Summary
OBJECTIVES
To describe temporal trends in baseline clinical characteristics, initial treatment regimens and monitoring of patients starting antiretroviral therapy (ART) in resource-limited settings.
METHODS
We analysed data from 17 ART programmes in 12 countries in sub-Saharan Africa, South America and Asia. Patients aged 16 years or older with documented date of start of highly active ART (HAART) were included. Data were analysed by calculating medians, interquartile ranges (IQR) and percentages by regions and time periods. Not all centres provided data for 2006 and 2005 and 2006 were therefore combined.
RESULTS
A total of 36 715 patients who started ART 1996–2006 were included in the analysis. Patient numbers increased substantially in sub-Saharan Africa and Asia, and the number of initial regimens declined, to four and five, respectively, in 2005–2006. In South America 20 regimes were used in 2005–2006. A combination of 3TC/D4T/NVP was used for 56% of African patients and 42% of Asian patients; AZT/3TC/ EFV was used in 33% of patients in South America. The median baseline CD4 count increased in recent years, to 122 cells/μl (IQR 53–194) in 2005–2006 in Africa, 134 cells/μl (IQR 72–191) in Asia, and 197 cells/μl (IQR 61–277) in South America, but 77%, 78% and 51%, respectively, started with <200 cells/μl in 2005–2006. In all regions baseline CD4 cell counts were higher in women than men: differences were 22 cells/μl in Africa, 65 cells/μl in Asia and 10 cells/μl in South America. In 2005–2006 a viral load at 6 months was available in 21% of patients Africa, 8% of Asian patients and 73% of patients in South America. Corresponding figures for 6-month CD4 cell counts were 74%, 77% and 81%.
CONCLUSIONS
The public health approach to providing ART proposed by the World Health Organization has been implemented in sub-Saharan Africa and Asia. Although CD4 cell counts at the start of ART have increased in recent years, most patients continue to start with counts well below the recommended threshold. Particular attention should be paid to more timely initiation of ART in HIV infected men.
Keywords: HIV/AIDS, antiretroviral therapy, highly active, gender identity, sub-Saharan Africa, Asia, Latin America
Introduction
The World Health Organization (WHO) estimates that as a result of the recent scaling up of antiretroviral combination therapy (ART) in low- and middle-income countries, two million people living with HIV/AIDS were receiving treatment at the end of 2006, representing 28% of the estimated 7.1 million people in urgent need of treatment in these countries. From January 2004 to 2006, the number of patients on ART increased to 1.3 million in Sub-Saharan Africa, to 280 000 in Asia, and to 355 000 in South America and the Caribbean (World Health Organization, UNAIDS, UNICEF 2007). There is now increasing evidence that the immunological and virological responses to treatment in resource-limited countries can equal those in high-income settings (Djomand et al. 2003; Coetzee et al. 2004; Ivers et al. 2005; Tuboi et al. 2005, 2007; Braitstein et al. 2006) and mortality in HIV-infected patients has decreased with the increasing provision of ART. Mortality of patients starting ART has, however, been substantially higher than in industrialised countries, particularly in the first few months of treatment (Braitstein et al. 2006; Ferradini et al. 2006).
The high early mortality rate is related to patients initiating treatment with very low CD4 cell counts and accompanying co-morbidities and opportunistic infections (Lawn et al. 2005, 2006; Bekker et al. 2006; Liechty et al. 2007). An earlier analysis of the ART in Lower-Income Countries (ART-LINC) Collaboration showed that the median CD4 cell count was 108 cells/μl compared to 234 cells/μl in Europe and North America (Braitstein et al. 2006). Because of the large number of HIV-infected patients with advanced disease who are not yet treated, starting treatment earlier is challenging during the scale-up of ART in many settings. Faced with limited resources and shortages of qualified medical personnel, it is unclear to what extent ART programmes in low- and middle-income countries have been able to raise awareness about the need to start ART before serious complications develop and to promote testing and counselling in order to enrol patients at an earlier stage of the infection. It is also unclear to what extent programmes have been able to monitor treatment response in rapidly growing patient populations. We examined time trends in the monitoring and characteristics of patients starting ART using data from the ART-LINC collaboration, a network of treatment programmes in resource-limited settings (Dabis et al. 2005; Braitstein et al. 2006).
Methods
The ART-LINC collaboration of IeDEA
The ART in Lower Income Countries collaboration of the International Databases to Evaluate AIDS (ART-LINC of IeDEA) is a large collaborative network of HIV/AIDS treatment programmes in low and middle income countries in Africa, South America and Asia. The collaboration has been described in detail previously (see also http://www.art-linc.org and http://www.iedea-hiv.org) (Dabis et al. 2005; Braitstein et al. 2006). In brief, the collaboration was set up in 2003 with the aim to define the prognosis of HIV-infected patients treated with HAART in resource-limited settings, to compare the experience between different settings, delivery modes and types of monitoring; and to compare outcomes with those observed in industrialized nations. The data collected at participating sites are transferred to data management and statistical teams at the universities of Bern and Bordeaux, where data are cleaned, merged and analysed according to agreed protocols. The collaborative database is updated regularly. The present analysis includes all data available up to 29 June 2007. At all sites, institutional review boards had approved the collection and transfer of data.
