Abstract
Purpose:
Acute upper respiratory infections (URI) are the second most common diagnosis in primary care offices. As treatments have limited effectiveness, patient counseling regarding expectations for the course of the URI is an important aspect of care. It is unknown how accurate patients, clinicians or questionnaires such as the Wisconsin Upper Respiratory Symptom Survey (WURSS) instrument are at predicting URI severity and duration, and whether these predictions should be used to counsel patients.
Methods:
Seven hundred and nineteen individuals with recent onset cold in community clinic settings participated. Participants and clinicians predicted the severity and duration of the URI and participants completed the WURSS instrument at initial visit. Subsequent URI global severity was calculated as area under the curve using an average of twice-daily WURSS-21 self-reports as the y-axis and illness duration as the x-axis. URI duration was determined by self-report of beginning and end of illness. Linear regression analysis was used to correlate baseline predictions with subsequent outcomes. Analyses by gender, age and income were also performed.
Results:
There was no significant association between participant and clinician predictions of severity or duration. Initial WURSS values explained 0.119 (95% CI: 0.074–0.163) of the variance in subsequent severity outcomes. There were no significant differences in associations by age, gender or income.
Conclusions:
Clinicians should not use their predictive assessments or their patients’ predictions when advising patients on the expected course of a URI. This study also suggests that the WURSS instrument could give some predictive information, but whether this is clinically useful is uncertain.
Keywords. Decision making, evidence-based medicine, family practice, patient-centered care, prognosis, upper respiratory tract infections.
Introduction
On average, adults experience an acute upper respiratory infection (URI) from two to four times a year, and children from six to eight times a year.1 Symptoms last from few to many days and vary greatly in severity.2 While most URIs are self-limited, they account for 42 million days of absence from work and school each year3 and are the second most common diagnosis in the primary care clinic.4 Currently, treatments aimed at shortening the duration or lessening the severity of symptoms have shown limited or no effectiveness.5–7 Therefore, an important aspect of care is providing patients with realistic expectations and reassurance regarding their illness by giving patients and families estimates on expected symptom duration and severity.8,9 While clinicians and patients might make predictions on the course of their illness based on their initial presentation, little is known about the accuracy of these predictions.
Clinical predictions can be made based on the clinician’s clinical experience, or by using clinical prediction instruments and models. Clinical predictions, subjective and objective, have been studied for several diseases,10–13 but the data for URIs are limited. Severity scoring instruments exist for URIs,14–16 but these instruments have not been used to predict the course of the illness. In one pediatric study, an objective scoring system for severity did not correlate well with physician’s clinical judgment of severity at the initial visit but did correlate with nurse and parent assessment of the child’s illness.14 Another study showed that two clinician-recorded items, age and presence of cough, and two instrument score items, fever and low energy, were associated, in combination, with a prolonged URI course in children. A third study reported that parents were better at predicting the duration of their child’s cough than was the child’s physician. However, neither prediction was better than fair, as physicians tended to overestimate recovery and parents to underestimate recovery after the first 2 weeks of cough.17
Considering this, our study aimed to assess the degree of association between patient, clinician and instrument predictions and the measured outcomes of the URI. Knowing how accurate clinicians, patients and instruments are in these predictions could help guide the use of clinical course predictions when educating patients on what to expect from the common cold.
Methods
The study design is a prospective cohort in which URI severity and duration were predicted by both the participant and the clinician at the beginning of the illness. URI symptoms and functional impact were self-reported at baseline and twice daily throughout the duration of the illness using the Wisconsin Upper Respiratory Symptom Survey (WURSS), a validated illness-specific outcomes instrument.16,18 The associations between baseline predictions and subsequent self-reported outcomes were then assessed for predictive ability.
