Abstract
Purpose
World Trade Center (WTC) exposure caused airflow obstruction years after exposure. Chitinases and IgE are innate and humoral mediators of obstructive airway disease. We investigated if serum expression of chitinases and IgE early after WTC exposure predicts subsequent obstruction.
Methods
With a nested case-control design, 251 FDNY personnel had chitotriosidase, YKL-40 and IgE measured in serum drawn within months of 9/11/2001. The main outcome was subsequent Forced Expiratory Volume after one second/Forced Vital Capacity (FEV1/FVC) less than the lower limit of normal (LLN). Cases (N=125) had abnormal FEV1/FVC whereas controls had normal FEV1/FVC (N=126). In a secondary analysis, resistant cases (N=66) had FEV1 (≥107%) one standard deviation above the mean. Logistic regression adjusted for age, BMI, exposure intensity and post-exposure FEV1/FVC modeled the association between early biomarkers and later lung function.
Results
Cases and Controls initially lost lung function. Controls recovered to pre-9/11 FEV1 and FVC while cases continue to decline. Cases expressed lower serum chitotriosidase and higher IgE levels. Increase in IgE increased the odds of airflow obstruction and decreased the odds of above average FEV1. Alternately, increasing chitotriosidase decreased the odds of abnormal FEV1/FVC and increased the odds of FEV1≥107%. Serum YKL-40 was not associated with FEV1/FVC or FEV1 in this cohort.
Conclusions
Increased serum chitotriosidase reduces the odds of developing obstruction after WTC-particulate matter exposure and is associated with recovery of lung function. Alternately, elevated IgE is a risk factor for airflow obstruction and progressive lung function decline.
Keywords: Chitotriosidase, Immunoglobulin E, WTC Particulate Matter, Pulmonary Function Testing
INTRODUCTION
World Trade Center (WTC)-particulate matter (PM) exposure from the 9/11 disaster resulted in significant airflow obstruction and reactive airway disease in Fire Department of New York (FDNY) rescue workers, other exposed workers, lower Manhattan residents and children (1–7). A recent longitudinal study by FDNY reported that exposure to WTC dust led to a dramatic decline in forced expiratory volume in one second (FEV1) of FDNY rescue workers during the first year after exposure without subsequent recovery in a majority of those exposed (8). A minority of those exposed had accelerated decline of lung function suggesting greater than average susceptibility to lung injury (1, 8). Alternately, another subgroup recovered of FEV1 suggesting greater than average resistance to the long-term effects of PM exposure (9). It is unclear why some individuals’ lung function improved while others’ continued to deteriorate. We therefore characterized biomarkers of resistance and susceptibility to WTC PM-related lung injury. We previously described a set of biomarkers of inflammation, metabolic syndrome and vascular injury in serum collected within six months of 9/11/2001 that predicted future decline or improvement in FEV1 (9–11).
The ratio of FEV1/Forced Vital Capacity (FVC) is another well-validated spirometric measure. Reduction of FEV1/FVC indicates airflow obstruction that characterizes COPD and asthma. Genome wide association studies (GWAS) observed that a set of genetic variants are associated with only FEV1, variation at other loci predict only FEV1/FVC and variation at a third set of loci is associated with both FEV1 and FEV1/FVC (12, 13). This suggests independent but overlapping regulation of FEV1 and FEV1/FVC in health and disease. Biomarkers predicting abnormal FEV1/FVC may therefore be distinct from FEV1. Hence, we investigated if biomarkers expressed within 6 months of 9/11/2001 predicted future abnormal FEV1/FVC in this WTC exposed cohort.
The glycosyl hydrolase 18 gene family contains true chitinases that bind and cleave chitin. Other chitinase-like proteins (CLP) bind but do not cleave the chitin polysaccharide. Among these chitinases/CLPs, chitotriosidase is the major enzymatically active chitinase in humans and the best characterized chitinase from a biologic and clinical perspective (14, 15). Chitotriosidase is part of the innate host defense against bacterial and fungal infections since chitin is a major structural component in bacteria, fungi, insects and crustaceans, but not in mammals (16–20). YKL-40 is a CLP that is strongly associated with human diseases characterized by inflammation, remodeling and fibrosis (21–25). However, its biological function has not been clearly defined. Chitotriosidase is produced in mature monocyte-derived macrophages, lung macrophages and other specific subsets of tissue macrophages (26–29). Elevated chitotriosidase expression is associated with smoking induced and fibrotic lung disease (30, 31). The utility of chitotriosidase or YKL-40 as biomarkers of lung disease is under active investigation. However, their utility in particulate matter induced airway obstruction has not been elucidated.
Immunoglobulin E (IgE)-mediated humoral immunity is another important immune response mechanism in the respiratory tract (32). Elevated IgE is a key immune mediator in asthma. Children and adults with asthma have higher IgE than normal controls and anti-IgE antibody is an effective asthma treatment (33–36). Elevated serum IgE is strongly associated with low FEV1/FVC in patients with chronic obstructive lung disease (37).
Our studies have focused on the well phenotyped WTC exposed FDNY firefighter cohort. The intense PM exposure at the WTC site overwhelmed the lung’s normal protective barriers. We hypothesized that particulate matter exposure-induced lung injury is associated with immune mediators of asthma and COPD. We tested if serum chitinases and IgE, expressed soon after a massive PM exposure predict subsequent airway obstruction.
