Abstract
This study was designed to evaluate whether Lactobacillus rhamnosus strain GG (LGG), an extensively studied probiotic organism in humans, can colonize the intestines of adult horses and foals. Lactobacillus rhamnosus strain GG was administered to adult horses at doses of 1 × 109 CFU/50kg bodyweight (BW)/day (group 1, 7 horses), 1 × 1010 colony forming units/ 50kg BW/day (group 2, 7 horses) and 5 × 1010 colony forming units/50kg BW/day (group 3, 7 horses) for 5 d. Foals received 2 × 1010 colony forming units/50kg BW/day (group 1, 7 foals) or 1 × 1011 colony forming units/50kg BW/day (group 2, 7 foals) for 5 d. Fecal levels of L. rhamnosus strain GG in adult horses were low and variable in the 2 lower dose groups. Even in the high dose group, colonization was relatively low. In contrast, more consistent intestinal colonization was present in foals, and colonization persisted for up to 9 d following cessation of administration. No adverse effects were observed in any animal. Clinical studies evaluating this probiotic are indicated in foals. The presence of this organism in the feces of adult horses may only represent passive movement through the intestinal tract, not actual colonization. Consistent intestinal colonization in adults was only achieved with a prohibitively high dose.
Introduction
A probiotic can be defined as a living microorganism that upon ingestion in certain numbers exerts health effects beyond inherent basic nutrition (1). Elie Metchnikoff first discussed the concept of probiotics almost 100 y ago (2). He suggested that the longevity of certain ethnic groups was related to their ingestion of fermented milk products and that these products manipulated the intestinal microflora to maintain the normal balance between pathogenic and nonpathogenic bacteria.(2) Since this initial observation, it has been recognized that a number of factors beyond simple manipulation of the intestinal microflora can play a role in the success of probiotic therapy. Despite a relative paucity of research, probiotic therapy is generating increasing attention in veterinary medicine.
Appealing properties of probiotics include the ability to reduce antibiotic use, the apparently high index of safety, and the public's positive perception about “natural” or “alternative” therapies. Probiotics are “generally regarded as safe,” as opposed to antibiotics, which have a number of recognized adverse effects (3). Very little research has been performed in the field of equine probiotic therapy. Parraga et al (4) were unable to demonstrate any influence on the shedding of Salmonella spp., prevalence of postoperative diarrhea, length of antimicrobial therapy, and duration of hospitalization in horses at a teaching hospital as a result of the administration of 2 commercial probiotics. A second study reported no effect on Salmonella shedding in hospitalized horses with colic (5). However, there was no indication that the strains used in this study possessed any of the probiotic properties listed above or that an adequate dose was administered.
A variety of microorganisms, typically lactic acid bacteria, such as lactobacilli, bifidobacteria, and enterococci, have been evaluated as potential probiotics (6). A small number of yeasts have also been evaluated (7,8). Commercial probiotic preparations are available for human and animal use; however, there is little to no objective research available for many of them, particularly those intended for companion animals. Based on the definition (1) stated above, it is clear that adequate numbers of viable probiotic organisms must reach the intestinal tract. To do this, they must be able to survive transit through the acidic environment of the stomach and resist bile digestion. Organisms that survive acid and bile must possess a variety of other properties, including the ability to adhere to intestinal epithelial cells, colonize the intestinal tract, produce an antimicrobial factor, and inhibit enteric pathogens (9,10,11,12). Other properties, such as immunomodulation, modulation of metabolic activities, and inactivation of procarcinogens, are also desirable (13,14). An organism can only be considered to be a probiotic after these properties have been identified and a positive health effect has been documented.