Inclusion criteria and definitions
All patients aged 16 years or older with complete data on sex, date of birth and start of highly active antiretroviral therapy (HAART) were included in the study. HAART was defined as a minimum of three antiretroviral drugs and categorized into NNRTI-based regimen [two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI)], PI-based regimen [two NRTIs and one protease inhibitor (PI)] and other regimens. Advanced stage of disease was defined as WHO stages III or IV or Centers for Disease Control and Prevention (CDC) stage C. Underweight was defined as a body mass index (BMI) <18.5 kg /m2. Measurements of laboratory values closest to the starting date of ART (within 6 months before up to one week after the date of starting ART) were taken as the baseline levels. For very low CD4 values (<25 cells/μl) and high HIV viral loads (>100 000 copies/ml) the time window was extended to one year before starting therapy. To describe trends in monitoring after ART had been initiated, measurements of CD4 cell counts and viral loads nearest to 6 months (within a window of 3–9 months) after start of therapy were evaluated.
Statistical analysis
Descriptive analyses were stratified by region (Sub-Saharan Africa, South America and Asia) and time period. Not all centres provided data for the year 2006 and we therefore combined the years 2005 and 2006 in all analyses. Trends over time were evaluated using a test for trend. Year of starting ART was fitted first as a categorical variable to assess whether the data were compatible with a linear relationship. Logistic regression or linear regression was used for binary or continuous outcomes, respectively, with some analyses restricted to the years 2001 and later, the period ART was scaled up in sub-Saharan Africa and Asia. Stata software (version 9.2, Stata Corporation, College Station, TX, USA) was used for all analyses. Results are presented as medians with interquartile ranges (IQR) and number of patients (%).
Results
Site characteristics
Of 39 089 patients included in the ART-LINC database, 36 715 patients (94%) from 17 ART programmes in 12 countries were included in the analysis (Table 1). Patients with a missing or implausible date of HAART initiation (n = 1973, 5.1%), missing date of birth (n = 344, 0.9%) or missing information on sex (n = 57, 0.2%) were excluded. The majority of sites (11, 65%) were in Sub-Saharan Africa with the remainder from South America and Asia; the number of patients treated at each site ranged from 96 to 7484. Twelve sites were public clinics, two were research sites. One private for-profit clinic and one non-governmental organisation that provided family-based care were also included. All sites, except two, provided ART free of charge.
Table 1.
Characteristics of study sites in Sub-Saharan Africa, South-America and Asia
| Site† | Location | Site characteristics | Start of programme | Number of patients included in analysis | Scale-up site | Routine HIV-1 viral load testing |
|---|---|---|---|---|---|---|
| Sub-Saharan Africa | ||||||
| CEPREF | Abidjan, Côte d’Ivoire | Public, free treatment | 1999 | 2535 | Yes | No |
| 1290 ANRS | Dakar, Senegal | Research site, free treatment | 1998 | 393 | No | Yes |
| AMPATH | Eldoret, Kenya | Public, free treatment | 2002 | 7484 | Yes | No |
| AMU | Kampala, Uganda | Research site, fee-for-service | 2002 | 96 | No | Yes |
| ISS | Mbarara, Uganda | Public, free treatment | 2001 | 1337 | No | No |
| Lighthouse | Lilongwe, Malawi | Public, free treatment since June 2004 | 2000 | 7366 | Yes | No |
| Gugulethu | Cape Town, South Africa | Public, free treatment | 2002 | 2286 | Yes | Yes |
| Khayelitsha | Cape Town, South Africa | Public, free treatment | 2001 | 1730 | Yes | Yes |
| Themba Lethu | Johannesburg, South Africa | Public, free treatment | 1999 | 4464 | Yes | No |
| PHRU | Soweto, South Africa | Public, free treatment | 2004 | 750 | No | Yes |
| MTCT Plus‡ | Several | Family based care, free treatment | 2003 | 1615 | No | No |
| South America | ||||||
| PUMA | Buenos Aires, Argentina | Public, free treatment | 2003 | 390 | No | Yes |
| SOBRHIV | Porto Alegre, Brazil | Public, free treatment | 1996 | 1105 | No | Yes |
| RIOHIV | Rio de Janeiro, Brazil | Public, free treatment | 1996 | 618 | No | Yes |
| Asia | ||||||
| YRG Care | Chennai, India | Private, fee-for-service | 1996 | 4047 | Yes | No |
| HIVNAT | Bangkok, Thailand | Research site, free treatment | 2003 | 278 | No | No |
| MTCT Plus, Thailand | Bangkok, Thailand | Public, free treatment | 2003 | 221 | No | No |
Routine viral load monitoring was defined as at least one measurement between 3 and 9 months after starting HAART, in at least 50% of patients; the definition of scale-up site was based on the cumulative number of patients over time.
Abbreviations listed at the end of the article.
Network including sites in South Africa, Zambia, Kenya, Rwanda, Uganda, Côte d’ Ivoire.
