Abstract
Chronic stress exacerbates and can induce symptoms of depression and anxiety disorders. Chronic stress causes amygdala hyperactivity, which may contribute to these detrimental effects. One potential mechanism for amygdala hyperactivity is an increase of excitatory drive after stress. Excitatory inputs to the amygdala predominantly synapse upon dendritic spines, and repeated stress has been demonstrated to increase dendritic spines in the basolateral amygdala (BLA). However, the BLA is comprised of several nuclei, including the lateral nucleus (LAT) and the basal nucleus (BA), which exert functionally distinct roles in amygdala-dependent behaviors. Furthermore, while an increase of dendritic spines can impart significant functional ramifications, a shift of spine distribution can also exert significant impact. However, differences in the effects of repeated stress on LAT and BA have not been examined, nor differential effects on spine distribution. This study examined the effects of repeated restraint stress on dendritic structure of principal neurons from the LAT and BA in Golgi-stained tissue. This study found that repeated stress increased spine number in LAT and BA, but in very distinct patterns, with proximal increases in LAT neurons and non-proximal increases in BA neurons. Furthermore, repeated stress increased dendritic length in the BA, but not the LAT, leading to a global change of spine density in BA, but a focal change in LAT. These distinct effects of repeated stress in the LAT and BA may exert significant functional effects on fear behavior, and may underlie differences in the effects of repeated stress on acquisition, contextual modulation and extinction of fear behavior.
Keywords: amygdala, dendrite, spine, stress, Golgi, distribution
1.
Stress is a major contributing factor in the emergence of depression and anxiety disorders (Heim and Nemeroff, 2001; Lupien et al., 2009). Patients with anxiety and depression often display dysregulation of amygdala activity (Breiter et al., 1996; Davidson et al., 2003; Drevets et al., 1992; Sheline et al., 2001; Siegle et al., 2002; Thomas et al., 2001). Chronic stress in humans also causes hyperactivity of the amygdala (Armony et al., 2005; Bogdan et al., 2012; Dannlowski et al., 2012; Ganzel et al., 2007; Protopopescu et al., 2005; Shin et al., 1997; van Wingen et al., 2011), which may be a path through which stress precipitates the emergence of anxiety and depressive disorders.
In rodent models of chronic stress, neurons of the basolateral amygdala (BLA) display hyperactivity and increased responsiveness to excitatory inputs (Adamec et al., 2005; Correll et al., 2005; Mozhui et al., 2010; Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012). BLA neuronal hyperactivity could be caused by several factors, including a change in neuronal excitability and an increase of excitatory afferent drive. Consistent with the latter, repeated stress causes an increase in the number of dendritic spines and dendritic length of BLA principal neurons (Adamec et al., 2012; Hill et al., 2011; Hill et al., 2012; Mitra et al., 2005; Vyas et al., 2002; Vyas et al., 2006). The BLA is comprised of several nuclei, most notably the lateral nucleus (LAT) and basal nucleus (BA). These nuclei contribute to different aspects of fear behavior, with the LAT necessary for cued fear responses, while the BA imparts contextual specificity of conditioned fear responses (Calandreau et al., 2005; Onishi and Xavier, 2010; Orsini et al., 2011; Vlachos et al., 2011). However, previous studies of the effects of stress on BLA neuronal morphology have not compared the LAT and BA. Because these nuclei are functionally different, and they may be modulated in different manners by repeated stress, the effects of repeated stress on neuronal morphology was contrasted in these two nuclei.
Spines are the primary site of excitatory synaptic input onto BLA principal neurons (Brinley-Reed et al., 1995; Farb and Ledoux, 1999; Muller et al., 2006; Smith and Pare, 1994; Farb et al., 1992; Rademacher et al., 2010; Radley et al., 2007), and an increase of spines may reflect greater synaptic drive. However, the number of spines itself is not the only factor that influences the impact of synaptic input on neuronal activity. Synaptic inputs at different distances from the soma give rise to postsynaptic potentials (PSPs) of differing kinetics and amplitude (Andreasen and Lambert, 1998; Magee and Cook, 2000; Rosenkranz and Johnston, 2007; Turner, 1988). A change in the distribution of spines across the dendritic tree can lead to general differences in the integration of synaptic inputs. Furthermore, a more potent effect of repeated stress on spines at a specific range of distances from the soma may provide information about the influence of stress on subsets of excitatory input to the BLA. Previous studies have provided hints towards a greater impact of stress on specific segments of BLA dendrites (e.g. Mitra et al., 2005), but this has not been examined in depth. Therefore, the differential effects of repeated stress on the distribution of spines was also compared between LAT and BA nuclei. This study utilized Golgi-Cox staining of brain tissue from adult rats that were exposed to repeated restraint stress or control handling to test whether stress caused different effects in the LAT and BA on dendritic morphology, spine number or spine distribution.
2. Experimental Procedures
2.1. Subjects and groups
All procedures were approved by the Institutional Animal Care and Use Committee of Rosalind Franklin University of Medicine and Science, and followed the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Male Sprague-Dawley rats (Harlan; age 8–9 weeks at arrival) were group housed (2–3/cage) in a controlled climate animal facility. The housing room had a 12:12 light:dark schedule and food and water were available ad libitum. Repeated stress was performed by daily restraint stress. Rats were placed in a restraint hemi-cylinder for 20 minutes per session, one session per day, for 7 out of 9 consecutive days. This schedule of restraint exposure was previously shown to lead to increased adrenal gland weight and reduction of exploration in the elevated plus maze (Rosenkranz et al., 2010; Zhang and Rosenkranz, 2012); furthermore, it reduces inter-session habituation to restraint, which would otherwise be prominent (Kant et al., 1985; Stamp and Herbert, 1999). A control group was handled in the same manner as the restraint group, except that they remained in a transparent transport cage with bedding, instead of a restraint cylinder. The total amount of handling between groups was equivalent. All further experiments were performed one day after the final restraint or control session.
2.2. Golgi stain
Golgi-Cox staining of brain tissue was performed using the FD Rapid GolgiStain Kit (FD NeuroTechnologies, Columbia, MD), following the protocol suggested by the manufacturer. One day after the final restraint or control handling session, rodents were deeply anesthetized with chloral hydrate, (>400 mg/kg, i.p.), decapitated, and the brain was rapidly removed. The brain was blocked and placed immediately into Golgi impregnation solution in an opaque container. The impregnation solution was changed after 24 hours and brains were stored in the dark for 15 – 18 days at room temperature. Brain tissue was then transferred to solution C of the FD Rapid GolgiStain kit for 24 hours at 4° C. After 24 hours the solution was replaced with fresh solution C, and the brain was stored at 4o C for 7 days. Brains were sectioned (100 µm thickness, Leica SM 2000 R microtome), and slices were collected in 20% sucrose in 0.1 M phosphate buffer at room temperature. Slices were mounted on gelatinized slides and air dried (25 minutes – 1 hour), then rinsed in double distilled H2O (2 times, 4 minutes each rinse). Slides were dehydrated in 50%, 75% and 95% ethanol for 4 minutes each, then in 100% ethanol four times for 3 minutes each. Slides were cleared with xylene (3 times, 4 minutes each), then coverslipped with Permount. Slides were allowed to dry overnight. Throughout the staining and subsequent study, slides were protected from light.