One of the best-studied probiotics in human medicine is Lactobacillus rhamnosus strain GG (LGG). Lactobacillus rhamnosus GG has been shown to survive acid and bile digestion and to colonize the gastrointestinal tracts of humans (15,16,17,18). It also possesses powerful adhesive properties, suppresses bacterial enzyme activity, can displace or eliminate certain components of the normal intestinal flora, and produces an antimicrobial substance active against a variety of bacteria, including Escerichia coli, Salmonella spp., Clostridium spp., Streptococcus spp., and Bacteroides spp. (12). In humans, LGG has been shown to be effective in the treatment of several forms of diarrhea, including rotaviral diarrhea in children, acute nonrotaviral diarrhea in children, antibiotic-associated diarrhea in children and adults, “travellers” diarrhea, and relapsing Clostridium difficile diarrhea in placebo-controlled studies (12,19,20,21,22,23,24,25,26). Gastrointestinal disease is of serious concern in equine medicine and these results in humans suggest that probiotics, particularly LGG, might be treatment options.
Some authors believe that probiotic organisms should be naturally occurring in their target species to be effective (10). However, cross-species efficacy has been demonstrated for some probiotic strains, including LGG (27). Prior to evaluating the efficacy of any probiotic, it should be demonstrated that the organism has the ability to survive transit through the gastrointestinal tract of the intended host. This does not indicate that an organism will have probiotic properties in the given species; however, demonstration of intestinal survival and fecal presence are a prerequisite for studies evaluating efficacy. This study was designed to evaluate whether LGG can colonize the gastrointestinal tract of adult horses and foals, and do so without causing adverse effects.
Materials and methods
The study was approved by the University of Guelph Animal Care Committee. Twenty-one, clinically healthy, adult standardbred horses weighing between 450 and 500 kg were enrolled in the study. Fourteen, clinically healthy, pony foals, ranging from 1 to 3 d of age and weighing 18 to 25 kg were also used. Diet and management were not altered. Horses were individually housed and randomly allocated to treatment groups receiving L. rhamnosus GG at dosages of 1 × 109 colony forming units (CFU)/50kg bodyweight (BW) (group 1, n = 7), 1 × 1010 CFU/50kg BW (group 2, n = 7), or 5 × 1010 CFU/50kg BW (group 3, n = 7), once daily for 5 d (days 0 through 4). Lactobacillus rhamnosus GG was administered by opening capsules and mixing the contents with a small amount of moistened pelleted feed. Foals were housed as a group on pasture with their dams. Foals were divided into 2 groups. Group 1 (n = 7) received 2 × 1010 CFU/50kg BW and group 2 received 1 × 1011 CFU/50kg BW (n = 7), once daily for 5 d. Lactobacillus rhamnosus GG was administered either by opening the capsule and sprinkling contents directly into the mouth, or via a dosing syringe. Horses and foals were monitored daily for changes in clinical condition, appetite, and fecal consistency. Fecal samples were collected on days 0, 1, 3, 5, 6, 7, and 9, and every 48 h thereafter until day 15, or until 2 consecutive samples that were negative for LGG were obtained, whichever occurred first. Freshly passed fecal samples were obtained from adult horses. Fecal sampling from foals was performed directly per rectum. Samples were refrigerated and processed within 2 h or stored at −80°C until processed. Serial 10-fold dilutions of feces were performed in phosphate buffered saline (pH 7.2). Aliquots of the serial dilutions, from 102 to 108, were inoculated onto deMan, Rogosa, Sharp (MRS) agar and incubated in an anaerobic chamber at 37°C for 72 h. Colonies were identified as LGG, based on morphology (large, round, white, creamy colonies), Gram stain appearance (gram-positive uniform rods), and the inability to ferment lactose (28). Randomly selected isolates were confirmed as LGG by using the API 50 CHL (BioMerieux, St. Laurent, Quebec) biochemical identification assay.
The area under the curve of fecal LGG level versus day for each horse was analyzed using a Kruskal-Wallis 5 sample rank test. Multiple comparisons were based on a Tukey adjustment to control the overall experimentwise error rate. Shapiro Wilk test on the residuals of the areas confirmed that the data were normally distributed (P < 0.1301). Analysis was performed by using statistical analysis software (29).
Results
All horses but 1 consumed LGG readily in feed, and it appeared to have been completely consumed. The exception was in group 3, and this horse was administered the LGG via a dosing syringe after mixing it with water and corn syrup. No problems were encountered in the administration of LGG to foals, and it is believed that all was consumed. No adverse effects were identified in any horses or foals throughout the study period.