Trends in patient characteristics over time
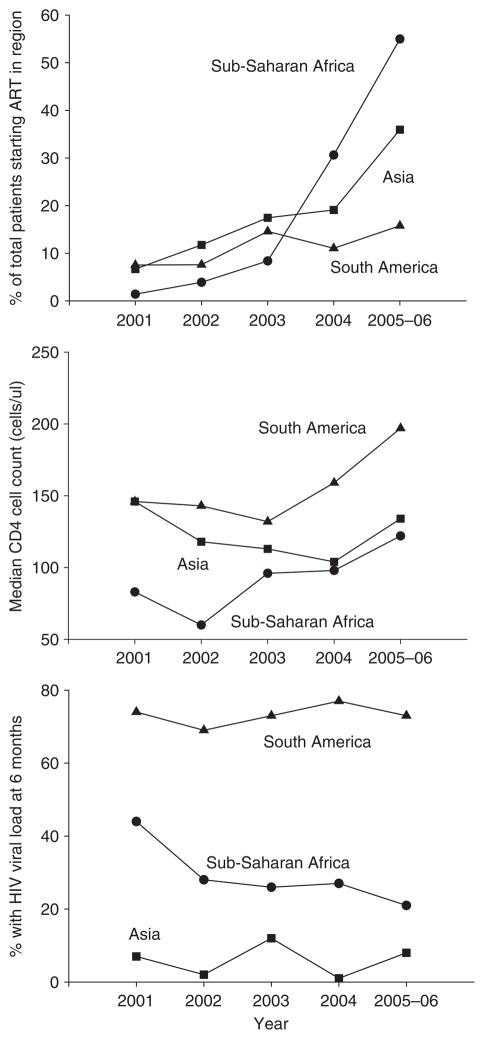
The mean number of patients receiving ART increased substantially in sites from sub-Saharan Africa and Asia, but not in South America where many patients started treatment 1996 to 2000 (Table 2). In sub-Saharan Africa, a 39-fold increase in the number of patients starting ART was observed since 2001 (Figure 1, top panel). The median age at the start of therapy was 35 years with little variation over time and across regions. Men were generally older than women at therapy initiation (median 37 vs. 33 years, P < 0.001), with little variation over time (Table 2). The proportion of women varied across regions: in sub-Saharan Africa 19 571 (65%) of 30 056 patients were women compared to 747 (35%) of 2113 patients in South America and 1415 (31%) of 4546 patients in Asia. In sub-Saharan Africa and Asia the proportion of women increased over time (P = 0.007 and P < 0.001, respectively). In all regions a great majority of patients were treatment naïve.
Table 2.
Demographic and clinical characteristics of patients starting HAART (12 countries, years 1996–2006)
| Region / calendar period | Number of patients | Number of women (%) | Median age in years (IQR)
|
Number of treatment naïve patients (%) | Type of ART regimen (%)
|
Number of regimens used to treat 95% of patients | |||
|---|---|---|---|---|---|---|---|---|---|
| Men | Women | NNRTI-based | PI-based | Other / unknown | |||||
| Sub-Saharan Africa | |||||||||
| 1996–2000 | 230 | 126 (55%) | 42 (35–47) | 34 (29–39) | 199 (87%) | 90 (39%) | 99 (43%) | 41 (18%) | 17 |
| 2001–2002 | 1590 | 940 (59%) | 38 (32–44) | 34 (29–40) | 1321 (83%) | 905 (57%) | 585 (37%) | 100 (6%) | 11 |
| 2003–2004 | 11 711 | 7695 (66%) | 38 (32–44) | 33 (28–39) | 10 287 (88%) | 9214 (79%) | 2122 (18%) | 375 (3%) | 4 |
| 2005–2006 | 16 525 | 10 810 (65%) | 38 (32–44) | 33 (28–40) | 15 475 (94%) | 14,744 (89%) | 1409 (9%) | 372 (2%) | 4 |
| South America | |||||||||
| 1996–2000 | 920 | 299 (33%) | 36 (32–43) | 35 (29–44) | 658 (72%) | 167 (18%) | 722 (78%) | 31 (3%) | 23 |
| 2001–2002 | 319 | 125 (39%) | 37 (32–44) | 35 (28–44) | 294 (92%) | 224 (70%) | 89 (28%) | 6 (2%) | 10 |
| 2003–2004 | 541 | 201 (37%) | 36 (31–42) | 34 (29–41) | 517 (96%) | 355 (66%) | 150 (28%) | 36 (7%) | 19 |
| 2005–2006 | 333 | 122 (37%) | 39 (33–46) | 37 (31–47) | 319 (96%) | 228 (68%) | 82 (25%) | 23 (7%) | 20 |
| Asia | |||||||||
| 1996–2000 | 416 | 102 (25%) | 34 (30–39) | 27 (22–34) | 88 (21%) | 38 (9%) | 49 (12%) | 329 (79%) | 8† |
| 2001–2002 | 837 | 179 (21%) | 34 (30–39) | 27 (23–33) | 623 (74%) | 614 (73%) | 10 (1%) | 213 (25%) | 4† |
| 2003–2004 | 1660 | 527 (32%) | 34 (30–39) | 30 (26–35) | 1521 (92%) | 1513 (91%) | 10 (1%) | 137 (8%) | 3 |
| 2005–2006 | 1633 | 607 (37%) | 35 (32–41) | 30 (27–36) | 1536 (94%) | 1481 (91%) | 43 (3%) | 109 (7%) | 5 |
This is probably an underestimate because data on regimens were missing for a substantial proportion of patients during this period.
