Abstract
Background.
In primary care, many consultations address symptom-based complaints. Recovery from these complaints seldom exceeds placebo effects. Patient participation, because of its supposed effects on trust and patient expectancies, is assumed to benefit patients’ recovery. While the idea is theoretically promising, it is still unclear what the effects of increased patient participation are on patient outcomes.
Aim.
To review the effects of controlled intervention studies aiming to improve patient participation in face-to-face primary care consultations on patient-oriented and/or disease-oriented outcomes.
Methods.
This study is a systematic review. A systematic search was undertaken for randomized controlled trials designed to measure the effects of interventions that aimed to improve adult patients’ participation in primary care visits. The CINAHL, Cochrane, EMBASE, PsycINFO and PubMed databases were searched.
Results.
Seven different trials fulfilled the inclusion criteria. Three of the studies were related to symptom-based complaints. Five studies measured patient-oriented outcomes, the primary outcome of interest for this review. All studies suffered from substantial bias. Studies varied widely in their aims, types of complaints/diseases, strength of the interventions and their outcomes. The effects on patient-oriented outcomes and disease-oriented outcomes were ambiguous.
Conclusion.
Little research has been performed on health outcomes of interventions aiming to increase patient participation in general practice visits among patients suffering from symptom-based complaints. The results still are non-conclusive. The quality of the trials has been weak, possibly due to the complexity of the concept. This weak quality may explain the lack of conclusive results. Proposals for future research designs are offered.
Keywords. Patient participation, patient-oriented outcomes, primary health care, shared decision-making, review (publication type).
Introduction
In primary care, patients consult their GP for complaints rather than for diseases.1 The effectiveness of the therapies for these illnesses seldom exceeds placebo effects,2,3 but placebo effects alone can be substantial.4,5 Usually these illnesses are evaluated by6–8 assessing patient-reported outcomes,8,9 which are highly dependent on patients’ perspectives.10
In 1999, Crow concluded that the evidence justifies techniques that facilitate patient participation in consultations.11 To encourage patient participation, providers need to recognize the patient’s ‘expert role’ in matters such as the patient’s preferences, concerns and self-efficacy.12–14 When earlier positive patient experiences are incorporated into the treatment plan, this can subconsciously lead patients to beneficial outcomes.15 In addition to the subconscious reaction, patients interpret this listening to their perspective as trust.16 Providing a caring, respectful and empowering context, thereby influencing the patient’s affective state, seems to be effective in producing positive health outcomes.10 Finally, incorporating patients’ preferences in medical decision-making will lead to higher treatment expectations and higher adherence to therapy.11,15 These expectations showed a positive effect on recovery17 although the literature shows some conflicting results.18
The most recent Cochrane review on the effect of patient-centred approaches on health-related outcomes concluded that there was limited proof of beneficial effects.19 The search was performed through 1999 using a restricted search strategy. Since then, shared decision-making (SDM), a technique in which patients are empowered to make health care choices jointly with the practitioner,13,20 has come to be considered crucial for patient-centred care.21 However, previous reviews on SDM do not consider health-related outcomes as primary outcomes.22,23
Given the limitations of earlier reviews and their inconclusive results, we performed a systematic review on the effects of controlled interventions that aimed to improve patient participation in complaint-driven face-to-face primary care consultations on patient-oriented and/or disease-oriented outcomes.
Methods
Search strategy
In March 2011, a pilot search was performed by MV in PubMed, using the search strategy developed by Légaré24 supplemented with terms from Lewin’s work.19 The pilot search was conducted backwards and forwards. This technique resulted in our definitive search strategy (see Appendix 1). A broad search strategy including the domain (providers, patients and provider–patient interactions) and determinant (promoting patient participation) was chosen to ensure that no applicable studies were missed. We used two filters (one to identify quasi-randomized and randomized controlled trials and one to restrict the search to primary care). The PubMed search strategy is shown in Appendix 1.
The following electronic databases were searched on 7 October 2012: CINAHL, Cochrane, EMBASE through Embase.com, PsycINFO and PubMed (incorporating MEDLINE and Old MEDLINE). The searches were not restricted by language or by date. The included studies were forward and backward searched.
Inclusion and exclusion criteria
Types of studies.
Randomized controlled intervention studies were included. We excluded controlled before-and-after studies, interrupted time series studies and all non-experimental studies. We did not exclude studies on the basis of allocation concealment or blinding.
Participants.
The patients were over 18 years. We excluded studies of people with serious psychiatric symptoms, defined as patients requiring help from secondary care.
The health care professionals were those responsible for patient care, including professionals in training.
Types of interventions.
We included all patient-centred interventions aimed at affecting patients’ ability to influence treatment decisions during primary care encounters. The interventions could occur before or during the clinical encounter.
Eligible interventions included educational meetings, audit and feedback (i.e. any summary of clinical health care performance over a specified period of time), reminders (i.e. information provided verbally, on paper or on a computer screen that prompts a professional to recall information), patient-mediated interventions (i.e. any intervention aimed at changing health care professionals’ behaviour through interactions with or information provided by or to patients, which could include providing patients with information about the effectiveness and/or appropriateness of particular health technologies) and the distribution of printed educational material (i.e. published or printed recommendations about clinical care and evidence to improve practices, including clinical practice guidelines, journals and monographs).24 Patient decision aids were considered patient-mediated interventions because one of their purposes is to foster patients’ participation in decisions made during the clinical encounter.25 Interventions conducted after the clinical encounter or studies that trained health care providers to achieve specific treatment or preventive goals (e.g. providers’ adherence to guidelines or behavioural changes by the patients) that were initiated by the health care provider were excluded. Routine consultations for controlling chronic diseases were included.
Types of outcome measures
Primary outcomes.
All patient-oriented outcomes, such as morbidity, mortality, symptoms, quality of life or personal costs were included.26
Secondary outcomes.
All disease-oriented outcomes, such as histopathologic, physiologic or surrogate indicators [e.g. clinical assessments, body mass index (BMI), blood pressure or blood glucose] that may reflect changes in the disease course or health risks were included.26
We excluded studies that did not include any of the outcomes listed above or that measured only the patient’s lifestyle behaviours; studies that measured only the provider’s knowledge, attitudes or intentions; and studies that used simulated patients.