Lactobacillus rhamnosus strain GG was not detected in the feces of any adult horse prior to administration (Figure 1). Intestinal colonization was identified in 5/7 (71%) of horses in group 1, 2/7 (29%) of horses in group 2, and 6/7 (86%) of horses in group 3. The mean number of positive samples was 1.0 in group 1 (s = 1.0, range 0 to 3), 0.28 in group 2 (s = 0.49, range 0 to 1), and 1.7 in group 3 (s = 0.95, range 0 to 3). Twenty-four hours after cessation of administration, LGG was still present in the feces of 1/7 (14%) horses in group 1, 1/7 (14%) in group 2, and 4/7 (57%) in group 3. By 48 h after cessation of administration, LGG was present in the feces of 1/7 (14%) horses in each of groups 1 and 2, and no horses in group 3. In adult horses, overall growth ranged from log10 2.6 to 6 CFU/g of feces. Among horses that were colonized with LGG, mean log10 levels detected during the administration period (days 0 to 4) were 3.9 CFU/g in group 1, 3.0 CFU/g in group 2, and 4.7 CFU/g in group 3. Based on area under the curve calculation, there was not a significant difference in intestinal level of LGG between the adult horse groups (P > 0.05).
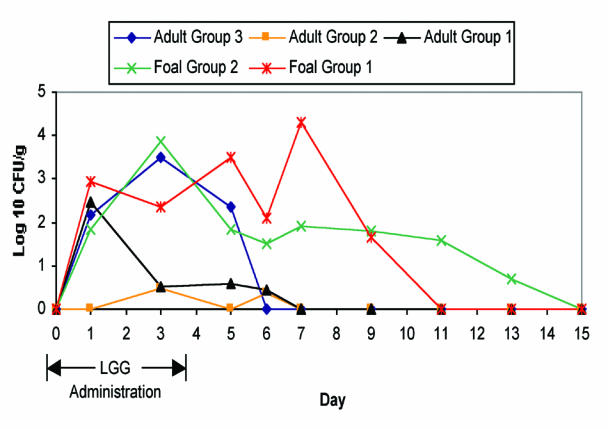
Figure 1. Mean fecal counts of Lactobacillus rhamnosus strain GG following oral administration in horses and foals. (Adult group 1: 1 × 109 CFU/50kg BW/d, adult group 2: 1 × 1010 CFU/50kg BW/d, adult group 3: 5 × 1010 CFU/50kg BW/d, foal group 1: 2 × 1010 CFU/50kg BW/d, foal group 2: 1 × 1011 CFU/50kg BW/d).
Lactobacillus rhamnosus GG was not present in any fecal samples from foals prior to administration. All foals in both groups were colonized with LGG on at least 1 d. The mean number of positive samples was 3.0 in group 1 (s = 1.73, range 1 to 5) and 2.7 in group 2 (s = 0.95, range 1 to 4). Intestinal colonization was somewhat intermittent, so not all samples were positive on all days. Among foals, fecal levels of LGG ranged from log10 3.7 to 7.5 CFU/g. Persistence of intestinal colonization was greater than for adults. The median day on which LGG was detected in feces after cessation of treatment was day 3, while LGG was not present in the feces of any adult horses on this day. One foal maintained detectable fecal levels of LGG for 9 d following cessation of administration. Among foals that were colonized, mean log10 fecal levels during the administration period were 5.3 CFU/g in group 1 and 5.4 CFU/g in group 2. The peak mean level of colonization was achieved at day 7 for group 1 (log10 4.2 CFU/g) and day 3 for group 2 (log10 3.9 CFU/g). There was not a significant difference in intestinal LGG colonization between foal groups. Intestinal LGG levels were, however, significantly higher in both foal groups compared with adult horse groups 1 and 2.