Figure 1.
Trends over time in the number of patients starting ART (expressed in per cent of the total number of patients in the region), the median CD4 cell at baseline and the percent of patients with HIV viral load tests at 6 months (12 countries, years 2001–2006).
ART regimens
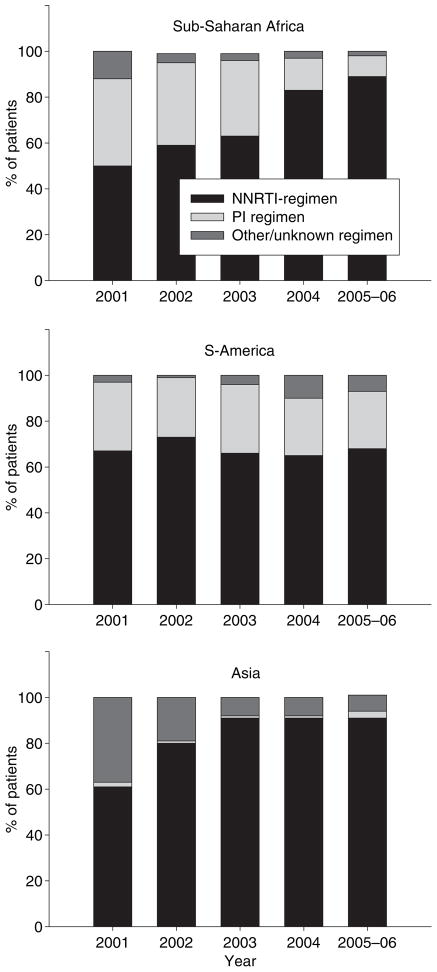
The number of regimens used to treat 95% of patients declined in sub-Saharan Africa, from 17 in the period 1996–2000 to four in 2005–2006. In South America this number declined initially but then rose to 20 different regimens in the most recent period. In Asia there were eight regimens in 1996–2000, but this is probably an underestimate because data on regimens were missing for many patients during this period. In 2005–2006 the number for Asia was five (Table 2). The use of NNRTI-based first-line regimens increased substantially during the study period, and dominated from 2001 onwards (Figure 2). In sub- Saharan Africa and Asia few patients started ART with a PI-based regimen in recent years, but PIs were used by over 25% of patients throughout the study period in South America. The most frequently used regimen in sub-Saharan Africa and Asia was lamivudine (3TC), stavudine (D4T) and nevirapine (NVP) and in South America it was zidovudine (AZT), lamivudine (3TC) and efavirenz (EFV). These regimens were used for 16 811 patients (56%), 1929 patients (42%) and 701 patients (33%) in Africa, Asia and South America, respectively. In the most recent period (2005–2006) 13 576 (82%) patients in sub-Saharan Africa started ART with a D4T-containing regimen compared to 1080 (66%) in Asia and 50 (15%) in South America.
Figure 2.
Type of HAART regimen used in ART-LINC centres in different regions (12 countries, years 2001–2006).
Availability of examinations at baseline and six months
The availability of laboratory measurements at baseline and 6 months after starting ART is summarised in Table 3. In sub-Saharan Africa, the proportion of patients with a baseline CD4 cell count dropped in the early phase of scaling up ART but increased thereafter. In the most recent period, 78% of patients had a CD4 count measured at baseline and 74% at 6 months. In South America the availability of CD4 counts at baseline decreased slightly, to 71% in 2005/06 whereas at 6 months it had remained constant at 81% since 2001–2002. In Asia, 90% and 77% had a baseline and 6-month CD4 count measured in 2005–2006. Viral load testing decreased in sub-Saharan Africa to 12% at baseline, and 21% at 6 months. No clear trends over time were observed for South America and Asia. In the most recent period (2005–2006), the proportion of patients with a viral load measurement at 6 months was 73% in South America and 8% in Asia.
Table 3.
Availability of laboratory measurements at baseline and 6 months after starting ART (12 countries, years 1996–2006)
| Baseline measurements
|
Measurements at 6 months
|
||||||
|---|---|---|---|---|---|---|---|
| Number of patients | CD4 count | HIV viral load | Haemoglobin | Number of patients† | CD4 count | HIV-1 viral load | |
| Sub-Saharan Africa | |||||||
| 1996–2000 | 230 | 188 (82%) | 181 (79%) | 186 (81%) | 216 | 165 (76%) | 166 (77%) |
| 2001–2002 | 1590 | 796 (50%) | 523 (33%) | 530 (33%) | 1332 | 561 (42%) | 438 (33%) |
| 2003–2004 | 11 711 | 7950 (68%) | 2206 (19%) | 5868 (50%) | 8812 | 6189 (70%) | 2357 (27%) |
| 2005–2006 | 16 525 | 12 888 (78%) | 1909 (12%) | 7344 (44%) | 4878 | 3596 (74%) | 1026 (21%) |
| South America | |||||||
| 1996–2000 | 920 | 726 (79%) | 471 (51%) | 9 (1%) | 890 | 651 (73%) | 581 (65%) |
| 2001–2002 | 319 | 246 (77%) | 149 (47%) | 116 (36%) | 308 | 250 (81%) | 220 (71%) |
| 2003–2004 | 541 | 407 (75%) | 311 (57%) | 134 (25%) | 447 | 360 (81%) | 334 (75%) |
| 2005–2006 | 333 | 236 (71%) | 160 (48%) | 192 (58%) | 129 | 105 (81%) | 94 (73%) |
| Asia | |||||||
| 1996–2000 | 416 | 309 (74%) | 58 (14%) | 248 (60%) | 312 | 153 (49%) | 27 (9%) |
| 2001–2002 | 837 | 629 (75%) | 91 (11%) | 624 (75%) | 625 | 312 (50%) | 24 (4%) |
| 2003–2004 | 1660 | 1446 (87%) | 142 (9%) | 1264 (76%) | 1182 | 723 (61%) | 77 (7%) |
| 2005–2006 | 1633 | 1475 (90%) | 143 (9%) | 1354 (83%) | 775 | 594 (77%) | 61 (8%) |
Number of patients (%) are shown.