This study is a secondary data analysis of the Physician, Echinacea and Placebo randomized controlled trial whose methods are described elsewhere.19 It was approved by the University of Wisconsin Health Sciences Institutional Review Board. The trial assessed possible influences of Echinacea, placebo and patient-oriented clinical interactions on URI outcomes. Assignment to placebo versus active Echinacea and regular office visit versus a longer, more empathetic encounter was concealed. Some participants purposely received open-label Echinacea. Findings from these studies showed little in the way of significant difference between intervention and control groups.20–22
Participants, setting and recruitment
Participants were recruited to the original RCT using advertisements inviting persons with cold symptoms to call the study telephone number. Callers were screened by structured telephone interview, and were eligible if they answered ‘Yes’ to ‘Do you think that you have a cold?’ or ‘Do you think you are coming down with a cold?’ and had at least one cold symptom (nasal discharge, nasal obstruction, sneezing, sore throat)23 with none beginning more than 36 hours prior to study entry. Participants over 18 years old and 12 years old with parental consent were included. Those with allergies or asthma (based on medical history or current symptoms), immune system dysfunction or current pregnancies were excluded, as well as those who would not refrain from taking over the counter or prescribed cold treatments. Participants were enrolled in person at University of Wisconsin Department of Family Medicine clinical sites. Participant demographics show a highly educated, mostly white population (Table 1).
Table 1.
Participant demographics at study intake
| Characteristic | Participants (n = 719) |
|---|---|
| Age, mean [SD], y | 33.7 [14.4] |
| Female gender, % | 64.1 |
| Education | |
| No college, % | 15.1 |
| Some college, % | 35.1 |
| Bachelor’s or higher, % | 43.7 |
| Ethnicity/race | |
| Non-White, % | 12.1 |
| Income | |
| <$25 000, % | 33.9 |
| $25 001–75 000, % | 33.9 |
| >$75 001, % | 26.1 |
| Current smoker, % | 12.4 |
Data collection and monitoring
At intake, trial participants (n = 719) gave consent and then were randomly assigned either to a clinician visit (n = 483) or no visit (n = 236). They had no previous relationship with the clinician (five family physicians and one family nurse practitioner).
The participants completed intake questionnaires describing their URI symptoms prior to learning of their assigned group. Baseline symptoms were assessed using the previously validated 44-item WURSS-44 instrument.16 The WURSS-44 measures 44 symptoms and quality-of-life indicators on seven-point scales and then provides a simple sum total excluding the global severity item (potential range 0–301). The patient’s baseline WURSS-44 score was used to predict subsequent severity and duration of this URI.
Participant predictions for this URI were assessed using an Expectation Scale for Severity and Duration with Likert-style responses: ‘Compared to prior colds, this cold feels like it will be: much milder= 1, milder=2, about the same=3, more severe=4, much more severe=5.’ Similarly, expected duration was assessed: ‘Compared to prior colds, this cold feels like it will last: much shorter than usual=1, shorter than usual=2, about the same as usual=3, somewhat longer than usual=4, much longer than usual=5’. Participants were asked to compare this cold to their previous colds as this would be their best frame of reference for a URI. After the participants completed their intake questionnaire, they were seen by a clinician (except for the participants randomized to not to see a clinician). The clinicians were not aware of the participants’ responses to the expectation questionnaires.
One clinician evaluated each participant who was randomized to see a clinician (n = 483) and then completed a Clinician Assessment Scale. Each clinician was asked to predict the participant’s illness course regarding severity and duration on a 100mm visual analog scale: ‘Please predict severity. Overall, this person’s cold will be: 0=extremely mild to 100=extremely severe’ and ‘Please predict duration. Overall, this person’s cold will be: 0=extremely short to 100=extremely long.’ The participants were not aware that the clinicians completed this assessment. Both the Participant Expectation Scale and the Clinician Assessment Scale were developed for the cold study, based on commonly used Likert-style and analog scales, respectively.
Twice daily during the study, participants completed the previously validated short form of the WURSS, the WURSS-21,18 to describe their symptoms. This shorter form is quicker for participants to complete and performs similarly to the longer WURSS-44.18 Severity of the URI subsequent to the initial visit and total duration of the URI were determined from the WURSS-21 reports. Subsequent global illness severity was calculated as area under the curve (AUC), using an average of the twice-daily WURSS-21 self-reports of URI symptom severity as the y-axis and illness duration as the x-axis. Trapezoidal approximations were used to produce a single measure representing global severity of the illness episode24 that incorporates the severity of the URI each day for the number of days the participant had symptoms. The initial WURSS-44 score was not included in the determination of subsequent severity. The duration of the URI was determined by total time with URI symptoms, ending at the last WURSS-21 report with URI symptoms before the participant was symptom-free for 2 days, or when 14 days had elapsed. Illness duration was assessed in hours and minutes and converted to decimalized days.
