Abstract
Twenty-three dogs with heart failure were evaluated in a 12-month study by measuring baseline plasma atrial natriuretic peptide (ANP) concentrations. Ten dogs were classified as having mild to moderate cardiac disease (group 1) and 13 dogs were classified as having severe cardiac disease (group 2). The mean plasma ANP concentration for the group 1 dogs was 64 ± 45 pg/mL and for the group 2 dogs, 328 ± 122 pg/mL. The median survival time (1095 d) for group 1 dogs was significantly greater (P < 0.05) than for group 2 dogs (58 d). A significantly (P , 0.05) greater median survival was noted for dogs with plasma ANP , 95 pg/mL (1095 d) compared with those with ANP . 95 pg/mL (58 d). Plasma ANP concentrations are a potential noninvasive predictor of survival in dogs with heart failure.
Introduction
In the late 1950s, granules comparable to those found in peptide-secreting hormone cells were found in the atria cordis of guinea pigs by Kisch (1). In 1981, deBold et al (2) demonstrated a profound diuresis and natriuresis in rats following IV administration of atrial muscle extracts, leading to the discovery of atrial natriuretic peptide (ANP). The amino terminus of the 126 amino acid prohormone (proANF-126) consists of amino acids 1-98 (proANF 1-98); ANP (99-126) is the 28 amino acid peptide that is also produced as part of the prohormone. At the cellular level, ANP release from atrial cardiocytes occurs in response to an increased volume of extracellular fluid (ECF) (3,4). Several studies have localized ANP production to specific granules in atrial cardiocytes (5,6,7). Lower levels are synthesized in other tissues, such as the brain (8).
Atriopeptins have been measured in various pathologic states and in normal dogs (9,10). Vollmar et al (9) showed that plasma ANP concentrations were increased in dogs with renal failure, congestive heart failure, and hyperadrenocorticism. Dogs with chronic renal failure had twice the ANP levels of healthy control dogs, while those dogs suffering from congestive heart failure had a 6-fold increase in plasma ANP concentrations compared with healthy controls (9).
In humans, plasma ANP concentrations have been correlated with left ventricular end-diastolic volume in patients with asymptomatic left ventricular dysfunction (11). Plasma ANP was significantly elevated in these patients before the appearance of clinical signs of heart disease. Atriopeptin concentrations were also found to decrease following medical therapy for congestive heart failure, suggesting the potential utility of atriopeptin quantitation as a noninvasive measure of prognosis and subsequent response to therapy (11,12). Gottlieb et al (13) reported that increased plasma concentrations of ANP were positively correlated with the degree of heart failure and with mortality in patients with congestive heart failure (13). Similarly, patients in the placebo group of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) showed significant positive correlation of increased plasma ANP concentrations with mortality (14). Hall et al (15) reported measurement of n-terminal ANP (1–98) concentrations in 136 human patients at baseline and after 6 wk of blinded treatment in a subset of the CONSENSUS patient population. Increased plasma n-terminal ANP (1–98) concentrations were positively correlated with mortality (15).
The purpose of this preliminary study was to determine if baseline plasma ANP (99–126) concentrations could be used as a prognostic indicator of survival in dogs suffering from mild to severe heart failure.
Materials and methods
Clinical study design
Ten clinically healthy dogs, based on physical examination and minimum database (complete blood cell (CBC) count, serum chemistry profile, urinalysis), aged 2 to 6 y old, were used to collect plasma samples for control values in the radioimmunoassay procedure. Twenty-three dogs with cardiovascular disease, selected from the canine patient population at the Colorado State University Veterinary Teaching Hospital, were used in a prospective study. Dogs were divided into 2 study groups: group 1 with mild to moderate signs of heart failure at rest and group 2 with advanced heart failure where home care was possible (16). Of the 10 dogs classified as group 1, 6 suffered from mitral insufficiency, 3 from dilated cardiomyopathy (DCM), and 1 from tricuspid insufficiency. Of the 13 dogs classified as group 2, 5 suffered from mitral insufficiency, 3 from both mitral and tricuspid insufficiency, and 5 from DCM. Exclusion criteria for the study included known concurrent systemic illness (hyperadrenocorticism, renal disease (urine specific gravity , 1.025 with concurrent azotemia)) other than cardiovascular disease, congenital cardiac conditions, and previous long-term drug therapy for cardiac disease.