Number of patients followed up to 6 months.
Baseline haemoglobin was measured in about half of patients in sub-Saharan Africa, with no increase in recent years. In contrast, haemoglobin measurements at baseline became more common over time in South America and Asia: 58% and 83%, respectively, had it measured in the most recent period (Table 3). Among patients with information on the initial HAART regimen, 4165 (14%) of 29 487 patients in sub-Saharan Africa were on an AZT containing regimen, compared to 1576 (76%) of 2086 patients in South America and 1050 (28%) of 3764 patients in Asia (P < 0.001 for differences across regions). Among patients starting an AZT containing regimen, 703 (35%) had haemoglobin measured at baseline in Africa, 356 (23%) in South America and 245 (61%) in Asia. In South America, the proportion of patients on an AZT containing regimen with a baseline haemoglobin increased over time, to 59% in 2005–2006 (P < 0.001). There was little evidence for a trend in this percentage in the other regions.
Finally, in sub-Saharan Africa the availability of the BMI at baseline increased from 556 (35%) patients with a BMI in 2001–2002 to 12 129 patients (73%) in 2005 and 2006. Similarly, 538 (64%) of patients had a BMI measured in 2001–2002 in Asia, compared to 1402 patients (86%) in 2005–2006. Few patients in South America had baseline height recorded.
CD4 counts and clinical stage at the start of ART
The middle panel of Figure 1 shows the median CD4 cell counts in patients starting ART 2001 to 2005–2006. Overall the median count was 114 cells/μl (IQR 48–89 cells/μl) but there was substantial variation by region and time: medians (IQR) were 111 cells/μl (45–184 cells/μl) in sub-Saharan Africa, 123 cells/μl (60–190 cells/μl) in Asia and 151 cells/μl (55–257 cells/μl) in South America. In Sub-Saharan Africa the median count was lowest in the year 2002 (60 cells/μl, IQR 18–147 cells/μl) and increased to 122 cells/μl (IQR 53–194 cells/μl) thereafter (P < 0.001). In South America, CD4 cell counts were consistently higher than in the other regions and reached 197 cells/μl (IQR 61–277 cells/μl) in 2005–2006 (P = 0.003). In Asia there was some increase in CD4 cell counts in recent years, to 134 cells/μl (IQR 72–191) in 2005–2006, but overall there was little evidence for a time trend (P = 0.32).
In 2005–2006 9883 (77%) of 12 888 patients with available measurements from sub-Saharan Africa started ART with a CD4 count below 200 cells/μl compared to 1150 (78%) of 1475 patients in Asia and 120 (51%) of 236 patients in South America. The corresponding figures for starting below 100 cells/μl were 5420 (42%), 539 (37%) and 81 (34%), respectively. In all regions baseline CD4 cell counts were higher in women than men. The mean difference was 22 cells/μl (95% CI 17–26) in sub-Saharan Africa, 65 cells/μl (95% CI 56–75) in Asia and 10 cells/μl (95% CI 5–25) in South America. Trends over time were similar for the two sexes (tests for interaction P > 0.50).
Information on baseline clinical stage was available for 27 391 patients (91%) from sub-Saharan Africa, 1452 patients (69%) from South America and 100% of patients from Asia. Data are strictly not comparable because some sites used the CDC clinical stages and others the WHO classification. Among patients with information on clinical stage, most started treatment with advanced disease, in CDC stage C or WHO stages III or IV: 16 975 (62%) in sub-Saharan Africa, 1148 (79%) in South America and 1986 (44%) in Asia. In Sub-Saharan Africa and Asia over 90% of patients starting HAART in the most recent period had information on WHO stage or a documented CD4 cell count. In Latin America this percentage was about 85% throughout the study period.