Data collection and analysis
Selection of trials.
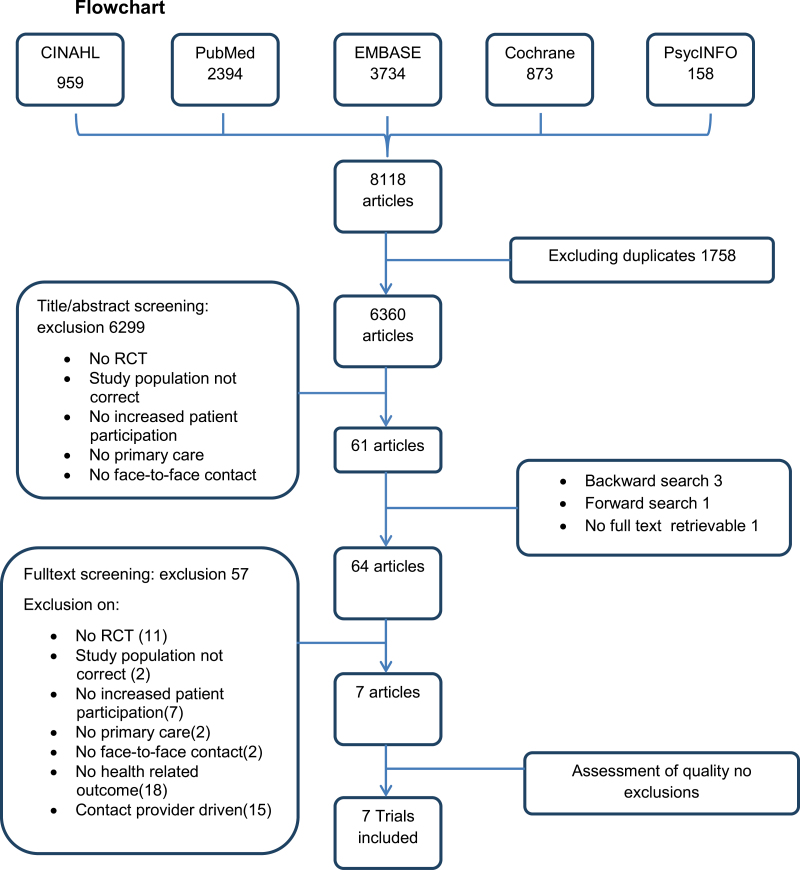
After excluding duplicates, two authors (IW and AS) screened the titles and abstracts of the articles obtained from the search and excluded studies according to the predetermined exclusion criteria (see Fig. 1). For studies that were questionable, one author (IW) scanned the full text before flagging the study for full text reading. The full text copies of all potentially relevant studies, except for one, were retrieved. The author of the study, which was not retrievable from several international libraries, was emailed but did not respond.
Figure 1.
Flow chart
Eligible trials were screened for quality, the types of interventions they included and outcomes. The relevant data were extracted from the eligible studies by one author (AS), and the data from a sample of the studies (one-third) were independently confirmed by the second author (IW).
Quality assessment.
All eligible trials were screened for the following characteristics: randomization procedure, allocation concealment, blinded assessment of the outcome(s), intention-to-treat (ITT) analysis, differences at baseline between the groups of professionals and patients, missing data for providers and patients and protection against contamination. We also determined whether there was potential for error related to the unit of analysis and, if so, whether it was acknowledged and adjustments were made.
Disagreements were resolved through discussion between AS and IW; in cases of persistent disagreement, consensus was reached with the entire research team. To reduce quality assessment to one criterion, all of the articles were assessed by two independent researchers (AS and JvdZ) using the SORT criteria.26 The SORT criteria for high-quality randomized controlled clinical trials (RCT) include concealed allocation, blinding, ITT analysis, adequate statistical power and adequate percentage of participants completing follow-up (greater than 80 per cent).
Data analysis.
We planned to combine the studies with common outcomes when possible and to conduct a subgroup analysis for trials about complaint-based consultations versus routine check-ups for chronic diseases. We assumed that the effects would be lower in the latter group because preventive visits do have different contextual effects.27 We estimated that there would be only moderate publication bias for the patient-oriented results because secondary patient-reported outcomes from studies that measured process outcomes as their primary outcomes were also included in the review.
Results
Study selection
The search identified a total of 8118 potentially relevant articles. After excluding duplicates, this number was reduced to a total of 6360 articles. The titles and abstracts were screened based on all of the exclusion criteria except for the outcomes and the purpose of contact. The correlation between both assessors was 0.645 (standard error = 0.032; 95% confidence interval = 0.582–0.708).
A total of 61 studies were flagged for full text screening. Three articles were added based on the backward search, and one was added from the forward search. Fifty-seven publications were excluded because they did not meet the inclusion criteria. Thirty-three of these were excluded based on the two criteria that were not applied during the screening of the titles and abstracts (i.e. they had no health-related outcomes or they included provider-driven contact; see Fig. 1). Ultimately, seven trials were included in this review.
Characteristics of the included studies
Two studies were conducted in England,28,29 two in Germany,30,31 one in the USA,32 one in Canada33 and one in France.34 The data collection occurred in various time periods between 1993 and 2012. All of the studies were cluster randomized trials; the unit of randomization was either the GP or the practice. The providers were predominantly GPs or primary care physicians (PCP). In four studies, PCP practice teams received the intervention training as a unit.28,29,32,33 The number of providers varied from 3031 to 162.33 The number of included patients ranged from 16529 to 926.30 The patients were seen for acute respiratory infections,33 osteoarthritis (OA),34 depression,31 diabetes,28,29 hypertension32 and cholesterol measurement.30 In three studies, patient complaints prompted contact with a provider31,33,34; the other four studies involved routine visits to control chronic diseases.28–30,32
Only in one study was the primary aim of the intervention to use patient-centred methods to relieve patients’ complaints.34 All others, except for one,31 used patient participation as an instrument to address a disease-oriented measure, such as antibiotic use,33 A1C, lipid levels, etc.28–30 Four of the studies combined this focus with process outcomes.29,30,32,33 Loh31 focused on the beneficial effects on process outcomes. Disease-oriented outcomes such as adherence to therapy were considered secondary outcomes. In all except one study,28 the control subjects received care as usual. Table 1 summarizes the characteristics of the included studies.