Statistical analysis
Primary outcomes of this study were (i) participants’ ability to predict the subsequent severity and overall duration of their URI; (ii) clinicians’ ability to predict the subsequent severity and duration of the participants’ URI; and (iii) the WURSS-44 instrument’s baseline score’s ability to predict the subsequent severity and duration of the participants’ URI.
Ordinary least squares (OLS) regression models were used to assess the strength of linear association between predictions and subsequently measured outcomes, creating six main association results. The ordinal predictor variables for patient predictions were treated as quasi-interval variables. Testing of the ordinal variables showed no significant difference compared with the quasi-interval variables, supporting the use of the ordinal data as quasi-interval in this regression. Association results were assessed visually and by R 2 values with 95% confidence intervals (CI). Analyses for each of the three predictors were kept separate due to scale differences in the predictions. Severity by AUC outcomes were Winsorized in the standard approach25 and then log transformed to improve regression fit. Grouped analyses by age, gender and income were done. Statistical analysis was done with NCSS 2007 version 7.1.20 and SAS 9.3. software.
Results
Of 719 participants enrolled in the study, six participants did not complete the study, all from the no-clinician visit group, and three participants did not complete one or both baseline predictions. Due to scheduling difficulties, 27 participants assigned to a clinician visit did not see a clinician. Therefore, participant and instrument predictions are available for 710 participants, and clinician predictions are available for 456 of these participants. For participants who completed the study, only 0.27% of WURSS-21 scores were missing. Participants who remained in the trial recovered from their URIs without major complications during the trial period. Illness outcomes for the six participants who did not complete the study are unknown.
Duration of URI symptoms in our sample ranged from 0.23 to 13.56 days (median of 6.25, mean 6.75). Subsequent severity by AUC in our sample ranged from 0.25 to 1238.75 (median 144.5). This means that severity of symptoms in our study ranged from almost gone the day after enrolling in the study to moderate symptoms for up to 14 days. The average severity was mild symptoms for 7 days.
Participant predictions
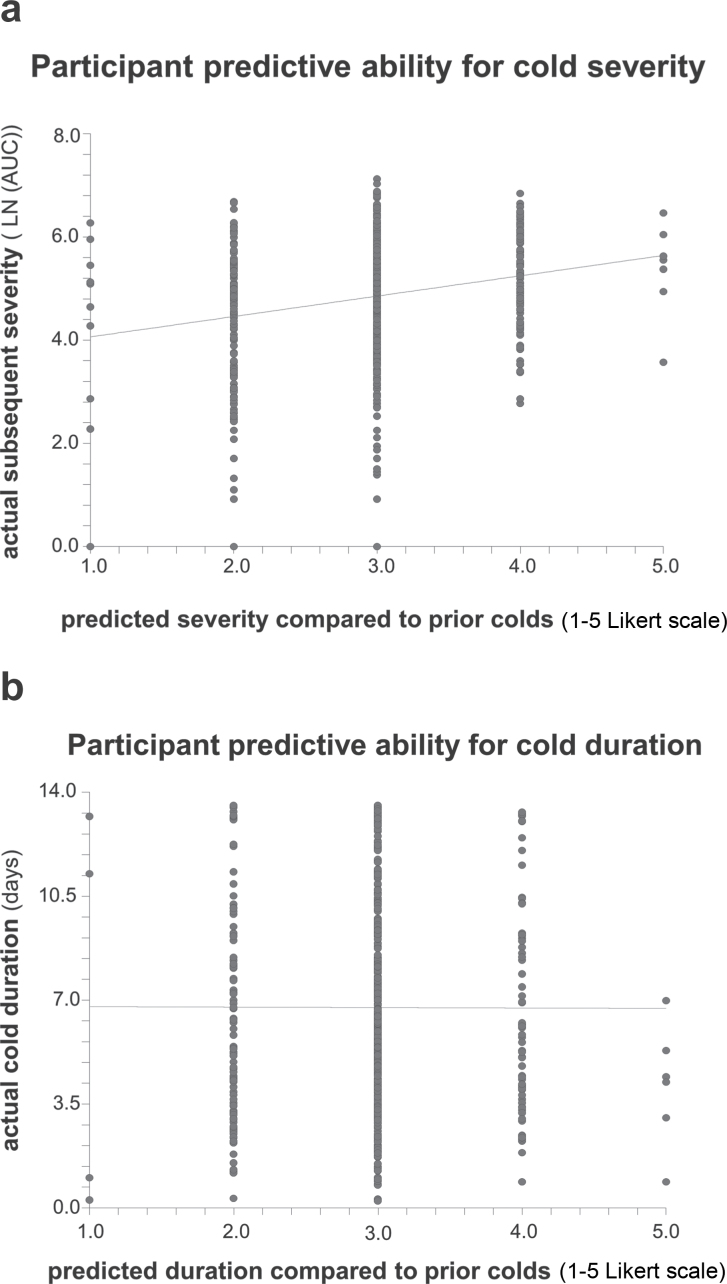
Participant severity predictions ranged from 1 to 5, with a mean of 2.95 (SD 0.63) and median of 3. Participant duration predications had the same range, mean and median, with an SD of 0.55. The linear associations between the participants’ predictions for their URIs and the subsequent illness outcomes are presented in Figure 1. The scatterplots visually show the lack of participants’ predictive abilities. R 2 values are used as numeric measures of strength of the associations. The R 2 for participants’ predictions for severity was 0.051 (95% CI: 0.020–0.082) and for duration was 0.00001 (95% CI: −0.00045–0.00047). These results are neither statistically significant, as determined by CI, nor clinically significant, as such low R 2 values could not provide helpful clinical information about the course of the URI.