METHODS
Study design
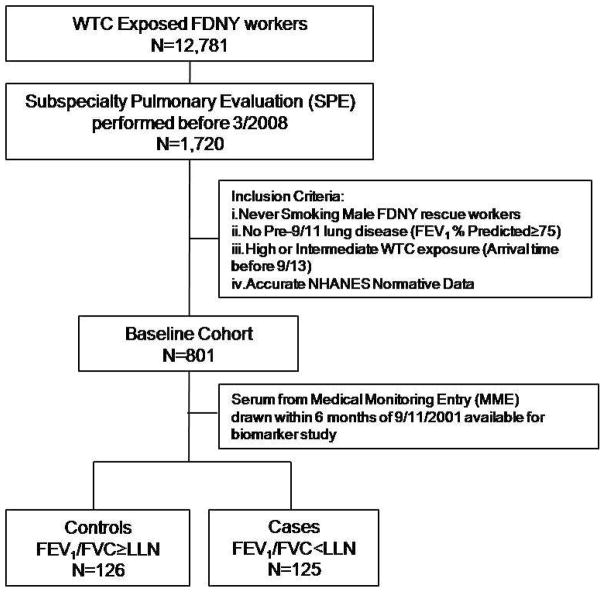
We preformed a nested case-control study using a homogeneous previously healthy baseline cohort of 801 WTC exposed rescue and recovery workers. The study group did not contain individuals with lung disease or tobacco use to eliminate these confounders. The baseline cohort was derived from 1,720 exposed workers who needed subspecialty pulmonary evaluation (SPE) and treatment within 6.5 years of 9/11/2001. The study cohort was derived from three subgroups of the baseline cohort: 1) A randomly selected a subgroup to represent biomarker expression in the total cohort (171/801). 2) Those with the worst FEV1 at SPE (100/801) to represent those susceptible to WTC induced lung injury. 3) Those with the best FEV1 at SPE (100/801) to represent those resistant to lung injury (9). Serum was available in 124/171 of the representative subgroup, 66/100 of the resistant subgroup and 68/100 of the susceptible subgroup. Since there was overlap between subgroups, the study group was 251/801 of the baseline cohort. Cases were defined by FEV1/FVC less than lower limit of normal (LLN, N=125) at SPE, whereas controls had FEV1/FVC greater than LLN (N=126).
Demographics
Age, race and years of service at FDNY were obtained from the FDNY-WTC-monitoring database. BMIs were calculated from height and weight measured at the time of medical monitoring evaluation (MME) and SPE. Degree of exposure was self-reported at the first FDNY-WTC-monitoring and was categorized using the FDNY-WTC Exposure Intensity Index (Arrival Time): i. Presented on the morning of 9/11/2001 ii. Arrived between afternoon on 9/11/2001 and 9/12/2001 (9–11). Those arriving after day three were excluded from analysis as a result of their low numbers in this sample.
Serum Sampling and Analysis
Blood drawn at the first post-9/11 FDNY-WTC monitoring exam was allowed to stand for one hour at room temperature before being centrifuged at 1,800g for ten minutes. Serum was stored at −80°C (Bio-Reference Laboratories, Inc. Elmwood Park, NJ). Serum was thawed once at four degrees and assayed using chitotriosidase (Quidel, USA), YKL-40 (MicroVue, USA) and IgE (R&D, Minnesota, USA) ELISA panels according to manufacturer’s instructions. Data was analyzed using Graphpad Prism V (San Diego, USA).
Statistical Analysis
We tested normality using the Shapiro-Wilk test and Q-Q plots. We used unpaired Student’s t-test or Wilcoxon rank-sum test for between group comparisons, as appropriate. Chi-squared test was used for inferences on proportions.
Given the dichotomous outcome of normal and abnormal FEV1/FVC ratio, we tested if serum biomarkers predicted airflow obstruction using logistic regression. In addition to demographic variables, we tested if FEV1/FVC ratio at the time serum was drawn altered the biomarker disease association. Equivalent analysis was done with the dichotomous outcome of FEV1.
The Hosmer-Lemeshow goodness-of-fit test was used to assess calibration of the model. The model discrimination was evaluated through the receiver operating characteristic area under the curve (AUC). To test the robustness of the models, internal validation was performed using bootstrap procedures (10,000 bootstrap samples). Data are expressed as mean (standard deviation, SD), median (interquartile range, IQR) or Odds Ratio (95% confidence interval), unless otherwise stated. A two-sided P-value less than 0.05 was considered significant. All analyses were performed with STATA/SE version 12.1 (StataCorp LP, College Station, TX) and SPSS version 20 (IBM, USA).
Results
Demographics
Derivation of cases and controls is described in Figure 1. The demographics of baseline cohort, the study cohort, cases and controls are shown in Table 1. A study cohort of 251/801 had biomarkers measured in serum drawn soon after 9/11 during the first medical monitoring exam (MME). The median time to the serum draw at MME was 2.6 months post 9/11. The baseline cohort, study cohort, cases and controls had no significant difference in the time post 9/11 that serum biomarkers were measured.