Table 1.
Characteristics of the included studies
| Author | Publication year | Country | Period of data collection | Unit of randomization | Provider participants | Number of providers (IV/controls) | Patient participants | Number patients (IV/controls) | Contact complaint drivena | Primary aimb | Patient participationc | Controlsd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chassany34 | 2006 | France | May 2001–April 2002 | GP | GPs | 180 (84/96) | Chronic complaints of osteoarthritis | 842 (414/428) | c | c | 1 | 1 |
| Cooper32 | 2011 | USA | January 2002–August 2005 | Practice | General internists and family physicians | 41 (22/19) | Hypertensive patients in underserved PC | 279 (83/57/84/55) | r | d + p | 1 | 1 |
| Kinmonth28 | 1998 | England | April 1994–June 1995 | Practice | PC practice teamse | 41 (21/20)f | Newly diagnosed NIDDM | 250 (142/108) | r | d | 0 | 2g |
| Krones30 | 2008 | Germany | May 2005–March 2006 | CME-group | PC practice teamse | 87 (44/47) | Cholesterol measurement | 926 (460/466) | r | d + p | 0 | 1 |
| Légaré33 | 2012 | Canada | July 2010-April 2011 | Practice | Family physicians | 149 (77/72) | Acute respiratory infections | 359 (181/178) | c | d + p | 1 | 1 |
| Loh31 | 2007 | Germany | October 2002–December 2004 | PCP | PCPsh | 30 (20/10) | Newly diagnosed depressive disorder | 405 (263/142) | c | p | 0 | 1 |
| Pill29 | 1998 | England | April 1993–April 1996 | Practice | PCPsi | 29 general practicesj | NIDDM | 165 (77/88) | r | d + p | 0 | 1 |
IV, intervention; NIDDM, non-insulin-dependent diabetes mellitus; CME, continuous medical education.
ac: face-to-face contact is complaint-driven; r: routine visit for controlling chronic diseases.
bc: primary aim to relieve patient-oriented outcomes (complaints); d: disease-oriented outcomes (disease); p: process outcomes.
c1: patient participation as an end in itself; 0: as an instrument to reach another goal.
d1: controls care as usual; 2: other.
ePC practice teams consisted of doctors and nurses.
f21 practices consisted of 23 doctors and 32 nurses; 20 practices consisted of 20 doctors and 32 nurses.
gThe nurses in the comparison group were offered similar support sessions focusing on the use of guidelines and materials.
hPCP: primary care physicians, all teaching practices.
iPCPs and their practice nurses committed for at least 2 years to an annual peer reviewed clinical audit of diabetic care.
jProvider number, discrimination between IV versus controls not made.
Quality assessment
All of the trials are cluster randomized controlled trials and therefore provided a moderate level of evidence according to the SORT criteria.26 However, a considerable risk of bias hampered all of the studies. For two studies,31,34 the randomization procedure could not be definitively determined from the article. Information about patient blinding and outcome blinding could not be retrieved from three of the articles. Only one trial31 had a low risk of bias based on missing outcomes. In the study by Loh, the follow-up time was short (2 weeks) and the GPs who did not include patients in the study were excluded from the analysis. Thus, the internal validity of the overall results of this review is low. For all of the trials except the study by Légaré, recruitment bias was considered high. Consequently, the external validity must also be considered low. Table 2 summarizes the quality assessment. Table 3 summarizes the interventions.
Table 2.
Quality assessment based on SORT-criteria 26
| Article | Randomizationa | Blinding providersb | Blinding patientsc | ITTd | Adequate size | Missing datae | Level of evidencef |
|---|---|---|---|---|---|---|---|
| Chassany34 | − | − | − | + | + | + | 2 |
| Cooper32 | + | − | + | + | − | + | 2 |
| Kinmonth28 | + | − | + | + | + | − | 2 |
| Krones30 | + | − | + | + | + | − | 2 |
| Légaré33 | + | − | + | + | + | − | 2 |
| Loh31 | − | − | + | − | − | − | 2 |
| Pill29 | + | − | − | − | + | − | 2 |
a+: low risk of bias; −: high risk of bias or insufficient information to judge the risk.
b+: providers blinded; −: providers not blinded or insufficient information to judge provider blinding.
c+: patients blinded; −: patients not blinded or insufficient information to judge patient blinding.
d+: ITT analysis performed; −: ITT analysis not performed or insufficient information available to judge ITT analysis.
e+: missing data <20%; −: missing data> 20%.
fQuality level based on the SORT criteria.26
Table 3.