All dogs selected underwent a complete history, physical examination, CBC count, serum chemistry profile, urinalysis, thoracic radiographs, echocardiography, and electrocardiography. Plasma was obtained for baseline ANP concentration measurement, prior to the initiation of therapy. Plasma ANP, echocardiography, electrocardiography, and thoracic radiographs were repeated every 3 mo for a period of 12 mo, or until the time of death or euthanasia. A postmortem examination was performed on all dogs that died within the study period. The status of all dogs (death due to heart failure or due to another cause) that survived beyond the study period (12 mo) was determined by phone calls to the owner or referring veterinarian for up to 3 y later.
Radioimmunoassay protocol
The following protocol, previously validated in dogs, was used (17). All standard peptides, antibodies, and assay reagents were obtained from a commercial radioimmunoassay (RIA) kit (ANF (RAT) RIA kit; Peninsula Laboratories, Belmont, California USA). Thirty-three study samples and 10 control samples were run in these assays. Blood samples were collected into vacuum tubes (BD Vacutainer; Becton Division, Franklin Lakes, New Jersey USA) containig the anticoagulan EDTA and centrifuged at 1500 g and 0°C for 20 min. Plasma was removed and stored at 220°C for 3 mo, until thawed for analysis. All assays were run in duplicate in 12 3 75 mm polystyrene tubes. A standard curve was generated for each assay by using serial dilutions of the standard ANP peptide.
To provide a background consistent with the plasma of the study samples, pooled plasma from several healthy dogs was obtained to add to the standard peptide dilutions. This pooled sample was first processed for removal of interfering substances (ANP degradation products, etc) that might cross-react with canine plasma ANP (17). This was accomplished by running the pooled plasma over a 5.02 3 0.52cm column of washed activated carbon. Fractions were combined after disposal of the first 10% of column eluent. The combined fraction sample was heated to 65°C in a water bath for 2 h and then centrifuged at 1400 g for 5 min to eliminate any remaining ANP. For removal of nonspecific binding factors, the cleared supernatant was diluted 1:1 with 0.1 M acetic acid, heated to 85°C in an oven for 10 min, and centrifuged at 1700 g for 4 min. This ANP-free preparation was then added to 50 mL of each concentration of the standard curve, so that final concentrations of 1, 10, 20, 40, 80, 160, 320, 640, and 1280 pg/mL were obtained.
Study samples were treated with 0.1 M acetic acid, as described above, for removal of nonspecific binding factors. First, a 50 mL aliquot of each treated sample was added in duplicate to individual assay tubes; 50 mL of rabbit anti-human ANP (Peninsula Laboratories) were then added to each tube. Assay tubes were shaken and incubated at 4°C for 24 h. Next, 50 mL of assay buffer containing approximately 13 000 counts/min of alpha-human125 I-ANP (Peninsula Laboratories) was added to all tubes, shaken and incubated at 4°C for another 24 h. Bound ANP was separated from free ANP by a double antibody precipitation technique: 50 mL each of goat-anti-rabbit IgG serum (Peninsula Laboratories) and normal rabbit serum (Peninsula Laboratories) were added to each assay tube, which was then incubated at room temperature for 90 min. After the bound ANP was precipitated with chilled assay buffer, each tube was centrifuged at 1500 g for 20 min at 4°C. Supernatant was removed by aspiration and bound ANP in the pellet was counted in a gamma scintillation counter (1414 Winspectral; Perkin Elmer Life Sciences Inc, Wellesley, Massachusetts USA) for 1 min. Linear regression analysis was applied to the standard curve for each ANP assay by using software (SAS/STAT User's Guide, release 6.03 edition; SAS Institute Inc., Cary, North Carolina USA). Concentrations of ANP peptide in the samples were determined by using logit transformation of the standard curve.
Statistical Analysis
For statistical comparisons between group 1 and group 2, a Student's t-test was performed on baseline ANP concentrations; ANP concentrations were reported as mean and standard deviation for the control and each study group. Group differences over time were examined by a Kruskall-Wallis test. A Kruskall-Wallis test was used because the 3-mo and 6-mo data were not normally distributed. The relation between ANP concentrations and mortality up to 36 mo was tested by using Kaplan-Meir survival plots. Survival was analyzed as a function of baseline ANP concentration (above or below mean control values plus 2 standard deviations (s)) expressed as a categorical entity (ANP , 92 pg/mL or ANP . 92 pg/mL) by using a commercial statistical software package (SPSS/PC1; Chicago SPSS Inc, SAS Institute). Group comparisons were made by using a logrank (Mantel-Cox) test.
