (Kelley et al. 2005).
Though there has been an increase in the number of studies assessing risk behavior among populations within MENA (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b), the representation, quality, and reproducibility of these data are open to question. Sexual measures in MENA tend to be limited due to minimal surveillance efforts, methodological limitations, conservative nature of MENA societies, and stigma and illegality associated with some forms of sexual practices (Abu-Raddad et al. 2010a). Several integrated bio-behavioral surveillance surveys were recently conducted in MENA among men who have sex with men (MSM), including male sex workers (MSWs), and among female sex workers (FSWs), and injecting drug users (IDUs) (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). Most surveys revealed low or absent HIV infection despite significant reported risk behaviors (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). Though these results might reinforce a perception that this region is immune to HIV due to its socio-cultural fabric (Gray 2004; Khawaja et al. 1997; Lenton 1997), these populations may be at risk for potentially extensive HIV epidemics. Current low HIV prevalence may be due to initial isolation of hard to reach populations in MENA from high-risk networks in other parts of the world. Indeed, several recent studies show rapidly escalating HIV epidemics among IDUs and MSM in MENA (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). Alternatively, different sexual networking patterns and universal circumcision status may be limiting the sexual spread of HIV.
Even when accurate risk behavior measures exist, they may not establish a precise assessment of true risk of HIV exposure. Questionnaires, interviews, and focus groups provide only indirect information regarding sexual activity (Obasi et al. 1999). The sensitive nature of sexual behavior, informational limitations of ego-centric data, and non-random social desirability and recall biases, can introduce invalidity or inaccuracy of estimates for associations or effects (Caldwell 1989; Lee et al. 1990; Morris 1993; Wadsworth et al. 1993). Women may underreport sexual activity while men may over-report (Catania et al. 1990). Validity of reported risk behaviors that are illegal is likewise difficult to ascertain (Pisani et al. 2003).
It is also challenging to precisely quantify the multitude of facets that define sexual behavior including rate of partnership formation, contact with sex workers, heterogeneity in partner change rates, and risk group and age cohort mixing (Abu-Raddad et al. 2006). Network structure and concurrency of partnerships play a major role in HIV transmission (Farjadian et al. 2003; Kretzschmar et al. 1996; Morris 1997; Watts et al. 1992): a monogamous member of a stable sexual partnership can be at high risk of infection because of connection through a primary partner, or a partner of a primary partner, to a high-risk sexual network. Conversely, a person with frequent partnership change remains at low risk if his/her network is closed with low risk of HIV penetration.
Faced with these barriers, population data for other sexually transmitted infections (STIs) take on importance as objective measures of HIV risk. Herpes simplex virus type-2 (HSV-2) is particularly relevant to the dynamics of HIV transmission (Corey et al. 2004). Genital herpes caused by HSV-2 is one of the most widespread sexually transmitted infections (O'Farrell 1999; Smith et al. 2002; Weiss 2004), and is currently the leading cause of genital ulcer disease (GUD) in developed and developing countries (Ahmed et al. 2003; Mertz et al. 1998; Morse 1999). An estimated 536 million people were living with this infection and 23.6 million new infections occurred in 2003 (Looker et al. 2008). HSV-2 is transmitted almost exclusively by sexual contact and results in production of lifelong antibodies (van de Laar et al. 2001). There is repeatedly a very strong observed correlation between HSV-2 infection and sexual risk behavior as well as HSV-2 infection and HIV infection (Abu-Raddad et al. 2008b; Cowan et al. 1994; Cunningham et al. 1993; Obasi et al. 1999; van de Laar et al. 2001). Measurement of HSV-2 antibodies in the blood is therefore a convenient method to assess hidden levels of sexual risk behavior (Nahmias et al. 1990).
There is also extensive observational evidence that HSV-2 infection substantially increases HIV acquisition and transmission risk due to enhanced genital inflammation in HSV-2 infected persons (Freeman et al. 2006). At the population level, HSV-2, may have played an important role in fueling the HIV epidemic in sub-Saharan Africa (Abu-Raddad et al. 2008b; Corey et al. 2004). The majority of HIV infected persons are infected with HSV-2, and at an individual level, HSV-2 is usually acquired before HIV (Corey et al. 2004). As such, HSV-2 spreads along the paths of sexual risk and delineates potential avenues of future sexual HIV spread in the population. In a sense, HSV-2 infection may act as a “tour guide” for future HIV infection, even when conventional behavioral measures such as partnership change rates fail to predict the risk posed by the structure of sexual networks (Nagelkerke et al. 2006). In a study of four cities in Africa, only circumcision and HSV-2 prevalence were determinants of large differences in HIV-1 prevalence (Buve et al. 2001). Because circumcision is widespread in MENA, HSV-2 may take on primary importance as a risk factor for HIV-1 infection in the region.
In this article, we use a mathematical model that estimates spread of HIV and HSV-2 to illustrate how the extent of future HIV epidemics can be inferred by measuring endemic prevalence of HSV-2 within different subpopulations. We also review HSV-2 prevalence data from MENA, and conclude that strategically implemented HSV-2 serosurveys are critical in mapping HIV-1 risk among different populations within MENA.
Methods
Mathematical modeling
We used a compartmental modeling formalism to simulate the potential course of HIV and HSV-2 epidemics in a high risk population in MENA (Online Supporting Appendix). The model is based on extensions of earlier HIV and HSV-2 transmission models (Abu-Raddad et al. 2008a; Abu-Raddad et al. 2006; Garnett et al. 1993), but is applied, with no loss of generality, to a prototype MSM population, that is a group of MSM individuals at heterogeneous levels of risk behavior and where there is mixing between the different risk groups through proportional as well as assortative components (Online Supporting Appendix).
The formalism was built accommodating both deterministic and stochastic solutions to allow for wider applicability of the modeling framework. The deterministic version of the framework consists of a system of four differential equations for each risk group of ten MSM risk groups in the population. The stochastic version of the framework assumes the same transition rates (hazard rates) as in the deterministic version, but uses these transition rates to generate the stochastic process. The deterministic framework can be used whenever the population size in each risk group is large enough so that stochastic effects are not influential. The stochastic framework can be used at any arbitrary population size, but the computing time increases exponentially with the population size leading to impracticality at large populations sizes. In this article we focused on the predictions for an MSM population where some of the risk groups within the MSM community can be small enough to necessitate the use of the stochastic framework. Accordingly, all reported results apart from Figure 1 were generated using the stochastic version of the model. Nevertheless, our modeling framework is general enough to be used for large populations using the deterministic version. We have also used the deterministic version to provide checks on the results generated using the stochastic framework.
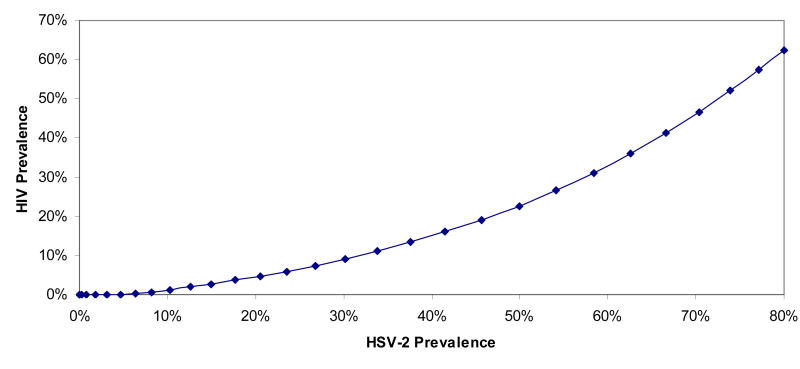
Figure 1.
The link between HIV and HSV-2 epidemiologies. The relationship between the endemic saturated levels of HIV prevalence and HSV-2 prevalence in a prototype high-risk population of men who have sex with men in the Middle East and North Africa. Both infections are spreading in the same sexual network, but reach different prevalence levels due to the biological differences between the two infections.
We chose model parameters for an MSM population since MSM is a leading group at risk of HIV sexual transmission in MENA (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b); but the model and its key inferences apply generally to other risk populations where sexual contact is the dominant mode of transmission. The model's biological input, including HIV and HSV-2 natural history and transmission probabilities, is described in the Online Supporting Appendix and in more detailed form elsewhere (Abu-Raddad et al. 2008a). Parameter values of the HSV-2 submodel are chosen according to the best available evidence of HSV-2 biology including detailed data about the pattern of HSV-2 reactivation in its clinical and subclinical forms (Abu-Raddad et al. 2008b; Mark et al. 2008) (supplementary information appendix for Ref (Abu-Raddad et al. 2008b)). Behavioral parameters in the model are informed by data from the MENA HIV/AIDS Epidemiology Synthesis Project described below (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b), and are chosen to provide a representative heterogeneous risk behavior structure typical of that of MSM as well as other risk groups (Garnett et al. 1993). We altered risk behavior in different simulations to account for discrepant values of HSV-2 prevalence in different populations and then observed the dynamics of HIV-1 spread. To disentangle sexual risk behavior from biological interactions of HIV and HSV-2, we assumed no enhanced acquisition or transmission probability of one infection due to the biology of the other infection. Therefore, the only assumed link between HIV and HSV-2 in this model is the common mode of transmission.
HSV-2 in MENA systematic review
We conducted a systematic review of HSV-2 epidemiology in MENA by undertaking a literature search of Medline (PubMed) using both free text and MeSH headings: (Herpesvirus 2, Human OR Herpes Genitalis) AND (Middle East OR Islam OR Arabs OR Arab World OR Africa, Northern OR Mauritania OR Sudan OR Somalia OR Djibouti OR Pakistan). No language or year limitation was imposed. The inclusion criterion was any publication with a serological measurement of HSV-2 prevalence in any population group. We identified 30 publications for inclusion as of August 17, 2010. The countries included in our systematic review are Afghanistan, Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, United Arab Emirates, and Yemen (Figure S1 of the Online Supporting Appendix shows a map of the region).