Discussion
Compared with other species, intestinal colonization of LGG in adult horses is sporadic and poor. The lack of a statistically significant difference between the adult groups was surprising. An explanation for the lower intestinal level of LGG in the intermediate dose group is not apparent. Even with a high level of supplementation (5 × 1010 CFU/50kg BW/d), the peak intestinal colonization was on day 3 when only 5/7 horses were colonized with a mean level of 3.5 log10 CFU/g of feces. In humans, levels of 5 to 7 log10 CFU/g have been reported following administration of 1 × 1010 CFU/d (18,28). Different dosage groups complicate direct comparison between adult horses and foals. However, it appears that LGG is better able to colonize the intestinal tract of foals. Being of human origin, it is possible that LGG is less adapted to successfully compete with the intestinal microflora of adult horses. Presumably, the immature nature of the gastrointestinal microflora in foals facilitated colonization. It is possible that LGG is not able to adhere well to equine intestinal epithelial cells. This would not explain the differences between adults and foals, unless age-related differences in enterocytes adhesion properties were present. This warrants further study.
Persistence of colonization following cessation of administration in adult horses was limited. Even transient colonization of LGG can have an effect on the resident microflora in humans; however, the inconsistent and generally low levels present in adult horses in this study may not be adequate for such an effect. Further, because LGG did not persist in adult fecal samples for more than 48 h after cessation of administration, even in the high dose group, it is not clear whether fecal levels were due to actual transient colonization of the intestinal tract or simply due to passive movement through the gastrointestinal tract. In foals, however, LGG persisted for a median of 3 d and a maximum of 9 d following cessation of administration. Campbell et al (30) reported complete intestinal clearance of barium within 36 h in 2 young foals, suggesting that LGG colonized the intestinal tract of foals, although it may not be reasonable to equate movement of barium to that of bacteria. In humans, LGG is reported to persist for longer periods of time. One study reported that 87% of humans shed LGG in feces 4 d following cessation of administration, while 33% shed LGG after 7 d (15).
The lack of a dose response in foals was interesting and unexpected. It was suspected that higher fecal levels would be present with the higher oral dose; however, this was not the case. Perhaps, the intestinal microflora in foals is quite susceptible to colonization and the dose used in group 1 was optimal.
Based on the sporadic colonization of LGG in adult horses, it is unlikely that this organism has significant probiotic potential in healthy horses. It is possible that colonization would be better in diarrheic horses or in those undergoing antimicrobial therapy because of disruption of the normal protective intestinal microflora. This should be evaluated. In contrast, LGG may have potential as a probiotic in foals. While peak intestinal levels were lower than those encountered in humans, LGG was able to colonize the majority of foals and persisted longer than in adults. Neonatal foals were used in this study. It is unclear at this point whether these results can be extrapolated to older foals, as maturation of the intestinal microflora in foals is poorly understood.
Lactobacillus rhamnosus GG cannot be considered an equine probiotic at this point. The poor intestinal colonization in adult horses is not encouraging. Unless improved colonization by LGG can be demonstrated in diarrheic or antibiotic-treated horses, further efficacy studies are likely not warranted. This situation may be different in foals. Adequate colonization of LGG in foals without any adverse effects was identified in this study. Efficacy studies should be performed to evaluate this organism in the prevention or treatment of disease in foals. It has been reported that LGG is able to affect antigen transport in the intestinal tract via closure of large molecular transport pores (12). It is unclear whether this property could interfere with passive transfer of maternal antibodies. As a result, it would be prudent to avoid administering LGG to foals less than 24 h of age. CVJ
Footnotes
This study was funded by the American Association of Equine Practitioners and the Ontario Horse Racing Industry Association.
Address all correspondence and reprint requests to Dr. J. Scott Weese; e-mail: jsweese@uoguelph.ca
References
- 1.Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol 1998;39:237–238. [DOI] [PubMed]
- 2.Metchnikoff E. The Prolongation of Life, London. William Heinemann 1907.
- 3.Reid G. In defense of probiotics. ASM News 2000;66:261.