Discussion
This study of more than 36 000 patients from 17 ART treatment sites in low- and middle-income countries found that the number of patients starting ART increased substantially in sub-Saharan Africa and Asia, but not in South America. An important finding is that in more recent years patients initiated treatment with less pronounced immunodeficiency, particularly in South America. However, even in 2005–2006 a majority of patients started with a CD4 count of less than 200 cells/μl: 80% in sub-Saharan Africa, 78% in Asia and 61% in South America. In all regions, but particularly in sub-Saharan African and Asia, NNRTI-based regimens dominated, and 82% of patients in sub-Saharan Africa, 66% in Asia and 15% in South America started with a D4T containing regimen. In recent years, viral load was no longer commonly measured at the start of treatment or at 6 months in sub-Saharan Africa and Asia whereas in Latin America about half of patients had their viral load determined at baseline and about 70% at 6 months.
Based on the experience with treating tuberculosis, WHO developed a public health approach to providing ART in resource-limited settings, taking into account the realities of weak health systems, costs and availability of drugs and the experience of pilot programmes (Gilks et al. 2006; WHO 2006). Key characteristics of this approach include the standardisation of first-line and second-line regimens, simplified clinical decision making and standardised clinical and laboratory monitoring (Gilks et al. 2006). Viral load monitoring is not considered essential, and individual drug resistance testing is not recommended. A survey of national guidelines from 43 middle and lower income countries showed that the WHO (2002) guidelines have been adopted widely (Beck et al. 2006). Our study shows that these guidelines have been implemented in sub- Saharan Africa and Asia: the number of regimens in use to treat 95% of patients declined to four and five, respectively. In Brazil and Argentina, national guidelines for ART include routine viral load measurements, and a larger number of first-line regimens are available and used in these countries (Ministry of Health 2004; Sociedad 2006). Indeed, treatment guidelines in Brazil allow for the use of more antiretroviral drugs than any other middle- or lowincome country (Nunn et al. 2007). Of note, the public health approach to ART also influenced the strategy of the pharmaceutical industry, including suppliers of generic drugs. Several competitively priced fixed dose combinations became widely available at the time the guidelines were disseminated, and this may have facilitated implementation in some settings.
The World Health Organization 2002 guidelines recommended ART only for patients with WHO stage IV disease or a CD4 cell count of less than 200 cells/μl (World Health Organization 2002). The revised 2003 guidelines, however, state that in patients with WHO stage III disease, treatment should be considered when the CD4 count is below 350 cells/μl and initiated before the CD4 count drops to below 200 cells/μl (WHO 2004). We found that a majority of patients continue to start treatment well below this threshold. The higher median baseline CD4 in Brazil reflects both the well established treatment programmes with little or no backlog and guidelines that recommend ART at higher CD4 counts. Disease progression and mortality in patients starting HAART is associated with more advanced immunodeficiency (Egger et al. 2002), and it is likely that an earlier start would largely prevent the excess mortality observed in lower income settings during the first few months of therapy (Braitstein et al. 2006). Studies have shown that delays in treatment initiation are related to unawareness of HIV status, costs and stigma, as well as waiting lists, shortage of personnel and organizational problems (Coetzee et al. 2004; Ehiri et al. 2005).
A majority of the patients receiving ART in the African sites were women, and this proportion increased during the study period. In contrast to sub-Saharan Africa, a larger number of men than women received ART in the Latin America and Asian sites. These differences probably reflect differences in the routes of HIV transmission, with heterosexual transmission dominating in Africa, and sex between men and intravenous drug use playing an important role in South America and Asia (UNAIDS 2006). We previously compared UNAIDS data on the gender distribution of HIV infection in the countries represented in ART-LINC with the proportions of women and men receiving ART in the different programmes participating in ART-LINC (Braitstein et al. 2008). We found that the proportion of female ART recipients was similar to, or higher than the estimated proportion of HIV-infected adults who are women in most sub-Saharan countries and the programmes in Brazil, Argentina and Thailand (Braitstein et al. 2008). These findings are supported by a systematic review, which found a female to male ratio of recipients of HAART of more than one in all but two of 21 published studies in seven countries in Southern Africa (Myer et al. 2005). The only ART-LINC centre in India, and one of the largest in the collaboration, requires some patients to pay for treatment. Women’s inferior economic position is an important barrier to health care in India (Go et al. 2003; Fikree & Pasha 2004). This might help explain why women were under-represented in this clinic when compared to the UNAIDS estimates (Braitstein et al. 2008). Note that in previous analyses from this collaboration, survival in the first year of HAART was similar in women and men (hazard ratio 0.84, 95% CI 0.58–1.23) (Braitstein et al. 2006).
Gender inequalities that inhibit men’s use of health care have received relatively little attention (Greig & Lang 2000). While traditional gender roles give men the power to deny women access to health care (Go et al. 2003), the physical and emotional strength associated with masculinity and power can also make it unacceptable for men to seek health care (Greig & Lang 2000). Also, women are often more likely than men to attend services because of dedicated provision of reproductive and child health clinics. Health services that address the needs of men remain under-developed and men are more likely to seek care in the informal sector, such as pharmacies (Collumbien & Hawkes 2000; Varga 2001).