Figure 1.
Study design. WTC=World Trade Center; FDNY=Fire Department City of New York; NHANES=National Health and Nutrition Examination Survey; PFT=Pulmonary Function Test
Table 1.
Demographics
| Baseline Cohort N=801 | Study Group N=251 | Controls N=126 | Cases N=125 | |
|---|---|---|---|---|
| High WTC Exposure*‡ | 197 (25) | 52 (21) | 30 (24) | 22 (18) |
| Intermediate WTC Exposure†‡ | 604 (75) | 199 (79) | 96 (76) | 103 (82) |
| Months to MME§ | 2.7 (2–3.8) | 2.6 (2.1–3.3) | 2.6 (2.1–3.3) | 2.7 (2.1–3.7) |
| Months to SPE§ | 33.8 (25–57) | 33.6 (24–54) | 32.0 (24–53) | 35.5 (24–57) |
| BMI at MME, kg/m2 § | 28.0 (26–30) | 27.9 (26–30) | 27.6 (26–30) | 28.1 (26–31) |
| BMI at SPE, kg/m2 § | 28.9 (27–31) | 28.8 (27–31) | 28.5 (26–31) | 29.1 (27–31) |
| Age at hire, years§ | 27 (24–29) | 27 (25–29) | 27 (25–30) | 27 (25–29) |
| Age at 9/11, years§ | 40 (36–45) | 41 (37–46) | 42 (38–46) | 40 (36–45) ll |
| Years of Service at 9/11§ | 13.9 (7–19) | 14.5 (8–18) | 15.3 (10–20) | 13.1 (7–17) ll |
| Race, Caucasian/African American‡ | 782 (98)/19 (2) | 242 (96)/9 (4) | 121 (96)/5 (4) | 121 (97)/4 (3) |
Definition of abbreviations: WTC = World Trade Center; MME = medical monitoring entry; SPE = subspecialty pulmonary evaluation; BMI = body mass index.
Arrival at WTC 9/11 Morning,
Arrival at WTC between noon of 9/11 and midnight of 9/12.
Expressed as N (%);
Values Expressed as Medians (IQR);
p<0.01 by Wilcoxon rank-sum test between cases and controls.
Case status was defined by FEV1/FVC ratio at subsequent subspecialty pulmonary evaluation (SPE). Cases of airflow obstruction (125/251) have had an abnormally low FEV1/FVC ratio at SPE, while controls (126/251) have a normal ratio. The median time to SPE was 33.8 months post 9/11. The baseline cohort, study cohort, cases and controls had no significant difference in the time post-9/11 that FEV1/FVC was measured. Cases and controls had similar WTC exposure intensity, BMI and racial distribution. Cases were two years younger at 9/11 and had 2.2 less years of firefighting service than controls (P<0.01).
Pulmonary Function
By design, cases and controls were markedly different from one another in FEV1/FVC ratio 34 months after 9/11/2001 on SPE spirometry (0.73 vs 0.82, Table 2). Cases and controls were also different from one another in pre-9/11 ratio (0.82 vs 0.87 P<0.001) and ratio at MME (0.81 vs 0.87 P<0.001). In a longitudinal analysis, the FEV1/FVC ratio did not change in cases or controls from pre-9/11 spirometry to MME. In the interval from MME to SPE, the ratio declined in both cases (0.81 to 0.73 P<0.001) and controls (0.87 to 0.82 P<0.01) but cases had more deterioration than controls. To confirm that the median FEV1/FVC in cases and controls represented individual deterioration, we used patients as their own control subjects. The mean individual reduction of the ratio from 9/11 to SPE was 9% (P=0.019) in cases and 4% in controls (P<0.001).
Table 2.