Censored observations occur in patients for whom the critical event (death due to heart failure) has not yet occurred. The time from the date of baseline data collection to the last date on which the live patient was examined is known as the censored survival time. Censored observations can occur in 3 ways: a) the patient is known to be still alive when the trial analysis is carried out; b) the patient was known to be alive at some past follow-up, but the investigator has since lost trace of the patient; or c) the patient has died of some cause totally unrelated to the disease in question. Most of the patients in this study were censored because of death due to another cause (18). If P , 0.05, groups were considered significantly different.
Results
Characteristics of the radioimmunoassay
The amount of unlabeled ANP required to displace 50% of bound 125I-ANP was 125 ± 5 pg/mL. With no unlabeled ANP present (B0), 38.2% ± 0.5% of the radiolabeled ANP was bound. Nonspecific binding of 125I-ANP was 4.6% ± 0.5%. The sensitivity of the assay was 1 pg/mL of sample. The intra and interassay coefficients of variation were 8.6% and 7.4%, respectively.
Plasma concentrations of ANP in control and study dogs
Twenty-three dogs were studied; 10 dogs were in group 1 and 13 dogs were in group 2. In group 1, 6 dogs had mitral insufficiency (MI), 3 had dilated cardiomyopathy (DCM), and 1 had tricuspid insufficiency (TI). In group 2, 5 dogs had MI, 5 dogs had DCM, and 3 dogs had both MI and TI. There was no significant difference in the number of dogs with MI versus DCM in the 2 groups; 3/10 dogs in group 1 had DCM, and 5/13 dogs in group 2 had DCM. The majority of the dogs in both groups had MI. Of the 3 dogs with DCM in group 1, 2 were boxers and 1 was a Doberman pinscher. In group 2, 2/5 were Doberman pinschers, 1 was a mixed breed dog, 1 was a Rottwieller, and 1 was a golden retriever. None of the dogs in group 1 died of heart failure; however, 8 dogs were censored after 12 mo because of death due to another cause. In group 1, 6 dogs returned for a 6-month recheck and 3 dogs returned for the 9-month recheck. In contrast, in group 2, 9 dogs died within the first 3 mo of the study; 1 dog survived to the 6-month recheck, and 3 dogs were censored (at days 358, 640, and 700).
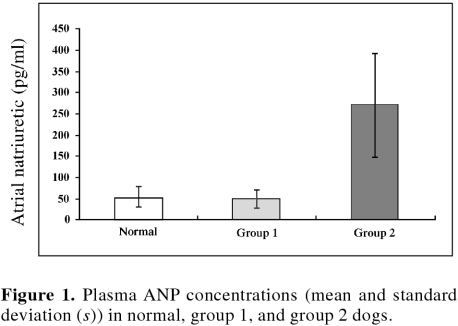
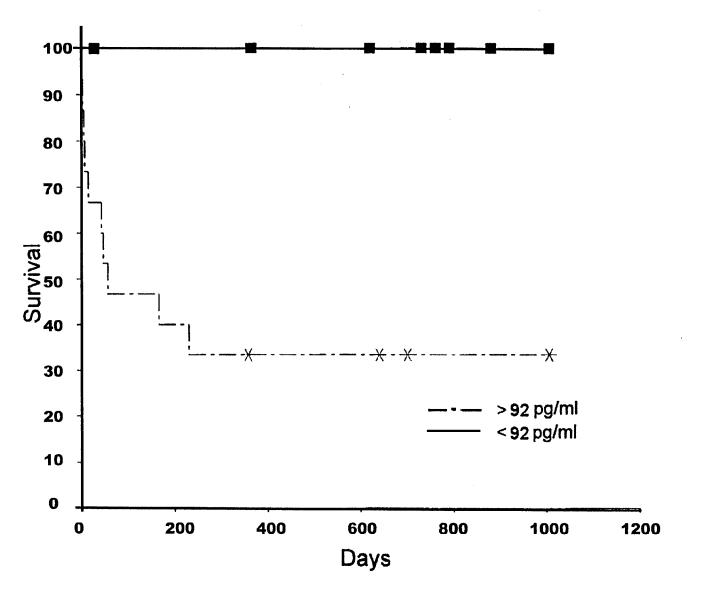
Results of baseline mean and standard deviation (s) plasma ANP concentrations for healthy control dogs and dogs with cardiac disease are summarized in Figure 1. There was no significant difference in the mean and (s) baseline plasma ANP concentration in clinically normal dogs (56.9, s = 17.4 pg/mL) versus dogs in group 1 (64.2, s = 45.0 pg/mL) (Figure 1). In contrast, dogs in group 2 had significantly (P , 0.05) greater plasma ANP concentrations (328.0, s = 122.0 pg/mL) than dogs in group 1 (64.2, s = 5.0 pg/mL). In group 1 dogs, there was no significant difference in plasma ANP concentrations at baseline (64.2, s = 45.0 pg/mL), compared at the 6 mo (median 70, range 54 to 94 pg/mL, n = 6) or at 9 mo (median 10, range 8 to 34 pg/mL, n = 3). In group 2, only 1 dog survived to the 6-month recheck; therefore, no statistical comparisons could be made. The median survival time for group 1 dogs (1095 d) was significantly greater (P , 0.05) than that for group 2 dogs (58 d) (Figure 2).