We supplemented our HSV-2 Medline systematic review by the data identified through the MENA HIV/AIDS Epidemiology Synthesis Project (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). The Synthesis Project mandate was to systematically collect, review, and synthesize all available data in MENA on HIV, STIs, and sexual behavior, and was conducted through a partnership with the World Bank, the MENA Regional Support Team (RST) of Joint United Nations Programme on HIV/AIDS (UNAIDS), and the Eastern Mediterranean Regional Office (EMRO) of the World Health Organization (WHO) (Abu-Raddad et al. 2010a). Detailed description of the Synthesis Project systematic review can be found in (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). The identified studies and data sources include, in addition to nearly 4,000 scientific sources of literature, hundreds of country-level reports, governmental studies and publications, non-governmental organizations studies and publications, international organizations' reports and databases, and other institutional reports related to HIV and STIs in MENA (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). We also searched the WHO/EMRO databases of notified HIV/AIDS cases and STIs (WHO/EMRO), and consulted with public health officials, and key experts in the region and beyond.
Results
HSV-2 prevalence as a predictor of the distribution, evolution, and size of future HIV epidemics
To illustrate the link between HIV and HSV-2 epidemiology, we calculated the relationship between HSV-2 prevalence and potential size of an HIV epidemic within an MSM sexual-risk contact structure of different risk groups mixing with each other. We varied HSV-2 infection prevalence by altering the average level of sexual risk behavior practiced in the sexual-risk contact structure. We achieved different levels of risk by varying the rate of partner change in the whole population by the same multiplicative factor. Both viral infections spread within the same contact structure, but at different rates reflecting biological differences between the two infections.
Our model demonstrates that HSV-2 prevalence is predictive of the size of future endemic HIV prevalence, but that the relationship is non-linear (Figure 1). A sexual risk contact structure where a low level of risk behavior supports an HSV-2 prevalence of about 10%, cannot sustain much endemic HIV transmission. Though HIV can be introduced into such a sexual risk contact structure, HIV prevalence is not likely to exceed 1%. In order for an HIV epidemic to reach greater than 5% in a population (a concentrated HIV epidemic (Pisani et al. 2003)), the level of sexual risk behavior in the population must support an HSV-2 prevalence of about 20%. In settings where level of risk behavior supports HSV-2 prevalence exceeding 60%, HIV prevalence is predicted to exceed 35%. At very high levels of HSV-2 prevalence, HIV prevalence values approach those of HSV-2. This non-linear relationship between the two total-population prevalence levels for each infection is also qualitatively seen for each risk group in the population (Figure S2). It must be noted here that these quantitative results provide rough estimates to demonstrate this concept of the link between the epidemiology of the two infections rather than strictly literal quantitative predictions.
Case scenarios illustrating HSV-2 prevalence as a biomarker of risk behavior and potential HIV expansion
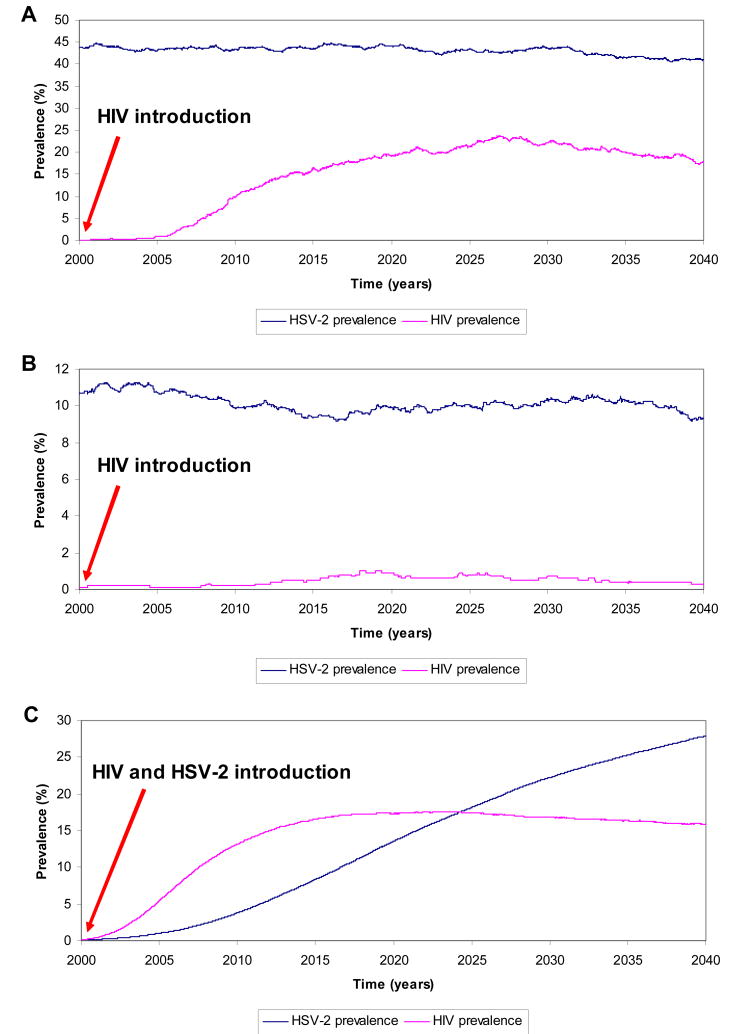
Figure 2A shows the time course of an HIV epidemic in a prototype MSM population whose endemic HSV-2 prevalence is 45%. HIV infection is introduced into this population in the year 2000: it takes several years before HIV infection sustains considerable transmission. The epidemic peaks at approximately 20% prevalence following two decades of expansion. In the simulation in Figure 2B, HSV-2 prevalence is 10%. When HIV is introduced into this population, the level of sexual risk behavior does not sustain infectious spread. Figure 2C shows the average of 100 simulations of HIV and HSV-2 epidemics assuming that the two infections are introduced concurrently into an MSM population. The HSV-2 infectious spread in its epidemic phase grows more slowly than that of HIV, but eventually achieves a larger endemic prevalence than that of HIV.
Figure 2.
A case scenario for HIV epidemic expansion in a prototype high-risk population of men who have sex with men in the Middle East and North Africa. HIV infection is introduced in the year 2000 by one infected person with latent infection. A) HSV-2 prevalence is at about 45% prevalence reflecting high levels of sexual risk practices in this population. B) HSV-2 prevalence is at about 10% prevalence reflecting relatively low levels of sexual risk practices in this population. C) The two infections are introduced concurrently in the year 2000 by one infected person with latent infection of both diseases. The HIV and HSV-2 prevalence curves shown reflect the average over 100 simulations for each epidemic.
HSV-2 prevalence in MENA
We identified 14 references including HSV-2 prevalence measures out of the 30 references identified through the Medline search. We also identified another 14 relevant references through the wide umbrella of the Synthesis Project search. Through this extensive review, we found that HSV-2 research remains limited in MENA. The majority of data were from the peer-reviewed literature, but some of which were not indexed in Medline. Table 1 lists HSV-2 prevalence measurements in MENA and related cultural settings. There is a pattern of low prevalence among the general population, but substantial prevalence among groups with identifiable risk factors such as FSWs, MSM, “bar girls”, and STD clinic attendees. HSV-2 prevalence is lower overall than in all other regions of the world (Table 2).
Table 1.
HSV-2 prevalence in different Middle East and North Africa populations as well as culturally related populations.
| Country | HSV-2 prevalence | Assay | Accuracy and reproducibility of measurement2 |
|---|---|---|---|
|
| |||
| Bangladesh | 12% (women attending primary care clinic) (Bogaerts et al. 2001) | HSV-2 specific IgG ELISA (Gull Laboratories Inc, Salt Lake City, USA) | Good |
|
| |||
| Djibouti | 2% (general population women), 5% (male blood donors), 49% (luxury bar FSWs1), 81% (street-based FSWs) (Marcelin et al. 2001) | Unknown | Unknown |
|
| |||
| Egypt | 32% (women, obstetric outpatient clinic) (el-Sayed Zaki et al. 2007) | HSV-2 qualitative specific IgM ELISA (Equipar Via G, Ferrari, Saronno, Italy) | Poor |
|
| |||
| Iran | 28% (women, primary health care centers) (Kasraeian et al.) | HSV-2 specific IgG ELISA (unknown; commercial test) | Unknown |
| 8.25% (pregnant women) (Ziyaeyan et al. 2007) | Serum neutralization test | Poor | |
| 23.3% (university students) (Tayyebi et al. 2010) | HSV-2 specific IgG ELISA (Dia-pro, Italy) | Unknown | |
|
| |||
| Israel | 9% (Arab & Jewish non-Soviet immigrants: pregnant women) (Dan et al. 2003) | HSV-2 specific IgG ELISA (Savyon Diagnostics Ltd, Ashdod, Israel) (Ohana et al. 2000) | Good |
| 2.4% (Arab STD1 clinic attendees) (Feldman et al. 2003) | HSV-2 specific IgG ELISA (EIA-gG; Gull, USA) (Ashley et al. 1998) | Good | |
|
| |||
| Jordan | 53% (male university students), 42% (female university students) (Abuharfeil et al. 2000) | HSV-2 IgG ELISA (Ismunit, Italian Institute of Immunology, Rome, Italy) | Fair |
|
| |||
| Lebanon | 0.027% (general population women) (Karam et al. 2007) | Unknown | Unknown |
|
| |||
| Morocco | 16.2% (ANC attendees), 13% (general population women), 10% (general population men), 6.7% (STD clinic attendees) (WHO/EMRO) | Unknown | Unknown |
| 26% (urban women with a median age of 40 years) (Patnaik et al. 2007) | Western blot (reference gold standard) (Ashley 1998; Ashley et al. 1988; Ashley et al. 1999) | Excellent | |
| 12.9% (ANC1 attendees), 9.2% (male HIV sentinel surveillance), 6.5% (military personnel) (Cowan et al. 2003) | HSV-2-specific IgG ELISA (HerpesSelect; Focus Technologies, CA, USA) | Good | |
|
| |||
| Pakistan | 3.4% (urban men) (Mir et al. 2009) | HSV-2 specific IgG ELISA (unknown; commercial test) | Unknown |
| 11.0% (IDUs1), 6.0% (IDUs) (Platt et al. 2009) | HSV-2-specific IgG ELISA (HerpesSelect; Focus Technologies, Cypress, CA, USA) | Good | |
| 8% (FSWs), 4.7% (FSWs), 7.4% (MSWs1; bantha†), 2.5% (MSWs; bantha), 14% (MSWs; khotki1), 25% (MSWs; khotki), 54% (MSWs; khusra1), 31.3% (MSWs; khusra) (Hawkes et al. 2009) | HSV-2-specific IgG ELISA (HerpesSelect; Focus Technologies, Cypress, CA, USA) | Good | |
|
| |||
| Saudi Arabia | 27% (pregnant women) (Ghazi et al. 2002) | HSV-2-specific IgG ELISA (Wampole Laboratories, New Jersey, USA) | Poor. |