Figure 1.
Scatterplots of relationship between participant predictions and actual outcomes with linear regression lines (a) Participant prediction of cold severity on x-axis compared with actual subsequent severity by AUC on y-axis with linear regression line. R 2=0.051 (95% CI: 0.020–0.082). (b) Participant prediction of cold duration on x-axis compared with actual duration in days on y-axis with linear regression line. R 2=0.00001 (95% CI: −0.00045–0.00047).
Clinician predictions
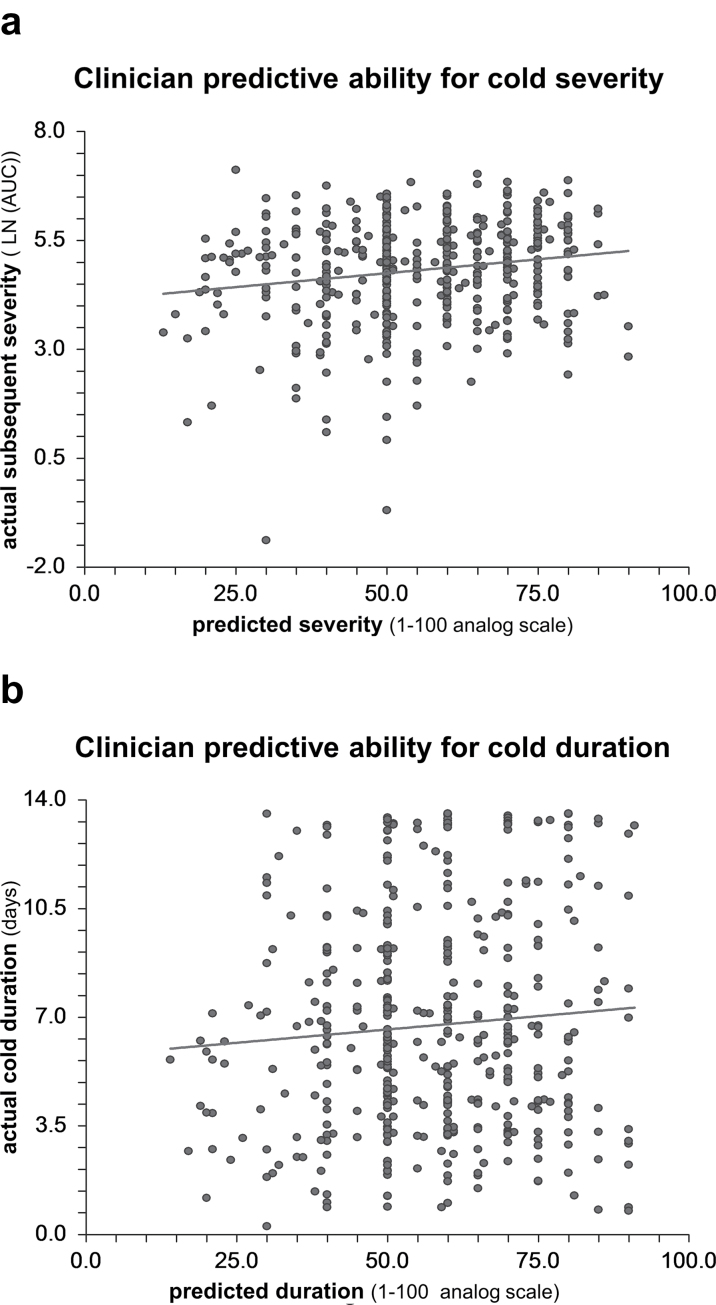
Clinician severity predictions ranged from 13 to 90, with a mean of 55.31 (SD 15.89) and median of 55. Clinician duration predications ranged from 14 to 91, with a mean of 57.22 (SD 15.80) and median of 60. The R 2 for clinicians’ prediction for severity was 0.031 (95% CI: −0.0003–0.062) and for duration was 0.0061 (95% CI: −0.008–0.020). Neither R 2 was statistically significant. The scatterplot (Figure 2) visually demonstrates the lack of association between clinical predictions and subsequent outcomes.