2.3. Neuronal reconstruction
Golgi-stained neurons from LAT and BA were reconstructed using Neurolucida software (MBF Bioscience, Williston, VT) under white field illumination using the 100x objective of a Nikon Eclipse E400 microscope. LAT and BA were defined based on previously established borders (Swanson and Petrovich, 1998; Paxinos, 2004). In initial studies, Golgi-stained tissue was lightly counterstained with Nissl to facilitate the identification of LAT and BA until proficiency was achieved at determination of LAT and BA borders based on fiber tracts and structural landmarks. Only neurons that appeared to be completely filled were utilized. Thus, secondary and tertiary dendrites had to be visible, and spines had to be visible. In addition, there could be no breaks in the dendrites. Neurons were selected based on morphology consistent with BLA principal neurons (e.g. obvious primary dendrites and spines; McDonald, 1982). Reconstructions were performed by an individual that was blind to treatment conditions. Aspiny neurons that displayed small somata with few dendrites or large somata with bipolar primary dendrites were not included in this analysis. Dendritic branching was quantified as the number of intersections with concentric circles at increasing diameters (10 µm steps) in Sholl analysis (Sholl, 1953). Similarly, dendritic length and spine number were analyzed at 10 µm steps in Sholl analyses. To further measure these parameters in functional dendritic subdivisions, instead of only distance, the dendritic length and total number of spines in each dendritic branch order were quantified for comparison. Branch order was measured centrifugally, such that at each dendritic branch point the branch order number of both the parent and branch were increased. In addition, the normalized distribution of spines across the dendritic tree was quantified as [(Spine NumberBr)÷(Spine NumberTot)] where Spine NumberBr = the number of spines at a specific branch order, and Spine NumberTot = the total number of spines on that neuron. This normalization to the neuronal total facilitated measurement of the shift of distributions across distance. Normalization of neuronal values to peak neuronal branch values was also performed in some instances to facilitate comparison across neurons; Normalization to peak = [(Spine NumberBr) ÷(Spine NumberPk)], where Spine NumberBr = the number of spines at a specific branch order, and Spine Numberppk = the number of spines at the branch order with the largest number of spines. To demonstrate the magnitude of the effects of stress across dendritic branch order, the mean spine number at each branch order of controls was subtracted from each neuron from the stress group (Difference score). Photographs were acquired at 10, 20 or 100X magnification from control and stress groups under similar light conditions. In the images displayed, the color was adjusted to grayscale and only the size of the images has been adjusted, and this was applied equally to all images.
2.4. Statistical analysis
Neurons were excluded from analysis if they did not display morphological aspects of BLA principal neurons (as described above), or if their mean length or spine density was >2 standard deviations (SD) from the mean. Comparisons of multiple factors were examined using a two-way ANOVA. An alpha level of 0.05 was considered significant. Multiple post hoc comparisons were performed using t-tests with Bonferroni corrections based on the number of comparisons. For planned comparisons of single parameters between two groups, two-tailed unpaired t-tests were used. Data were tested for normality of distribution (Kolmgorov and Smirnov test), and for equality of the standard deviation (Bartlett’s test). Statistical tests were performed using Prism 6 software (GraphPad Software, La Jolla, CA). All values are expressed as the mean ± S.E.M. A portion of the brain tissue used in this data set was used in a separate study.
3. Results
3.1. Neurons of the BLA
Neurons of the LAT and BA were reconstructed. Only neurons that displayed a morphology consistent with BLA principal neurons were included for analysis (Fig 1A.; Methods). Consistent with previous studies (McDonald, 1982), neurons of the BA had larger somata than neurons of the LAT (LAT 280.5 ± 15.3 µm2 , n=30; BA 332.0 ± 13.3 µm2, n=28, p=0.015, two-tailed unpaired t-test, t=2.52, df=56). However, there was no significant difference in the average dendritic length of neurons between the LAT and BA (Fig. 1B; LAT 1338 ± 123.9 µm, n=30; BA 1447 ± 95.23 µm, n=28, p=0.49, two-tailed unpaired t-test, t=0.69, df=56) nor dendritic length measured as a function of distance from the soma (i.e. Sholl analysis; Fig. 1B; 2-way repeated measures ANOVA, Nucleus × Distance, no significant main effect of Nucleus, p=0.54, F(1,56)=0.37). There was no significant difference in the total number of spines/neurons (Fig. 1C; LAT 331.8 ± 45.5 spines, n=30; BA 343.1 ± 37.8 spines, n=28, p=0.85, two-tailed unpaired t-test, t=0.19, df=56) or number of spines measured as a function of distance from the soma (Fig. 1C; Sholl analysis, 2-way repeated measures ANOVA, Nucleus × Distance, no significant main effect of Nucleus, p=0.85, F(1,56)=0.04). There was no significant difference in spine density between LAT and BA neurons (Fig. 1D; Sholl analysis, 2-way repeated measures ANOVA, p=0.974, F(1,56)=0.001; LAT 2.38 ± 0.21 spines/10 µm, n=30; BA 2.26 ± 0.16 µm, n=28, p=0.64). There was also no significant difference in branching across the dendritic tree between neurons from the LAT and BA (Fig. 1E; Sholl analysis, number of intersections, 2-way repeated measures ANOVA, Nucleus × Distance, no significant main effect of Nucleus, p=0.76, F(1,56)=0.09).
Figure 1. Morphology of principal neurons in the LAT and BA.
Neurons of the LAT and BA were reconstructed after Golgi-Cox staining. A) Boundaries of LAT and BA were defined by comparison to a brain atlas (left; in this section by comparison to −3.30 mm from bregma (Paxinos and Watson, 2001) and Nissl-stained sections (middle). The outline of the LAT and BA are drawn over the Golgi-stained section for comparison (right). B) Principal neurons of the LAT (left) and BA (right) that were reconstructed were spiny, with identifiable primary dendrites. C) Dendritic length of principal neurons in the LAT and BA across distances from the soma (Sholl analysis, left) and total dendritic length (right) were similar. D) The spine number on principal neurons in the LAT and BA were similar across distance (Sholl analysis, left) and in total spine number (right). E) The average spine density of principal neurons in the LAT and BA was similar. F) The number of intersections of neurons in Sholl analysis was similar between LAT and BA principal neurons. Here, and in all figures, LAT= lateral nucleus, BA = basal nucleus, BLA = lateral + basal nuclei.
When the BLA complex was examined overall (LAT + BA), repeated restraint stress caused a significant increase in dendritic length (Fig. 2A; Sholl analysis, 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0013, F(1,113)=10.8; significant interaction p<0.0001, F(32,3616)=4.1; control n=58, stress n=57) and dendritic branching (Fig. 2B; Sholl analysis, 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0007, F(1,113)=12.2; significant interaction p<0.0001, F(32,3616)=5.1). Repeated stress also had a significant effect on spine number (Fig. 2C; Sholl analysis, 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p<0.0001, F(1,113)=12.9; significant interaction p<0.0001, F(32,3616)=4.2) and spine density (Fig. 2D; Sholl analysis, 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0021, F(1,113)=9.91; significant interaction p<0.0001, F(32,3616)=2.5).
Figure 2. Repeated stress increases BLA neuronal length and spine number.