Summary of interventions
| Author | Description of intervention and supportive material(s) | Training strategies | Conceptual basisa | Multi-faced interventionb | Trainers | Behaviour change c | Duration of trainingd | Supportive materiale | Strength of interventionf | How manipulation was measuredg | Manipulation check resulth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chassany34 | Pragmatic, interactive, centred on the patient–physician relationship and based on the specific biopsychosocial model of chronic pain; the training focused on 3 themes: the patient–physician relationship, analysis and evaluation of pain and prescribing and the negotiation of the therapeutic contract with the patient | Workshops, group discussion, reminders | 1 | 1 | Facilitators and experts | 1 | 240 | 0 | 2 | − | − |
| Cooper32 | Physician training focused on increasing patient engagement and pre-visit patient coaching to improve patient communication with clinicians and outcomes | Physicians: feedback on simulated patient contact and workbook or CD-ROM exercise; patients: coaching and telephone | 1 | 1 | Physicians: unknown; patients: by community health workers | 1 | 1480 +? | 1 | 2 | Videotape and PQ, PQ on patient ratings of physicians’ participatory decision-making style, PICS50 | +? |
| Kinmonth28 | Doctor training: didactic only; nurses training: didactic instruction and skills, including active listening and negotiation of behaviour change; for patients: a booklet that encouraged patients to ask questions and an optional leaflet for patients encouraging discussion of complications and concerns and a booklet for practitioners describing approaches to behaviour change | Doctors and nurses receive theory and nurses practiced skills and were supported by a facilitator | 1 | 1 | Experienced facilitator | 1 | 270 | 1 | 2 | PQ recall of supportive material and DQ on use of skills | + |
| Krones30 | Training for family doctors on SDM, script-like decision aid, booklet for doctors, individual summary sheet for the patient | CME groups in which family doctors were trained to moderate the training for the participants | 1 | 1 | Moderators of CME group and members of the research team | 1 | 240 | 1 | 2 | PQ50–53 | + |
| Légaré33 | Online self-tutorial and an interactive workshop addressing key components of the clinical decision-making process about antibiotic treatment for acute respiratory infections in PC | Videos, exercises, decision aid | 1 | 1 | Facilitators trained during the pilot trial | 1 | 240 | 1 | 2 | PQ: the modified Control Preference Scale54–56 and a single question with a Likert scale to assess the quality of the decision made | + |
| Loh31 | Multi-faceted intervention, physician training, decision aid and patient information leaflet | Theory, role play, discussions, modelling | 1 | 1 | Unclear | 1 | Unknown | 1 | 2 | PQ (PICS)50 results | + |
| Pill29 | Training at surgery was tailored to the needs of the practice but at least 2 sessions of 3 hours with simple visual aids designed to assist the clinician in encouraging active patient participation, newsletters every 3–4 months | Discussion, demonstration of technology and often role play, continuing support by a research nurse, who mostly visited the practice nurses, 2 group meetings during the course of the study | 1 | 1 | Intervention team (GP, research nurse and clinical psychologist) | 1 | >360 | 0 | 3 | Audiotape and telephone interview | +/− |
PICS=patient perceived involvement in care scale.
aConceptual basis—1: yes; 0: no;?: unclear
bMulti-faced intervention—1: yes; 0: no;?: unclear.
cIntended target of behaviour change—1: provider; 0: patient.
dDuration of training in minutes.
eSupportive material for patient—1: yes; 0: no.
fStrength of the intervention—1: weak (1 session, 1 day, teaching didactics); 2: intermediate (all other interventions with training sessions between sessions); 3: strong (3≥ sessions, >1 day, opportunity to practice skills between sessions and at least one of next 3 items: follow-up support, additional materials or a supportive tool); 0: no trained intervention.
gHow manipulation was measured? 0: no manipulation check; PQ: patient questionnaire after the encounter; DQ: provider questionnaire after the encounter.
hManipulation check results—0= no manipulation check;? = unclear because the manipulation check failed; + = increased patient participation in the intervention group; − = less patient participation in the intervention group compared with the control group.
Outcome measures
The outcome measures varied across different studies (see Table 4). All health-related outcomes were included. The patient-oriented outcomes varied from pain intensity34 to perceived health (overall or disease-related) measured with questionnaires.28,29,31,33,34 Most of the instruments were validated. The disease-oriented outcomes varied from biochemical results28,29 to physical measurements28,29,32 to risk scores.30,34 The outcomes were measured between 2 weeks33,34 and 1 year32 after the intervention.
Table 4.
Summary of patient-oriented outcomes (primary review outcome) and disease-oriented outcomes (secondary review outcome)
| Author | Outcome | Instrument | Study duration in weeks | Outcomea | Significanceb | Adverse effectsc | Summary of results |
|---|---|---|---|---|---|---|---|
| Chassany34 | Pain intensity with motion (change from baseline over 2 weeks) | VAS-scale expressed as the sum of pain intensity differences | 2 | + | <0.00001 | None | Overall significant positive effect on the primary review outcome measurement point after 2 weeks only |
| Pain intensity with motion by VAS (change from baseline to study end) | VAS-scale | 2 | + | 0.01 | |||
| Functional disability | WOMAC index58 | 2 | + | <0.0001 | |||
| Global perception | A 7-point Likert scale | 2 | + | 0.002 | |||
| Cooper32 | Blood pressure change, systolic and diastolic | Automatic oscillometric monitor (Omron HEM 907) | 52 | 0 | ns | None | No primary review outcome; no effect on secondary review outcomes; positive effect on process outcomes |
| Kinmonth28 | Quality of life | ADDQoL57 | 52 | + | ns | Knowledge score below 0.03 | Of 3 primary review outcomes one showed a significant positive effect, 2 were positive but non-significant. Secondary review outcomes: all patients reached good clinical A1C levels. 2 out of 6 showed significant negative effects. Combined, all other clinical measures (i.e., cardiovascular risk factors) showed a negative trend. Both groups showed improved performances on process and health outcomes |
| Depressed well-being | Depressed well-being questionnaire57 | 52 | + | ns | |||
| Well-being | Bradley well-being questionnaire57 d | 52 | + | 0.03 | |||
| A1C | Percentage of glycated haemoglobin 30 | 52 | + | ns | |||
| Total cholesterol | Cholesterol oxidase concentration | 52 | + | ns | |||
| Triglycerides | Plasma triglyceride concentration | 52 | − | 0.02 | |||
| Body mass index (kg/m²) | Harpenden pocket stadiometer and Seca 835 electronic scales | 52 | − | 0.03 | |||
| Blood pressure, systolic and diastolic | Omron electronic | 52 | − | ns | |||
| Urinary albumin/creatinine ratio | Jaffé reaction | 52 | + | ns | |||
| Krones30 | Mean change in cardiovascular disease risk (%) | Framingham scoring system calibrated for European populations | 26 | − | ns | None | No primary review outcome. Secondary review outcome: no significant difference between groups. Cardiovascular disease risk status decreased in both groups |
| Légaré33 | Self-reported health status | Short form SF-12 questionnaire59 | 2 | 0 | ns | None | No effect on primary review outcome. There was a positive effect on process outcomes |
| Loh31 | Decline in depression severity (PHQ baseline − PHQ treatment/PHQ baseline × 100%) | Measured by brief PHQ-D | 6–8 | − | −0.078 | None | Primary review outcome: negative non-significant decrease in depression, but controls had a worse starting point depression scores decreased in both groups |
| Pill29 | Self-reported health status | Short form SF-36 questionnaire60 | 39 | −? | 1 of 8 was significant— 0.02 | None | Primary review outcome: 1 out of 8 showed a significant negative effect. Others showed no effect. Positive effect on secondary review outcomes (not significant). Low self-reported ability to maintain behaviour over time |
| Glyco-Hb | Lab test | 39 | + | ns | |||
| Body mass index (kg/m²) | Routinely collected audit data | 39 | + | ns | |||
| Blood pressure, systolic and diastolic | Routinely collected audit data | 39 | + | ns | |||
| Other clinical complications e | Routinely collected audit data | 39 | 0 | ns |
PHQ= the short form of the patient health questionnaire.