Figure 2.
Scatterplots of relationship between clinician predictions and actual outcomes with linear regression lines (a) Clinician prediction of cold severity on x-axis compared with actual subsequent severity by AUC on y-axis with linear regression line. R 2=0.031 (95% CI: −0.0003–0.062). (b) Clinician prediction of cold duration on x-axis compared with actual duration in days on y-axis with linear regression line. R 2=0.0061 (95% CI: −0.008–0.020).
Instrument predictions
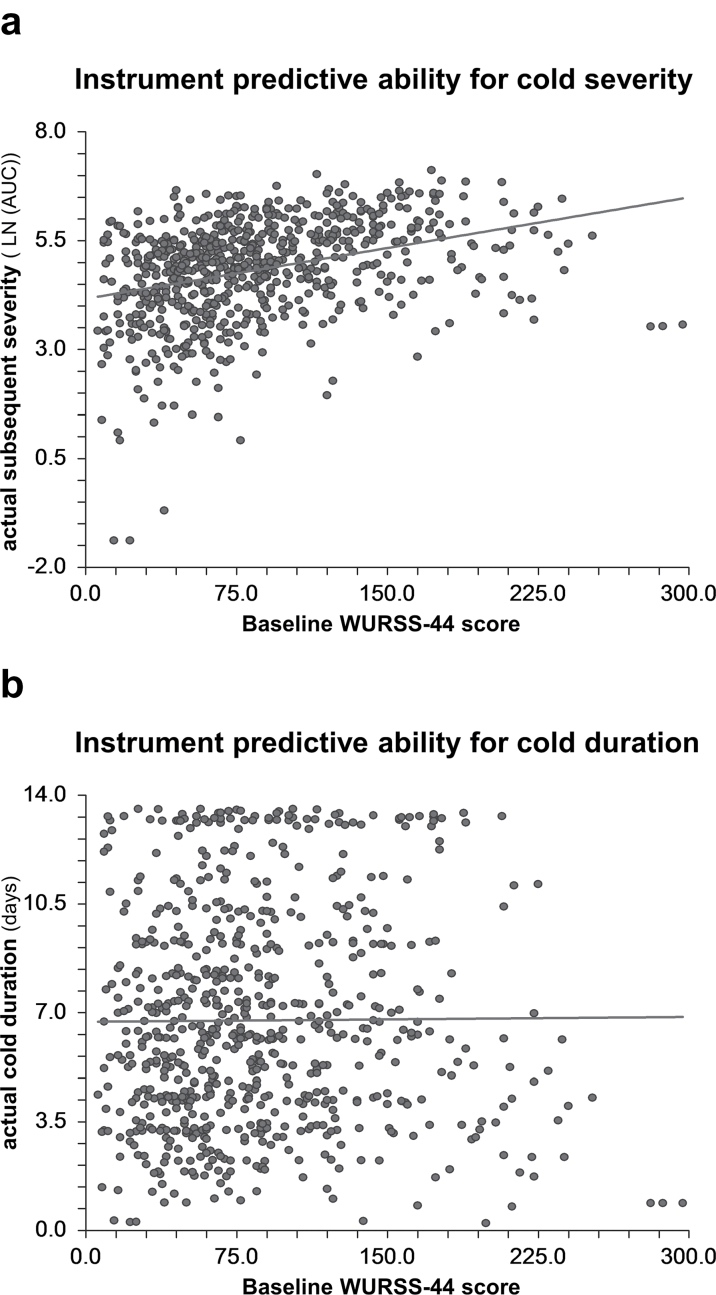
The baseline WURSS-44, as the instrument’s severity and duration prediction, ranged from 6 to 297, with a mean of 85.45 (SD 51.39) and a median of 74. The R 2 for the instrument’s prediction for severity was 0.119 (95% CI: 0.074–0.163) and for duration was 0.00006 (95% CI: −0.001–0.001). The scatterplots (Figure 3) visually demonstrate the associations. The initial WURSS-44 instrument score explains 11.9% of the variation in the subsequent severity of the URI and was statistically significant by CI. The instrument prediction for duration was not statistically or clinically significant.