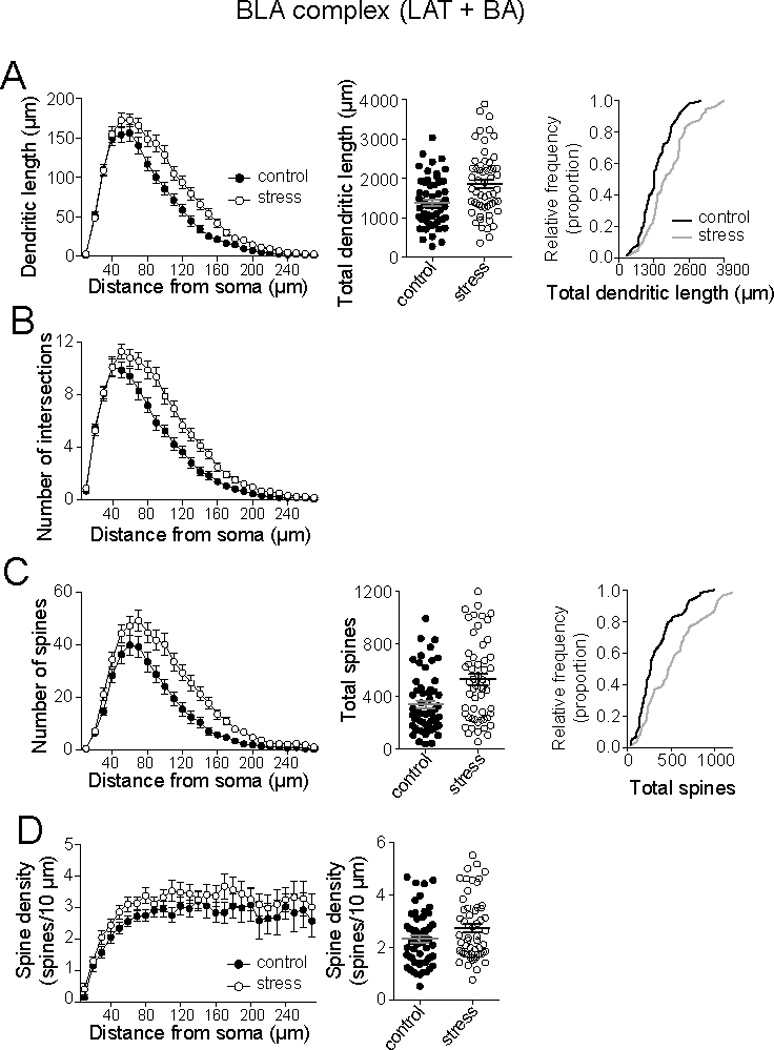
BLA neurons (LAT and BA) were grouped together for initial analysis. A) Repeated restraint increased the dendritic length of BLA neurons in Sholl analysis (left) and total dendritic length (middle). There was a rightward shift in the cumulative frequency of BLA principal neurons when total dendritic length was measured (right). B) Repeated stress increased the number of intersections of BLA principal neurons in Sholl analysis. C) Repeated stress increased the number of spines in BLA principal neurons (Sholl analysis, left), increased total number of spines (middle), and caused a rightward shift in the cumulative frequency of total spines in neurons (right). D) Repeated stress increased spine density of BLA principal neurons, demonstrated in Sholl analysis (left), and average neuronal spine density (right).
3.2. Effects of repeated restraint stress on LAT neurons
Repeated restraint stress did not significantly increase the dendritic length of pyramidal-like neurons in the LAT when examined by Sholl analysis (Fig. 3A; 2-way repeated measures ANOVA, Stress × Distance, no significant main effect of Stress, p=0.17, F(1,57)=2.0, control 1338 ± 123.9 µm, n=30, stress 1646 ± 133.6 µm, n=29, 123% of control). Repeated stress did not cause a significant increase in dendritic branching in neurons from the LAT (Fig. 3B; Sholl analysis, 2-way repeated measures ANOVA, Stress × Distance, no significant main effect of Stress, p=0.11, F(1,57)=2.66). However, repeated restraint stress caused a significant increase of spine number in neurons of the LAT (Fig. 3C; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.047, F(1,57)=4.14; control 331.8 ± 45.5, n=30, stress 475.3 ± 61.5, n=29, 143% of control). The increased spine number without significantly increased dendritic length would be expected to lead to a measured increase of spine density. However, the spine density measured by Sholl analysis did not reach statistical significance, yet there was a clear trend towards increased spine density, noticeable at proximal locations (Fig. 3D; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, no significant main effect of Stress, p=0.088, F(1,57)=3.01; control 2.4 ± 0.21 spines/10 µm, n=30, stress 2.7 ± 0.22 spines/10 µm, n=29, t=0.99, df=57, 113% of control).
Figure 3. Repeated stress increased LAT neuronal spine number.

A) When principal neurons of the LAT were analyzed there was no significant increase in dendritic length, measured by Sholl analysis (left) or total dendritic length (right). B) Repeated stress did not cause a significant increase of dendritic intersections in Sholl analysis. C) Repeated stress caused a significant increase in the number of spines, measured by Sholl analysis (left) and total spines (right). D) Repeated stress did not cause a significant change in spine density in Sholl analysis (left) or average spine density of neurons (right).
3.3. Effects of repeated restraint stress on BA neurons
Unlike effects in the LAT, repeated restraint stress significantly increased dendritic length in BA principal neurons (Fig. 4A; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0016, F(1,54)=11.0; control 1447 ± 95.2, n=28, stress 2082 ± 165.9, n=28, 144% of control) and dendritic branching (Fig. 4B; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0012, F(1,54)=11.7). There was also a significant increase in spine number (Fig. 4C; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0021, F(1,54)=10.5; control 331.8 ± 45.5, n=28, stress 505.3 ± 66.8, n=28, 152% of control), and a significant effect of stress on spine density (Fig. 4E; Sholl analysis 2-way repeated measures ANOVA, Stress × Distance, significant main effect of Stress, p=0.0082, F(1,54)=7.53; control 2.26 ± 0.16, n=28, stress 2.77 ± 0.23, n=28, 123% of control). This indicated that repeated stress increased spine number and increased dendritic length, but the increase of spine number outpaced the increase of dendritic length, leading to increased spine density.
Figure 4. Repeated stress increased BA neuronal dendritic length and spine number.
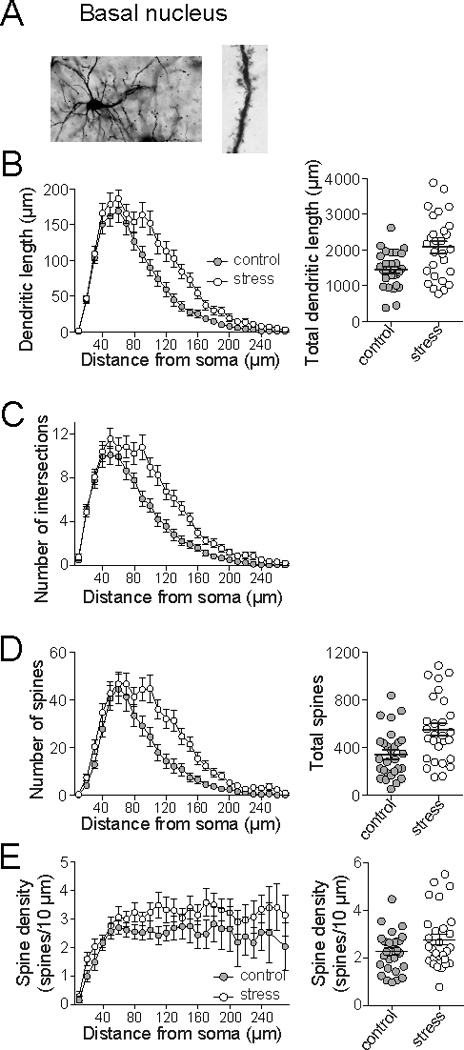
A) When principal neurons of the BA were analyzed, repeated stress caused a significant increase of dendritic length measured by Sholl analysis (left) and total dendritic length (right). B) Repeated stress caused an increase of intersections measured by Sholl analysis. C) Repeated stress increased the number of spines measured in Sholl analysis (left) and total spines (right). D) There was a significant effect of repeated stress on spine density (Sholl analysis, left) and average neuronal spine density (right).