Pulmonary Function Testing
| Study Group N=251 | Controls N=126 | Cases N=125 | P-value* | ||
|---|---|---|---|---|---|
| Pre-9/11 Spirometry | FEV1/FVC, % | 84 (81–87) | 87 (85–89) | 82 (79–84) | <0.001 |
| FEV1, L | 4.3 (3.8–4.9) | 4.5 (3.9–5.1) | 4.1 (3.7–4.7) | <0.001 | |
| FEV1, % Predicted | 104 (91–115) | 109 (100–119) | 93 (86–107) | <0.001 | |
| FVC, L | 5.0 (4.6–5.8) | 5.1 (4.5–5.8) | 4.9 (4.6–5.7) | 0.564 | |
| FVC, % Predicted | 96 (87–108) | 100 (91–110) | 93 (85–106) | 0.011 | |
|
| |||||
| MME Spirometry | FEV1/FVC, % | 84 (80–87) | 87 (84–89) | 81 (77–84) | <0.001 |
| FEV1, L | 3.8 (3.3–4.3) | 4.0 (3.6–4.5) | 3.6 (3.2–4.2) | <0.001 | |
| FEV1, % Predicted | 92 (81–103) | 97 (89–107) | 86 (77–97) | <0.001 | |
| FVC, L | 4.6 (4.1–5.2) | 4.6 (4.1–5.2) | 4.7 (4.1–5.1) | 0.776 | |
| FVC, % Predicted | 88 (81–97) | 90 (82–99) | 86 (78–95) | 0.034 | |
|
| |||||
| SPE Spirometry | FEV1/FVC, % | 76 (73–80) | 82 (79–84) | 73 (69–76) | <0.001 |
| FEV1, L | 3.8 (3.2–4.5) | 4.2 (4.1–5.2) | 3.4 (3.0–4.0) | <0.001 | |
| FEV1, % Predicted | 95 (76–109) | 108 (94–114) | 81 (73–95) | <0.001 | |
| FVC, L | 5.0 (4.3–5.7) | 5.1 (4.3–5.8) | 4.9 (4.3–5.5) | 0.162 | |
| FVC, % Predicted | 96 (86–108) | 101 (89–108) | 91 (83–103) | 0.003 | |
|
| |||||
| SPE Plethysmograph and Diffusion | TLC% Predicted | 103 (93–110) | 102 (85–106) | 104 (96–112) | 0.031 |
| FRC% Predicted | 95 (84–107) | 86 (72–103) | 100 (87–114) | 0.212 | |
| RV, % Predicted | 123 (108–141) | 114 (98–128) | 132 (115–157) | <0.001 | |
| DLCO, % Predicted | 107 (15) | 106 (21) | 109 (15) | 0.793 | |
|
| |||||
| SPE Airway Reactivity | MCT Slope, % ΔFEV1/mg | 0.054 (0.03–0.14) | 0.038 (0.02–0.07) | 0.092 (0.04–0.42) | <0.001 |
| BD Response, % | 8 (3–15) | 4 (2–8) | 12 (5–18) | <0.001 | |
Definition of abbreviations: MME = medical monitoring entry; SPE = subspecialty pulmonary evaluation. MCT = methacholine challenge test
All Values Expressed as Median (IQR) or Mean (SD);
Significance assessed by Wilcoxon rank-sum test or unpaired Student t-test between cases and controls. SPE Plethysmograph and Diffusion: Total N=121; Controls N=55; Cases N=66. BD Response: Total N=130; Controls N=56; Cases N=74. MCT Slope: Total N=182; Controls N=97; Cases N=85.
Similar to FEV1/FVC ratio, FEV1 % predicted was consistently lower in cases than controls at all time points. Surprisingly, in longitudinal analysis, cases lost 7% of their pre-9/11 FEV1 while controls lost 11% at MME. Cases continued to lose FEV1 from MME to SPE (86% to 81%), whereas FEV1 in controls increased between two time points (97% to %108) returning to pre-9/11 values. Individual longitudinal lung function demonstrated the mean FEV1 increased by 5% from 9/11 to SPE in cases (P=0.019) and decreased by 5% in controls (P=0.001). The mean individual FVC increased by 6% in cases (P<0.001) and 8% in controls (P<0.001).
At the time of SPE, cases had more air trapping and airway reactivity than controls. Cases had higher residual volume (RV) than controls (130% vs 111% P<0.001) without changes in functional residual capacity (FRC) or diffusing capacity for carbon monoxide (DLCO). Cases had more airway reactivity defined by FEV1 increase in response to bronchodilator (9, IQR 4–16 in cases vs 4, IQR 0–8 in controls P<0.001) and FEV1 decline in response to methacholine (MCT slope of cases 0.092, IQR 0.04–0.42 vs controls 0.038, IQR 0.02–0.07 P<0.001).
Serum Biomarkers
A total of 251 cases and controls had serum chitotriosidase, YKL-40 and IgE measured an average of 3 months after September 11, 2001. Compared to controls, cases had significant reduction of chitotriosidase (24.5ng/mL vs 32.2ng/mL, P<0.001) and significant elevation of IgE (50IU/mL vs 35.7IU/mL, P=0.01) (Table 3). There was no difference in YKL-40 between cases and controls (41.0ng/mL vs 44.0ng/mL, P=0.621). The range of chitotriosidase concentration was 1 to 174ng/mL and the range of IgE was 1.47 to 641IU/ml. Since the major source of chitotriosidase is phagocytes, we assessed if controls and cases had different leukocyte concentrations and differentials to rule out confounding effect. There was no significant difference in total leukocyte concentration, absolute neutrophil count and absolute lymphocyte count (P=0.549, 0.942 and 0.057 respectively) between cases and controls.
Table 3.
Serum Levels of Analytes
| Serum Analyte | Total | Controls | Cases | P-value* |
|---|---|---|---|---|
| Chitotriosidase (ng/mL) | 29.9 (18–46) | 32.2 (20–52) | 24.5 (15–40) | <0.001 |
| IgE (IU/mL) | 44.6 (17–116) | 35.7 (11–81) | 50 (24–148) | 0.010 |
| YKL-40 (ng/mL) | 42.1 (30–61) | 41.0 (31–65) | 44.0 (30–59) | 0.621 |
All Values Expressed as Median (IQR);
Significance assessed by Wilcoxon rank-sum test between cases and controls.
Logistic model predicting abnormal FEV1/FVC
In an initial series of univariable analysis we observed significant association of abnormal FEV1/FVC ratio at SPE with chitotriosidase, IgE, age and FEV1/FVC ratio at the time serum was drawn. Increasing age, chitotriosidase and FEV1/FVC ratio at MME reduced the odds of abnormal FEV1/FVC ratio at SPE while increasing IgE increased the odds of airflow obstruction (Table 4). There was no association between YKL-40 and airflow obstruction (data not shown). In this small cohort, there was no significant association of WTC exposure intensity or BMI with abnormal FEV1/FVC ratio at SPE (Table 4).