Figure 3.
Scatterplots of relationship between instrument predictions and actual outcomes with linear regression lines (a) Instrument prediction of cold severity on x-axis compared with actual subsequent severity by AUC on y-axis with linear regression line. R2=0.119 (95% CI: 0.074–0.163). (b) Instrument prediction of cold duration on x-axis compared with actual duration in days on y-axis with linear regression line. R 2=0.00006 (95% CI: −0.001–0.001).
Grouped analyses
Analyses by age group, gender, household income, clinician visit or no visit and original RCT pill assignment showed no statistical significance or difference in predictive ability by participants, clinicians or the instrument, for severity or duration.
Discussion
In this study, we assess participant and clinician predictions of the subsequent severity and duration of an acute URI episode. We also assess predictive abilities of a baseline score of a URI-specific instrument. We found that neither participant nor clinician predictions of severity and duration were significantly correlated with subsequent measured outcomes. The data suggest that the initial WURSS instrument score can weakly predict subsequent overall severity, but not duration, of illness.
To our knowledge, this is the first study to ask cold-sufferers and clinicians to directly predict subsequent severity and duration of an acute URI episode. Previous studies have asked parents of young children and clinicians to predict cough duration,17 or assessed the patient’s chart and initial instrument score compared with URI duration.15 In contrast, our study asked the cold-sufferer to make predictions about their URI, and to predict both the duration and the severity. We believe that this information is relevant in that knowing what to tell a patient regarding the expected severity and duration of a URI would be expected to be helpful in supporting a patient through an illness episode for which there is no specific treatment. Adults have been shown to expect the duration of an acute cough to be roughly half the actual average cough duration.26 Accurate patient education on the course of the URI could help reduce unnecessary repeat office visits,26 help the patient plan for a reduced work load or time off work, or alleviate anxiety and improve a sense of control in the patient regarding their short-term health. While clinicians and patients commonly consider duration in a discussion of the expected course of a URI, global severity by AUC may be a better measure for describing the course of the URI, as it incorporates suffering-over-time into a single measure.24
Patient self-rating of health is a significant predictor of long-term health.27 In this study, we attempted to assess participants’ ability to predict their short-term health with an acute, self-limited disease. While participants were unable to directly predict the severity of their illnesses in our study, their responses to the WURSS instrument were predictive of their URIs’ subsequent global severity. The WURSS incorporates multiple elements of URI symptoms and functional impact and might account for aspects not considered by participants when making predictions. Alternatively, a comprehensive questionnaire may account for all symptoms more evenly than a participant who might be more strongly influenced by certain elements of illness, perhaps those that are most bothersome at the time of the prediction, while disregarding less irritating but more predictive symptoms.
Although statistically significant, the R 2 value of 0.119 for the severity prediction is modest, suggesting that URI symptoms as scored by the WURSS provide only a small piece of the puzzle in predicting the course of the URI. The WURSS is a research-oriented outcomes instrument and may need to be modified and tested in a clinical environment before clinical prognostic use.