Note: gray shade = consultations based on patients’ complaints. Secondary review outcomes are in italics.
VAS-scale, visual analogue scale; WOMAC, Western Ontario and McMaster Universities osteoarthritis index.
a+ = in favour of the intervention group; − = in favour of the control group; 0 = no difference.
bSignificance expressed in P-values, if possible.
cAdverse effect = effects not mentioned as primary or secondary review outcomes but (potentially) harmful other effects (i.e. patient withdrawal from care), expressed in P-values.
dDiabetes specific measures of well-being and satisfaction with treatment.
eClinical complications, 1 point for each complication: amputation, foot ulcer, cardio vascular accident, ischemic heart disease, retinopathy and neuropathy.
Effects
A meta-analysis on effect estimates could not be performed because of the wide variety of effects. There were both positive and negative effects for intervention groups, but only a few were significant (see Table 4).
Chassany found significant positive results for all outcome measures (pain, disability and global perceptions of osteoarthritis). Cooper found no effect on blood pressure control. Kinmonth found positive effects on all three primary outcomes (quality of life, depression and well-being) in newly diagnosed diabetic patients. The impact on well-being, however, was the only significant result. The effects on secondary outcomes were positive in some cases (A1C and total cholesterol) and negative in other cases (triglycerides, BMI and blood pressure). The impact on two indicators, BMI and triglycerides, was significantly negative; overall, there was a non-significant negative trend. Krones only measured secondary outcomes. The mean change in cardiovascular risk for patients who had their cholesterol measured tended to be negative, albeit non-significant. Légaré found no effect on perceived health status. Loh found only a non-significant negative effect on depression as a primary review outcome. Pill found a significant negative effect on one out of eight primary outcomes (self-reported health status for diabetic patients); there was no effect on the other seven measures. The effect on secondary outcomes (glyco-haemoglobin, BMI, blood pressure and cardio vascular complications) was positive but not significant.
In summary, three studies showed positive results for both groups.28,30,31 Four studies suffered from recruitment bias, which led to a very good level of performance by all of the providers,28–31 or differences in the baseline characteristics of providers31 or patients.28,30 Of all five studies that measured primary outcomes, two found positive effects: one all significant34 and one partly significant.28 One study found a negative result for one of eight included outcomes,29 and one study showed a non-significant negative result.31 The remaining study showed no effect.33 The measurements in the significant positive study34 were taken over a short time span.
Of the three studies with consultations based on patients’ complaints,31,33,34 two of them31,34 measured patient-related outcomes as primary outcomes. One showed a significant positive effect,34 and one found a non-significant negative effect.31
Subgroup analysis.
Four studies were based on routine visits for controlling chronic diseases.28–30,32 Two of them measured patient-oriented outcomes as primary outcomes. One study found a significant positive effect out of three positive effects,28 and one study found one significant negative effect out of eight outcomes.29 For the secondary review outcomes, non-significant positive effects were observed in one study.29 In addition to these effects, there were significant negative effects in Kinmonth’s study and a non-significant negative effect in Krones’ study. There were no effects observed in the study by Cooper (see Table 4).
Discussion
Main findings
Despite an elaborate search strategy, we found only seven controlled intervention studies that related to our study aim despite the growing emphasis on patient participation in the literature.10,15,36 The seven studies in this review show ambiguous results. Despite the underlying theory, we see no significant effect (a suggestion of a positive impact at most) of patient participation on patient-related outcomes. This is similar to or even weaker than the results of other reviews.10,19,24,37 For disease-related outcomes, no overall effect of patient participation can be demonstrated; some studies even revealed deterioration in disease-oriented outcomes. Moreover, in several studies, the control patients also improved sometimes even more than the patients in the experimental condition. These results deserve some reflection.
First, why did we find so few studies in this area even though few people will deny the importance of the subject? In the literature, many different terms are used for patient participation. We may have missed certain terms and consequently missed trials. Backward and forward searches and a review of the reference lists of the selected publications resulted in only three new publications. Thus, this will be not the main reason.
A more convincing reason might be that there simply are not many controlled intervention studies on the effect of patient participation on health outcomes. The concepts used can be considered ‘fuzzy concepts’19,38: everyone understands what is meant by the concept generally, but there is no precise definition, which hampers the operationalization of the term.38 Therefore, researchers may shy away from choosing this topic for an RCT on health outcomes rather than process outcomes. Many other topics are easier to research.
The yield of this review suggest that researchers show more interest in disease-specific goals such as adherence,31 antibiotic use or lowering risk factors,28,30 using patient participation as an instrument. Training physicians hampers blinding them. Yet, well-performed, state-of-the-art empirical research is needed to discriminate between sense and nonsense of specific approaches to improve patient participation.