Figure 1. Plasma ANP concentrations (mean and standard deviation (s)) in normal, group 1, and group 2 dogs.
Figure 2. Survival curves for 2 groups of dogs with plasma ANP concentrations greater or less than 92 pg/mL.
Discussion
The mean plasma ANP concentration in this study was similar to that reported by Verburg et al (10). As in previous studies, there was a significant increase in ANP concentration in dogs with severe clinical signs of heart failure (group 2) compared with that in normal dogs (9); however, dogs with only mild clinical signs of heart failure (group 1) did not have elevated ANP concentrations when compared with those of the control dogs. The nature of the heart disease was similar between the 2 groups, the only variable being the baseline ANP concentrations.
Dogs with increased plasma ANP concentrations (more than 2 standard deviations over control) had a significantly shorter median survival time (58 d) than did those dogs with lower plasma ANP concentrations (1095 d), findings that are similar to those previously reported in humans (12,14). All dogs with low ANP concentrations (, 92 pg/mL) were either still alive 3 y later or censored because of death due to another cause. In contrast, only 4 dogs with high ANP concentrations were censored because of death due to another cause; the other 9 died of heart failure, findings that are consistent with those reported in humans (15).
Increased ventricular secretion of atrial peptides has been shown to occur with increased wall stress in rats (19,20). The plasma level of atrial peptides may therefore reflect the extent and progression of cardiac pathology. Changes in immunoreactive (ir) ANP (1–98) presage later mortality and may be used as an index of the efficacy of medical interventions in humans (21); therefore, the use of ANP (1–98) peptide as a routine clinical biochemical parameter in the identification and treatment of such patients has been suggested (15). However, the radioimmunoassay used in our study was much more technically demanding than the analysis for irANP (1–98) directly in plasma (21); in fact, the irANP (1–98) assay was not available at the time of this study. Before ANP concentrations could be used by veterinarians to routinely screen and monitor dogs with heart failure, the irANP (1–98) assay would have to be validated in canine plasma. Recent studies in humans have shown that brain natriuretic peptide (BNP), a related peptide to ANP, is an even stronger predictor of mortality in patients with left ventricular dysfunction (22).
It should be noted that, in humans, several factors have been demonstrated to predict survival, including the New York Heart Association class of congestive heart failure (13), exercise capacity, heart rate at rest, systolic blood pressure, pulse pressure, left ventricular ejection fraction, systemic vascular resistance, degree of heart enlargement on chest radiographs or echocardiography, serum electrolytes (Na, K, and Mg), neuroendocrine elevations (epinephrine, renin, vasopressin, ANP, big endothelin, and aldosterone), frequency of ventricular ectopy, complexity of ventricular arrhythmias, and atrial fibrillation. In dogs, cause of heart failure (MI versus DCM), breed of dog with DCM, age, gender, presence of pleural effusion, and presence of pulmonary edema have also been used as predictors of survival. In this study, there was no significant difference in the number of dogs with MI versus DCM in the 2 groups.
This study was designed as a pilot study to evaluate the utility of plasma ANP in the prognosis of heart failure in dogs; funding was not available to evaluate a large number of dogs. Larger sample size (50 dogs per group) would have provided enough statistical power to perform multivariate analysis and, therefore, to determine which factors were independent predictors of survival. Either plasma ANP concentrations or severity of heart failure (or both) was a predictor of survival in this study. Therefore, it is impossible to determine if ANP was an independent predictor of survival in this study. Further refinement of this technique, using the (1–98) irANP assay and increased numbers of samples/cases, might allow multivariate analysis to determine if certain factors, such as ANP, are independent predictors of survival in dogs suffering from heart failure.
Footnotes
Dr. Greco's current address is The Animal Medical Center, 510 East 62nd Street, New York, New York 10021, USA.
Address all correspondence and reprint requests to Dr. Greco.