|
| |||
| Sudan | 27% (women Sudanese refugees in Ethiopia), 26% (men Sudanese refugees in Ethiopia) (Holt et al. 2003) | Glycoprotein G-based immunoblot assays (Centers for Disease Control and Prevention laboratory, Atlanta, USA) (Schmid et al. 1999) | Fair |
| 5.5% (household cluster survey; South Sudan),4.5% (household cluster survey; South Sudan),6.1% (household cluster survey; South Sudan) (Kaiser et al. 2006) | Glycoprotein G-based immunoblot assays (Centers for Disease Control and Prevention laboratory, Atlanta, USA). | Fair | |
|
| |||
| Syria | 0% (pregnant women), 0% (general population women), 0.3% (general population men), 0% (neonates), 9.5% (STD clinic attendees), 8.0% (women with cervical cancer), 20% (“bar girls”), 34% FSWs (Ibrahim et al. 2000) | HSV-2-specific IgG ELISA (Radim company, Sulzbach, Germany) | Good |
|
| |||
| Turkey | 89% (women with pregnancy complication) (Cengiz et al. 1993b) | HSV-2 IgG ELISA (Unknown) | Unknown |
| 63.1% (pregnant women) (Duran 2004; Duran et al. 2004) | IgG antibodies | Poor | |
| 5.0% (pregnant women) 5.5% (blood donors), 4.8% (sexually active adults), 8.3% (hotel staff), 17.3% (patients with genital warts), 60% (FSWs) (Dolar et al. 2006) | HSV-2-specific IgG ELISA (Euroimmun, Germany) (Aksozek et al. 2004; Eing et al. 2002) | Good | |
| 26% (MSM) (Cengiz et al. 1992; Cengiz et al. 1993a) | HSV-2-specific IgG ELISA (Unknown) | Unknown | |
| 53.5% (rural general population women) (Maral et al. 2009) | EIAgen Herpes Simplex 2 IgG Code 08.1007.2 (ADALTIS ItaliaS.p.A. Via Magnanelli, 2-40033 Casalecchio di Reno) | Unknown. | |
| 80% (FSWs) (Gul et al. 2008) | HSV-2-specific IgG ELISA (Euroimmun, Germany) (Aksözek et al. 2004; Eing et al. 2002) | Good | |
|
| |||
| United Arab Emirates | 17.7% (blood donors including migrant workers), 7.3% (migrant workers), 9.7% (migrant workers) [N.J. Nagelkerke, personal communication] | HSV-2-specific IgG ELISA (Kalon Biological, Ltd., Surrey, United Kingdom) | Good, but relatively insensitive to new diagnosis. |
FSW = female sex worker, MSW = male sex workers, MSM = men who have sex with men, ANC = ante-natal clinic, STD = sexually transmitted diseases, IDU = injecting drug user, bantha = biological males with a male gender identity, khotki = biological males who dress as men but have “female soul” and feminized traits, khusra = transgenders who dress as women (also known as hijra).
Criteria used to judge the confidence in the serology measurements were the robustness of the serology test used, procedures used to conduct the serology test, and amount of available information on the serology test. We labeled any test with greater than 95% specificity and sensitivity excellent; tests with greater than 90% sensitivity and specificity good; tests with greater than 75% sensitivity and specificity fair; all other tests were considered poor. Poor tests tend to suffer from cross-reactivity with other infections, particularly HSV-1. We also judged a test to be poor if authors used or described tests that have not been validated in the medical literature. In several cases, we were not able to identify the test used from manuscripts and numerous attempts to contact authors: these were labeled unknown.
Table 2.
HSV-2 prevalence in the general population in different regions of the world (O'Farrell 1999; Paz-Bailey et al. 2007; Pebody et al. 2004; Smith et al. 2002; Weiss 2004).
| Region | HSV-2 Prevalence |
|---|---|
| Asia | 10% to 30% |
| Europe | 4% to 24% |
| Latin America | 20% to 40% |
| Middle East and North Africa | 0% to 15% |
| North America | 18% to 26% |
| Sub-Saharan Africa | 10% to 80% |
Several studies found higher HSV-2 prevalence levels (27% - 54%) among the general population, but there is reason to doubt the accuracy of these studies. Diagnostic tests used in these publications often suffered from high levels of cross reactivity with herpes simplex virus type 1 (HSV-1) antibodies (Ashley et al. 1991). In other studies, we were unable to clarify, despite repeated attempts to contact the authors, the type of HSV-2 serology test performed. Thus, actual prevalence of HSV-2 in these reports is in doubt.
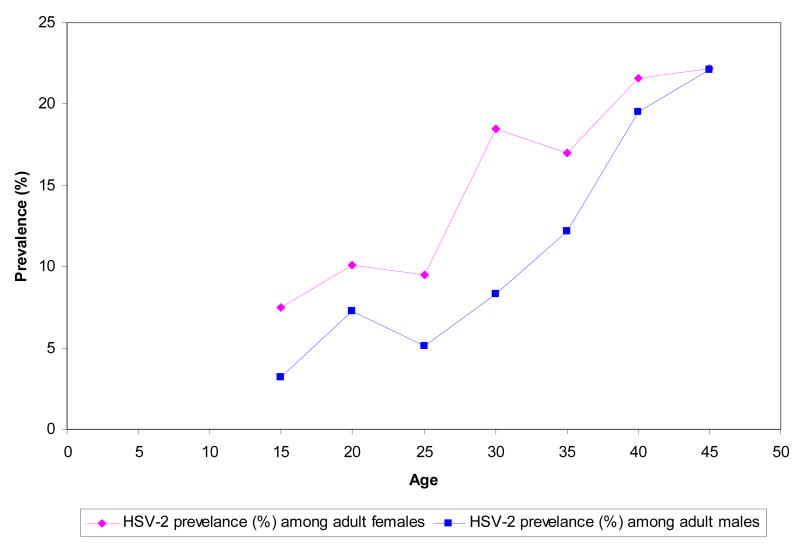
In certain countries, HSV-2 seroprevalence among certain age groups achieves reasonably considerable levels. Figure 3 shows age stratified prevalence in a study from Morocco (Cowan et al. 2003). Prevalence grows slowly for males and females in comparison to other regions (see multiple data in (Cowan et al. 2003; Smith et al. 2002)) suggesting that it takes a long time after sexual debut for the risk of exposure to this STI to become appreciable.
Figure 3.
HSV-2 prevalence for selected populations by age group in Morocco (Cowan et al. 2003).
Discussion
HSV-2 versus other biomarkers of risk
HSV-2 serology is a powerful marker of sexual risk that bypasses shortcomings of risk behavior measures gathered through surveys, and can be used in a similar manner to the use of hepatitis C infection as a proxy for HIV risk of acquisition among IDUs (Vickerman et al. 2010). HSV-2 is also a more powerful marker of sexual risk behavior than other STIs in relation to HIV epidemic potential. Bacterial STIs tend to cluster among populations at highly elevated risk behavior and therefore are not as representative as HSV-2 to HIV infectious spread which can affect a larger segment of the population (Abu-Raddad et al. 2008b; Boily et al. 1997; Brunham et al. 1990; Yorke et al. 1978). On the other hand, other viral STIs such as human papilloma virus (HPV), are much more infectious than HIV and HSV-2, and can affect a much larger part of the population than HIV and HSV-2 (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2008b; Trottier et al. 2006). HSV-2 epidemiology is the most relevant of all STIs to HIV epidemiology. This fact is manifested in the consistently strong association between these two infections in multiple studies in diverse settings and populations (Corey et al. 2004; Freeman et al. 2006). Other infections such hepatitis B and hepatitis C have modes of transmission other than sexual intercourse and their epidemiology reflect multiple risk factors beyond sexual behavior.
Prevalence of HSV-2 in MENA and prediction of HIV epidemic potential
Using a mathematical model, we described how forthcoming HSV-2 prevalence data could help identify the potential size of HIV epidemics in MENA. Our literature review documents low levels of HSV-2 infection in the general population suggesting that although sexual risk practices are present, they may be at low levels compared to other regions (Table 2). Low levels of cervical cancer (Abu-Raddad et al. 2010a; Drain et al. 2002), which suggests low circulating levels of oncogenic human papilloma virus, as well as the very low HIV prevalence among the general population (Abu-Raddad et al. 2010a), attest to this conclusion. Yet, HSV-2 prevalence appears to be considerably higher among core groups for sexually transmitted infections, indicating a higher level of risk behavior.
MENA populations, for cultural reasons, are unlikely to self-identify as high risk for STIs or HIV infection. Tables 1, 3, and 4 illustrate how HSV-2 prevalence can identify populations with elevated sexual risk behavior such as MSM, FSWs, “bar girls”, and STD clinic attendees. In order to prioritize the limited HIV prevention resources in this region, it is critical to finely map elevated risk behaviors. In Pakistan, surveillance work among different types of MSWs shows widely variable HSV-2 prevalence, but very limited HIV prevalence (Table 5) (Hawkes et al. 2009). The khusra MSWs (often known as hijra) have much higher HSV-2 prevalence than the bantha or khotki MSWs, and must be prioritized for HIV prevention interventions. Though HIV prevalence continues to be low among hijra MSWs in Pakistan (Hawkes et al. 2009; Pakistan National AIDS Control Program 2005; Pakistan National AIDS Control Program 2006-07; Pakistan National AIDS Control Program 2008), it is probably only a matter of few years before a substantial upsurge in HIV incidence occurs in this population leading to a prevalence that possibly could reach 20% (Figure 2A). Recent rapid rise in HIV prevalence among hijras in Pakistan affirms this conjecture (HIV prevalence increased from 0.8% in 2005 to 1.8% in 2006, to 6.4% in 2008 (Pakistan National AIDS Control Program 2005; Pakistan National AIDS Control Program 2006-07; Pakistan National AIDS Control Program 2008)). Meanwhile, HIV prevalence is more likely to stay at low levels among bantha and FSWs where HSV-2 prevalence is below 10% (Figure 2B). Any group in MENA with high HSV-2 seroprevalence should be prioritized for HIV prevention interventions.