This study has several limitations. First, predictive abilities between clinicians, participants and the WURSS could not be directly compared as these predictions were done using different scales and question stems. These scales are based off commonly accepted and used Likert-style and analog scales, and as there is no gold standard for URI predictions, no questionnaire validation was done. Additionally, participants were asked to use their previous URIs as their frame of reference for their predictions. We believe that a participant’s prior experience would be their best frame of reference. As URI duration and severity vary across the population in reasonably predictable ways, and people experience many episodes across the lifetime, it seemed reasonable that each individual would have a broad base for comparison. This question stem could influence the assessment of their predictive ability; however, we believe that it is likely that patients use their own experiences as their referent in making predictions on their health. Second, similar instruments were used to both predict the severity of the URI and to describe the subsequent severity of the URI. We recognize that the use of the WURSS-44 to predict severity as measured by WURSS-21 might seem problematic. However, both the WURSS-44 and the WURSS-21 have been independently validated by comparison to two other instruments that measure health and URI symptoms, the SF-8 and the Jackson criteria,16,18 and have been shown to correlate with biomarkers of disease severity, including interleukin-8, nasal neutrophils, mucus weight and viral titer.28 Finally, another possible limitation comes from the use of several experimental treatment groups in our study population as the data for this study comes from a randomized trial for URI treatments. However, previous analyses of these data did not show clinically significant differences among experimental groups20–22 and our analyses showed no difference in predictive abilities by RCT experimental groups. This approach has been used previously.15 It is also possible that participants, and even clinicians, would make more optimistic predictions for their URIs if they expected that the trial treatment would improve their symptoms. However, the participants and clinicians also knew that the trial therapies were not yet proven to be beneficial, and that many would be in control groups, and so it is also possible that predictions, especially from clinicians who should understand the RCT process, were unchanged or minimally changed by inclusion in an RCT.
We interpret the results of this study to suggest that clinicians should not use their own predictions or their patients’ predictions when making decisions or advising patients on the expected course of an acute URI episode. Patient-centered shared decision making starts with evidence-based information. We recommend that patient education and counseling on expectations for symptom severity and duration be based on population averages for URI symptom duration and severity but not the individual patient’s presentation or perception of their illness. The mean duration of URI is 7–10 days, with a minority of patients experiencing symptoms for more than 3 weeks.2 Severity varies greatly but typically is mild to moderate for the duration of symptoms. Patients also should be informed that clinicians and patients cannot accurately predict the course of a URI based on a patient’s initial symptoms. As we cannot accurately predict when the URI will end or how bad it will be, our best clinical tools for patients with URIs are empathy, reassurance and education on the self-limited, short-duration nature of viral upper respiratory tract infections.
Declaration
Funding: The work presented here was carried out while EL was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department Of Family Medicine and with academic support from the Clinical and Translational Science Award program, previously through the National Center for Research Resources (1UL1RR025011), and now by the National Center for Advancing Translational Sciences (9U54TR000021). The PEP trial was sponsored by the National Center for Complementary and Alternative Medicine (NCCAM) at the National Institutes of Health (NIH NCCAM 1-RO1-AT-1428). BB was supported by a NCCAM patient-oriented career development grant (K23 AT00051) during the development of the proposal and by the Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program during the conduct of the trial. Additional support was provided by the University of Wisconsin School of Medicine and Public Health and the University of Wisconsin Department of Family Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical approval: This RCT on which this study was based was approved by the University of Wisconsin Health Sciences Institutional Review Board.
Conflict of interest: Two authors (BB, RB) are partial copyright holders of the WURSS questionnaire and receive limited royalty funds for its rare commercial use.
Acknowledgements
The authors thank Tola Ewers for data management, Heidi Marleau for literature search assistance, Mindy Smith MD, MS, for comments and edits, and Terry Little and the University of Wisconsin Department of Family medicine for administrative support. The authors also thank the study participants for volunteering to advance science while feeling ill.