Table 4.
Analysis Predicting FEV1/FVC<LLN at SPE
| Model | Predictor | OR (95% CI) |
|---|---|---|
| Univariable | Age (yr) | 0.94 (0.90, 0.98) |
| BMI (kg/m2) | 1.02 (0.97, 1.08) | |
| Exposure High* | 0.68 (0.40, 1.27) | |
| FEV1/FVC at MME | 0.78 (0.72, 0.83) | |
| Chitotriosidase† | 0.84 (0.75, 0.94) | |
| IgE‡ | 1.23 (1.01, 1.50) | |
|
| ||
| Multivariable§ | Chitotriosidase† | 0.82 (0.71, 0.95) |
| IgE‡ | 1.36 (1.09, 1.71) | |
Definition of abbreviations: LLN = lower limit of normal; SPE = subspecialty pulmonary evaluation; OR = odds ratio; BMI = body mass index; MME = medical monitoring evaluation.
Reference is intermediate exposure group.
10 ng/mL of Chitotriosidase
100 IU/mL of IgE.
Adjusted for age at 9/11/2001, BMI at SPE, exposure group and FEV1/FVC at MME. χ2 (6) = 49.465, P = <0.001. Hosmer and Lemeshow’s goodness-of-fit test P=0.588. Area under ROC curve=0.86.
Multivariable logistic regression analysis showed that the biomarker-disease association remained after adjusting the model for potential confounders (Table 4). For each 10ng/mL increase of serum chitotriosidase the odds airflow obstruction at SPE declined by 18% (95%CI: 0.71, 0.95). We added the range of chitotriosidase to the adjusted logistic model and calculated the probability of abnormal FEV1/FVC ratio (Figure 2). When holding all other variables in the model constant the probability of obstruction declined from 60.2% to 15.5% as the concentration of chitotriosidase increased from 1 to 174ng/mL (Figure 2 panel A). For each 100IU/mL increase of IgE, the odds of having a FEV1/FVC less than LLN increased by 36% (95%CI: 1.09, 1.71). The probability of obstruction increased from 44.8% to 74.9% as the concentration of IgE increased from 1.47 to 641IU/mL (Figure 2 panel B). We then used receiver operating characteristic analysis to assess the sensitivity and specificity of the model for predicting airflow obstruction. The model had excellent performance with an AUC of 0.86 (Figure 2 panel C). The interaction term between chitotriosidase and IgE was not significant (data not shown).
Figure 2.
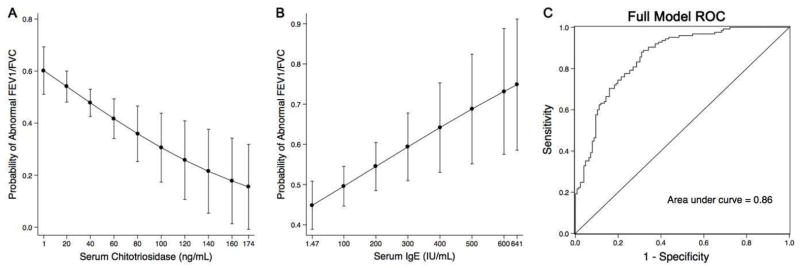
Final logistic model prediction A. Calculated probability of FEV1/FVC<LLN with 95% CI as the concentration of chitotriosidase increased over the observed biomarker range with all other covariates held constant. B. Calculated probability of FEV1/FVC<LLN as IgE increases C. Receiver operating characteristic (ROC) of the final model to predict abnormal FEV1/FVC. Area under the curve (AUC)=0.86.
FEV1 as an outcome
FEV1 is another well-validated spirometric measure to identify airway obstruction (9). We repeated the analysis with 66 individuals who had FEV1≥107% at SPE. These cases represent individuals with above average lung function who were resistant to the damaging effects of WTC exposure. We have previously reported the demographics and PFT of these resistant cases (9). Resistant cases had higher chitotriosidase than controls (26.2ng/mL vs 35.3ng/mL, P=0.007) and significant reduction of IgE (50.0IU/mL vs 35.7IU/mL, P=0.026). There was no difference in YKL-40 between cases and controls (43.2ng/mL vs 41.8ng/mL, P=0.621). For each 10ng/mL increase of serum chitotriosidase the odds of being in resistant group at SPE increased by 14% (95%CI: 1.012, 1.292). There was no association between YKL-40 and improvement in lung function after WTC exposure (95% CI: 0.991, 1.009). For each 100 IU increase in IgE the OR for resistance was 0.887 (95% CI: 0.71, 1.11). In addition, we tested if previously reported biomarkers of FEV1 (9–11) were correlated with chitotriosidase or IgE. There was significant correlation between chitotriosidase and soluble vascular cell adhesion molecule (sVCAM) (R=0.197, P=0.002). Macrophage-derived chemokine (MDC), granulocyte-macrophage colony-stimulating factor (GM-CSF), leptin, amylin, ApoAII, C-reactive protein (CRP), mMacrophage inflammatory protein (MIP) were not associated with either chitotriosidase or IgE.