Table 3.
HSV-2 prevalence in different population groups in a study from Syria (Ibrahim et al. 2000).
| Population | Population size | Prevalence HSV-2 |
|---|---|---|
| Healthy men | 305 | 0.3% |
| Healthy women | 349 | 0.0% |
| Pregnant women | 55 | 0.0% |
| Neonates | 101 | 0.0% |
| Sexually transmitted disease patients | 21 | 9.5% |
| Cervical cancer patients | 51 | 8.0% |
| Bargirls | 125 | 20.0% |
| Female sex workers | 101 | 34.0% |
Table 4.
HSV-2 prevalence in different population groups in a study from Turkey (Dolar et al. 2006).
| Population | Population size | Prevalence of HSV-2 |
|---|---|---|
| Sexually active adults | 725 | 4.8% |
| Pregnant women | 300 | 5.0% |
| Blood donors | 200 | 5.5% |
| Patients with genital warts | 110 | 17.3% |
| Hotel staff | 264 | 8.3% |
| Female sex workers | 483 | 60.0% |
Table 5.
Prevalence of HSV-2 and HIV in different high-risk populations in a study from Pakistan (Hawkes et al. 2009).
| Type of population | Rawalpindi | Abbottabad | ||
|---|---|---|---|---|
| HSV-2 prevalence (%) | HIV prevalence (%) | HSV-2 prevalence (%) | HIV prevalence (%) | |
| Bantha MSWs | 7.4 | 0.5 | 2.5 | 0 |
| Khotki MSWs | 14 | 0 | 25 | 0 |
| Khusra MSWs | 54 | 2.4 | 31.3 | 0 |
| FSWs | 8.0 | 0 | 4.7 | 0 |
Despite overall low levels of HSV-2 prevalence, there is considerable heterogeneity with some sub-regions showing higher prevalence (such as Sudan and Morocco). Age stratified analyses of a Moroccan cohort reveals a typical increase in prevalence with age, though infection appears to be less commonly acquired during adolescence than in other regions of the world (Cowan et al. 2003; Smith et al. 2002). Increasing prevalence in successive age cohorts reflects cumulative acquisition of HSV-2 through adulthood. Men in particular could be engaging in repeated episodes of high-risk practices throughout their sexual activity lifespan, such as contacts with FSWs. An alternative explanation might be a changing likelihood of successive birth cohorts to be exposed to HSV-2 infection during different eras (Burchell et al. 2006), or a low force of infection that increases average age at infection (Anderson et al. 1991). The former explanation seems unlikely as recent trends suggest increasing sexual risk behavior in the young population (Abu-Raddad et al. 2010a).
HSV-2 research and trends in sexual risk behavior in MENA
The use of age cohorts will be critical to study the nature of HSV-2 prevalence in MENA. Rates of HSV-2 prevalence often change most rapidly among adolescents and young adults, suggesting that HSV-2 can be used to gauge recent changes in risk behavior among young age cohorts (Obasi et al. 1999; Slomka 1996; van de Laar et al. 2001; Xu et al. 2006). MENA is characterized by a massive youth bulge. One-fifth of the population, 95 million people, are in the 15-24 years age group (Assaad et al. 2007; Roudi-Fahimi et al. 2008; UNAIDS RST MENA 2007) which is normally the age of initiation of sexual activities (Roudi-Fahimi et al. 2008). This youth cohort represents the largest potentially-vulnerable population to HIV in MENA (Assaad et al. 2007). Youth are experiencing high rates of unemployment, delayed marital age, increased premarital sex, conflict morbidity, increased mobility, peer pressure to engage in risk behavior, and changing lifestyle norms (Busulwa 2003; UNAIDS RST MENA 2007; UNAIDS RST MENA 2008). Population-based data of HSV-2 among youth collected sequentially in time would be valuable to determine trends of sexual risk behavior.
Epidemic growth and endemicity of HSV-2 and HIV infections
Though overall HSV-2 transmission probability per coital act during shedding is larger than that of HIV (Abu-Raddad et al. 2008b; Wald et al. 2006; Wald et al. 2001), HSV-2 transmission occurs only during HSV-2 primary infection and reactivations, thereby slowing growth of HSV- 2 epidemics. HIV transmission probability is considerable throughout the natural history of infection (Wawer et al. 2005) allowing for more rapid epidemic expansion. However, the infectious period for HIV is limited by disease mortality after about ten years, whereas the infectious period of HSV-2 persists for the duration of sexual activity of the infected host. Therefore, HIV cannot sustain as high a prevalence as that of HSV-2 in the population. HSV-2 prevalence reaches high levels but may need as much as several decades to reach its peak. Once HSV-2 reaches its endemic saturation, the HSV-2 force of infection becomes substantially larger than that of HIV and it is much more likely for a susceptible entering the sexually active population to be infected with HSV-2 prior to HIV.
There are no longitudinal data of HSV-2 prevalence in MENA and it is not known whether HSV-2 prevalence has saturated its epidemic potential among the different populations in the region. Based on data from other regions (Korenromp et al. 2002a; Korenromp et al. 2002b; Korenromp et al. 2002c; O'Farrell 1999; Smith et al. 2002; Weiss 2004), HSV-2 prevalence may have reached endemic levels within MSM in MENA. In most of Africa for example, the HSV-2 epidemic started no later than the first half of the twentieth century (Nahmias et al. 1990), and reached its peak prior to the HIV epidemic (Abu-Raddad et al. 2008b). However some high-risk populations in MENA have HSV-2 prevalence measures (Table 1) that are considerably lower than those in corresponding populations in other regions (Corey et al. 2004; O'Farrell 1999; Smith et al. 2002; Weiss 2004). One cannot exclude that HSV-2 could be in the midst of early epidemic expansion in some high-risk populations in MENA.
If HSV-2 has only recently been introduced into a high-risk population in MENA, its use as a proxy to map levels of existing risk behaviors may be limited. Indeed, if risk behavior is increasing within certain subpopulations in MENA, then this may allow for conditions that promote both HSV-2 and HIV expansion. Because our model suggests that HSV-2 prevalence is best predictive of HIV spread when an endemic HSV-2 level is established, serial measures of HSV-2 prevalence will be necessary to rule out an enlarging HSV-2 epidemic. A related conclusion of our simulations among MSM is that a cross-sectional study that reveals an HIV-1 prevalence greater than or equivalent to that of HSV-2 suggests that both HSV-2 and HIV prevalence may be in an expansion phase.
Potential study limitations and limitations of available HSV-2 evidence in MENA
Our study has several limitations. First, our model's quantitative predictions are generated for a specific sexual risk contact structure and cannot be generalized for all populations. Sexual networks vary in structure (Ghani et al. 2000; Morris 1997) and this variation may affect HIV and HSV-2 infectious spread differently. It is not our aim to explore the complexity of links between HIV and HSV-2 epidemiology. For this reason, we also did not include biologic interactions between HSV-2 and HIV in our model. Rather, we explored generic conclusions regarding the predictive nature of HSV-2 prevalence as a measure of risk for HIV epidemic propagation. These findings do not apply for an HIV epidemic fueled by non-sexual modes of transmission such as injecting drug use. We also did not incorporate overlap of risk behaviors such as part of the MSM population engaging in sexual risk behavior and injecting drug use at the same time. Existing evidence in MENA suggests considerable overlap of risk factors between different risk groups (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b). For example, often as much as 15% of MSM inject drugs and 30% report anal sex with injecting drug users (Mumtaz et al. 2010).
HSV-2 seroprevalence is a marker of lifetime sexual risk, not necessarily of current risk behaviors, and therefore HSV-2 prevalence may reflect current or recent risk behavior only among youth (Obasi et al. 1999; Slomka 1996; van de Laar et al. 2001; Xu et al. 2006). However, this limitation should not affect the utility of using HSV-2 prevalence as a powerful marker of risk behavior and HIV epidemic potential in MENA. The populations for which this methodology is suggested are either young in age (high risk populations), or populations with variable age structure (general population) but whose HSV-2 prevalence appears, given the above data, to be at low levels. A low HSV-2 prevalence in a population suggests that this population, irrespective of its age structure, may not have practiced high levels of sexual risk behaviors in the past.
Multiple studies on MSM in MENA in different countries have shown that the majority of sexually active MSM are young in their 20s and that these young MSM changed their sexual partners most often (Abu-Raddad et al. 2010a; El-Rahman 2004; El-Sayyed et al. 2008; Elrashied 2006; Mishwar 2008). In Pakistan, MSWs had an average age of 22.3 (Pakistan National AIDS Control Program 2005), 21.3 (Pakistan National AIDS Control Program 2006-07), and 21.7 (Pakistan National AIDS Control Program 2008) years, and started commercial sex at an average age of 16.9 (Pakistan National AIDS Control Program 2005), 15.9 (Pakistan National AIDS Control Program 2006-07), and 16.2 (Pakistan National AIDS Control Program 2008) years. Furthermore, the vast majority of FSWs in MENA have been identified in multiple studies in different countries to be well below age 30, and young FSWs most often had the highest client volume (Abu-Raddad et al. 2010a; ACCORD 2005; ACCORD 2006; Ati 2005; Syria National AIDS Programme 2004; Yousif 2006). Lastly, multiple studies in different countries show also that the dominant profile of IDUs, who engage in multiple sexual risk behaviors (Abu-Raddad et al. 2010a; Abu-Raddad et al. 2010b), is that of young men raised in large families shattered by unemployment, economic hardship, and urban-rural migration (Abu-Raddad et al. 2010a; Afifi et al. 2004; Bolhari et al. 2002; Michael et al. 2003; Mishwar 2008; 2006; Pakistan National AIDS Control Program 2005; Pakistan National AIDS Control Program 2006-07; Shareef et al. 2006; Tiouiri et al. 1999; Zamani et al. 2006).