References
- 1. Monto AS. Epidemiology of viral respiratory infections. Am J Med 2002; 112 (Suppl 6A): 4S–12S [DOI] [PubMed] [Google Scholar]
- 2. Heikkinen T, Järvinen A. The common cold. Lancet 2003; 361: 51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med 2003; 163: 487–94 [DOI] [PubMed] [Google Scholar]
- 4. Hsiao CJ, Cherry DK, Beatty PC, Rechtsteiner EA. National Ambulatory Medical Care Survey: 2007 summary. National health statistics reports. Centers for Disease Control and Prevention, 2010; 27: 1–32 [PubMed] [Google Scholar]
- 5. Simasek M, Blandino DA. Treatment of the common cold. Am Fam Physician 2007; 75: 515–20 [PubMed] [Google Scholar]
- 6. Arroll B. Common cold. Am Fam Physician 2011; 84: 1390–1 [PubMed] [Google Scholar]
- 7. Wat D. The common cold: a review of the literature. Eur J Intern Med 2004; 15: 79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee GM, Friedman JF, Ross-Degnan D, Hibberd PL, Goldmann DA. Misconceptions about colds and predictors of health service utilization. Pediatrics 2003; 111: 231–6 [DOI] [PubMed] [Google Scholar]
- 9. Sanchez-Menegay C, Hudes ES, Cummings SR. Patient expectations and satisfaction with medical care for upper respiratory infections. J Gen Intern Med 1992; 7: 432–4 [DOI] [PubMed] [Google Scholar]
- 10. Copeland-Fields L, Griffin T, Jenkins T, Buckley M, Wise LC. Comparison of outcome predictions made by physicians, by nurses, and by using the Mortality Prediction Model. Am J Crit Care 2001; 10: 313–9 [PubMed] [Google Scholar]
- 11. Sinuff T, Adhikari NK, Cook DJ, et al. Mortality predictions in the intensive care unit: comparing physicians with scoring systems. Crit Care Med 2006; 34: 878–85 [DOI] [PubMed] [Google Scholar]
- 12. Chow E, Harth T, Hruby G, Finkelstein J, Wu J, Danjoux C. How accurate are physicians’ clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol) 2001; 13: 209–18 [DOI] [PubMed] [Google Scholar]
- 13. Glare P, Virik K, Jones M, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ 2003; 327: 195–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shepperd S, Perera R, Bates S, et al. A children’s acute respiratory illness scale (CARIFS) predicted functional severity and family burden. J Clin Epidemiol 2004; 57: 809–14 [DOI] [PubMed] [Google Scholar]
- 15. Butler CC, Hood K, Kinnersley P, Robling M, Prout H, Houston H. Predicting the clinical course of suspected acute viral upper respiratory tract infection in children. Fam Pract 2005; 22: 92–5 [DOI] [PubMed] [Google Scholar]
- 16. Barrett B, Brown R, Mundt M, et al. The Wisconsin Upper Respiratory Symptom Survey is responsive, reliable, and valid. J Clin Epidemiol 2005; 58: 609–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hay AD, Wilson A, Fahey T, Peters TJ. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract 2003; 20: 696–705 [DOI] [PubMed] [Google Scholar]
- 18. Barrett B, Brown RL, Mundt MP, et al. Validation of a short form Wisconsin Upper Respiratory Symptom Survey (WURSS-21). Health Qual Life Outcomes 2009; 7: 76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barrett B, Rakel D, Chewning B, et al. Rationale and methods for a trial assessing placebo, echinacea, and doctor-patient interaction in the common cold. Explore (NY) 2007; 3: 561–72 [DOI] [PubMed] [Google Scholar]
- 20. Barrett B, Brown R, Rakel D, et al. Placebo effects and the common cold: a randomized controlled trial. Ann Fam Med 2011; 9: 312–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rakel D, Barrett B, Zhang Z, et al. Perception of empathy in the therapeutic encounter: effects on the common cold. Patient educ couns 2011; 85: 390–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barrett B, Brown R, Rakel D, et al. Echinacea for treating the common cold: a randomized trial. Ann Intern Med 2010; 153: 769–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jackson GG, Dowling HF, Muldoon RL. Acute respiratory diseases of viral etiology. VII. Present concepts of the common cold. Am J Public Health Nations Health 1962; 52: 940–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lydick E, Epstein RS, Himmelberger D, White CJ. Area under the curve: a metric for patient subjective responses in episodic diseases. Qual Life Res 1995; 4: 41–5 [DOI] [PubMed] [Google Scholar]
- 25. Dixon WJ. Simplified Estimation from Censored Normal Samples. Ann Math Stat 1960; 31: 385–91 [Google Scholar]
- 26. Ebell MH, Lundgren J, Youngpairoj S. How long does a cough last? Comparing patients’ expectations with data from a systematic review of the literature. Ann Fam Med 2013; 11: 5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Idler EL, Russell LB, Davis D. Survival, functional limitations, and self-rated health in the NHANES I Epidemiologic Follow-up Study, 1992. First National Health and Nutrition Examination Survey. Am J epidemiol 2000; 152: 874–83 [DOI] [PubMed] [Google Scholar]
- 28. Barrett B, Brown R, Voland R, Maberry R, Turner R. Relations among questionnaire and laboratory measures of rhinovirus infection. Eur Respir J 2006; 28: 358–63 [DOI] [PubMed] [Google Scholar]