We must also conclude that the results are contradictory. The lack of actual behavioural differences between the experimental groups and the control groups may have contributed to the non-significant results. Informing participating GPs about the aim of the study might affect communication in both groups. In addition, conflicting motives (i.e. increasing patient participation versus changing the patients’ unhealthy behaviours) might have counteracted the providers’ performance of the skills they had been taught. Changing provider behaviour, especially related to patient participation,19,24,39,40 is not easily achieved.41,42 All 4 trials28,30–32 that measured a positive intervention effect used questionnaires, but the results of videotaped or audiotaped encounters were ambiguous.29,32
Patient-related elements, such as patients’ former failed attempts to change harmful behaviours, can lead to negative effects on health-related outcomes such as blood pressure.11,43
Learning from the pitfalls mentioned above, we offer several recommendations for future research in this field.
We recommend choosing an illness rather than a disease and measuring patient-oriented outcomes rather than disease-oriented outcomes. To design a properly blinded RCT, one could consider training participants in task-oriented behaviour (i.e. guideline adherence) instead of providing care as usual. The patients could be recruited in proximity to each training session, but there should be sufficient time between trainings to prevent problematic carry-over effects. To measure training effects, one could use baseline measurements before both patient recruitment phases. We assume carry-over effects are lower than generally accepted because the persistence of trained behaviours is repetitively lower than expected.6,29,39,44 To prevent the influence of affective behaviour, providers should not be informed about the aims of health-oriented trials, only about process goals. This can best be accomplished during the recruitment of professionals because a particular interest in the topic affects associated outcomes. It is generally recommended to assess the feasibility of the trial with a pilot test,45–47 but it seems wise to pay extra attention to the behavioural effects of the trainings and observe subsequent behaviour for changes.
Limitations of the study
We only found one trial32 in which an intervention (decision aid) was performed prior to consulting a physician. This might be due to logistic problems implementing this type of design in primary care.48 However, if an intervention preceding a clinical encounter was not described in the abstract, we may have missed it.
For the sake of time, we did not contact researchers in the field. Instead, we based our search on well-established search strategies with proven merit. We piloted the search strategy in PubMed. When leading articles were not identified, we broadened the strategy using terms that cover these non-identified articles. Many studies in this field focus on process outcomes and do not mention health-related outcomes in the abstract. Therefore, we did not exclude outcomes during the abstract screening.
Publication bias may have influenced the results, but we believe that this risk was low because studies that had secondary outcomes relevant to the review were included. There were no restrictions on time or language.
Authors were not contacted, which may have led to an underestimation of trial quality.49 Overall, more than 570 providers and 3244 patients were included, and the primary outcome was measured in 1824 patients.
Conclusion
The trials were heterogeneous in their populations, interventions and measures. The theoretical concept that patient participation has a beneficial effect on patient-reported health outcomes has still not been proven. The trials that concentrated on relieving patient complaints were scarce and suffered from quality issues. The results of studies on disease-oriented health outcomes were ambivalent. However, including patients in trials aimed at improving patient participation tends to benefit all patients. Research on the effects of improved patient participation should devote extra attention to developing unbiased designs and invest more in changing the affective behaviour of providers.
Declaration
Funding: ZonMw (project numbers 42011009 and 808330098030) (the Netherlands organisation for health research and development) and the Vocational Training Institute for GPs at the University of Utrecht. Open Access charge was funded by The Netherlands Organisation for Scientific Research.
Ethical approval: none.
Conflict of interest: none.
Acknowledgements
The authors wish to thank Jouke van der Zee for critically appraising all included articles.
Appendix 1
Search strategy
| No. | Synonyms |
|---|---|
| Domain: providers, patients and provider–patient interactions | |
| #1 | patient OR patient[MeSH] OR subject OR subjects OR participant* OR client* OR inpatient* OR outpatient* OR hospitalized* OR institutionalized* OR institutionalised* OR survivor* OR men OR woman OR women OR man OR consumer* OR people |
| #2 | health personnel[MeSH] OR doctor* OR physician* OR provider* OR practitioner* OR gp OR gps OR health-professional* OR nurse* OR carer* OR caregiver* OR clinician* OR health-care-professional OR health-care-professionals OR healthcare professional* OR health-care-worker* OR healthcare-worker* OR hospitalist* OR resident* NOT (veterinarian*) |
| #3 | communication[MeSH] OR interact* OR communicat* OR relation* OR instruct* OR verbal* OR nonverbal OR smiling OR “facial expression” OR advis* OR talk* OR contact* OR conversation* OR consult OR consultation |
| #4 | Professional-Patient Relations[MeSH] OR Physician’s Role[MeSH] OR nurse’s role[MeSH] OR “Professional Patient” OR “patient professional” OR “therapeutic alliance” OR doctor-patient OR patient-doctor OR clinician-patient OR patient-clinician OR physician-patient OR patient-physician OR nurse-patient OR patient-nurse OR patient-practitioner OR practitioner-patient OR biopsychosocial* |
| #5 | (#1 AND #2 AND #3) OR #4 |
| Determinant: promoting patient participation | |
| #6 | decision making[MeSH] OR decision support techniques[MeSH:noexp] OR decision support systems, clinical[MeSH] OR choice behaviour[MeSH] OR choice behavior[MeSH] OR decision making[tiab] OR decision counselling[tiab] OR decision support[tiab] OR choice-behaviour*[tiab] OR choice-behavior*[tiab] OR ((decision*[ti] OR choice*[ti]) AND (making*[ti] OR support*[ti] OR behaviour*[tiab] OR behavior*[tiab])) OR shared-decision[tiab] OR sharing-decision*[tiab] OR informed-decision*[tiab] OR informed-choice*[tiab] OR treatment-choice*[tiab] OR decision-autonomy[tiab] OR decisional-autonomy[tiab] |