DISCUSSION
The WTC collapse produced a massive acute exposure to PM leading to a 439mL reduction of FEV1 over the first six months in never smoking firefighters (8). FVC declined in parallel producing a pseudo-restrictive pattern on spirometry that was due to air trapping (38). Some individuals recovered while others had progressive lung function decline. To better understand intrinsic susceptibility and resistance to the damaging effects of WTC exposure we conducted a series of nested case-control studies (9–11). The study cohort was 801 never smokers with normal pre-9/11 lung function who sought subspecialty pulmonary evaluation (SPE) prior to March 2008. We have reported that levels of serum biomarkers of inflammation, metabolic syndrome and cardiovascular risk expressed within 6 months of September 11, 2011 were associated with increased risk of abnormal FEV1 years later, with higher levels of some associated with increased risk and others associated with reduced risk. We now report early elevation of serum chitotriosidase reduces the risk of airway obstruction defined by abnormal FEV1/FVC. It also predicts above average FEV1 in the years following WTC exposure. Therefore chitotriosidase is a biomarker of resistance to lung injury. Alternately elevated IgE is a risk factor for abnormal FEV1/FVC and reduces the odds of above average lung function post WTC exposure. Finally, there was no association of YKL-40 and any measure of lung function. This suggests that YKL-40 regulation in PM-induced asthma is significantly different than YKL-40 in allergic asthma (21–25).
The study cohort was nested within a larger, longitudinally followed population of symptomatic FDNY firefighters who were exposed to particulate matter from the WTC disaster. We narrowed 1720 subjects to a baseline cohort of 801 individuals. We chose to exclude active and prior smokers to eliminate a major confounder of abnormal lung function and chitotriosidase expression (30).
This investigation used FEV1/FVC and FEV1 as two separate outcomes in the analysis. We chose FEV1/FVC below LLN to define lung injury since this cut point is derived from large cross sectional population studies. We chose FEV1≥107% to define resistance to lung injury since this value is one standard deviation above the mean at SPE in this WTC exposed cohort. Prior studies of this cohort used only FEV1 as a measure of susceptibility or resistance to lung injury. Adding an explicit measure of airflow obstruction to the analysis enabled us to overcome the limitations of using of FEV1 as a single measure of lung injury in this cohort. Using only FEV1 can result in misclassification of disease since FEV1 is reduced in both restrictive and obstructive lung disease. Furthermore patients who starting with pre-9/11 FEV1 well above 107%, could suffer large, clinically significant decline in FEV1 and remain above the cut point defining resistance to lung injury. Therefore FEV1/FVC, when used in conjunction with FEV1, provides a more complete measure of the resistance and susceptibility to the damaging effects of WTC exposure.
Both serum chitotriosidase and IgE expressed early after exposure are biomarkers for abnormal FEV1/FVC and above average FEV1 years later. The concept that FEV1/FVC and FEV1 may reflect distinct but overlapping pathophysiologic mechanisms of lung disease is supported by GWAS findings (12, 13). Some genetic polymorphisms are associated with both FEV1 and FEV1/FVC, whereas other variants are associated with one or the other. To date chitotriosidase and IgE are the only biomarkers that predict susceptibility or resistance to FEV1/FVC. Previously reported biomarkers of FEV1(9–11); MDC, GM-CSF, leptin, amylin and apolipoproteins are not associated with airflow obstruction.
Patients with normal FEV1/FVC and above average FEV1 years after WTC exposure had higher serum chitotriosidase than controls. Multivariable logistic regression showed that patients with increased serum chitotriosidase soon after exposure had significantly reduced odds of subsequent airflow obstruction defined by abnormal FEV1/FVC. They also had increased odds of above average FEV1. In fact, these resistant individuals returned to their pre-9/11 FEV1 and FVC on average 32 months post-9/11. Chitotriosidase is therefore a biomarker of recovery from lung injury. This suggests the group with high chitotriosidase levels had the capacity to repair irritant induced lung injury. We have previously observed sVCAM also predicts average FEV1. Interestingly sVCAM expression and chitotriosidase expression are correlated raising the possibility that they are separate components of protective pathways.
Our nested case-control biomarker study has the advantage that serum biomarkers were measured on average 3 months after the acute exposure during the process of disease evolution. The outcome occurred on average 32 months after exposure. Because the biomarkers were measured prior to case definition it is unlikely that its expression is a consequence of good outcome. Chitotriosidase plays an important role in innate immunity and is associated with smoking, sarcoidosis, pulmonary infections, as well as interstitial lung disease (30, 31, 39–42). These cross sectional studies are unable to distinguish if elevated chitotriosidase cause disease, are a reparative response to disease or are a consequence of disease with no impact on lung function. The potential mechanism of chitotriosidase’s role in protecting from WTC-PM induced lung injury is unknown. Our data are consistent with the hypothesis that chitotriosidase plays a beneficial role in injury repair after WTC-PM induced lung. Testing this hypothesis requires experiments in model systems in combination with larger longitudinal studies.