References
- 1.Kisch B. Electronmicroscopy of the atrium of the heart. I. Guinea pig. Exp Med Surg 1956;14:99–100. [PubMed]
- 2.deBold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and important natriuretic response to intravenous injection of atrial myocardial extracts in rats. Life Sci 1981;28:89–94. [DOI] [PubMed]
- 3.Ackermann U, Irizawa TG, Milojevic S, et al. Cardiovascular effects of atrial extracts in anesthetized rats. Can J Physiol Pharmacol 1984;62:819–826. [DOI] [PubMed]
- 4.Genest J, Cantin M. Atrial natriuretic factor. Circulation 1987;75:118–124. [PubMed]
- 5.Garcia R, Cantin M, Thibault G, et al. Relationship of specific granules to the natriuretic and diuretic activity of rat atria. Experientia 1982;38:1071–1073. [DOI] [PubMed]
- 6.deBold AJ. Tissue fractionation studies on the relationship between an atrial natriuretic factor and specific atrial granules. Can J Physiol Pharmacol 1982;60:324–330. [DOI] [PubMed]
- 7.Cantin M, Gutkowska J, Thibault G, et al. Immunochemical localization of atrial natriuretic factor in the heart and salivary glands. Histochemistry 1984;89:113–127. [DOI] [PubMed]
- 8.Jacobowitz DM, Skofitsch G, Keiser HR, et al. Evidence for the existence of atrial natriuretic factor-containing neurons in the rat brain. Neuroendocrinology 1985;40:92–94. [DOI] [PubMed]
- 9.Vollmar AM, Reusch C, Kraft W, Schulz R. Atrial natriuretic peptide concentration in dogs with congestive heart failure, chronic renal failure, and hyperadrenocorticism. Am J Vet Res 1991;52:1831–1834. [PubMed]
- 10.Verburg KM, Freeman RH, Davis JO, et al. Control of atrial natriuretic factor release in conscious dogs. Am J Physiol 1986;251:R947–R956. [DOI] [PubMed]
- 11.Burnett JC. Atrial natriuretic peptide in congestive heart failure. Adv Atrial Peptide Res, vol II. New York, Raven Pr, 1988:141–153.
- 12.Burnett JC. Atrial natriuretic factor: implications in congestive heart failure and hypertension. J Human Hypertens 1989;3:41–46. [PubMed]
- 13.Gottleib SS, Kukin ML, Ahern D, Packer M. Prognostic importance of atrial natriuretic peptide in patients with chronic heart failure. J Am Coll Cardiol 1989;13:1534–1539. [DOI] [PubMed]
- 14.Swedburg K, Eneroth P, Kjekshus J, Wilhilmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation 1990;82:1730–1736. [DOI] [PubMed]
- 15.Hall C, Kjekshus J, Eneroth P, Snapinn S. The plasma concentration of n-terminal proatrial natriuretic factor ANP (1–98) is related to prognosis in severe heart failure. Clin Cardiol 1994;17:191–195. [DOI] [PubMed]
- 16.Fox PR, Sisson D, Moise NS. Canine and Feline Cardiology. 2nd ed, Philadelphia, WB Saunders, 1999:885.
- 17.Radin MJ, McClure SA. The effects of atrial natriuretic peptide infusion on renal hemodynamics and plasma lipoproteins in puromycin aminonucleoside nephrosis in rats. Clin Expt Pharmacol Physiol 1993;20:245–251. [DOI] [PubMed]
- 18.Campbell MJ, Machin D. Medical Statistics: A Commonsense Approach. New York: John Wiley & Sons, 3rd ed, 1999:122.
- 19.Dietz JR, Nazian SJ, Vesely DL. Release of ANP, pro-ANP 1–98, and pro ANP 31–67 from isolated rat atria by atrial distension. Am J Physiol 1991;260:H1774–H1778. [DOI] [PubMed]
- 20.Rusknoaho H, Kinnunen P, Mantymaa P, et al. Ventricular stretch releases atrial natriuretic peptide from hypertrophied rat myocardium. Circulation 1990;186:82.
- 21.Sundsfjord JA, Thibault G, Larochelle P, Cantin M. Identification and plasma concentrations of the N-terminal fragment of proatrial natriuretic factor in man. J Clin Endocrinol Metab 1988;66:605–610. [DOI] [PubMed]
- 22.McDonagh TA, Cunningham AD, Morrison CE, Ford I, Morton JJ, Dargie HJ. Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 2001;86:21–26. [DOI] [PMC free article] [PubMed]