| #7 | consumer participation[MeSH] OR patient participation[MeSH] OR patient-participation*[tiab] OR consumer-participation*[tiab] OR patient involvement*[tiab] OR consumer-involvement[tiab] OR ((patient*[ti] OR consumer*[ti]) AND (involvement*[ti] OR involving*[ti] OR participation*[ti] OR participating*[ti])) OR patient-centered[tiab] OR patient-centred[tiab] OR patient-oriented[tiab] OR patient-focused[tiab] OR client-focused[tiab] OR client-oriented[tiab] OR patient preference[MeSH] OR patient-centered care[MeSH] OR patient preference[tiab] OR patient-centered care[tiab] |
| #8 | decision-aid*[tiab] OR consultation-leaflet[tiab] OR patient education handout[tiab] OR patient education as topic[MeSH Terms] OR “patient education”[All Fields] OR decision trees[MeSH] OR decision-support[tiab] OR audiotape*[tiab] OR brochure[tiab] OR booklet[tiab] OR flyer[tiab] OR folder[tiab] OR handout[tiab] OR leaflet[tiab] OR pamphlet[tiab] OR guide[tiab] |
| #9 | #6 OR #7 OR #8 |
| RCT-filter | |
| #10 | (randomized-controlled-trial OR controlled-clinical-trial OR randomized[tiab] OR randomised[tiab] OR placebo[tiab] OR drug-therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (animals[mh] AND humans[mh])) |
| General practice | |
| #11 | primary health care[MeSH] OR physicians, primary care[MeSH] OR primary care nursing[MeSH] OR general practice[MeSH] OR general practitioners[MeSH] OR primary-care[tiab] OR general-practice[tiab] OR family-practice[tiab] OR primary-health-care[tiab] OR primary-care-physician*[tiab] |
| Domain AND Determinant AND RCT AND General Practice | |
| #12 | #5 AND #9 AND #10 AND #11 |
References
- 1. NIVEL, Netherlands institute for health services and research. Incidentie- en prevalentiecijfers in de huisartsenpraktijk; incidentie alle symptomen en klachten ICPC (R01-R29). Afkomstig uit LINH. 2012. http://www.nivel.nl/incidentie-en-prevalentiecijfers-in-de-huisartsenpraktijk (accessed on 22 June 2012). [Google Scholar]
- 2. Biondi DM. Physical treatments for headache: a structured review. Headache 2005; 45: 738–46. [DOI] [PubMed] [Google Scholar]
- 3. Montgomery P, Dennis J. Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev 2003; 1: CD003161. [DOI] [PubMed] [Google Scholar]
- 4. Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet 2001; 357: 757–62. [DOI] [PubMed] [Google Scholar]
- 5. Enck P, Horing B, Weimer K, Klosterhalfen S. Placebo responses and placebo effects in functional bowel disorders. Eur J Gastroenterol Hepatol 2012; 24: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Jellema P, van der Windt DA, van der Horst HE, Twisk JW, Stalman WA, Bouter LM. Should treatment of (sub)acute low back pain be aimed at psychosocial prognostic factors? Cluster randomised clinical trial in general practice. BMJ 2005; 331: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Windt DA, Kuijpers T, Jellema P, van der Heijden GJ, Bouter LM. Do psychological factors predict outcome in both low-back pain and shoulder pain? Ann Rheum Dis 2007; 66: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan AA, Khan A, Harezlak J, Tu W, Kroenke K. Somatic symptoms in primary care: etiology and outcome. Psychosomatics 2003; 44: 471–8. [DOI] [PubMed] [Google Scholar]
- 9. Ursin H, Eriksen H. Cognitive activation theory of stress, sensitization, and common health complaints. Ann N Y Acad Sci 2007; 1113: 304–10. [DOI] [PubMed] [Google Scholar]
- 10. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ 1995; 152: 1423–33. [PMC free article] [PubMed] [Google Scholar]
- 11. Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H. The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review. Health Technol Assess 1999; 3: 1–96. [PubMed] [Google Scholar]
- 12. Ong LM, de Haes JC, Hoos AM, Lammes FB. Doctor-patient communication: a review of the literature. Soc Sci Med 1995; 40: 903–18. [DOI] [PubMed] [Google Scholar]
- 13. Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ 1999; 319: 766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect 2005; 8: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bensing JM, Verheul W. The silent healer: the role of communication in placebo effects. Patient Educ Couns 2010; 80: 293–9. [DOI] [PubMed] [Google Scholar]
- 16. Mechanic D, Meyer S. Concepts of trust among patients with serious illness. Soc Sci Med 2000; 51: 657–68. [DOI] [PubMed] [Google Scholar]
- 17. Thomas KB. General practice consultations: is there any point in being positive? Br Med J 1987; 294: 1200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Knipschild P, Arntz A. Pain patients in a randomized trial did not show a significant effect of a positive consultation. J Clin Epidemiol 2005; 58: 708–13. [DOI] [PubMed] [Google Scholar]
- 19. Lewin SA, Skea ZC, Entwistle V, Zwarenstein M, Dick J. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database Syst Rev 2001; 4: CD003267. [DOI] [PubMed] [Google Scholar]
- 20. Rockenbauch K, Schildmann J. Shared decision making (SDM): a systematic survey of terminology use and concepts. Gesundheitswesen 2011; 73: 399–408. [DOI] [PubMed] [Google Scholar]
- 21. Weston WW. Informed and shared decision-making: the crux of patient-centered care. CMAJ 2001; 165: 438–9. [PMC free article] [PubMed] [Google Scholar]
- 22. Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 2008; 77: 219–26. [DOI] [PubMed] [Google Scholar]
- 23. Légaré F, Turcotte S, Stacey D, Ratté S, Kryworuchko J, Graham ID. Patients’ perceptions of sharing in decisions: a systematic review of interventions to enhance shared decision making in routine clinical practice. Patient 2012; 5: 1–19. [DOI] [PubMed] [Google Scholar]
- 24. Légaré F, Bekker H, Desroches S, et al. Effective continuing professional development for translating shared decision making in primary care: A study protocol. Implement Sci 2010; 5: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O’Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2009; 3: CD001431. [DOI] [PubMed] [Google Scholar]
- 26. Ebell MH, Siwek J, Weiss BD, et al. Simplifying the language of evidence to improve patient care: Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in medical literature. J Fam Pract 2004; 53: 111–20. [PubMed] [Google Scholar]