3.4. Repeated stress shifts distribution of spines
While stress increased the spines in both LAT and BA principal neurons, visual examination of Sholl plots indicate that it appears to exert its greatest impact in proximal dendrites in the LAT, but more intermediate and distal dendrites in the BA. Therefore, the distribution of spines was measured. The distribution of spines across the dendritic tree was quantified as the proportion of spines at branches of increasing order from the soma (normalized to the peak proportion; branch order was used instead of distance to control for differences in dendritic length). The effects of stress were determined by comparison of best-fit Gaussian curves to the distribution. When examined across the BLA (LAT and BA), there was a shift in the distribution of spines towards more distal distribution after repeated restraint stress (Fig. 5A, Table 1; different best-fit for data sets, p<0.001, F(3,914)=7.2; mean branch order, control 2.9 ± 0.07, stress 3.3 ± 0.09). To demonstrate the region of highest stress effects on distribution, the average number of spines at each branch order of the control group was subtracted from the stress group (difference score; Fig. 5A). Repeated stress significantly changed the distribution of dendrites towards greater dendritic length in more distal branch orders when BLA overall was examined (p=0.0002, F(7,392)=4.12, one-way repeated measures ANOVA; significantly different than 0 at 5th and 6th branch after Bonferroni corrections for multiple comparisons).
Figure 5. Repeated stress shifts the dendritic spine distribution in BA principal neurons, but not LAT.
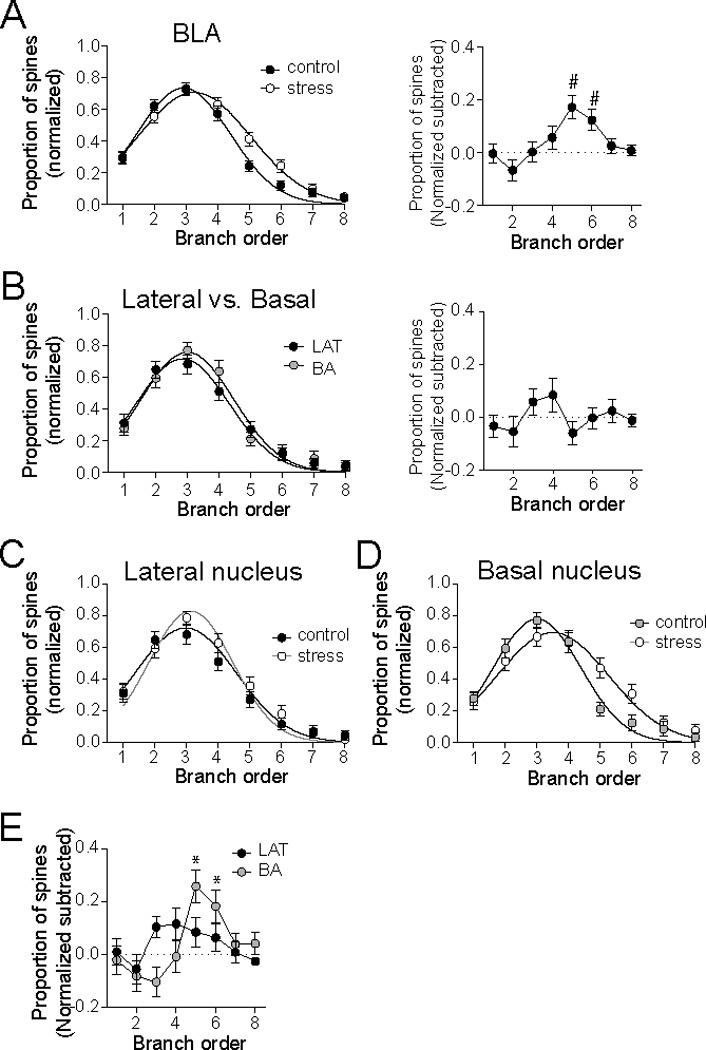
A) There was a significant difference in the normalized distribution of spines across the dendritic tree of BLA principal neurons after repeated stress (left). Subtraction of the average control spine distribution demonstrates that the peak effects of repeated stress lie between the 4th – 6th dendritic branch orders. B) There was no significant difference in the distribution of spines between LAT and BA neurons (left), and no significant difference when LAT and BA spine distribution was subtracted. C) Repeated stress did not significantly change the normalized distribution of spines in LAT principal neurons. D) Repeated stress caused a significant shift in the distribution of spines across the dendritic tree of BA principal neurons. E) Repeated stress did not lead to any significant changes in the subtracted effects of repeated stress in LAT neurons (black), but did significantly change the subtracted effects across branch orders in BA neurons (grey). # indicates BA group significantly different than 0. * indicates BA group significantly different than 0.
Table 1.
Best-fit values to curves to the distribution of spines across dendrites.
| Nucleus | Treatment | Best-fit values | ||
|---|---|---|---|---|
| Amplitude | Branch order | Standard deviation | ||
| All BLA | Control | 0.733 ± 0.03 | 2.93 ± 0.06 | 1.48 ± 0.07 |
| Stress | 0.705 ± 0.03 | 3.27 ± 0.08a | 1.78 ± 0.09 a | |
| LAT | Control | 0.695 ± 0.04 | 2.84 ± 0.10 | 1.57 ± 0.11 |
| Stress | 0.757 ± 0.04 | 3.09 ± 0.09 | 1.62 ± 0.10 | |
| BA | Control | 0.779 ± 0.04 | 3.00 ± 0.08 | 1.38 ± 0.09 |
| Stress | 0.667 ± 0.04 | 3.49 ± 0.13ab | 1.93 ± 0.14 a | |
significantly different than control (within nucleus comparison)
significantly different than LAT - Stress
BA vs. LAT
Control: Amplitude – n.s. p=0.13, F(1,458)=2.29
Branch order – n.s. p=0.21, F(1,458)=1.59
SD – n.s. p=0.22, F(1,458)=1.54
Stress: Amplitude – n.s. p=0.088, F(1,450)=2.92
Branch order – p=0.011, F(1,450)=6.54
SD – n.s. p=0.054, F(1,450)=3.73
Control vs. stress
BLA: Amplitude – n.s. p=0.48, F(1,914)=0.50
Branch order – p=0.0008, F(1,914)=11.3
SD – p=0.0099, F(1,914)=6.68
LAT: Amplitude – n.s. p=0.24, F(1,466)=1.41
Branch order – n.s. p=0.062, F(1,466)=3.51
SD – n.s. p=0.75, F(1,466)=0.10
BA: Amplitude – p=0.04, F(1,442)=4.0
Branch order – p=0.002, F(1,442)=9.95
SD – p=0.0012, F(1,442)=10.59
Post-hoc correction – for significance p=0.05/3 = 0.0167
To determine whether the shift of spine distribution was similar across BLA nuclei, the effects of stress on spine distribution were examined separately in the LAT and BA, and these effects were compared. In control rats, the distribution of spines was not significantly different between LAT and BA principal neurons (Fig. 5B, Table 1; best-fit curves not significantly different, p=0.28, F(3,458)=1.29; mean branch order, LAT 2.8 ± 0.1, BA 3.0 ± 0.09). Similarly, the subtracted difference between LAT and BA spine distributions was not significant at any branch order (Fig. 5B; p=0.11, F(8,216)=1.65, one-way repeated measures ANOVA).