- 4.Parraga ME, Spier SJ, Thurmond M, Hirsh D. A clinical trial of probiotic administration for prevention of Salmonella shedding in the postoperative period in horses with colic. J Vet Int Med 1997;11:36–41. [DOI] [PubMed]
- 5.Kim L, Morley PS, Traub-Dargatz JL, Salman MD, Gentry-Weeks C. Factors associated with Salmonella shedding among colic patients at a veterinary teaching hospital. J Am Vet Med Assoc 2001;218:740–748. [DOI] [PubMed]
- 6.Fuller R. Probiotics in human medicine. Gut 1991;32:439–442. [DOI] [PMC free article] [PubMed]
- 7.Filho-Lima JVM, Vieira EC, Nicoli JR. Antagonistic effect of Lactobacillus acidophilus, Saccharomyces boulardii and Escherichia coli combinations against experimental infections with Shigella flexneri and Salmonella enteritidis subsp. typhimurium in gnotobiotic mice. J Appl Microbiol 2000;88:365–370. [DOI] [PubMed]
- 8.McFarland LV, Surawicz CM, Greenburg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994;271:1913–1918. [PubMed]
- 9.Dunne C, O'Mahoney L, Murphy L, et al. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr 2001;73:386S–392S. [DOI] [PubMed]
- 10.Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 2000;78:80–88. [DOI] [PubMed]
- 11.Ouwehand AC, Niemi P, Salminen SJ. The normal faecal microflora does not affect the adhesion of probiotic bacteria in vitro. FEMS Microbiol Lett 1999;177:35–38. [DOI] [PubMed]
- 12.Gorbach SL. Probiotics and gastrointestinal health. Am J Gastroenterol 2000;95:S1–S4. [DOI] [PubMed]
- 13.Gibson GR, Fuller R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J Nutr 2000;130:391S–395S. [DOI] [PubMed]
- 14.Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotech 2000;84:197–215. [DOI] [PubMed]
- 15.Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S. Survival of Lactobacillus species (Strain GG) in human gastrointestinal tract. Dig Dis Sci 1992;37:121–128. [DOI] [PubMed]
- 16.Alander M, Satokari R, Korpela R, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Env Microbiol 1999;65:351–354. [DOI] [PMC free article] [PubMed]
- 17.Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microbiol Ecol Health Dis 1993;6:119–122.
- 18.Saxelin M, Elo S, Salminen S, Vapaatalo H. Dose response colonisation of faeces after oral administration of Lactobacillus casei strain GG. Microbiol Ecol Health Dis 1991;4:209–214.
- 19.Armuzzi A, Cremonin F, Ojetti V, et al. Effect of Lactobacillus GG supplementation on antibiotic-associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 2001;63:1–7. [DOI] [PubMed]
- 20.Oberhelman RA, Gilman RH, Sheen P, et al. A placebo-controlled trial of Lactobacillus GG to prevent diarrhea in undernourished Peruvian children. J Pediatr 1999;134:15–20. [DOI] [PubMed]
- 21.Saavedra J. Probiotics and infectious diarrhea. Am J Gastroenterol 2000;95:S16–S18. [DOI] [PubMed]
- 22.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 1999;135:564–568. [DOI] [PubMed]
- 23.Oksanen PJ, Salminen S, Saxelin M, et al. Prevention of travellers' diarrhoea by Lactobacillus GG. Ann Med 1990;22:53–56. [DOI] [PubMed]
- 24.Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter Euopean trial. J Pediatr Gastrenterol Nutr 2000;30:54–60. [DOI] [PubMed]
- 25.Raza S, Graham SM, Allen SJ, Sultana S, Cuevas L, Hart CA. Lactobacillus GG promotes recovery from acute nonbloody diarrhea in Pakistan. Pediatr Infect Dis J 1995;14:107–111. [DOI] [PubMed]
- 26.Isolauri E, Juntunen M, Pautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991;88:90–97. [PubMed]
- 27.Lee DJ, Drongowski RA, Coran AG, Harmon CM. Evaluation of probiotic treatment in a neonatal animal model. Pediatr Surg Int 2000;16:237–242. [DOI] [PubMed]
- 28.Saxelin M, Pessi T, Salminen S. Fecal recovery following oral administration of Lactobacillus Strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int J Food Microbiol 1995;25:199–203. [DOI] [PubMed]
- 29.Anonymous. SAS/STAT Software: changes and enhancements through release 6.12, Cary, North Carolina, USA.
- 30.Campbell ML, Ackerman N, Peyton LC. Radiographic gastrointestinal anatomy of the foal. Vet Radiol 1984;25:194–204.