We assumed no enhanced acquisition or transmission probability of one infection due to the biology of the other infection in order to disentangle sexual risk behavior from biological interactions of HIV and HSV-2. In light of the fact that recent clinical trials have failed to document effecpts of HSV-2 suppressive therapy on HIV incidence, the degree of epidemiological synergy between HSV-2 and HIV-1 incidence remains uncertain (Celum et al. 2008; Lingappa et al. 2010; Watson-Jones et al. 2008). If such biological synergy between HIV and HSV-2 exists (Abu-Raddad et al. 2008b), it can affect epidemic spread and heterogeneity of disease burden, but at the same time, it would strengthen our argument for using HSV-2 as a predictor of the HIV epidemic potential since HSV-2 prevalence would reflect levels of risk behavior as well as the additional biological susceptibility to HIV infection arising from the biology of HSV-2 infection. HIV mortality may also affect, as a matter of principle, HSV-2 prevalence complicating the relationship between the epidemiology of the two infections. However earlier work has suggested that this effect is very minor (Abu-Raddad et al. 2008b).
Our focus in this article was on the qualitative findings linking the epidemiology of HIV and HSV-2 rather than on precise quantitative estimates. Therefore we did not incorporate uncertainty analyses on the parameters of the model. Incorporation of such analyses would produce a range of estimate around the point estimates of our predictions presented here. Though we presented results for an MSM population, our qualitative results are of general nature and apply to heterosexual sex networks. Heterogeneities in HIV transmission process due to variability in risk behavior, transmission probability and gender, can influence the distribution of the infection burden, but maintain the predictive effect of HSV-2 as a proxy of the risk of HIV acquisition for each sub-population as can be seen in Figure S2 of the Online Supporting Appendix.
Many HSV-2 seroprevalence studies to date in MENA suffer from methodological limitations due to use of inappropriate whole virus based assays that do not distinguish antibodies to HSV-2 from those against HSV-1. Due to the wide burden of HSV-1 infection in the region (Cowan et al. 2003; Smith et al. 2002), assays that accurately differentiate chronic HSV-1 from HSV-2 infection are imperative. HSV-1 infection is usually acquired during childhood through oral but not sexual contact, has a very high prevalence in MENA (Cowan et al. 2003; Smith et al. 2002), and shows extensive sequence homology with HSV-2 (Gentry et al. 1988). HSV-1 causes a high proportion of incident genital herpes in some populations due to oro-genital as opposed to purely genital transmission (Lafferty et al. 2000; Langenberg et al. 1999; Lowhagen et al. 2000; Nilsen et al. 2000; Ribes et al. 2001; Roberts et al. 2003; Scoular et al. 2002; Tran et al. 2004). This trend has been observed regionally only in Israel (Samra et al. 2003), but could become more widespread if there is a trend of increased oral sex. Clinical exam is of no utility in differentiating HSV-1 and HSV-2 lesions, and up to 90% of HSV-2 seropositive persons do not recall having genital herpes (Corey et al. 2000; Cowan et al. 1994; Fleming et al. 1997). For these reasons, monitoring of HSV-2 infection must include accurate serologic assays. Several available serological assays have high specificity and sensitivity, though their results still need to be validated by population with Western blot assay (WBA) because of variable performance characteristics in different regions (Ashley-Morrow et al. 2004; Ashley et al. 1999). For these reasons our classification of serology tests as excellent, good, fair or poor should be interpreted cautiously as assay utility may vary regionally.
Finally, HSV-1 infection could hypothetically have a protective effect against HSV-2 acquisition (Cowan et al. 1994; Mertz et al. 1985), thereby partially contributing to lower regional prevalence of HSV-2. However, evidence for a protective effect is conflicting (Brown et al. 1997; Cowan et al. 2003; Langenberg et al. 1999), and in many settings, high HSV-1 prevalence (Cowan et al. 2003; Smith et al. 2002) has not tainted the predictive power of HSV-2 as a proxy for sexual risk behavior. In fact, overwhelming evidence affirms the utility of HSV-2 as a marker of risk behavior irrespective of HSV-1 prevalence (Cowan et al. 2003; Cowan et al. 1994; Cunningham et al. 1993; Dan et al. 2003; Obasi et al. 1999; van de Laar et al. 2001). Near universal male circumcision coverage in MENA is also not likely to explain low HSV-2 prevalence as male circumcision reduces HSV-2 sero-incidence by at most 30% (Bailey 2007; Tobian et al. 2009; Weiss et al. 2006).
Summary of findings
In conclusion, we identify HSV-2 serology as a powerful marker of sexual risk behavior that should be a standard component in any proposed HIV surveillance efforts in MENA. This is particularly true for studies incorporating integrated bio-behavioral surveillances surveys among high-risk populations. At present, HSV-2 prevalence in the general population in MENA is among the lowest globally (Table 2) (O'Farrell 1999; Paz-Bailey et al. 2007; Pebody et al. 2004; Smith et al. 2002; Weiss 2004). This provides an indication that sexual risk behavior in the general population in MENA is low, and that HIV infection will have limited inroads into this population. Yet, HSV-2 prevalence levels in MENA populations with identifiable risk behaviors are substantial and on occasion comparable to those in other regions (Smith et al. 2002). Our model suggests that in a population where HSV-2 is already at a relatively high endemic level, HIV prevalence may continue at low levels for many years. However, there exists potential for rapid exponential spread. Such populations should be a priority for early public health interventions including surveillance mechanisms for more intensive data collection to track potential for further spread.
Supplementary Material
Acknowledgments
The MENA HIV/AIDS Epidemiology Synthesis Project was funded through a joint partnership of the World Bank, the MENA Regional Support Team (RST) of United Nations Programme on HIV/AIDS (UNAIDS), and the Eastern Mediterranean Regional Office (EMRO) of the World Health Organization (WHO). LJA and GM are also grateful for the Qatar National Research Fund for supporting this work (NPRP 08-068-3-024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abu-Raddad L, Akala FA, Semini I, Riedner G, Wilson D, Tawil O, Fall F, Victor-Ahuchogu J. Characterizing the HIV/AIDS epidemic in the Middle East and North Africa. Time for Strategic Action. Middle East and North Africa HIV/AIDS Epidemiology Synthesis Project. World Bank/UNAIDS/WHO. 2010a. [Google Scholar]
- Abu-Raddad LJ, Hilmi N, Mumtaz G, Benkirane M, Akala FA, Riedner G, Tawil O, Wilson D. Epidemiology of HIV infection in the Middle East and North Africa. AIDS. 2010b;24(Suppl 2):S5–23. doi: 10.1097/01.aids.0000386729.56683.33. [DOI] [PubMed] [Google Scholar]
- Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008a;22(9):1055–1061. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- Abu-Raddad LJ, Magaret AS, Celum C, Wald A, Longini IM, Jr, Self SG, Corey L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE. 2008b;3(5):e2230. doi: 10.1371/journal.pone.0002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314(5805):1603–1606. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- Abuharfeil N, Meqdam MM. Seroepidemiologic study of herpes simplex virus type 2 and cytomegalovirus among young adults in northern Jordan. New Microbiol. 2000;23(3):235–239. [PubMed] [Google Scholar]
- ACCORD. Socio Economic Research on HIV/AIDS Prevention among Informal Sex Workers. Agency for Co-operation and Research in Development Federal Ministry of Health, Sudan National AIDS Control Program, and the World Health Organization. 2005. [Google Scholar]
- ACCORD. Qualitative Socio Economic Research on Female Sex Workers and their Vulnerability to HIV/AIDS in Khartoum State. Agency for Co-operation and Research in Development. 2006. [Google Scholar]
- Afifi M, El-Sousi S. Drug Abuse and Related Behaviors among High School Student Children in Palestine Authority (2002-2004) 2004. [Google Scholar]
- Ahmed HJ, Mbwana J, Gunnarsson E, Ahlman K, Guerino C, Svensson LA, Mhalu F, Lagergard T. Etiology of genital ulcer disease and association with human immunodeficiency virus infection in two tanzanian cities. Sex Transm Dis. 2003;30(2):114–119. doi: 10.1097/00007435-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Aksözek A, Özdamar M, Ergin S. Comparison of three commercial glycoprotein G-based enzyme immunoassays for detection of HSV-1/HSV-2 IgG antibodies in pregnant women and sex workers. Clinical Microbiology and Infection. 2004;11:216. [Google Scholar]
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford: Oxford University Press; 1991. [Google Scholar]
- Ashley-Morrow R, Nollkamper J, Robinson NJ, Bishop N, Smith J. Performance of focus ELISA tests for herpes simplex virus type 1 (HSV-1) and HSV-2 antibodies among women in ten diverse geographical locations. Clin Microbiol Infect. 2004;10(6):530–536. doi: 10.1111/j.1469-0691.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- Ashley R, Cent A, Maggs V, Nahmias A, Corey L. Inability of enzyme immunoassays to discriminate between infections with herpes simplex virus types 1 and 2. Ann Intern Med. 1991;115(7):520–526. doi: 10.7326/0003-4819-115-7-520. [DOI] [PubMed] [Google Scholar]
- Ashley RL. Genital herpes. Type-specific antibodies for diagnosis and management. Dermatol Clin. 1998;16(4):789–793. xiii–xiv. doi: 10.1016/s0733-8635(05)70048-6. [DOI] [PubMed] [Google Scholar]
- Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26(4):662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley RL, Wald A. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin Microbiol Rev. 1999;12(1):1–8. doi: 10.1128/cmr.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley RL, Wu L, Pickering JW, Tu MC, Schnorenberg L. Premarket evaluation of a commercial glycoprotein G-based enzyme immunoassay for herpes simplex virus type-specific antibodies. J Clin Microbiol. 1998;36(1):294–295. doi: 10.1128/jcm.36.1.294-295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad R, Roudi-Fahimi F, Bureau PR. Youth in the Middle East and North Africa: Demographic Opportunity Or Challenge? Population Reference Bureau 2007 [Google Scholar]
- Ati HA. HIV/AIDS/STIs Social and Geographical Mapping of Prisoners, Tea Sellers and Commercial Sex Workers in Port Sudan Town, Red Sea State. Ockenden International, Sudan. Draft 2. 2005. [Google Scholar]
- Bailey R. Scaling up circumcision Programmes: The Road from Evidence to Practice; 4th IAS Conference on HIV Pathogenesis, Treatment & Prevention; July 22-25 2007; Sydney, Australia. 2007. [Google Scholar]
- Bogaerts J, Ahmed J, Akhter N, Begum N, Rahman M, Nahar S, Van Ranst M, Verhaegen J. Sexually transmitted infections among married women in Dhaka, Bangladesh: unexpected high prevalence of herpes simplex type 2 infection. Sex Transm Infect. 2001;77(2):114–119. doi: 10.1136/sti.77.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon J. Science in Libya. From pariah to science powerhouse? Science. 2005;308(5719):182–184. doi: 10.1126/science.308.5719.182. [DOI] [PubMed] [Google Scholar]
- Boily MC, Masse B. Mathematical models of disease transmission: a precious tool for the study of sexually transmitted diseases. Can J Public Health. 1997;88(4):255–265. doi: 10.1007/BF03404793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhari J, Mirzamani SM. Assessment of Substance Abuse in Iran's Prisons. United Nations Drug Control Program in Cooperation with the Drug Control Headquarters. UNDCP. 2002. [Google Scholar]
- Brown ZA, Selke S, Zeh J, Kopelman J, Maslow A, Ashley RL, Watts DH, Berry S, Herd M, Corey L. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337(8):509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- Brunham RC, Plummer FA. A general model of sexually transmitted disease epidemiology and its implications for control. Med Clin North Am. 1990;74(6):1339–1352. doi: 10.1016/s0025-7125(16)30484-9. [DOI] [PubMed] [Google Scholar]
- Burchell AN, Winer RL, de Sanjose S, Franco EL. Chapter 6: Epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Busulwa R. HIV/AIDS Situation Analysis study, conducted in Hodeidah, Taiz and Hadhramut, Republic of Yemen.The Detailed Report of the Findings. 2003. [Google Scholar]
- Buve A, Carael M, Hayes RJ, Auvert B, Ferry B, Robinson NJ, Anagonou S, Kanhonou L, Laourou M, Abega S, Akam E, Zekeng L, Chege J, Kahindo M, Rutenberg N, Kaona F, Musonda R, Sukwa T, Morison L, Weiss HA, Laga M. The multicentre study on factors determining the differential spread of HIV in four African cities: summary and conclusions. Aids. 2001;15(Suppl 4):S127–131. doi: 10.1097/00002030-200108004-00014. [DOI] [PubMed] [Google Scholar]
- Caldwell C. Quiggin (1989). J. Caldwell, P. Caldwell and P. Quiggin, The social context of AIDS in Sub-Saharan Africa. Population and Development Review. 1989;15(2):185–234. [Google Scholar]
- Catania JA, Gibson DR, Chitwood DD, Coates TJ. Methodological problems in AIDS behavioral research: influences on measurement error and participation bias in studies of sexual behavior. Psychol Bull. 1990;108(3):339–362. doi: 10.1037/0033-2909.108.3.339. [DOI] [PubMed] [Google Scholar]
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany-Moretlwe S, Cowan F, Casapia M, Ortiz A, Fuchs J, Buchbinder S, Koblin B, Zwerski S, Rose S, Wang J, Corey L. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9630):2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz AT, Kendi O, Kiyan M, Bilge Y, Ugurel S, Tumer AR. Detection of herpes simplex virus 2 (HSV) IgG and IgM using ELISA in transsexuals and homosexuals. Mikrobiyol Bul. 1992;26(1):41–49. [PubMed] [Google Scholar]
- Cengiz AT, Kendi O, Kiyan M, Bilge Y, Ugurel S, Tumer AR. Demonstration of herpes simplex virus (HSV)-2 IgG and IgM using ELISA in transsexuals and homosexuals. Mikrobiyol Bul. 1993a;27(1):46–51. [PubMed] [Google Scholar]
- Cengiz L, Kiyan M, Cengiz AT, Kara F, Ugurel MS. Detection of herpes simplex virus 1 and 2 (HSV-1 and HSV-2) IgG and IgM by ELISA in cord blood and sera of mothers with pregnancy complications. Mikrobiyol Bul. 1993b;27(4):299–307. [PubMed] [Google Scholar]
- Corey L, Handsfield HH. Genital herpes and public health: addressing a global problem. Jama. 2000;283(6):791–794. doi: 10.1001/jama.283.6.791. [DOI] [PubMed] [Google Scholar]
- Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35(5):435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- Cowan FM, French RS, Mayaud P, Gopal R, Robinson NJ, de Oliveira SA, Faillace T, Uuskula A, Nygard-Kibur M, Ramalingam S, Sridharan G, El Aouad R, Alami K, Rbai M, Sunil-Chandra NP, Brown DW. Seroepidemiological study of herpes simplex virus types 1 and 2 in Brazil, Estonia, India, Morocco, and Sri Lanka. Sex Transm Infect. 2003;79(4):286–290. doi: 10.1136/sti.79.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan FM, Johnson AM, Ashley R, Corey L, Mindel A. Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. Bmj. 1994;309(6965):1325–1329. doi: 10.1136/bmj.309.6965.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Lee FK, Ho DW, Field PR, Law CL, Packham DR, McCrossin ID, Sjogren-Jansson E, Jeansson S, Nahmias AJ. Herpes simplex virus type 2 antibody in patients attending antenatal or STD clinics. Med J Aust. 1993;158(8):525–528. doi: 10.5694/j.1326-5377.1993.tb121867.x. [DOI] [PubMed] [Google Scholar]
- Dan M, Sadan O, Glezerman M, Raveh D, Samra Z. Prevalence and risk factors for herpes simplex virus type 2 infection among pregnant women in Israel. Sex Transm Dis. 2003;30(11):835–838. doi: 10.1097/01.OLQ.0000086608.07893.1A. [DOI] [PubMed] [Google Scholar]
- Dolar N, Serdaroglu S, Yilmaz G, Ergin S. Seroprevalence of herpes simplex virus type 1 and type 2 in Turkey. J Eur Acad Dermatol Venereol. 2006;20(10):1232–1236. doi: 10.1111/j.1468-3083.2006.01766.x. [DOI] [PubMed] [Google Scholar]
- Drain PK, Holmes KK, Hughes JP, Koutsky LA. Determinants of cervical cancer rates in developing countries. Int J Cancer. 2002;100(2):199–205. doi: 10.1002/ijc.10453. [DOI] [PubMed] [Google Scholar]
- Duran N. Serological Evaluation of HSV-1 and HSV-2 Infection In Pregnancy. Tr J of Med Sci. 2004;34:97–101. [Google Scholar]
- Duran N, Yarkin F, Evruke C, Koksal F. Asymptomatic herpes simplex virus type 2(HSV-2) infection among pregnant women in Turkey. Indian J Med Res. 2004;120(2):106–110. [PubMed] [Google Scholar]
- Egypt Ministry of Health and Population National AIDS Program. HIV/AIDS biological and behavioral surveillance survey, Summary report Egypt 2006. 2006. [Google Scholar]
- Eing BR, Lippelt L, Lorentzen EU, Hafezi W, Schlumberger W, Steinhagen K, Kuhn JE. Evaluation of confirmatory strategies for detection of type-specific antibodies against herpes simplex virus type 2. J Clin Microbiol. 2002;40(2):407–413. doi: 10.1128/JCM.40.2.407-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Rahman A. Risky behaviours for HIV/AIDS infection among a sample of homosexuals in Cairo city, Egypt. XV International AIDS Conference; Bangkok. 11-16 July.2004. Abstract WePeC6146. [Google Scholar]
- el-Sayed Zaki M, Goda H. Relevance of parvovirus B19, herpes simplex virus 2, and cytomegalovirus virologic markers in maternal serum for diagnosis of unexplained recurrent abortions. Arch Pathol Lab Med. 2007;131(6):956–960. doi: 10.5858/2007-131-956-ROPBHS. [DOI] [PubMed] [Google Scholar]
- El-Sayyed N, Kabbash IA, El-Gueniedy M. Risk behaviours for HIV/AIDS infection among men who have sex with men in Cairo, Egypt. East Mediterr Health J. 2008;14(4):905–915. [PubMed] [Google Scholar]
- Elrashied SM. Generating Strategic Information and assessing HIV/AIDS Knowledge, Attitude and Behaviour and Practices as well as Prevalence of HIV1 among MSM in Khartoum State, 2005. A draft report subitted to Sudan National AIDS Control Programme. Together Against AIDS Organization (TAG). Khartoum, Sudan. 2006. [Google Scholar]
- Farjadian S, Asadi E, Doroudchi M, Dehaghani AS, Tabei SZ, Kumar VP, Ghaderi A. High risk HPV types in southern Iranian patients with cervical cancer. Pathol Oncol Res. 2003;9(2):121–125. doi: 10.1007/BF03033756. [DOI] [PubMed] [Google Scholar]
- Feldman PA, Steinberg J, Madeb R, Bar G, Nativ O, Tal J, Srugo I. Herpes simplex virus type 2 seropositivity in a sexually transmitted disease clinic in Israel. Isr Med Assoc J. 2003;5(9):626–628. [PubMed] [Google Scholar]
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, Lee FK, St Louis ME. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337(16):1105–1111. doi: 10.1056/NEJM199710163371601. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Garnett GP, Anderson RM. Factors controlling the spread of HIV in heterosexual communities in developing countries: patterns of mixing between different age and sexual activity classes. Philos Trans R Soc Lond B Biol Sci. 1993;342(1300):137–159. doi: 10.1098/rstb.1993.0143. [DOI] [PubMed] [Google Scholar]