In contrast to chitotriosidase, IgE is critical for humoral immune function in the lung. IgE’s association with airway obstruction and atopy is well known (32–37). As expected, increased IgE predicted subsequent airway obstruction in our WTC exposed cohort. We also observed that increasing IgE reduced the odds of above average FEV1 post exposure. Importantly, no member of this cohort had atopic asthma prior to exposure since the presence of asthma precludes active firefighting duty. Also most of the population had IgE levels in the normal range. IgE’s association with WTC-PM induced lung injury implies a complex pathophysiologic response to irritant induced lung injury reflected in the humoral immune system. Further investigation is required to assess if antigen specific antibodies are responsible for the observed phenotype or variation of IgE within the normal range reflects other attributes of the immune response to respiratory irritants that increases the risk for lung injury.
This study has several limitations. Our FDNY firefighters cohort is unique as they had massive acute exposure to WTC dusts. This limits the generalizability of these finding to other study populations with lower level PM exposure produced by ambient air pollution. We did not have an unexposed control group to compare and therefore we could not determine the direct effect of WTC-PM exposure on chitotriosidase or IgE levels. Replication of these findings in other longitudinally followed populations with and without PM exposure will be important to demonstrate the generalizability of these findings.
In this study, increased serum chitotriosidase levels reduced the risk of developing airway obstruction after WTC-PM exposure and predicted above average FEV1 post exposure. Alternately, elevated IgE was a risk factor for abnormal FEV1/FVC and reduced the odds of above average FEV1. These results suggest that both innate and humoral immune mediators are involved in the pathogenesis of WTC-PM mediated lung injury. Further investigation is required to define if chitotriosidase is a mediator of lung repair after PM exposure.
Acknowledgments
Funding: K23HL084191 (AN), K24A1080298 (MDW), RO1HL057879; (MDW), HL090316, Al080298A, TL1RR029892; T32 ES007267 (BN, SJC); U01CA008617, RO1HL090316 (WNR), NIOSH/CDC (U10-OH008243, U10-OH008242), and 1 UL1RR029893. This work was also partially funded by the NYU-HHC Clinical and Translational Science Institute, supported in part by grant UL1TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health. The funding agencies did not participate in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Author Contributions: conception and design, S.J.C, M.D.W., and A.N; analysis and interpretation, S.J.C., M.D.W., G.C.E., A.N., S.K.; drafting the manuscript for important intellectual content, S.J.C., M.D.W., A.N., S.K., E.S., J.T., D.J.P., W.N.R.
Disclosures: The authors of this manuscript have no actual or potential conflicts of interest to disclose.
References
- 1.Prezant DJ, Weiden M, Banauch GI, McGuinness G, Rom WN, Aldrich TK, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. The New England journal of medicine. 2002 Sep 12;347(11):806–15. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 2.Banauch GI, Dhala A, Alleyne D, Alva R, Santhyadka G, Krasko A, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Critical care medicine. 2005 Jan;33(1 Suppl):S102–6. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 3.Banauch GI, Hall C, Weiden M, Cohen HW, Aldrich TK, Christodoulou V, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. American journal of respiratory and critical care medicine. 2006 Aug 1;174(3):312–9. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman DM, Baron SL, Bernard BP, Lushniak BD, Banauch G, Arcentales N, et al. Symptoms, respirator use, and pulmonary function changes among New York City firefighters responding to the World Trade Center disaster. Chest. 2004 Apr;125(4):1256–64. doi: 10.1378/chest.125.4.1256. [DOI] [PubMed] [Google Scholar]
- 5.Banauch GI, Alleyne D, Sanchez R, Olender K, Cohen HW, Weiden M, et al. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. American journal of respiratory and critical care medicine. 2003 Jul 1;168(1):54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 6.Reibman J, Lin S, Hwang SA, Gulati M, Bowers JA, Rogers L, et al. The World Trade Center residents’ respiratory health study: new-onset respiratory symptoms and pulmonary function. Environmental health perspectives. 2005 Apr;113(4):406–11. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(CDC) CfDCaP. Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center-Manhattan, New York. JAMA. 2002;288(12):1466–7. [PubMed] [Google Scholar]
- 8.Aldrich TK, Gustave J, Hall CB, Cohen HW, Webber MP, Zeig-Owens R, et al. Lung function in rescue workers at the World Trade Center after 7 years. The New England journal of medicine. 2010 Apr 8;362(14):1263–72. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiden MD, Naveed B, Kwon S, Jung Cho S, Comfort AL, Prezant DJ, et al. Cardiovascular disease biomarkers predict susceptibility or resistance to lung injury in World Trade Center dust exposed firefighters. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2012 Aug 16; [Google Scholar]
- 10.Nolan A, Naveed B, Comfort AL, Ferrier N, Hall CB, Kwon S, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest. 2012 Aug;142(2):412–8. doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naveed B, Weiden MD, Kwon S, Gracely EJ, Comfort AL, Ferrier N, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. American journal of respiratory and critical care medicine. 2012 Feb 15;185(4):392–9. doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot RG, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. The Journal of allergy and clinical immunology. 2008 Nov;122(5):944–50. e3. doi: 10.1016/j.jaci.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminuddin F, Akhabir L, Stefanowicz D, Pare PD, Connett JE, Anthonisen NR, et al. Genetic association between human chitinases and lung function in COPD. Human genetics. 2012 Jul;131(7):1105–14. doi: 10.1007/s00439-011-1127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007 Oct;177(2):959–70. doi: 10.1534/genetics.107.075846. Epub 2007/08/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Funkhouser JD, Aronson NN., Jr Chitinase family GH18: evolutionary insights from the genomic history of a diverse protein family. BMC Evol Biol. 2007;7:96. doi: 10.1186/1471-2148-7-96. Epub 2007/06/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 1993 Apr;41(4):571–8. doi: 10.1177/41.4.8450196. [DOI] [PubMed] [Google Scholar]