There was no significant effect of repeated stress on the distribution of spines on LAT neurons (Fig. 5C, Table 1; p=0.068, F(3,466)=2.39, best fit with same polynomial to both data sets), even when the subtracted difference score was measured (Fig. 5E, p=0.36, F(7,203)=1.12, one-way repeated measures ANOVA). However, the distribution of spines across the dendritic tree was significantly different after repeated restraint in BA neurons, with a greater proportion of spines at higher branch orders compared to controls (Fig. 5D, Table 1; best fit to different polynomials, p<0.0001, F(3,442)=7.75). When the subtracted difference score was measured, a significant difference was found at the 5th and 6th branch orders (p<0.0001, F(7,189)=5.19, one-way repeated measures ANOVA, one-sample t-tests significantly different than 0 at 5th and 6th branch after Bonferroni corrections for multiple comparisons). Furthermore, the impact of stress (measured as the difference score) was significantly different in the LAT and BA (Fig. 5E; significant interaction Nucleus × Branch order, two-way repeated measures ANOVA, p=0.0056, F(7,385)=2.91). This indicates that repeated stress exerted nucleus-specific effects on the distribution of spines across the dendritic tree.
It is expected that nucleus-specific effects on spine number and dendritic length would lead to differences in spine density (density = spines/dendritic length). However, while stress appeared to exert some effect on spine density in the LAT, it did not reach statistical significance in Sholl analysis (Fig. 3E). Therefore, spine density was examined across dendritic branch order, which subdivides the dendritic tree into subunits based on a functional consideration (branch points instead of distance). There was a significant effect of stress on spine density across branch order in the LAT (Fig. 6A; 2-way ANOVA, Stress × Branch order, no significant main effect of Stress, p=0.045, F(1,315)=4.07). There was also a significant effect of repeated stress on spine density across branch order in the BA (Fig. 6A; 2-way ANOVA, Stress × Branch order, significant main effect of Stress, p=0.0048, F(1,309)=8.06). To measure whether repeated stress caused a shift in the spine density across the dendritic tree, or if the increased spine density was localized to one region, spine density was normalized (to peak spine density), and the best fit curves were compared. Repeated stress significantly shifted the spine density across branch orders in the LAT (Fig. 6B; best-fit curves significantly different, p=0.030, F(3,309)=3.01) but not in the BA (Fig. 6B; best-fit curves not significantly different, p=0.80, F(3,306)=0.33). When the subtracted effects of stress were measured across branch order (as above, stress minus averaged control), there was a significant effect of stress on normalized spine density proximally in the LAT, but no effect on BA spine density distribution (Fig. 6C; two-way ANOVA F(1,312)=3.93, p=0.048; LAT one-way ANOVA: p=0.041, F(6,153)=2.26, one-sample t-tests with post-hoc Bonferroni corrections, 2nd and 3rd branch orders significantly different than 0; BA one-way ANOVA: p=0.653, F(5,159)=0.495). This is consistent with a proximal increase of spines in the LAT that is accompanied by minimal dendritic growth, leading to an increase of spine density and a shift in the region of highest relative spine density. However, in the BA, spines were increased globally across the dendritic tree, and regions of particularly high spinogenesis are overlaid upon an increase of dendritic length, leading to equilibration of the increase of spine density across the dendritic tree.
Figure 6. Repeated stress changes spine density patterns in LAT and BA neurons.
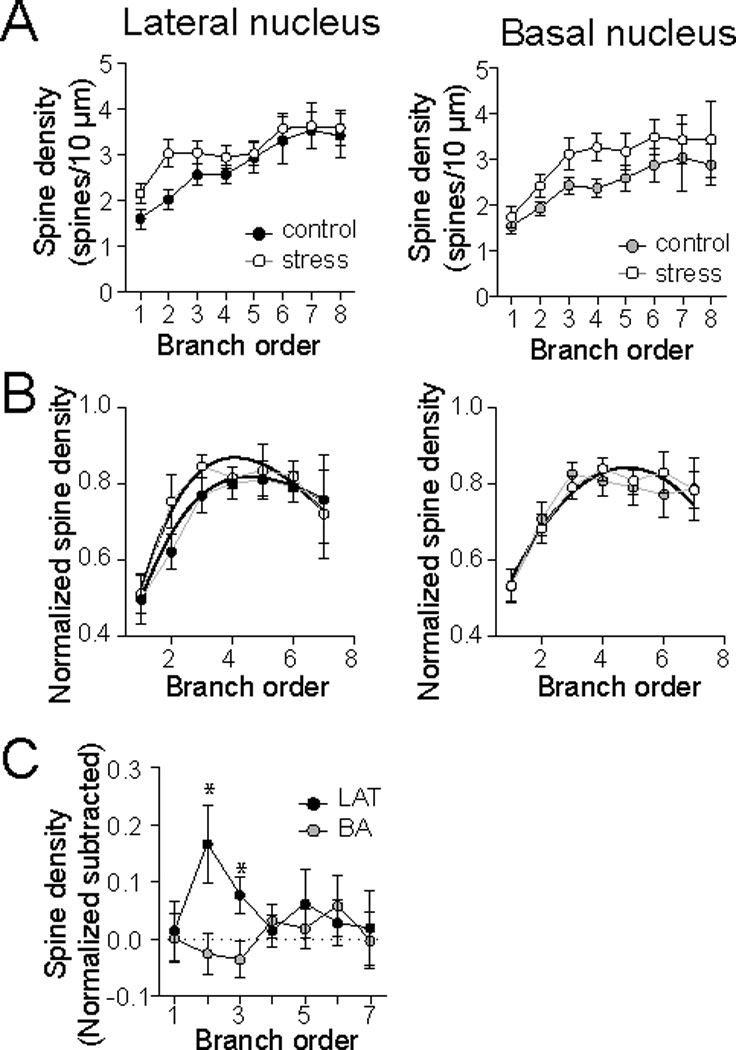
A) When measured over branch order, repeated stress increased spine density in LAT (left) and BA (right) principal neurons. B) When spine density data is normalized, different patterns emerged in the effects of stress. Repeated stress significantly shifted the distribution of spine densities across dendritic branches in the LAT (left) but not the BA (right). C) Subtraction of the spine densities across dendritic branches indicates that repeated stress increased spine density in low order branches of LAT neurons with minimal impact on spine density in BA neurons. * indicates LAT group significantly different than 0.