- Gentry GA, Lowe M, Alford G, Nevins R. Sequence analyses of herpesviral enzymes suggest an ancient origin for human sexual behavior. Proc Natl Acad Sci U S A. 1988;85(8):2658–2661. doi: 10.1073/pnas.85.8.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghani AC, Garnett GP. Risks of acquiring and transmitting sexually transmitted diseases in sexual partner networks. Sex Transm Dis. 2000;27(10):579–587. doi: 10.1097/00007435-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Ghazi HO, Telmesani AM, Mahomed MF. TORCH agents in pregnant Saudi women. Med Princ Pract. 2002;11(4):180–182. doi: 10.1159/000065813. [DOI] [PubMed] [Google Scholar]
- Gray PB. HIV and Islam: is HIV prevalence lower among Muslims? Soc Sci Med. 2004;58(9):1751–1756. doi: 10.1016/S0277-9536(03)00367-8. [DOI] [PubMed] [Google Scholar]
- Gul U, Kilic A, Sakizligil B, Aksaray S, Bilgili S, Demirel O, Erinckan C. Magnitude of sexually transmitted infections among female sex workers in Turkey. J Eur Acad Dermatol Venereol. 2008;22(9):1123–1124. doi: 10.1111/j.1468-3083.2007.02548.x. [DOI] [PubMed] [Google Scholar]
- Hawkes S, Collumbien M, Platt L, Lalji N, Rizvi N, Andreasen A, Chow J, Muzaffar R, ur-Rehman H, Siddiqui N, Hasan S, Bokhari A. HIV and other sexually transmitted infections among men, transgenders and women selling sex in two cities in Pakistan: a cross-sectional prevalence survey. Sex Transm Infect. 2009;85(Suppl 2):ii8–16. doi: 10.1136/sti.2008.033910. [DOI] [PubMed] [Google Scholar]
- Holt BY, Effler P, Brady W, Friday J, Belay E, Parker K, Toole M. Planning STI/HIV prevention among refugees and mobile populations: situation assessment of Sudanese refugees. Disasters. 2003;27(1):1–15. doi: 10.1111/1467-7717.00216. [DOI] [PubMed] [Google Scholar]
- Ibrahim AI, Kouwatli KM, Obeid MT. Frequency of herpes simplex virus in Syria based on type-specific serological assay. Saudi Med J. 2000;21(4):355–360. [PubMed] [Google Scholar]
- Jahani MR, Kheirandish P, Hosseini M, Shirzad H, Seyedalinaghi SA, Karami N, Valiollahi P, Mohraz M, McFarland W. HIV seroconversion among injection drug users in detention, Tehran, Iran. AIDS. 2009;23(4):538–540. doi: 10.1097/QAD.0b013e3283269e3c. [DOI] [PubMed] [Google Scholar]
- Kaiser R, Kedamo T, Lane J, Kessia G, Downing R, Handzel T, Marum E, Salama P, Mermin J, Brady W, Spiegel P. HIV, syphilis, herpes simplex virus 2, and behavioral surveillance among conflict-affected populations in Yei and Rumbek, southern Sudan. Aids. 2006;20(6):942–944. doi: 10.1097/01.aids.0000218564.20521.51. [DOI] [PubMed] [Google Scholar]
- Karam W, Aftimos G, Jurjus A, Khairallah S, Bedrossian N. Prevalence of sexually transmitted infections in Lebanese women as revealed by Pap smear cytology: A cross sectional study from 2002-2006. WHO/EMRO. 2007. [PubMed] [Google Scholar]
- Kasraeian M, Movaseghii M, Ghiam AF. Seroepidemiological Study of Herpes Simplex Virus Type 2 (HSV-2) Antibody in Shiraz, Iran. [Google Scholar]
- Kelley LM, Eberstadt N. The Muslim face of AIDS. Foreign Policy. 2005;149:42–48. [Google Scholar]
- Khattabi H, Alami K. Surveillance sentinelle du VIH, Resultats 2004 et tendance de la seroprevalence du VIH. Ministry of health. UNAIDS Morocco. 2005. [Google Scholar]
- Khawaja ZA, Gibney L, Ahmed AJ, Vermund SH. HIV/AIDS and its risk factors in Pakistan. Aids. 1997;11(7):843–848. doi: 10.1097/00002030-199707000-00002. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Bakker R, de Vlas SJ, Gray RH, Wawer MJ, Serwadda D, Sewankambo NK, Habbema JD. HIV dynamics and behaviour change as determinants of the impact of sexually transmitted disease treatment on HIV transmission in the context of the Rakai trial. Aids. 2002a;16(16):2209–2218. doi: 10.1097/00002030-200211080-00014. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Bakker R, De Vlas SJ, Robinson NJ, Hayes R, Habbema JD. Can behavior change explain increases in the proportion of genital ulcers attributable to herpes in sub-Saharan Africa? A simulation modeling study. Sex Transm Dis. 2002b;29(4):228–238. doi: 10.1097/00007435-200204000-00008. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Bakker R, Gray R, Wawer MJ, Serwadda D, Habbema JD. The effect of HIV, behavioural change, and STD syndromic management on STD epidemiology in sub-Saharan Africa: simulations of Uganda. Sex Transm Infect. 2002c;78(Suppl 1):i55–63. doi: 10.1136/sti.78.suppl_1.i55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar M, Morris M. Measures of concurrency in networks and the spread of infectious disease. Mathematical Biosciences. 1996;133(2):165–195. doi: 10.1016/0025-5564(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Kriitmaa K, Testa A, Osman M, Bozicevic I, Riedner G, Malungu J, Irving G, Abdalla I. HIV prevalence and characteristics of sex work among female sex workers in Hargeisa, Somaliland, Somalia. AIDS. 2010;24(Suppl 2):S61–67. doi: 10.1097/01.aids.0000386735.87177.2a. [DOI] [PubMed] [Google Scholar]
- Lafferty WE, Downey L, Celum C, Wald A. Herpes simplex virus type 1 as a cause of genital herpes: impact on surveillance and prevention. J Infect Dis. 2000;181(4):1454–1457. doi: 10.1086/315395. [DOI] [PubMed] [Google Scholar]
- Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N Engl J Med. 1999;341(19):1432–1438. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- Lee RM, Renzetti CM. The problems of researching sensitive topics. American Behavioral Scientist. 1990;33(5):510–528. [Google Scholar]
- Lenton C. Will Egypt escape the AIDS epidemic? Lancet. 1997;349(9057):1005. doi: 10.1016/s0140-6736(05)62906-6. [DOI] [PubMed] [Google Scholar]
- Lingappa JR, Baeten JM, Wald A, Hughes JP, Thomas KK, Mujugira A, Mugo N, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, de Bruyn G, Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Magaret AS, Wang RS, Kidoguchi L, Barnes L, Ridzon R, Corey L, Celum C. Daily aciclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet. 2010;375(9717):824–833. doi: 10.1016/S0140-6736(09)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ. 2008;86(10):805–812, A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowhagen GB, Tunback P, Andersson K, Bergstrom T, Johannisson G. First episodes of genital herpes in a Swedish STD population: a study of epidemiology and transmission by the use of herpes simplex virus (HSV) typing and specific serology. Sex Transm Infect. 2000;76(3):179–182. doi: 10.1136/sti.76.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfoud Z, Afifi R, Ramia S, El Khoury D, Kassak K, El Barbir F, Ghanem M, El- Nakib M, DeJong J. HIV/AIDS among female sex workers, injecting drug users and men who have sex with men in Lebanon: results of the first biobehavioral surveys. AIDS. 2010;24(Suppl 2):S45–54. doi: 10.1097/01.aids.0000386733.02425.98. [DOI] [PubMed] [Google Scholar]
- Maral I, Biri A, Korucuoglu U, Bakar C, Cirak M, Ali Bumin M. Seroprevalences of herpes simplex virus type 2 and Chlamydia trachomatis in Turkey. Arch Gynecol Obstet. 2009 doi: 10.1007/s00404-009-0998-z. [DOI] [PubMed] [Google Scholar]
- Marcelin AG, Grandadam M, Flandre P, Koeck JL, Philippon M, Nicand E, Teyssou R, Agut H, Huraux JM, Dupin N. Comparative study of heterosexual transmission of HIV-1, HSV-2 and KSHV in Djibouti. 2001. [Google Scholar]
- Marcelin AG, Grandadam M, Flandre P, Nicand E, Milliancourt C, Koeck JL, Philippon M, Teyssou R, Agut H, Dupin N, Calvez V. Kaposi's sarcoma herpesvirus and HIV-1 seroprevalences in prostitutes in Djibouti. J Med Virol. 2002;68(2):164–167. doi: 10.1002/jmv.10184. [DOI] [PubMed] [Google Scholar]
- Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang ML, Corey L. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis. 2008;198(8):1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz GJ, Schmidt O, Jourden JL, Guinan ME, Remington ML, Fahnlander A, Winter C, Holmes KK, Corey L. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985;12(1):33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- Mertz KJ, Trees D, Levine WC, Lewis JS, Litchfield B, Pettus KS, Morse SA, St Louis ME, Weiss JB, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald GA, Novotny J, Weisfuse I, Goldberg M, O'Donnell JA, Knaup R. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. The Genital Ulcer Disease Surveillance Group. J Infect Dis. 1998;178(6):1795–1798. doi: 10.1086/314502. [DOI] [PubMed] [Google Scholar]
- Michael T, Ahmed M, Lemma W. HIV/AIDS behavioral surveillance survey (BSS): round one, Djibouti, Ministry of Health. 2003. [Google Scholar]
- Mir AM, Wajid A, Reichenbach L, Khan M. STI prevalence and associated factors among urban men in Pakistan. Sex Transm Infect. 2009;85(3):199–200. doi: 10.1136/sti.2008.034165. [DOI] [PubMed] [Google Scholar]
- Mishwar . An integrated bio-behavioral surveillance study among four vulnerabl groups in lebanon: men who have sex with men; prisoners; commercial sex workers and intravenous drug users. Final Report. November 2008. 2008. [Google Scholar]
- Morris M. Telling tails explain the discrepancy in sexual partner reports. Nature. 1993;365(6445):437–440. doi: 10.1038/365437a0. [DOI] [PubMed] [Google Scholar]
- Morris M. Sexual networks and HIV. Aids 11. 1997:S209–S216. [PubMed] [Google Scholar]
- Morse SA. Etiology of genital ulcer disease and its relationship to HIV infection. Sex Transm Dis. 1999;26(1):63–65. doi: 10.1097/00007435-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Mumtaz G, Hilmi N, Kaplan R, Akala FA, Semini I, Riedner G, Tawil O, Wilson D, Abu-Raddad LJ. Nascent HIV epidemics among men who have sex with men appear to be emerging in the Middle East and North Africa. 2010. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke NJ, Bernsen RM, Sgaier SK, Jha P. Body mass index, sexual behaviour, and sexually transmitted infections: an analysis using the NHANES 1999-2000 data. BMC Public Health. 2006;6:199. doi: 10.1186/1471-2458-6-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmias AJ, Lee FK, Beckman-Nahmias S. Sero-epidemiological and - sociological patterns of herpes simplex virus infection in the world. Scand J Infect Dis Suppl. 1990;69:19–36. [PubMed] [Google Scholar]