- 17.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annual review of microbiology. 1994;48:471–97. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 18.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Molecular and biochemical parasitology. 1985 Oct;17(1):93–104. doi: 10.1016/0166-6851(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 19.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. Journal of cell science. 1976 Jun;21(1):73–82. doi: 10.1242/jcs.21.1.73. [DOI] [PubMed] [Google Scholar]
- 20.Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Experimental parasitology. 1994 Aug;79(1):85–8. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- 21.Ober C, Tan Z, Sun Y, Possick JD, Pan L, Nicolae R, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. The New England journal of medicine. 2008 Apr 17;358(16):1682–91. doi: 10.1056/NEJMoa0708801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. The New England journal of medicine. 2007 Nov 15;357(20):2016–27. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 23.Sohn MH, Lee JH, Kim KW, Kim SW, Lee SH, Kim KE, et al. Genetic variation in the promoter region of chitinase 3-like 1 is associated with atopy. American journal of respiratory and critical care medicine. 2009 Mar 15;179(6):449–56. doi: 10.1164/rccm.200809-1422OC. [DOI] [PubMed] [Google Scholar]
- 24.Lee CG, Elias JA. Role of breast regression protein-39/YKL-40 in asthma and allergic responses. Allergy, asthma & immunology research. 2010 Jan;2(1):20–7. doi: 10.4168/aair.2010.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. American journal of respiratory and critical care medicine. 2012 Apr 1;185(7):692–4. doi: 10.1164/rccm.201202-0203ED. [DOI] [PubMed] [Google Scholar]
- 26.Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clinical chemistry and laboratory medicine: CCLM/FESCC. 2005;43(5):499–502. doi: 10.1515/CCLM.2005.088. [DOI] [PubMed] [Google Scholar]
- 27.Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. Journal of clinical laboratory analysis. 2005;19(3):128–32. doi: 10.1002/jcla.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. The Journal of biological chemistry. 1995 Feb 3;270(5):2198–202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 29.van Eijk M, van Roomen CP, Renkema GH, Bussink AP, Andrews L, Blommaart EF, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. International immunology. 2005 Nov;17(11):1505–12. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 30.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. American journal of respiratory cell and molecular biology. 2009 Oct;41(4):379–84. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Letuve S, Kozhich A, Humbles A, Brewah Y, Dombret MC, Grandsaigne M, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. The American journal of pathology. 2010 Feb;176(2):638–49. doi: 10.2353/ajpath.2010.090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Postma DS, Bleecker ER, Amelung PJ, Holroyd KJ, Xu J, Panhuysen CI, et al. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. The New England journal of medicine. 1995 Oct 5;333(14):894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 33.Beeh KM, Ksoll M, Buhl R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2000 Oct;16(4):609–14. doi: 10.1034/j.1399-3003.2000.16d07.x. [DOI] [PubMed] [Google Scholar]
- 34.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. The New England journal of medicine. 1991 Oct 10;325(15):1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 35.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. The New England journal of medicine. 2011 Mar 17;364(11):1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Annals of internal medicine. 2011 May 3;154(9):573–82. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 37.Renkema TE, Kerstjens HA, Schouten JP, Vonk JM, Koeter GH, Postma DS. The importance of serum IgE for level and longitudinal change in airways hyperresponsiveness in COPD. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1998 Oct;28(10):1210–8. doi: 10.1046/j.1365-2222.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiden MD, Ferrier N, Nolan A, Rom WN, Comfort A, Gustave J, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010 Mar;137(3):566–74. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bargagli E, Maggiorelli C, Rottoli P. Human chitotriosidase: a potential new marker of sarcoidosis severity. Respiration; international review of thoracic diseases. 2008;76(2):234–8. doi: 10.1159/000134009. [DOI] [PubMed] [Google Scholar]
- 40.Bargagli E, Bennett D, Maggiorelli C, Di Sipio P, Margollicci M, Bianchi N, et al. Human Chitotriosidase: a Sensitive Biomarker of Sarcoidosis. Journal of clinical immunology. 2012 Aug 10; doi: 10.1007/s10875-012-9754-4. [DOI] [PubMed] [Google Scholar]
- 41.Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-beta1 signaling. Journal of immunology. 2012 Sep 1;189(5):2635–44. doi: 10.4049/jimmunol.1201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Labadaridis I, Dimitriou E, Theodorakis M, Kafalidis G, Velegraki A, Michelakakis H. Chitotriosidase in neonates with fungal and bacterial infections. Archives of disease in childhood Fetal and neonatal edition. 2005 Nov;90(6):F531–2. doi: 10.1136/adc.2004.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]