4. Discussion
Repeated stress has been demonstrated to increased spine number in the BLA (Hill et al., 2012; Mitra et al., 2005). However, it was not known whether repeated stress exerts different actions in the LAT and BA subdivisions of the BLA. The current study demonstrates that repeated restraint stress leads to a significant increase of spines across the dendritic tree in BLA neurons, but especially at intermediate and distal sites. When analyzed further, this shift in distribution of spines was largely due to the effects of stress in the BA. Indeed, the changes observed at the level of the BLA overall could be largely explained by effects of stress in the BA. This may indicate that the effects of stress on neuronal morphology of BLA neurons in other studies may also have been driven by changes in the BA. Repeated stress increased spines in both the LAT and BA, but to different degrees and in different patterns. In BA neurons the increase of spine number was most prominent at intermediate and distal distances from the soma (Fig. 7B), leading to a significant shift of spine distribution in the BA. In LAT neurons the increase of spine number was most prominent at locations proximal to the soma (Fig. 7A), without significantly shifting the overall spine distribution. Furthermore, repeated stress significantly increased the dendritic length in BA, but not in LAT. The growth across dendrites in BA neurons almost, but not quite, kept pace with the spinogenesis, such that there was a general increase in the spine density across BA dendrites (Fig. 7B). However, in LAT neurons there was not significant dendritic growth, leading to a focal increase of spine density in LAT neurons in proximal regions of spinogenesis (Fig. 7A). These data demonstrate that repeated stress exerts actions that may be functionally different in the BA and LAT. The data with Sholl analysis and branch order largely agree in supporting these conclusions. One discrepancy emerges with examination of spine density. When compared by branch order, repeated restraint significantly increased spine density in LAT principal neurons, however, when compared with Sholl analysis it did not (p=0.088). Potential explanations for this discrepancy are that Sholl analysis does not analyze the dendrite by functional units (unlike branch order analysis), and small focal effects can be missed in Sholl analysis. However, even in the Sholl plot an effect of repeated stress on spine density begins to emerge.
Figure 7. Summary of different effects of repeated stress on LAT and BA neurons.
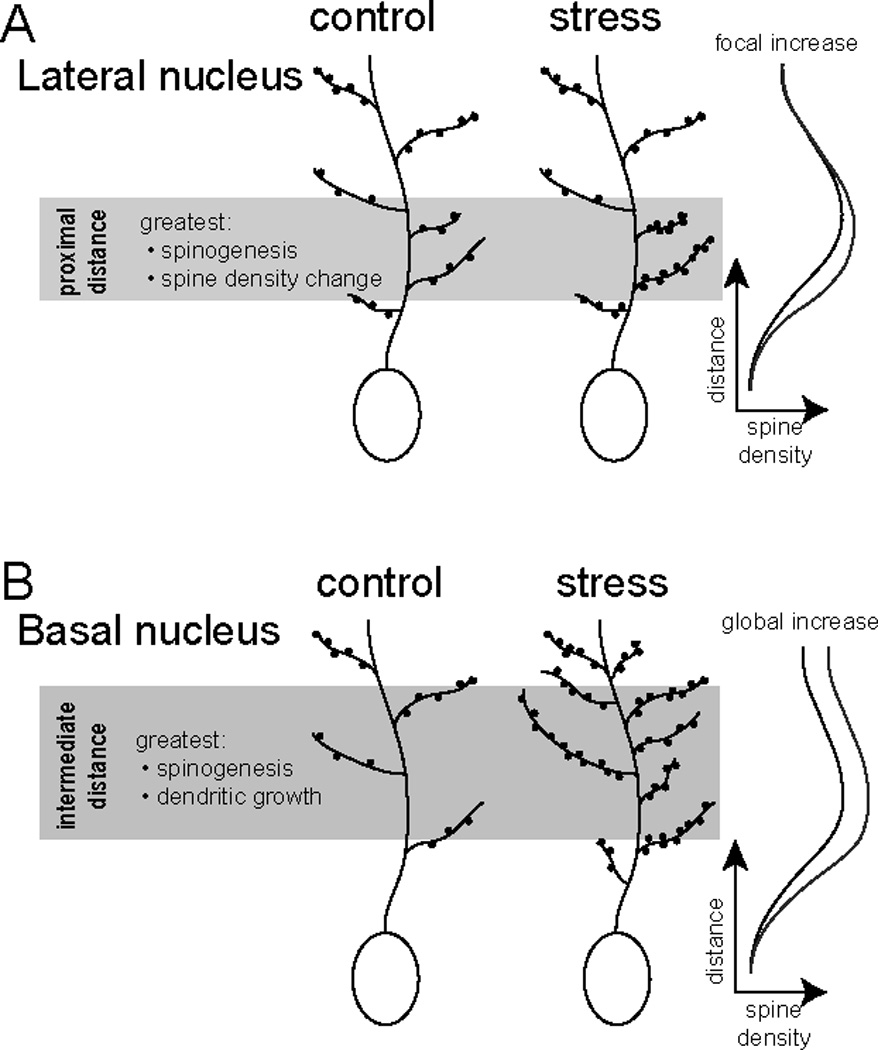
A) In principal neurons of the LAT, repeated stress causes an increase of spine number at distances relatively proximal to the soma. This is not accompanied by increased dendritic length, but does lead to a focal increase in spine density at distances proximal to the soma. B) In principal neurons of the BA, repeated stress causes an increase of spine number at relatively intermediate and distal distances from the soma. This is accompanied by increased dendritic length. The increased dendritic length is apparently not enough to compensate for the increased spine number, as there is an increase of spine density across the dendritic tree.
There are a number of possible outcomes of the effects of repeated restraint observed here. An overall increase of dendritic spines can represent an increase of excitatory synaptic input. In many brain regions, there is a topography for the location of inputs across the dendrites (Amaral and Witter, 1989; Bollmann and Engert, 2009; French and Totterdell, 2002; Ishizuka et al., 1990; Jia et al., 2010; Markram et al., 1997; Petreanu et al., 2009; Richardson et al., 2009; Steward, 1976; Triplett et al., 2009). If a similar principle holds true in the BLA, a shift in the distribution of spines points to a relative increase in a subset of excitatory afferents. In addition, dendrites filter synaptic inputs. This filtering results in distance-dependent effects on the amplitude and kinetics of excitatory post-synaptic potentials (EPSPs) that reach the soma (Andreasen and Lambert, 1998; Magee, 2000; Turner, 1988). Relative increases in the abundance of excitatory input at a particular distance may result in an overall shift in the shape and amplitude of EPSPs that arrive at the soma (e.g. Rall, 1967; Rall et al., 1967). Functionally, a shift in the decay time and amplitude of EPSPs exerts a large impact on the integration of inputs, and ultimately impacts action potential firing. Furthermore, a general increase of ongoing synaptic activity at proximal and distal locations exerts functionally distinct effects on synaptic integration and plasticity (Schaefer et al., 2003; Larkum et al., 2004; Oviedo and Reyes, 2005; Williams, 2005). Most directly, however, is demonstration that the dendritic structure contributes to computational ability of neurons (Jaffe and Carnevale, 1999; Mainen and Sejnowski, 1996; Vetter et al., 2001), and the presence and density of spines itself contributes to propagation of signals (Baer and Rinzel, 1991; Jaslove, 1992; Segev and Rall, 1998; Shepherd et al., 1985). Those studies point to different functional consequences of a change in spine number and a change in spine density.