- 27. Brownson RC, Haire-Joshu D, Luke DA. Shaping the context of health: a review of environmental and policy approaches in the prevention of chronic diseases. Annu Rev Public Health 2006; 27: 341–70. [DOI] [PubMed] [Google Scholar]
- 28. Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998; 317: 1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pill R, Stott NC, Rollnick SR, Rees M. A randomized controlled trial of an intervention designed to improve the care given in general practice to Type II diabetic patients: patient outcomes and professional ability to change behaviour. Fam Pract 1998; 15: 229–35. [DOI] [PubMed] [Google Scholar]
- 30. Krones T, Keller H, Sönnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med 2008; 6: 218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loh A, Simon D, Wills CE, Kriston L, Niebling W, Härter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns 2007; 67: 324–32. [DOI] [PubMed] [Google Scholar]
- 32. Cooper LA, Roter DL, Carson KA, et al. A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med 2011; 26: 1297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ 2012; 184: E726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chassany O, Boureau F, Liard F, et al. Effects of training on general practitioners’ management of pain in osteoarthritis: a randomized multicenter study. J Rheumatol 2006; 33: 1827–34. [PubMed] [Google Scholar]
- 35. Kinmonth AL, Spiegal N, Woodcock A. Developing a training programme in patient-centred consulting for evaluation in a randomised controlled trial; diabetes care from diagnosis in British primary care. Patient Educ Couns 1996; 29: 75–86. [DOI] [PubMed] [Google Scholar]
- 36. Salzburg Global Seminar. Salzburg statement on shared decision making. BMJ 2011; 342: d1745. [DOI] [PubMed] [Google Scholar]
- 37. Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med 2004; 2: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Entwistle VA, Watt IS. Patient involvement in treatment decision-making: the case for a broader conceptual framework. Patient Educ Couns 2006; 63: 268–78. [DOI] [PubMed] [Google Scholar]
- 39. Edwards A, Elwyn G. Involving patients in decision making and communicating risk: a longitudinal evaluation of doctors’ attitudes and confidence during a randomized trial. J Eval Clin Pract 2004; 10: 431–7. [DOI] [PubMed] [Google Scholar]
- 40. Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995; 153: 1423–31. [PMC free article] [PubMed] [Google Scholar]
- 41. Lee RG, Garvin T. Moving from information transfer to information exchange in health and health care. Soc Sci Med 2003; 56: 449–64. [DOI] [PubMed] [Google Scholar]
- 42. Gysels M, Richardson A, Higginson IJ. Communication training for health professionals who care for patients with cancer: a systematic review of training methods. Support Care Cancer 2005; 13: 356–66. [DOI] [PubMed] [Google Scholar]
- 43. Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychol Bull 2004; 130: 324–40. [DOI] [PubMed] [Google Scholar]
- 44. Elwyn G, Edwards A, Hood K, et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract 2004; 21: 337–46. [DOI] [PubMed] [Google Scholar]
- 45. Campbell M, Fitzpatrick R, Haines A, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000; 321: 694–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Caldwell PH, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med 2010; 7: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Treweek S, Pitkethly M, Cook J, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010; 4: MR000013. [DOI] [PubMed] [Google Scholar]
- 48. van der Wouden JC, Blankenstein AH, Huibers MJ, van der Windt DA, Stalman WA, Verhagen AP. Survey among 78 studies showed that Lasagna’s law holds in Dutch primary care research. J Clin Epidemiol 2007; 60: 819–24. [DOI] [PubMed] [Google Scholar]
- 49. Soares HP, Daniels S, Kumar A, et al. Bad reporting does not mean bad methods for randomised trials: observational study of randomised controlled trials performed by the Radiation Therapy Oncology Group. BMJ 2004; 328: 22–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lerman CE, Brody DS, Caputo GC, Smith DG, Lazaro CG, Wolfson HG. Patients’ Perceived Involvement in Care Scale: relationship to attitudes about illness and medical care. J Gen Intern Med 1990; 5: 29–33. [DOI] [PubMed] [Google Scholar]
- 51. Man-Son-Hing M, Laupacis A, O’Connor AM, et al. A patient decision aid regarding antithrombotic therapy for stroke prevention in atrial fibrillation: a randomized controlled trial. JAMA 1999; 282: 737–43. [DOI] [PubMed] [Google Scholar]
- 52. Krones T, Keller H, Sönnichsen AC, Sadowski EM, Baum E, Donner-Banzhoff N. Partizipative Entscheidungsfindung in der Cardiovasculären Risicoprävention: Ergebnisse der Pilotstudie von ARRIBA-Hertz, einer consultationbezogenen Entscheidungshilfe für die allgemeinmedizinische Praxis [Shared decision-making in cardiovasculair prevention: results from the pilotting of ARRIBA-Herz, a transactional decision aid for general practice]. Z Med Psychol 2006; 15: 61–70. [Google Scholar]
- 53. Loh A, Leonhart R, Wills CE, Simon D, Härter M. The impact of patient participation on adherence and clinical outcome in primary care of depression. Patient Educ Couns 2007; 65: 69–78. [DOI] [PubMed] [Google Scholar]
- 54. Strull WM, Lo B, Charles G. Do patients want to participate in medical decision making? JAMA 1984; 252: 2990–4. [PubMed] [Google Scholar]
- 55. Melbourne E, Roberts S, Durand MA, Newcombe R, Légaré F, Elwyn G. Dyadic OPTION: Measuring perceptions of shared decision-making in practice. Patient Educ Couns 2011; 83: 55–7. [DOI] [PubMed] [Google Scholar]
- 56. Melbourne E, Sinclair K, Durand MA, Légaré F, Elwyn G. Developing a dyadic OPTION scale to measure perceptions of shared decision making. Patient Educ Couns 2010; 78: 177–83. [DOI] [PubMed] [Google Scholar]
- 57. Bradley C. (ed.). Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Management. Chur, Zwitserland: Harwood Academic, 1994. [Google Scholar]
- 58. López Alonso SR, Martínez Sánchez CM, Romero Cañadillas AB, Navarro Casado F, González Rojo J. Metric properties of WOMAC questionnaires-original and reduced versions-to measure symptoms and Physical Functional Disability. Aten Primaria 2009; 41: 613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996; 34: 220–33. [DOI] [PubMed] [Google Scholar]
- 60. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–83. [PubMed] [Google Scholar]