Several studies from North America and Europe (Saah et al. 1994; Moore et al. 1998; Sullivan et al. 1998; Mocroft et al. 1999) showed that anaemia in HIV-infected patients is associated with higher rates of disease progression and death, independently of the CD4 cell count and other prognostic factors. Studies from Ethiopia (Mekonnen et al. 2003) and Asia (Zhou & Kumarasamy 2005) have also shown an association of anaemia with mortality. Similarly, total lymphocyte count and body mass index are independent predictors of disease progression and death (Mekonnen et al. 2003; Anastos et al. 2004). A recent analysis of the of IeDEA cohorts in Southern and West Africa showed that in patients starting ART, a prognostic model that included haemoglobin and total lymphocyte count was similarly powerful compared to a model including the CD4 cell count. This suggests that haemoglobin and total lymphocyte count could potentially be used instead of CD4 in resource-constrained settings (May et al. 2008). However, with the exception of sites in Asia, we found that at present haemoglobin is not widely available in patients starting ART.
Strengths of our study include its large size, the wide geographic coverage, and longitudinal design. ART-LINC of IeDEA is one of the largest databases of HIV-infected patients followed up in resource-limited settings. Many of the sites have provided ART over extended periods of time, which allowed analyses of trends over time. The type and size of the centres vary considerably and include large scale-up cohorts but also smaller research-oriented sites and private clinics. We acknowledge that the clinics and programmes who participate in ART-LINC are not representative of all sites providing ART in the country. The ART-LINC sites are mainly urban and capture data in electronic databases. Furthermore, not all sites have contributed data for all time periods: only relatively few sites were operating in the earlier years before the scale-up of therapy began.
In conclusion, the public health approach to providing ART has been implemented in many ART-LINC sites. However, most patients start ART at advanced disease, with CD4 cell counts well below the recommended thresholds. Further research is urgently needed to better understand the reasons why many patients start with such advanced disease and how this could be effectively addressed (Egger et al. 2005; Lawn et al. 2006). There were some improvements in recent years and it will be important to document to what extent the trend towards higher CD4 cell counts will translate into improved outcomes. Similarly, it will be important to compare outcomes of programmes with and without access to viral load monitoring, and further work is required on the possible role of haemoglobin, total lymphocyte count and BMI in deciding on when to start ART and monitoring of treatment response. Gender equity in access to care and treatment for HIV infection is a complex issue. Women are more vulnerable than men to becoming infected with HIV and continued efforts are needed to empower women and secure their rights to treatment and care for HIV infection. Attention, however, should also be paid to HIV-infected men to ensure that gender stereotypes do not prevent them from accessing ART.
Acknowledgments
We are grateful to all treatment programmes and patients participating in ART-LINC. The ART-LINC collaboration of IeDEA is funded by the US National Institutes of Health (Office of AIDS Research and National Institute of Allergy and Infectious Diseases) and the French Agence Nationale de Recherches sur le Sida (ANRS).
References
- Anastos K, Shi Q, French AL, et al. Total lymphocyte count, hemoglobin, and delayed-type hypersensitivity as predictors of death and AIDS illness in HIV-1-infected women receiving highly active antiretroviral therapy. Journal of 1 Acquired Immune Deficiency Syndrome. 2004;35:383–392. doi: 10.1097/00126334-200404010-00008. [DOI] [PubMed] [Google Scholar]
- Beck E, Vitoria JM, Mandalia S, et al. National adult antiretroviral therapy guidelines in resource-limited countries: concordance with 2003 WHO guidelines? AIDS. 2006;20:1497–1502. doi: 10.1097/01.aids.0000237365.18747.13. [DOI] [PubMed] [Google Scholar]
- Bekker LG, Myer L, Orrell C, et al. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. South African Medicne Journal. 2006;96:315–320. [PubMed] [Google Scholar]
- Braitstein P, Brinkhof MWG, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Boulle A, Nash D, et al. Gender and the use of potent antiretroviral treatment in resource-constrained settings: Findings from a multi-centre collaboration. Journal of Women’s Health. 2008;17:47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- Coetzee D, Hildebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- Collumbien M, Hawkes S. Missing men’s messages: does the reproductive health approach respond to men’s sexual health needs? Cult Health Sex. 2000;2:135–150. doi: 10.1080/136910500300769. [DOI] [PubMed] [Google Scholar]
- Dabis F, Balestre E, Braitstein P, et al. Cohort Profile: Antiretroviral Therapy in Lower Income Countries (ART-LINC): international collaboration of treatment cohorts. International Journal of Epidemiology. 2005;34:979–986. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- Djomand G, Roels T, Ellerbrock T, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d’Ivoire. AIDS. 2003;17(Suppl 3):S5–S15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- Egger M, May M, Chene G, et al. Prognosis of HIV-1- infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- Egger M, Boulle A, Schechter M, et al. Antiretroviral therapy in resource-poor settings: scaling up inequalities? International Journal of Epidemiology. 2005;34:509–512. doi: 10.1093/ije/dyi110. [DOI] [PubMed] [Google Scholar]