- Nilsen A, Myrmel H. Changing trends in genital herpes simplex virus infection in Bergen, Norway. Acta Obstet Gynecol Scand. 2000;79(8):693–696. [PubMed] [Google Scholar]
- O'Farrell N. Increasing prevalence of genital herpes in developing countries: implications for heterosexual HIV transmission and STI control programmes. Sex Transm Infect. 1999;75(6):377–384. doi: 10.1136/sti.75.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obasi A, Mosha F, Quigley M, Sekirassa Z, Gibbs T, Munguti K, Todd J, Grosskurth H, Mayaud P, Changalucha J, Brown D, Mabey D, Hayes R. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis. 1999;179(1):16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- Ohana B, Lipson M, Vered N, Srugo I, Ahdut M, Morag A. Novel approach for specific detection of herpes simplex virus type 1 and 2 antibodies and immunoglobulin G and M antibodies. Clin Diagn Lab Immunol. 2000;7(6):904–908. doi: 10.1128/cdli.7.6.904-908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman Ministry of Health. HIV Risk among Heroin and Injecting Drug Users in Muscat, Oman. Quantitative Survey Preliminary Data. 2006. [Google Scholar]
- Pakistan National AIDS Control Program. HIV Second Generation Surveillance In Pakistan. National Report Round 1. National Aids Control Program, Ministry Of Health, Pakistan. Canada-Pakistan HIV/AIDS Surveillance Project. 2005. [Google Scholar]
- Pakistan National AIDS Control Program. HIV Second Generation Surveillance In Pakistan. National Report Round II. National Aids Control Program, Ministry Of Health, Pakistan. Canada-Pakistan HIV/AIDS Surveillance Project. 2006-07. [Google Scholar]
- Pakistan National AIDS Control Program. HIV Second Generation Surveillance In Pakistan. National Report Round III. National Aids Control Program, Ministry Of Health, Pakistan. Canada-Pakistan HIV/AIDS Surveillance Project. 2008. [Google Scholar]
- Patnaik P, Herrero R, Morrow RA, Munoz N, Bosch FX, Bayo S, El Gueddari B, Caceres E, Chichareon SB, Castellsague X, Meijer CJ, Snijders PJ, Smith JS. Type-specific seroprevalence of herpes simplex virus type 2 and associated risk factors in middle-aged women from 6 countries: the IARC multicentric study. Sex Transm Dis. 2007;34(12):1019–1024. [PubMed] [Google Scholar]
- Paz-Bailey G, Ramaswamy M, Hawkes SJ, Geretti AM. Herpes simplex virus type 2: epidemiology and management options in developing countries. Sex Transm Infect. 2007;83(1):16–22. doi: 10.1136/sti.2006.020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pebody RG, Andrews N, Brown D, Gopal R, De Melker H, Francois G, Gatcheva N, Hellenbrand W, Jokinen S, Klavs I, Kojouharova M, Kortbeek T, Kriz B, Prosenc K, Roubalova K, Teocharov P, Thierfelder W, Valle M, Van Damme P, Vranckx R. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004;80(3):185–191. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani E, Lazzari S, Walker N, Schwartlander B. HIV surveillance: a global perspective. J Acquir Immune Defic Syndr. 2003;32(Suppl 1):S3–11. doi: 10.1097/00126334-200302011-00002. [DOI] [PubMed] [Google Scholar]
- Platt L, Vickerman P, Collumbien M, Hasan S, Lalji N, Mayhew S, Muzaffar R, Andreasen A, Hawkes S. Prevalence of HIV, HCV and sexually transmitted infections among injecting drug users in Rawalpindi and Abbottabad, Pakistan: evidence for an emerging injection-related HIV epidemic. Sex Transm Infect. 2009;85(Suppl 2):ii17–22. doi: 10.1136/sti.2008.034090. [DOI] [PubMed] [Google Scholar]
- Ribes JA, Steele AD, Seabolt JP, Baker DJ. Six-year study of the incidence of herpes in genital and nongenital cultures in a central Kentucky medical center patient population. J Clin Microbiol. 2001;39(9):3321–3325. doi: 10.1128/JCM.39.9.3321-3325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30(10):797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- Roudi-Fahimi F, Ashford L. Sexual & Reproductive Health in the Middle East and North Africa. Population Reference Bureau. A Guide for Reporters. 2008. [Google Scholar]
- Samra Z, Scherf E, Dan M. Herpes simplex virus type 1 is the prevailing cause of genital herpes in the Tel Aviv area, Israel. Sex Transm Dis. 2003;30(10):794–796. doi: 10.1097/01.OLQ.0000079517.04451.79. [DOI] [PubMed] [Google Scholar]
- Schmid DS, Brown DR, Nisenbaum R, Burke RL, Alexander D, Ashley R, Pellett PE, Reeves WC. Limits in reliability of glycoprotein G-based type-specific serologic assays for herpes simplex virus types 1 and 2. J Clin Microbiol. 1999;37(2):376–379. doi: 10.1128/jcm.37.2.376-379.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoular A, Norrie J, Gillespie G, Mir N, Carman WF. Longitudinal study of genital infection by herpes simplex virus type 1 in Western Scotland over 15 years. BMJ. 2002;324(7350):1366–1367. doi: 10.1136/bmj.324.7350.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shareef A, Burqan AJ, Abed A, Kalloub E, Alaiwi A. Drug Abuse situation, and ANGA needs study. Power Point presentation. 2006. [Google Scholar]
- Slomka MJ. Seroepidemiology and control of genital herpes: the value of type specific antibodies to herpes simplex virus. Commun Dis Rep CDR Rev. 1996;6(3):R41–45. [PubMed] [Google Scholar]
- Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- Syria National AIDS Programme. HIV/AIDS Female Sex Workers KABP Survey in Syria. 2004. [Google Scholar]
- Tayyebi D, Tabatabaee M, Rahsaz M. Seroepidemiology of infection with Herpes Simplex Virus type 2 (HSV2) among asymptomatic students attending Islamic Azad University of Kazeroun, southwest of Iran. Retrovirology. 2010;7(Suppl 1):P88. [Google Scholar]
- Tiouiri H, Naddari B, Khiari G, Hajjem S, Zribi A. Study of psychosocial factors in HIV infected patients in Tunisia. East Mediterr Health J. 1999;5(5):903–911. [PubMed] [Google Scholar]
- Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, Laeyendecker O, Charvat B, Ssempijja V, Riedesel M, Oliver AE, Nowak RG, Moulton LH, Chen MZ, Reynolds SJ, Wawer MJ, Gray RH. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360(13):1298–1309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T, Druce JD, Catton MC, Kelly H, Birch CJ. Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex Transm Infect. 2004;80(4):277–279. doi: 10.1136/sti.2004.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- UNAIDS RST MENA. Notes on HIV and AIDS in the Middle East and North Africa. 2007. [Google Scholar]
- UNAIDS RST MENA. Notes on AIDS in the Middle East and North Africa. 2008. [Google Scholar]
- van de Laar MJW, Termorshuizen F, Slomka MJ, van Doornum GJJ, Ossewaarde JM, Brown DWG, Coutinho RA, van den Hoek JAR. Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioural risk factors. International Journal of Epidemiology. 2001;27(1):127–134. doi: 10.1093/ije/27.1.127. [DOI] [PubMed] [Google Scholar]
- Vickerman P, Hickman M, May M, Kretzschmar M, Wiessing L. Can hepatitis C virus prevalence be used as a measure of injection-related human immunodeficiency virus risk in populations of injecting drug users? An ecological analysis. Addiction. 2010;105(2):311–318. doi: 10.1111/j.1360-0443.2009.02759.x. [DOI] [PubMed] [Google Scholar]
- Wadsworth J, Field J, Johnson AM, Bradshaw S, Wellings K. Methodology of the National Survey of Sexual Attitudes and Lifestyles. J R Stat Soc Ser A Stat Soc. 1993;156(3):407–421. [PubMed] [Google Scholar]
- Wald A, Krantz E, Selke S, Lairson E, Morrow RA, Zeh J. Knowledge of partners' genital herpes protects against herpes simplex virus type 2 acquisition. J Infect Dis. 2006;194(1):42–52. doi: 10.1086/504717. [DOI] [PubMed] [Google Scholar]
- Wald A, Langenberg AG, Link K, Izu AE, Ashley R, Warren T, Tyring S, Douglas JM, Jr, Corey L. Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. Jama. 2001;285(24):3100–3106. doi: 10.1001/jama.285.24.3100. [DOI] [PubMed] [Google Scholar]
- Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, Tanton C, Ross D, Everett D, Clayton T, Balira R, Knight L, Hambleton I, Le Goff J, Belec L, Hayes R. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358(15):1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math Biosci. 1992;108(1):89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- Weiss HA, Thomas SL, Munabi SK, Hayes RJ. Male circumcision and risk of syphilis, chancroid, and genital herpes: a systematic review and meta-analysis. Sex Transm Infect. 2006;82(2):101–109. doi: 10.1136/sti.2005.017442. discussion 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/EMRO. Regional database on HIV/AIDS. WHO Regional Office for the Eastern Mediterranean. [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Yorke JA, Hethcote HW, Nold A. Dynamics and control of the transmission of gonorrhea. Sex Transm Dis. 1978;5(2):51–56. doi: 10.1097/00007435-197804000-00003. [DOI] [PubMed] [Google Scholar]
- Yousif MEA. Health Education Programme among Female Sex workers in Wad Medani Town- Gezira State. Final Report 2006 [Google Scholar]
- Zamani S, Kihara M, Gouya MM, Vazirian M, Nassirimanesh B, Ono-Kihara M, Ravari SM, Safaie A, Ichikawa S. High prevalence of HIV infection associated with incarceration among community-based injecting drug users in Tehran, Iran. J Acquir Immune Defic Syndr. 2006;42(3):342–346. doi: 10.1097/01.qai.0000219785.81163.67. [DOI] [PubMed] [Google Scholar]
- Ziyaeyan M, Japoni A, Roostaee MH, Salehi S, Soleimanjahi H. A serological survey of Herpes Simplex Virus type 1 and 2 immunity in pregnant women at labor stage in Tehran, Iran. Pak J Biol Sci. 2007;10(1):148–151. doi: 10.3923/pjbs.2007.148.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.