Several caveats should be considered when interpreting these data. Not all excitatory inputs synapse onto spines. A proportion of asymetrical contacts are formed at the dendritic shaft (~3 – 15%; Brinley-Reed et al., 1995; Farb and Ledoux, 1999; Muller et al., 2006; Rademacher et al., 2010; Smith and Pare, 1994). Furthermore, not all of the new spines necessarily receive any excitatory input, or may receive weak synaptic input.
The repeated stress regimen used here is shorter than many previous studies. In hippocampal CA3, a prolonged period (21 d) is required for the emergence of the effects of stress (Magarinos and McEwen, 1995). However, shorter periods of repeated stress (10 d) change the morhphology of neurons in the prefrontal cortex (Brown et al., 2005) and BLA (Mitra et al., 2005; Vyas et al., 2002). However, when longer periods (21 d) of repeated restraint are used the effects of repeated stress on the morphology of BLA neurons may be somewhat greater (25% compared to 30% increase Vyas et al., 2006). Our results indicate that use of an intermittent stress over an even shorter period of time can lead to a similar magnitude of effect on BLA morphology as the longer (21 d) period.
The LAT and BA are involved in partially overlapping behaviors. The BA imparts contextual confines to conditioned fear, while the LAT has a more significant role in formation of cued fear memory. Similarly, ther are differences in synaptic input and projection targets of the LAT and BA. The LAT and BA are targeted by a large number of structures. Differences in afferent inputs are largely a matter of degree. LAT receives more sensory and perirhinal cortical input (LeDoux et al., 1991; Turner and Herkenham, 1991; Romanski and LeDoux, 1993; Mascagni et al., 1993), while the BA receives more hippocampal (e.g. subiculum; Canteras and Swanson, 1992) input. LAT and BA also display many similar outputs (Pitkanen et al., 1997). Both project to the lateral portion of the central amygdala (CeA), while BA also projects to the medial CeA, and more extensively targets extended amygdala structures, such as the bed nucleus of the stria terminalis (BNST; Bienkowski and Rinaman, 2013; Savander et al., 1997; Savander et al., 1995). The circuit from sensory input to LAT to CeA plays a critical role in cued phasic fear. The circuit that includes hippocampal inputs to BA to BNST plays a key role in contextual fear and prolonged anxiety (Davis et al., 2010; Duvarci et al., 2009; Waddell et al., 2006; Zimmerman and Maren, 2011). The increase in excitatory inputs to LAT neurons reflected by increased spines may be expected to increase the affective response to specific stimuli. Consistent with this, repeated restraint stress increased the fear response to conditioned cues (Atchley et al., 2012). An increase in spine number coupled with a shift in the distribution of spines in the BA after repeated stress may indicate that a specific set of afferents to the BA can now disproportionately drive BA activity and anxiety behavior. Repeated stress increases anxiety-like behavior in novel contexts (e.g. open field and elevated plus maze; Atchley et al., 2012; Vyas and Chattarji, 2004; Shoji and Mizoguchi, 2010; Katz et al., 1981; Beck and Luine, 2002), and generally increases social anxiety (Doremus-Fitzwater et al., 2009; Barsy et al., 2010; Green et al., 2012). However, there is mixed evidence of whether repeated stress increases contextual fear responses (Baran et al., 2009; Sanders et al., 2010; Sandi et al., 2001; Conrad et al., 1999). Based on these effects of repeated stress on context-related behaviors, it is tempting to speculate that repeated stress increases context-related hippocampal drive of the BA, producing greater affective responses to ambiguous or fear-conditioned contexts.
The current study demonstrates that repeated stress causes an increase of spines in both the LAT and BA, but in different patterns. Furthermore, it causes elongation of dendrites in the BA but not the LAT. This is expected to lead to different effects of repeated stress on the physiology of the LAT and BA. Repeated stress does in fact lead to a slightly greater impact on the firing of neurons in the BA compared to the LAT (Zhang and Rosenkranz, 2012). The behavioral importance of this may emerge in greater impact of repeated stress on BA-dependent behaviors, such as contextual modulation of fear. In human patients this may be displayed as disorganized contextual regulation of fear after repeated stress.
Highlights.
Repeated restraint stress increases spine number in basolateral amygdala complex.
Increased spines found on principal neurons in lateral (LAT) and basal (BA) nuclei.
Dendritic hypertrophy observed in LAT but not BA.
Spinogenesis is localized to LAT proximal dendrites but global on BA dendrites.
Acknowledgements
Supported by NIH (MH084970) and The Brain Research Foundation. The funding sources had no role in study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Abbreviations
- ANOVA
Analysis of variance
- BA
basal nucleus of the basolateral complex
- BLA
basolateral complex of the amygdale
- LAT
lateral nucleus of the basolateral complex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
References
- Adamec R, Blundell J, Burton P. Role of NMDA receptors in the lateralized potentiation of amygdala afferent and efferent neural transmission produced by predator stress. Physiol Behav. 2005;86:75–91. doi: 10.1016/j.physbeh.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Adamec R, Hebert M, Blundell J, Mervis RF. Dendritic morphology of amygdala and hippocampal neurons in more and less predator stress responsive rats and more and less spontaneously anxious handled controls. Behav Brain Res. 2012;226:133–146. doi: 10.1016/j.bbr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD. Factors determining the efficacy of distal excitatory synapses in rat hippocampal CA1 pyramidal neurones. J Physiol. 1998;507:441–462. doi: 10.1111/j.1469-7793.1998.441bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Atchley D, Hankosky ER, Gasparotto K, Rosenkranz JA. Pharmacological enhancement of calcium-activated potassium channel function reduces the effects of repeated stress on fear memory. Behav Brain Res. 2012;232:37–43. doi: 10.1016/j.bbr.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer SM, Rinzel J. Propagation of dendritic spikes mediated by excitable spines: a continuum theory. J Neurophysiol. 1991;65:874–890. doi: 10.1152/jn.1991.65.4.874. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsy B, Leveleki C, Zelena D, Haller J. The context specificity of anxiety responses induced by chronic psychosocial stress in rats: a shift from anxiety to social phobia? Stress. 2010;13:230–237. doi: 10.3109/10253890903296389. [DOI] [PubMed] [Google Scholar]
- Beck KD, Luine VN. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav. 2002;75:661–673. doi: 10.1016/s0031-9384(02)00670-4. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Common and distinct neural inputs to the medial central nucleus of the amygdala and anterior ventrolateral bed nucleus of stria terminalis in rats. Brain Struct Funct. 2013;218:187–208. doi: 10.1007/s00429-012-0393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Engert F. Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron. 2009;61:895–905. doi: 10.1016/j.neuron.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O'Sullivan RL, Savage CR, Jenike MA, Rosen BR. Functional magnetic resonance imaging of symptom provocation in obsessivecompulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Brinley-Reed M, Mascagni F, McDonald AJ. Synaptology of prefrontal cortical projections to the basolateral amygdala: an electron microscopic study in the rat. Neurosci Lett. 1995;202:45–48. doi: 10.1016/0304-3940(95)12212-5. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–1722. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Calandreau L, Desmedt A, Decorte L, Jaffard R. A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learn Mem. 2005;12:383–388. doi: 10.1101/lm.92305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–391. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: longterm consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Social and non-social anxiety in adolescent and adult rats after repeated restraint. Physiol Behav. 2009;97:484–494. doi: 10.1016/j.physbeh.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D. The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci. 2009;29:10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb C, Aoki C, Milner T, Kaneko T, LeDoux J. Glutamate immunoreactive terminals in the lateral amygdaloid nucleus: a possible substrate for emotional memory. Brain Res. 1992;593:145–158. doi: 10.1016/0006-8993(92)91303-v. [DOI] [PubMed] [Google Scholar]