- Ehiri JE, Anyanwu CE, Donath E, et al. AIDS-related stigma in sub-Saharan Africa: its contexts and potential intervention strategies. AIDS Public Policy Journal. 2005;20:25–39. [PubMed] [Google Scholar]
- Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- Fikree FF, Pasha O. Role of gender in health disparity: the South Asian context. British Medical Journal. 2004;328:823–826. doi: 10.1136/bmj.328.7443.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilks CF, Crowley S, Ekpini R, et al. The WHO publichealth approach to antiretroviral treatment against HIV in resource- limited settings. Lancet. 2006;368:505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- Go VF, Sethulakshmi CJ, Bentley ME, et al. When HIVprevention messages and gender norms clash: the impact of domestic violence on women’s HIV risk in slums of Chennai, India. AIDS Behavior. 2003;7:263–272. doi: 10.1023/a:1025443719490. [DOI] [PubMed] [Google Scholar]
- Greig A, Lang J. Gender in Development Monograph Series No. 10. UNDP; Geneva: 2000. Men, Masculinities & Development: Broadening Our Work Towards Gender Equality. [Google Scholar]
- Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clinical Infectious Diseases. 2005;41(2):217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Bekker LG, Myer L, et al. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2052. doi: 10.1097/01.aids.0000191232.16111.f9. [DOI] [PubMed] [Google Scholar]
- Lawn SD, Myer L, Harling G, et al. Determinants of mortality and nondeath losses from an antiretroviral treatment service in South Africa: implications for program evaluation. Clinical Infectious Diseases. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- Liechty CA, Solberg P, Were W, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Tropical Medicine and International Health. 2007;12:929–935. doi: 10.1111/j.1365-3156.2007.01874.x. [DOI] [PubMed] [Google Scholar]
- May M, Boulle A, Messou E, et al. Mortality of HIV-1 infected patients starting antiretroviral therapy (ART) in sub- Saharan Africa: the IeDEA Prognostic Model 15th Conference on Retroviruses and Opportunistic Infections; Boston. 2008. [accessed 17 March 3 2008]. Available at: http://www.retroconference.org/2008. [Google Scholar]
- Mekonnen Y, Dukers NH, Sanders E, et al. Simple markers for initiating antiretroviral therapy among HIV-infected Ethiopians. AIDS. 2003;17:815–819. doi: 10.1097/00002030-200304110-00006. [DOI] [PubMed] [Google Scholar]
- Ministry of Health. Recommendations on Antiretroviral Therapy in HIV-infected Adults and Adolescents. MoH; Brasília: 2004. Health Surveillance Secretariat. National STD/AIDS Programme. [Google Scholar]
- Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- Moore RD, Keruly JC, Chaisson RE, et al. Anemia and survival in HIV infection. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology. 1998;19:29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- Myer L, Rabkin M, Abrams EJ, et al. Focus on women: linking HIV care and treatment with reproductive health services in the MTCT-Plus Initiative. Reproduction and Health Matters. 2005;13:136–146. doi: 10.1016/s0968-8080(05)25185-6. [DOI] [PubMed] [Google Scholar]
- Nunn AS, Fonseca EM, Bastos FI, et al. Evolution of Antiretroviral Drug Costs in Brazil in the Context of Free and Universal Access to AIDS Treatment. PLoS Medicine. 2007;4:e305. doi: 10.1371/journal.pmed.0040305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saah AJ, Hoover DR, He Y, et al. Factors influencing survival after AIDS: report from the Multicenter AIDS Cohort Study (MACS) Journal of Acquired Immune Deficiency Syndrome. 1994;7:287–295. [PubMed] [Google Scholar]
- Sociedad Argentina de Infectiologia. Actualización SADI. SAI; Buenos Aires: 2006. Recomendaciones Sobre Tratamiento Antiretroviral. [Google Scholar]
- Sullivan PS, Hanson DL, Chu SY, et al. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- Tuboi SH, Harrison LH, Sprinz E, et al. Predictors of virologic failure in HIV-1-infected patients starting highly active antiretroviral therapy in Porto Alegre, Brazil. Journal of Acquired Immune Deficiency Syndrome. 2005;40:324–328. doi: 10.1097/01.qai.0000182627.28595.01. [DOI] [PubMed] [Google Scholar]
- Tuboi SH, Brinkhof MW, Egger M, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resourceconstrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. Journal of Acquired Immune Deficiency Syndrome. 2007;45:52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Report on the Global AIDS Epidemic. Joint United Nations Programme on AIDS; Geneva: 2006. [Google Scholar]
- Varga CA. The forgotten fifty per cent: a review of sexual and reproductive health research and programs focused on boys and young men in sub-Saharan Africa. African Journal of Reproduction and Health. 2001;5:175–195. [PubMed] [Google Scholar]
- World Health Organization. Scaling up Antiretroviral Therapy in Resource-limited Settings: Guidelines for a Public Health Approach. WHO; Geneva: 2002. [PubMed] [Google Scholar]
- World Health Organization. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach. WHO; Geneva: 2004. 2003 Revision. [Google Scholar]
- World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents in Resource-limited Settings: Towards Universal Access. Recommendations for a Public Health Approach. WHO; Geneva: 2006. [Google Scholar]
- World Health Organization, UNAIDS, UNICEF. Towards Universal Access: Scaling up Priority HIV/AIDS Interventions in the Health Sector: Progress Report, April 2007. WHO; Geneva: 2007. [Google Scholar]
- Zhou J, Kumarasamy N. Predicting short-term disease progression among HIV-infected patients in Asia and the Pacific region: preliminary results from the TREAT Asia HIV Observational Database (TAHOD) HIV Medicine. 2005;6:216–223. doi: 10.1111/j.1468-1293.2005.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]