- Farb CR, Ledoux JE. Afferents from rat temporal cortex synapse on lateral amygdala neurons that express NMDA and AMPA receptors. Synapse. 1999;33:218–229. doi: 10.1002/(SICI)1098-2396(19990901)33:3<218::AID-SYN6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- Ganzel B, Casey BJ, Glover G, Voss HU, Temple E. The aftermath of 9/11: effect of intensity and recency of trauma on outcome. Emotion. 2007;7:227–238. doi: 10.1037/1528-3542.7.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2012 doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, McEwen BS. Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb Cortex. 2011;21:2056–2064. doi: 10.1093/cercor/bhq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Kumar SA, Filipski SB, Iverson M, Stuhr KL, Keith JM, Cravatt BF, Hillard CJ, Chattarji S, McEwen BS. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Carnevale NT. Passive normalization of synaptic integration influenced by dendritic architecture. J Neurophysiol. 1999;82:3268–3285. doi: 10.1152/jn.1999.82.6.3268. [DOI] [PubMed] [Google Scholar]
- Jaslove SW. The integrative properties of spiny distal dendrites. Neuroscience. 1992;47:495–519. doi: 10.1016/0306-4522(92)90161-t. [DOI] [PubMed] [Google Scholar]
- Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010;464:1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Eggleston T, Landman-Roberts L, Kenion CC, Driver GC, Meyerhoff JL. Habituation to repeated stress is stressor specific. Pharmacol Biochem Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Milner TA. Ultrastructure and synaptic associations of auditory thalamo-amygdala projections in the rat. Exp Brain Res. 1991;85:577–586. doi: 10.1007/BF00231742. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci. 2000;3:895–903. doi: 10.1038/78800. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neurons in the developing rat neocortex. J Physiol. 1997;500:409–440. doi: 10.1113/jphysiol.1997.sp022031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ, Coleman JR. Corticoamygdaloid and corticocortical projections of the rat temporal cortex: a Phaseolus vulgaris leucoagglutinin study. Neuroscience. 1993;57:697–715. doi: 10.1016/0306-4522(93)90016-9. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212:293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J Comp Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi BK, Xavier GF. Contextual, but not auditory, fear conditioning is disrupted by neurotoxic selective lesion of the basal nucleus of amygdala in rats. Neurobiol Learn Mem. 2010;93:165–174. doi: 10.1016/j.nlm.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo H, Reyes AD. Variation of input-output properties along the somatodendritic axis of pyramidal neurons. J Neurosci. 2005;25:4985–4995. doi: 10.1523/JNEUROSCI.0562-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The rat nervous system. xvii. 2004:1309. [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, Epstein J, Yang Y, Gorman J, LeDoux J, Silbersweig D, Stern E. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–473. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Rosenkranz JA, Morshedi MM, Sullivan EM, Meredith GE. Amphetamine-associated contextual learning is accompanied by structural and functional plasticity in the basolateral amygdala. J Neurosci. 2010;30:4676–4686. doi: 10.1523/JNEUROSCI.6165-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967;30:1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Smith TG, Nelson PG, Frank K. Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol. 1967;30:1169–1193. doi: 10.1152/jn.1967.30.5.1169. [DOI] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–6417. doi: 10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb Cortex. 1993;3:515–532. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Johnston D. State-dependent modulation of amygdala inputs by dopamine-induced enhancement of sodium currents in layer V entorhinal cortex. J Neurosci. 2007;27:7054–7069. doi: 10.1523/JNEUROSCI.1744-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdale hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders MJ, Stevens S, Boeh H. Stress enhancement of fear learning in mice is dependent upon stressor type: Effects of sex and ovarian hormones. Neurobiol Learn Mem. 2010;94:254–262. doi: 10.1016/j.nlm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- Savander V, Go CG, LeDoux JE, Pitkanen A. Intrinsic connections of the rat amygdaloid complex: projections originating in the basal nucleus. J Comp Neurol. 1995;361:345–368. doi: 10.1002/cne.903610211. [DOI] [PubMed] [Google Scholar]
- Savander V, Ledoux JE, Pitkanen A. Interamygdaloid projections of the basal and accessory basal nuclei of the rat amygdaloid complex. Neuroscience. 1997;76:725–735. doi: 10.1016/s0306-4522(96)00371-5. [DOI] [PubMed] [Google Scholar]
- Schaefer AT, Larkum ME, Sakmann B, Roth A. Coincidence detection in pyramidal neurons is tuned by their dendritic branching pattern. J Neurophysiol. 2003;89:3143–3154. doi: 10.1152/jn.00046.2003. [DOI] [PubMed] [Google Scholar]
- Segev I, Rall W. Excitable dendrites and spines: earlier theoretical insights elucidate recent direct observations. Trends Neurosci. 1998;21:453–460. doi: 10.1016/s0166-2236(98)01327-7. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Brayton RK, Miller JP, Segev I, Rinzel J, Rall W. Signal enhancement in distal cortical dendrites by means of interactions between active dendritic spines. Proc Natl Acad Sci U S A. 1985;82:2192–2195. doi: 10.1073/pnas.82.7.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, Macklin ML, Pitman RK. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- Shoji H, Mizoguchi K. Acute and repeated stress differentially regulates behavioral, endocrine, neural parameters relevant to emotional and stress response in young and aged rats. Behav Brain Res. 2010;211:169–177. doi: 10.1016/j.bbr.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Smith Y, Pare D. Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol. 1994;342:232–248. doi: 10.1002/cne.903420207. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker MP, Feldheim DA. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139:175–185. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Herkenham M. Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol. 1991;313:295–325. doi: 10.1002/cne.903130208. [DOI] [PubMed] [Google Scholar]
- Turner DA. Waveform and amplitude characteristics of evoked responses to dendritic stimulation of CA1 guinea-pig pyramidal cells. J Physiol. 1988;395:419–439. doi: 10.1113/jphysiol.1988.sp016927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Roth A, Hausser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Vlachos I, Herry C, Luthi A, Aertsen A, Kumar A. tContext-dependent encoding of fear and extinction memories in a large-scale network model of the basal amygdala. PLoS Comput Biol. 2011;7 doi: 10.1371/journal.pcbi.1001104. e1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Chattarji S. Modulation of different states of anxiety-like behavior by chronic stress. Behav Neurosci. 2004;118:1450–1454. doi: 10.1037/0735-7044.118.6.1450. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with longduration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Williams SR. Encoding and decoding of dendritic excitation during active states in pyramidal neurons. J Neurosci. 2005;25:5894–5902. doi: 10.1523/JNEUROSCI.0502-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Repeated restraint stress increases basolateral amygdala neuronal activity in an age-dependent manner. Neuroscience. 2012;226:459–474. doi: 10.1016/j.neuroscience.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol Learn Mem. 2011;95:199–205. doi: 10.1016/j.nlm.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]



